INTRODUCTION
This chapter makes treatment recommendations for primary IgAN. Secondary IgAN will not be discussed. The cost implications for global application of this guideline are addressed in Chapter 2.
- 10.1 Initial evaluation including assessment of risk of progressive kidney disease
- 10.1.1: Assess all patients with biopsy-proven IgAN for secondary causes of IgAN. (Not Graded)
- 10.1.2: Assess the risk of progression in all cases by evaluation of proteinuria, blood pressure, and eGFR at the time of diagnosis and during follow-up. (Not Graded)
- 10.1.3: Pathological features may be used to assess prognosis. (Not Graded)
BACKGROUND
IgAN is diagnosed by kidney biopsy and is defined as dominant or codominant staining with IgA in glomeruli by immunohistology.470 LN should be excluded. The intensity of IgA staining should be more than trace. The distribution of IgA staining should include presence in the mesangium, with or without capillary loop staining. IgG and IgM may be present, but not in greater intensity than IgA, except that IgM may be prominent in sclerotic areas. C3 may be present. The presence of C1q staining in more than trace intensity should prompt consideration of LN.
IgAN is the most common primary GN in the world. The prevalence rate varies across different geographical regions. Typically, it is 30–35% of all primary glomerular diseases in Asia, but can be up to 45%.471 In Europe, this is about 30–40%. Recently in the USA, IgAN was also reported to be the most common primary glomerulopathy in young adult Caucasians.472
Secondary IgAN is uncommon. Cirrhosis, celiac disease, and HIV infection are all associated with a high frequency of glomerular IgA deposition. IgAN has been infrequently associated with a variety of other diseases, including dermatitis herpetiformis, seronegative arthritis (particularly ankylosing spondylitis), small-cell carcinoma, lymphoma (Hodgkin lymphoma and T-cell lymphomas, including mycosis fungoides), disseminated tuberculosis, bronchiolitis obliterans, and inflammatory bowel disease (Crohn's disease and ulcerative colitis). These are usually clinically evident at the time of biopsy. Investigations can include viral serologies (HIV, HBV, and HCV), liver function tests, and electrophoreses of serum immunoglobulins.
IgAN has a wide spectrum of clinical presentations, varying from isolated hematuria to rapidly progressive GN. Thorough risk assessment is essential to determine management and ensure that the risks of therapy are balanced by the selection of patients at highest risk of progression. Definitive outcomes in IgAN are kidney survival and the rate of kidney function decline. Determinants of mortality in IgAN have not been addressed in previous studies, although it is reasonable to assume that CKD increases cardiovascular morbidity and mortality in these patients, as in others with CKD.473
RATIONALE
There is moderate-quality evidence to suggest that accelerated decline in kidney function is associated with proteinuria ≥1 g/d in a dose-dependent fashion and independently of other risk factors.474, 475, 476, 477
There is moderate-quality evidence to suggest a favourable outcome when time-averaged proteinuria is reduced to <1 g/d.477 Whether long-term outcome differs in adult patients with a proteinuria between 0.5 and 1.0 g/d compared to <0.5 g/d remains uncertain. In children, expert opinion suggests a goal of proteinuria <0.5 g/d per 1.73 m2.478
There is moderate-quality evidence to recommend strict blood pressure control, as it is associated with better kidney survival in chronic proteinuric nephropathies, including IgAN.
There is low-quality evidence to suggest GFR at presentation is associated with the risk of ESRD. However, studies that have assessed the rate of change of kidney function have questioned its association with initial GFR. Proteinuria, blood pressure, and kidney biopsy findings at presentation have been associated with both risk of ESRD and doubling of SCr.
There is low-quality evidence to suggest kidney biopsy findings associated with a worse prognosis are the presence and severity of mesangial and endocapillary proliferation, extensive crescents, focal and segmental as well as global glomerulosclerosis, tubular atrophy, and interstitial fibrosis.470, 479 However, no single approach to the objective evaluation of biopsy findings has yet been validated or evaluated prospectively.
There is moderate-quality evidence to suggest that IgAN that presents with hematuria and minimal proteinuria is a progressive disease, and that life-long follow-up with regular monitoring of blood pressure and proteinuria is recommended.480
Proteinuria is the strongest prognostic factor in IgAN and has a “dose-dependent” effect that is independent of other risk factors in multiple large observational studies, as well as prospective trials. The threshold above which the risk develops in adults is uncertain; some studies indicate 0.5 g/d481 while others could only demonstrate a higher risk of ESRD and a more rapid rate of decline in kidney function when time-averaged proteinuria was above 1 g/d.474, 477 A large observational study demonstrated that a reduction of proteinuria to <1 g/d carried the same favorable impact on long-term outcome, whether the initial value was 1–2 g/d, 2–3 g/d, or >3 g/d.477 Other surrogates of long-term outcome, such as a 50% decline in proteinuria, have been used.482 In children, observational studies have also consistently shown a relationship between the level of proteinuria and outcome, but did not assess a threshold value. Expert opinions in children advocate a cut-off of 0.5 g/d per 1.73 m2 for partial remission, and 0.16 g/d per 1.73 m2 for complete remission; these thresholds have been used in RCTs.478, 483
Uncontrolled hypertension during follow-up is associated with greater proteinuria and predicts a faster GFR decline.484, 485 As in other proteinuric chronic glomerulopathies, a blood pressure goal <130/80 mm Hg in patients with proteinuria >0.3 g/d, and <125/75 mm Hg when proteinuria is >1 g/d, is recommended.486, 487
The GFR at presentation has consistently been related to the risk of ESRD. Whether a lower GFR is also accompanied by a faster rate of kidney function decline is questionable; two observational studies have failed to show this relationship.475, 488 Proteinuria, blood pressure, and pathological features should take precedence over initial GFR in the estimation of the future rate of kidney function decline.
Numerous studies have addressed the predictive value of pathology findings. Mesangial489, 490 and endocapillary proliferation,479, 491 extensive crescents,492, 493, 494, 495 FSGS,496, 497 global glomerulosclerosis, tubular atrophy, and interstitial fibrosis479, 491, 493, 496 are associated with a more rapid rate of deterioration and lower kidney survival using univariate and, at times, multivariate analysis adjusting for clinical assessment. The recent Oxford Classification of IgAN has demonstrated the importance of (i) mesangial hypercellularity; (ii) segmental glomerulosclerosis; (iii) endocapillary hypercellularity; and (iv) tubular atrophy/interstitial fibrosis, as independent pathological variables predicting kidney outcome.479 This may become the standard, but requires validation before it can be recommended in routine clinical practice. Whether classification of the disease in this manner should impact treatment choice has also not been determined.
Obesity has been identified as an independent risk factor for the appearance of ESRD,498 and weight loss induces a significant decrease in proteinuria.499 Some observational studies500, 501 have reported an increased risk of greater proteinuria, more severe pathological lesions, and ESRD among IgAN patients who are overweight (BMI >25 kg/m2).
Other risk factors have also been studied. Outcomes do not differ between sexes.217 Children are less likely to reach ESRD compared to adults, but this may be because GFR is higher at presentation in children, even though there is a similar rate of kidney function decline. Different biopsy and treatment practices in the pediatric population limit comparisons to adults. Since the risk factors presented above have been validated in both children and adults, clinicians should consider these before the age of the patient. Similarly, it is uncertain whether geographical or ethnic variations in outcomes are secondary to different biopsy and treatment practices or variations in disease severity.475 Macroscopic hematuria is more frequent in children, and some studies have associated its presence with a favorable outcome, while others have shown this benefit to be confounded by a higher initial GFR and earlier detection with no independent value.502, 503
- 10.2 Antiproteinuric and antihypertensive therapy
- 10.2.1: We recommend long-term ACE-I or ARB treatment when proteinuria is >1 g/d, with up-titration of the drug depending on blood pressure. (1B)
- 10.2.2: We suggest ACE-I or ARB treatment if proteinuria is between 0.5 to 1 g/d (in children, between 0.5 to 1 g/d per 1.73 m2). (2D)
- 10.2.3: We suggest the ACE-I or ARB be titrated upwards as far as tolerated to achieve proteinuria <1 g/d. (2C)
- 10.2.4: In IgAN, use blood pressure treatment goals of <130/80 mm Hg in patients with proteinuria <1 g/d, and <125/75 mm Hg when initial proteinuria is >1 g/d (see Chapter 2). (Not Graded)
RATIONALE
Many of the trials using ACE-I/ARBs in IgAN recruited patients with proteinuria ≥1 g/d478, 504 while some recruited patients with proteinuria ≥0.5 g/d.505
In registry data,477 the rate of decline of function increased with the amount of proteinuria; those with sustained proteinuria ≥3 g/d lost kidney function 25-fold faster than those with proteinuria <1 g/d. Patients who presented with ≥3 g/d who achieved proteinuria <1 g/d had a similar course to patients who had <1 g/d throughout, and fared far better than patients who never achieved this level. There is, as yet, no evidence in IgAN that reducing proteinuria below 1 g/d in adults gives additional benefit.
Several RCTs478, 504, 505, 506 have shown that ACE-I and ARBs can reduce proteinuria and improve kidney function (assessed by reduction of the slope of GFR deterioration; Online Suppl Table 44). However, there is, as yet, no definitive study of sufficient duration to show the benefit of either ACE-I or ARBs in reducing the incidence of ESRD.
There are no data to suggest preference of ACE-I over ARBs, or vice versa, except in terms of a lesser side-effect profile with ARBs compared to ACE-I.
One study507 suggested the combination of ACE-I and ARBs induced a 73% greater reduction of proteinuria than monotherapy (ACE-I 38% and ARB 30%, respectively). A small study of seven pediatric IgAN patients also showed some benefits508 with a combination of ACE-I and ARB. However, more studies are needed to determine whether the definite benefit of combination therapy is effective, leading to a better kidney outcome.
RESEARCH RECOMMENDATION
RCTs are needed to compare the efficacy in proteinuric IgAN of combination therapy using ACE-I and ARBs to monotherapy using either alone.
- 10.3 Corticosteroids
- 10.3.1: We suggest that patients with persistent proteinuria ≥1 g/d, despite 3–6 months of optimized supportive care (including ACE-I or ARBs and blood pressure control), and GFR >50 ml/min per 1.73 m2, receive a 6-month course of corticosteroid therapy. (2C)
RATIONALE
There is low-quality evidence that corticosteroids provide an additional benefit to optimized supportive care (Online Suppl Table 47).
A 6-month corticosteroid regimen can follow either of two regimens, which have been used in published trials (see Table 26).
There is no evidence to suggest the use of corticosteroids in patients with GFR <50 ml/min.
The available studies do not allow recommendations for preferred dosage regimens. The studies did not report serious side-effects. However, there are other studies with similar regimens in non-IgAN patients that suggest more side-effects with high-dose pulse corticosteroids, including hypothalamic-pituitary-adrenal axis suppression and acute myopathies.
Table 26. Corticosteroid regimens in patients with IgAN.
| References | Pozzi C et al.509 | Manno C et al.510; Lv J et al.511 |
|---|---|---|
| Regimen | i.v. bolus injections of 1 g methylprednisolone for 3 days each at months 1, 3, and 5, followed by oral steroid 0.5 mg/kg prednisone on alternate days for 6 months | 6-month regime of oral prednisonea starting with 0.8–1 mg/kg/d for 2 months and then reduced by 0.2 mg/kg/d per month for the next 4 months |
IgAN, immunoglobulin A nephropathy.
Prednisone and prednisolone are equivalent and can be used interchangeably with the same dosing regimen.
Few RCTs so far have tested the efficacy of a corticosteroid regimen vs. no immunosuppressive therapy. In an Italian trial,509 a 6-month course of corticosteroids led to better clinical disease remission and long-term outcome512 than no steroids. However, only about 15% of the patients had received an ACE-I at randomization,509 and blood pressure control was not optimal by contemporary standards.513
Two more recent RCTs510, 511, 514 used oral prednisone added to an ACE-I and compared this to an ACE-I alone. In the Italian study,510 the mean annual GFR loss was reduced from about −6 ml/min to −0.6 ml/min, and in the Chinese study511 the proportion of patients with a 50% increase in SCr decreased from 24% to 3% with corticosteroid therapy. A major limitation of both studies is that all ACE-I and ARBs had to be halted for 1 month prior to study inclusion, and then an ACE-I was started together with corticosteroids in the combination group. Therefore, a number of low-risk patients may have been included, who would have achieved proteinuria <1 g/d with ACE-I therapy alone. A further potential confounder is that both studies included patients who had received prior immunosuppression. An American trial in adults and children, all of whom received an ACE-I, also noted reduced proteinuria with corticosteroids (60 mg/m2 prednisone every other day tapered to 30 mg/m2 at 12 months) but no difference in kidney function was observed at 2 years.515
A Japanese RCT that used low-dose corticosteroids (20 mg/d prednisolone, tapered to 5 mg/d by 2 years) observed no benefit on kidney function, despite reduced proteinuria with the corticosteroid regimen.516
Subjects with IgAN and GFR<50 ml/min were either excluded from these trials509, 514 or were few in number,511 so that currently, there are no data to assess the value of corticosteroids in this population.
A recent meta-analysis517 concluded that corticosteroids reduce doubling of SCr. However, in that analysis, 85% of the weight was contributed by two studies,509, 518 both of which lacked optimal antiproteinuric and antihypertensive therapy based on contemporary standards. Of note, an American RCT in children and adults with IgAN515 noted no difference in reaching the endpoint (>40% decrease in GFR) between a group receiving ACE-I only vs. ACE-I plus prednisone (60 mg/m2 per 48 hours for 3 months, reduced to 30 mg/m2 per 48 hours at month 12). However, few end-points were reached in this trial; thus, it was underpowered to detect small differences.
RESEARCH RECOMMENDATION
Studies using immunosuppressive agents should always include rigorous blood pressure control and antiproteinuric therapy. This is currently being tested in the STOP-IgAN trial.519 Newer immunosuppressives (alone or in combination) should be compared in RCTs to a “control” group receiving corticosteroids alone.
- 10.4 Immunosuppressive agents (cyclophosphamide, azathioprine, MMF, cyclosporine)
- 10.4.1: We suggest not treating with corticosteroids combined with cyclophosphamide or azathioprine in IgAN patients (unless there is crescentic IgAN with rapidly deteriorating kidney function; see Recommendation 10.6.3). (2D)
- 10.4.2: We suggest not using immunosuppressive therapy in patients with GFR <30 ml/min per 1.73 m2 unless there is crescentic IgAN with rapidly deteriorating kidney function (see Section 10.6). (2C)
- 10.4.3: We suggest not using MMF in IgAN. (2C)
RATIONALE
Please consult Online Suppl Tables 51–60
There is very low–quality evidence from a single RCT in high-risk adults to use a combination of prednisolone (40 mg/d reduced to 10 mg/d by 2 years) and cyclophosphamide (1.5 mg/kg/d) for 3 months, followed by azathioprine (1.5 mg/kg/d) for a minimum of 2 years. This study showed a better kidney survival over controls in a highly selected group of patients.
There is insufficient evidence that immunosuppressive agents other than steroids used as first-line therapy offer an advantage or equivalence compared to steroids.
The risk-benefit assessment is strongly impacted by the potential for severe adverse effects of these drugs.
Despite retrospective studies in IgAN supporting the use of immunosuppressive therapy other than corticosteroids, few RCTs have demonstrated a benefit. An RCT using corticosteroids combined with cyclophosphamide, followed by azathioprine, included a highly selected group of patients with SCr >1.47–2.83 mg/dl (>130–250 μmol/l) with a 15% increase within the last year, and initial proteinuria 3.9±0.8 and 4.6±0.4 g/d in the treatment and control groups, respectively. The active treatment group achieved lower proteinuria, a 4-fold lower rate of kidney function decline, and a much greater kidney survival (72% 5-year survival compared to 6% in controls, P=0.006). There are limitations in the applicability of the findings: (i) there was no steroid monotherapy arm; (ii) the use of RAS blockade was not detailed but these agents could not be initiated after the start of the trial; (iii) the follow-up blood pressure was higher than recommended by current guidelines.
Two RCTs compared cyclophosphamide, dipyridamole, and warfarin to controls and found no benefit.520, 521 Given these results and the potential side-effects, we do not suggest the use of cyclophosphamide monotherapy.
Azathioprine
Two RCTs, one in children and another in children and adults, tested azathioprine and corticosteroids in patients with preserved kidney function. They demonstrated a reduction in chronic lesions compared to controls on repeat biopsy.483, 522 Monotherapy with steroids has been shown to preserve kidney function (a plausible surrogate for reduced chronic lesions). In a recent trial in patients with IgAN,523 adding low-dose azathioprine for 6 months did not increase the benefit of corticosteroids alone, but did increase the occurrence of adverse events.
A study in 80 children with newly diagnosed IgAN524 compared the effects of the combination of prednisolone, azathioprine, warfarin, and dipyridamole with those of prednisolone alone. There was complete remission of proteinuria (<0.1 g/m2/d) in 36 (92.3%) of the 39 patients who received the combination and 29 (74.4%) of the 39 who received prednisolone alone (P=0.007). Some side-effects were observed including leucopenia, glaucoma, and aseptic necrosis. The percentage of sclerosed glomeruli was unchanged in the patients who received the combination, but increased in the prednisolone group. In summary of these studies, we do not suggest the addition of azathioprine to corticosteroids for the treatment of IgAN.
An RCT compared 6 months of treatment with corticosteroids plus azathioprine or corticosteroids alone in 207 IgAN patients with plasma creatinine ≤2.0 mg/dl (≤177 μmol/l) and proteinuria ≥1 g/d. After a median follow-up of 4.9 years, a 50% increase in plasma creatinine from baseline occurred in 13% of the combination group and 11% of the monotherapy group (P=0.83); effects on proteinuria and 5-year cumulative kidney survival were also similar in both groups (88% vs. 89% P=0.83). Treatment-related adverse events were more frequent in the combination group (17%) as compared to the monotherapy group (6% P=0.01). Thus, in this study, 6 months of treatment with azathioprine did not increase the benefit obtained from steroids alone, but increased the occurrence of adverse events.523
MMF
The findings from RCTs studying MMF in IgAN are variable. A Belgian study525 assessed MMF 2 g/d for 3 years vs. placebo in 34 patients with an average initial inulin clearance of 70 ml/min per 1.73 m2 and proteinuria of 1.8 g/d. No difference in proteinuria reduction or preservation of GFR was observed. Similarly, a North American study482 found no benefits over 24 months using a 1-year regimen of MMF 2 g/d vs. placebo in 32 patients with an initial GFR of 40 ml/min per 1.73 m2 and a proteinuria of 2.7 g/d. In contrast, a Chinese study in 40 patients with a mean initial GFR of 72 ml/min per 1.73 m2 and mean proteinuria 1.8 g/d found a significant reduction in proteinuria at 18 months with MMF given for 6 months over controls.526 A 6-year follow-up of the same cohort demonstrated a kidney survival benefit.527 No steroid was given in these trials, and all patients received ACE-I. The results of these studies are too heterogeneous to suggest the use of MMF at the present time. The reasons for heterogeneity of outcome require further investigation, but different ethnicity or differences in drug levels achieved may be contributory factors. Of note is a retrospective cohort study that suggested delayed severe pneumonia could occur in MMF-treated patients with IgAN.528 The potential side-effects of using MMF and the heterogeneity of outcomes from these data require better-performed studies before this drug can be recommended as first-line therapy.
Steroid Resistance
The approach to patients, who have no benefit in response to corticosteroids added to optimal antihypertensive and antiproteinuric therapy is unknown; no relevant RCTs have been conducted.
RESEARCH RECOMMENDATIONS
An RCT is needed comparing MMF and corticosteroids vs. corticosteroids alone in patients receiving optimal antihypertensive and antiproteinuric therapy.
An RCT is needed to investigate the different efficacy of MMF in Asians vs. Caucasians, including evaluation of drug and metabolite levels.
- 10.5 Other treatments
- 10.5.1: Fish oil treatment
- 10.5.1.1: We suggest using fish oil in the treatment of IgAN with persistent proteinuria ≥1 g/d, despite 3–6 months of optimized supportive care (including ACE-I or ARBs and blood pressure control). (2D)
BACKGROUND
Fish oil supplements have shown a number of beneficial cardiovascular effects, including systolic blood pressure and triglyceride lowering, reduced resting heart rate, improvement in several markers of endothelial damage, and reduction in the risk of sudden cardiac death in patients with established coronary heart disease. Several RCTs have evaluated the effect of fish oil in IgAN.
RATIONALE
Please consult Online Suppl Tables 61–64.
There is mostly low-quality evidence that suggests using fish oil supplements in patients with IgAN, but the RCTs evaluating this therapy have reported conflicting results. However, given the very low risk profile and the potentially beneficial cardiovascular effects, fish oil can be considered a very safe treatment.
In a trial that included 106 patients, fish oil treatment (12 g/d) improved kidney survival and retarded the rate of kidney function loss, without significant reduction of proteinuria.529 Of note, the outcome of the control group, treated with 12 g/d of olive oil was poor (cumulative incidence of death or ESRD after 4 years was 10% in fish oil–treated patients, 40% in the control group). Longer follow-up confirmed the beneficial influence of fish oil treatment in this study.530 Another RCT including 34 patients reported a beneficial influence of fish oil (3 g/d) on two end-points: the risk of ESRD, and ≥50% increase in SCr.531 In this study, fish oil reduced proteinuria significantly. In a short-term (6-month) RCT, a significant proteinuria reduction was observed in patients treated with a combination of ACE-I and ARB plus fish oil (3 g/d) in comparison to a control group that received only ACE-I and ARB (percentage of patients with ≥50% proteinuria reduction, 80% and 20%, respectively).532
In contradiction to these studies, other RCTs failed to detect a significant benefit of fish oil treatment.533, 534 A meta-analysis535 concluded that fish oils are not beneficial in IgAN, although another meta-analysis that combined clinical trials focused on IgAN, diabetes, lupus nephritis, and other glomerular diseases showed a greater proteinuria decrease in patients treated with fish oil, without changes in renal function.536 A more recent RCT compared steroids (33 patients), fish oil (4 g/d, 32 patients), and placebo (31 patients) for 2 years.515 Neither treatment group showed benefit over the placebo group. However, patients in the placebo group had a statistically significant lower degree of proteinuria at baseline.
In trying to explain these discordant results, some authors have proposed that the effects of fish oil in IgAN patients could be dosage-dependent.537 However, another prospective trial reported that high (6.7 g/d) and low (3.3 g/d) doses of fish oil were similar in slowing the rate of kidney function loss in high-risk IgAN patients.538 At present, there is no evidence to support the use of high-dose fish oil in IgAN.
We suggest fish oil (3.3 g/d) can be considered in the treatment of IgAN with persistent proteinuria ≥1 g/d, despite 3–6 months of optimized supportive care (including ACE-I or ARBs and blood pressure control).
RESEARCH RECOMMENDATION
An RCT is needed of fish oil in IgAN to examine preserved kidney function with persistent significant proteinuria, despite optimal antihypertensive and antiproteinuric therapy.
- 10.5.2: Antiplatelet agents
- 10.5.2.1: We suggest not using antiplatelet agents to treat IgAN. (2C)
RATIONALE
Please consult Online Suppl Table 65.
There is low-quality evidence to recommend not using antiplatelet therapy in IgAN.
A meta-analysis539 based on seven studies, most of them performed in Japan, concluded that antiplatelet therapy resulted in reduced proteinuria and protected kidney function in patients with moderate to severe IgAN. However, there were significant limitations of the evidence in this meta-analysis, due to suboptimal quality of individual controlled trials. Importantly, the effect of antiplatelet agents alone could not be discerned because patients received other concomitant therapies. Thus, in three studies, both treatment and control groups received other agents, including cytotoxics, steroids, antihypertensive agents, and anticoagulants. In three other studies, the intervention group received warfarin (two studies) and aspirin (one study) in addition to the antiplatelet agent (dipyridamole). Dipyridamole was the most commonly used antiplatelet agent (five studies) followed by trimetazidine and Dilazep (one study each).
RESEARCH RECOMMENDATION
A multicenter RCT is needed to address the role of antiplatelet therapy in IgAN.
- 10.5.3: Tonsillectomy
- 10.5.3.1: We suggest that tonsillectomy not be performed for IgAN. (2C)
RATIONALE
There is low-quality evidence to suggest not using tonsillectomy as treatment for IgAN. No RCT has been performed of tonsillectomy for IgAN.
Tonsillectomy may be indicated in those with IgAN for conventional reasons, e.g., recurrent bacterial tonsillitis. Clinical judgment needs to be exercised to decide whether to perform tonsillectomy in a very selected group of patients with a close relationship between paroxysm of gross hematuria and tonsillitis. However, only retrospective analyses540, 541 as well as one nonrandomized trial542 have reported a better outcome for IgAN after tonsillectomy. In these studies, tonsillectomy was often combined with other—in particular, immunosuppressive—treatment;540, 541, 542 thus, the specific value of tonsillectomy is not always apparent. Furthermore, in other retrospective series, investigators failed to note a benefit from tonsillectomy.543
RESEARCH RECOMMENDATION
A multicenter RCT is needed to address the role of tonsillectomy in IgAN.
- 10.6 Atypical forms of IgAN
- 10.6.1: MCD with mesangial IgA deposits
- 10.6.1.1: We recommend treatment as for MCD (see Chapter 5) in nephrotic patients showing pathological findings of MCD with mesangial IgA deposits on kidney biopsy. (2B)
BACKGROUND
Patients with IgAN can present with proteinuria within the nephrotic range (>3.5 g/d), and it portends a poor prognosis if this high-grade proteinuria persists during follow-up. However, the typical accompanying findings of complete nephrotic syndrome (edema, hypoalbuminemia, hyperlipidemia) are uncommon. Rarely, some patients with nephrotic syndrome have been identified in whom kidney biopsy shows minimal glomerular changes by light microscopy, diffuse podocyte foot process effacement on electron microscopy, and predominant mesangial deposits of IgA on immunofluorescence. A coincidence of two different glomerular diseases (minimal-change nephrotic syndrome and IgAN) has been proposed as the most likely explanation for such cases.
RATIONALE
There is low-quality evidence to recommend that patients with nephrotic syndrome and coincidental histological findings of MCD and IgAN should be treated like patients with MCD.
Several series544, 545 have described prompt, complete remissions after corticosteroid therapy in a majority of patients with nephrotic syndrome and a pathological diagnosis of coincidental MCD and IgAN. This initial treatment response and the following clinical course, with a frequent appearance of nephrotic syndrome relapses, are very reminiscent of that of patients with pure MCD. An RCT in IgAN patients with nephrotic proteinuria546 also showed a high percentage of complete remission in patients with such characteristics.
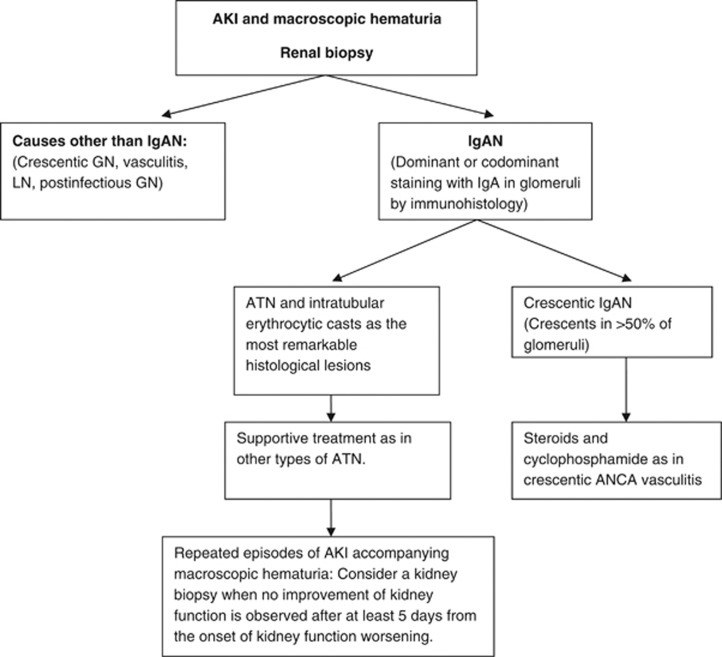
- 10.6.2: AKI associated with macroscopic hematuria
- 10.6.2.1: Perform a repeat kidney biopsy in IgAN patients with AKI associated with macroscopic hematuria if, after 5 days from the onset of kidney function worsening, there is no improvement. (Not Graded)
- 10.6.2.2: We suggest general supportive care for AKI in IgAN, with a kidney biopsy performed during an episode of macroscopic hematuria showing only ATN and intratubular erythrocyte casts. (2C)
BACKGROUND
Episodic macroscopic hematuria coinciding with mucosal (usually upper respiratory) infections are typical of IgAN. The macroscopic hematuria usually resolves spontaneously in a few days, but in some cases it can persist for several weeks.547 The development of AKI during macroscopic hematuria episodes is uncommon547, 548 but represents the first manifestation of IgAN in some patients.
RATIONALE
ATN and intratubular erythrocyte casts are the most common pathological findings in kidney biopsies during AKI accompanying macroscopic hematuria episodes in IgAN.
Kidney function usually, but not always, recovers completely after the disappearance of macroscopic hematuria.
Kidney biopsy allows differentiation of tubular damage and tubular occlusion by erythrocyte casts from crescentic IgAN or other coincidental causes of AKI.
Kidney biopsies performed during an episode of macroscopic hematuria typically show mesangial proliferation and occasional segmental crescents.549 In those cases with AKI coincidental with gross hematuria, the glomerular changes are usually insufficient to account for the AKI. Hematuria by itself may be responsible for the AKI, through tubular injury that is induced by intratubular erythrocytic casts and a possible nephrotoxic effect of the hemoglobin that is released from these casts. Features of ATN and tubules filled by red blood cells are the most relevant histological findings. In a majority of patients, kidney function returns to baseline after the disappearance of macroscopic hematuria,547, 548, 549 but incomplete recovery of kidney function has been described in up to 25% of affected patients.547 Duration of macroscopic hematuria longer than 10 days is the most significant risk factor for persistent kidney impairment.547 Continuous supportive care, as in other types of ATN, is the recommended therapeutic approach to these patients. There is no information about the usefulness of corticosteroids in patients with more severe forms of AKI or longer duration of macroscopic hematuria.
However, some patients with AKI and macroscopic hematuria exhibit a crescentic form of IgAN (crescents affecting >50% of glomeruli), whose prognosis is considerably worse.492, 493, 494, 495 A repeat kidney biopsy is suggested in patients with known IgAN who present a protracted AKI accompanying a new episode of gross hematuria, in order to differentiate ATN from crescentic IgAN or other types of AKI (Algorithm 1).
- 10.6.3: Crescentic IgAN
- 10.6.3.1: Define crescentic IgAN as IgAN with crescents in more than 50% of glomeruli in the renal biopsy with rapidly progressive renal deterioration. (Not Graded)
- 10.6.3.2: We suggest the use of steroids and cyclophosphamide in patients with IgAN and rapidly progressive crescentic IgAN, analogous to the treatment of ANCA vasculitis (see Chapter 13). (2D)
Algorithm 1.
Management algorithm of patients with AKI associated with macroscopic hematuria.
BACKGROUND
Crescentic IgAN has a poor prognosis. In a historical group of 12 untreated crescentic IgAN patients, about 42% reached ESRD in 36 months.495 Another Japanese study showed that patients with >50% crescents developed ESRD in 75% of patients by 10 years follow-up.550 In this study, the patients were divided into four groups: group 1, absence of crescents and fibrous adhesion of glomerular tufts to Bowman's capsule; group 2, less than 25% group 3, 25-50% group 4, more than 50%. Ten-year renal survival rates were 100% in group 1, 94.3% in group 2, 81.8% in group 3, and 25.5% in group 4, respectively, indicating that patients with >50% crescents in glomeruli had a much worse survival than those with ≤50% glomerular crescents.
The outcome of IgAN with diffuse crescent formation has also been studied in 25 Chinese IgAN patients.551 Most of them showed rapidly progressive GN associated with more severe pathological changes, including glomerular, tubular interstitial, and vascular lesions, than in patients with general IgAN. The infiltrates in glomeruli may contribute to the crescentic formation. Diffuse crescent formation was defined by 50% or more of the glomeruli affected.551 The pathological diagnosis of crescentic IgAN has not been unified among these studies. While some use crescents involving over 50% of glomeruli as the definition,551 others use the presence of incipient to fulminant cellular crescents, with or without segmental endocapillary proliferation in >10% of glomeruli.495 Although there is insufficient evidence for a unifying definition, we suggest a definition of crescentic IgAN as both a pathological finding of over 50% glomeruli having crescents and the clinical feature of rapidly progressive deterioration of renal function.
A recent study of 67 patients552 with vasculitic IgAN (33 HSP, 34 IgAN) showed that three factors significantly affected kidney outcome: kidney function, blood pressure at presentation, and the amount of chronic damage in the biopsy.
RATIONALE
There is no RCT of treatment in crescentic IgAN.
The three largest observational studies495, 551, 552 all concluded that immunosuppression is potentially useful. In a study of 25 patients with diffuse crescentic IgAN treated with immunosuppression, 67% of patients maintained sufficient kidney function to avoid renal replacement therapy, four had SCr <1.4 mg/dl (<124 μmol/l), and only five were dialysis-dependent. In another study, although an improved outcome was seen in those receiving immunosuppression, the conclusions were cautious, as the treated and untreated groups were not comparable. The third study also suggested positive effects of immunosupression.495 This study used i.v. methylprednisolone 15 mg/kg/d for 3 days and monthly i.v. cyclophosphamide 0.5 g/m2 for 6 months. Twelve treated patients were compared to 12 historical controls. After 36 months, the rate of ESRD in the treated group was lower (one out of 12) than in the historical controls (five out of 12).
Recommended therapeutic regimens in these reports are varied, but initial therapy has usually included high-dose oral or i.v. corticosteroids plus oral or i.v. cyclophosphamide. In one study, some patients were changed from cyclophosphamide to azathioprine at 3 months. Durations of treatment in these three series varied from 3 to 24 months.
There is only poor-quality evidence to support the use of plasma exchange. One anecdotal report indicated benefit in five patients using plasma exchange in a combination of immunosuppressive therapies.553
RESEARCH RECOMMENDATION
RCTs are needed to investigate the benefits of cyclophosphamide, MMF, and azathioprine in crescentic IgAN.
DISCLAIMER
While every effort is made by the publishers, editorial board, and ISN to see that no inaccurate or misleading data, opinion or statement appears in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor, copyright holder, or advertiser concerned. Accordingly, the publishers and the ISN, the editorial board and their respective employers, office and agents accept no liability whatsoever for the consequences of any such inaccurate or misleading data, opinion or statement. While every effort is made to ensure that drug doses and other quantities are presented accurately, readers are advised that new methods and techniques involving drug usage, and described within this Journal, should only be followed in conjunction with the drug manufacturer's own published literature.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Table 44: Evidence profile of RCTs examining ACE-I or ARB in biopsy-proven IgA nephropathy.
Supplementary Table 45: Summary table of RCTs examining ACE-I or ARB in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 46: Summary table of RCTs examining ACE-I or ARB in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 47: Evidence profile of RCTs examining steroid regimens in biopsy-proven IgA nephropathy.
Supplementary Table 48: Meta-analyses and systematic reviews on immunosuppression for IgA nephropathy.
Supplementary Table 49: Summary table of RCTs examining steroid regimens in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 50: Summary table of RCTs examining steroid regimens in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 51: Meta-analyses and systematic reviews on immunosuppression for IgA nephropathy.
Supplementary Table 52: Summary table of RCTs examining steroid and immunosuppressive regimens in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 53: Summary table of RCTs examining steroid and immunosuppressive regimens in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 54: Evidence profile of RCTs examining AZA in combination vs: AZA alone in biopsy-proven IgA nephropathy.
Supplementary Table 55: Summary table of RCT examining AZA in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 56: Summary table of RCT examining AZA in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 57: Evidence profile of RCTs examining MMF in biopsy-proven IgA nephropathy.
Supplementary Table 58: Meta-analyses and systematic reviews on MMF therapy for IgA nephropathy.
Supplementary Table 59: Summary Table of RCTs examining MMF in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 60: Summary Table of RCTs examining MMF in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 61: Evidence profile of studies examining omega-3 fatty acid treatment in IgA nephropathy.
Supplementary Table 62: Meta-analyses and systematic reviews on fish oil treatment in IgA nephropathy.
Supplementary Table 63: Summary table of RCTs examining omega-3 fatty acids in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 64: Summary table of RCTs examining omega-3 fatty acids in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 65: Meta-analyses and systematic reviews on antiplatelet therapy for IgA nephropathy.
Supplementary Table 66: Summary table of RCT examining immunosuppression and anti-platelets in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 67: Summary table of RCT examining immunosuppression and anti-platelets in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary Table 68: Summary table of RCT examining antiplatelet treatments in biopsy-proven IgA nephropathy (continuous outcomes)*.
Supplementary Table 69: Summary table of RCTs examining miscellaneous treatments in biopsy-proven IgA nephropathy (categorical outcomes).
Supplementary Table 70: Summary table of RCTs examining miscellaneous treatments in biopsy-proven IgA nephropathy (continuous outcomes).
Supplementary material is linked to the online version of the paper at http://www.kdigo.org/clinical_practice_guidelines/GN.php
Supplementary Material
References
- Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- Li PK, Ho KK, Szeto CC, et al. Prognostic indicators of IgA nephropathy in the Chinese--clinical and pathological perspectives. Nephrol Dial Transplant. 2002;17:64–69. doi: 10.1093/ndt/17.1.64. [DOI] [PubMed] [Google Scholar]
- Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA. Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Bergstralh EJ, Grande JP, et al. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant. 2002;17:1197–1203. doi: 10.1093/ndt/17.7.1197. [DOI] [PubMed] [Google Scholar]
- Geddes CC, Rauta V, Gronhagen-Riska C, et al. A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541–1548. doi: 10.1093/ndt/gfg207. [DOI] [PubMed] [Google Scholar]
- Goto M, Wakai K, Kawamura T, et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant. 2009;24:3068–3074. doi: 10.1093/ndt/gfp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich HN, Troyanov S, Scholey JW, et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- Coppo R, Peruzzi L, Amore A, et al. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18:1880–1888. doi: 10.1681/ASN.2006040347. [DOI] [PubMed] [Google Scholar]
- Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- Szeto CC, Lai FM, To KF, et al. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med. 2001;110:434–437. doi: 10.1016/s0002-9343(01)00659-3. [DOI] [PubMed] [Google Scholar]
- Coppo R, D'Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18:503–512. [PubMed] [Google Scholar]
- Frisch G, Lin J, Rosenstock J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–2145. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- Harmankaya O, Ozturk Y, Basturk T, et al. Efficacy of immunosuppressive therapy in IgA nephropathy presenting with isolated hematuria. Int Urol Nephrol. 2002;33:167–171. doi: 10.1023/a:1014424723466. [DOI] [PubMed] [Google Scholar]
- Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–735. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- Kanno Y, Okada H, Saruta T, et al. Blood pressure reduction associated with preservation of renal function in hypertensive patients with IgA nephropathy: a 3-year follow-up. Clin Nephrol. 2000;54:360–365. [PubMed] [Google Scholar]
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49:12–26. doi: 10.1053/j.ajkd.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Fellin G, Gentile MG, Duca G, et al. Renal function in IgA nephropathy with established renal failure. Nephrol Dial Transplant. 1988;3:17–23. [PubMed] [Google Scholar]
- Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int. 1991;40:1050–1054. doi: 10.1038/ki.1991.313. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Minetti L, Ponticelli C, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;59:363–378. [PubMed] [Google Scholar]
- Boyce NW, Holdsworth SR, Thomson NM, et al. Clinicopathological associations in mesangial IgA nephropathy. Am J Nephrol. 1986;6:246–252. doi: 10.1159/000167171. [DOI] [PubMed] [Google Scholar]
- Freese P, Norden G, Nyberg G. Morphologic high-risk factors in IgA nephropathy. Nephron. 1998;79:420–425. doi: 10.1159/000045087. [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Silva FG, Wyatt RJ, et al. Prognostic indicators in children with IgA nephropathy—report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol. 1994;8:15–20. doi: 10.1007/BF00868251. [DOI] [PubMed] [Google Scholar]
- Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant. 2003;18:1321–1329. doi: 10.1093/ndt/gfg081. [DOI] [PubMed] [Google Scholar]
- Katafuchi R, Oh Y, Hori K, et al. An important role of glomerular segmental lesions on progression of IgA nephropathy: a multivariate analysis. Clin Nephrol. 1994;41:191–198. [PubMed] [Google Scholar]
- Packham DK, Yan HD, Hewitson TD, et al. The significance of focal and segmental hyalinosis and sclerosis (FSHS) and nephrotic range proteinuria in IgA nephropathy. Clin Nephrol. 1996;46:225–229. [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Deprele C, Sassolas A, et al. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37:720–727. doi: 10.1016/s0272-6386(01)80120-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tsujii T, Komiya T, et al. Clinicopathological influence of obesity in IgA nephropathy: comparative study of 74 patients. Contrib Nephrol. 2007;157:90–93. doi: 10.1159/000102309. [DOI] [PubMed] [Google Scholar]
- Coppo R, Troyanov S, Camilla R, et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–927. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- Rauta V, Finne P, Fagerudd J, et al. Factors associated with progression of IgA nephropathy are related to renal function—a model for estimating risk of progression in mild disease. Clin Nephrol. 2002;58:85–94. doi: 10.5414/cnp58085. [DOI] [PubMed] [Google Scholar]
- Li PK, Leung CB, Chow KM, et al. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Praga M, Gutierrez E, Gonzalez E, et al. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol. 2003;14:1578–1583. doi: 10.1097/01.asn.0000068460.37369.dc. [DOI] [PubMed] [Google Scholar]
- Horita Y, Tadokoro M, Taura K, et al. Prednisolone co-administered with losartan confers renoprotection in patients with IgA nephropathy. Ren Fail. 2007;29:441–446. doi: 10.1080/08860220701260511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D, Pisani A, Balletta MM, et al. Additive antiproteinuric effect of converting enzyme inhibitor and losartan in normotensive patients with IgA nephropathy. Am J Kidney Dis. 1999;33:851–856. doi: 10.1016/s0272-6386(99)70416-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ohta K, Shimizu M, et al. Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol. 2005;64:35–40. doi: 10.5414/cnp64035. [DOI] [PubMed] [Google Scholar]
- Pozzi C, Bolasco PG, Fogazzi GB, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353:883–887. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- Manno C, Torres DD, Rossini M, et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694–3701. doi: 10.1093/ndt/gfp356. [DOI] [PubMed] [Google Scholar]
- Lv J, Zhang H, Chen Y, et al. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2009;53:26–32. doi: 10.1053/j.ajkd.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- Pozzi C, Locatelli F. Corticosteroids in IgA nephropathy (letter) Lancet. 1999;353:2159–2160. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- Manno C, Gesualdo L, D'Altri C, et al. Prospective randomized controlled multicenter trial on steroids plus ramipril in proteinuric IgA nephropathy. J Nephrol. 2001;14:248–252. [PubMed] [Google Scholar]
- Hogg RJ, Lee J, Nardelli N, et al. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1:467–474. doi: 10.2215/CJN.01020905. [DOI] [PubMed] [Google Scholar]
- Katafuchi R, Ikeda K, Mizumasa T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis. 2003;41:972–983. doi: 10.1016/s0272-6386(03)00194-x. [DOI] [PubMed] [Google Scholar]
- Strippoli GF, Maione A, Schena FP, et al. IgA nephropathy: a disease in search of a large-scale clinical trial to reliably inform practice. Am J Kidney Dis. 2009;53:5–8. doi: 10.1053/j.ajkd.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hiki Y, Kokubo T, et al. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–242. doi: 10.1159/000188848. [DOI] [PubMed] [Google Scholar]
- Eitner F, Ackermann D, Hilgers RD, et al. Supportive Versus Immunosuppressive Therapy of Progressive IgA nephropathy (STOP) IgAN trial: rationale and study protocol. J Nephrol. 2008;21:284–289. [PubMed] [Google Scholar]
- Walker RG, Yu SH, Owen JE, et al. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: a two-year prospective trial. Clin Nephrol. 1990;34:103–107. [PubMed] [Google Scholar]
- Woo KT, Lee GS. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin. Clin Nephrol. 1991;35:184. [PubMed] [Google Scholar]
- Yoshikawa N, Ito H, Sakai T, et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol. 1999;10:101–109. doi: 10.1681/ASN.V101101. [DOI] [PubMed] [Google Scholar]
- Pozzi C, Andrulli S, Pani A, et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol. 2010;21:1783–1790. doi: 10.1681/ASN.2010010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa N, Honda M, Iijima K, et al. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1:511–517. doi: 10.2215/CJN.01120905. [DOI] [PubMed] [Google Scholar]
- Maes BD, Oyen R, Claes K, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004;65:1842–1849. doi: 10.1111/j.1523-1755.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- Tang S, Leung JC, Chan LY, et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–812. doi: 10.1111/j.1523-1755.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- Tang SC, Tang AW, Wong SS, et al. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 2010;77:543–549. doi: 10.1038/ki.2009.499. [DOI] [PubMed] [Google Scholar]
- Lv J, Zhang H, Cui Z, et al. Delayed severe pneumonia in mycophenolate mofetil-treated patients with IgA nephropathy. Nephrol Dial Transplant. 2008;23:2868–2872. doi: 10.1093/ndt/gfn161. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Bergstralh EJ, Offord KP, et al. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Grande JP, Bergstralh EJ, et al. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10:1772–1777. doi: 10.1681/ASN.V1081772. [DOI] [PubMed] [Google Scholar]
- Alexopoulos E, Stangou M, Pantzaki A, et al. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a ″very low dose″ regimen. Ren Fail. 2004;26:453–459. doi: 10.1081/jdi-200026763. [DOI] [PubMed] [Google Scholar]
- Ferraro PM, Ferraccioli GF, Gambaro G, et al. Combined treatment with renin-angiotensin system blockers and polyunsaturated fatty acids in proteinuric IgA nephropathy: a randomized controlled trial. Nephrol Dial Transplant. 2009;24:156–160. doi: 10.1093/ndt/gfn454. [DOI] [PubMed] [Google Scholar]
- Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentanoic acid (EPA): a two-year prospective trial. Clin Nephrol. 1989;31:128–131. [PubMed] [Google Scholar]
- Pettersson EE, Rekola S, Berglund L, et al. Treatment of IgA nephropathy with omega-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol. 1994;41:183–190. [PubMed] [Google Scholar]
- Strippoli GF, Manno C, Schena FP. An “evidence-based” survey of therapeutic options for IgA nephropathy: assessment and criticism. Am J Kidney Dis. 2003;41:1129–1139. doi: 10.1016/s0272-6386(03)00344-5. [DOI] [PubMed] [Google Scholar]
- Miller ER, III, Juraschek SP, Appel LJ, et al. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: meta-analysis of clinical trials. Am J Clin Nutr. 2009;89:1937–1945. doi: 10.3945/ajcn.2008.26867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RJ, Fitzgibbons L, Atkins C, et al. Efficacy of omega-3 fatty acids in children and adults with IgA nephropathy is dosage- and size-dependent. Clin J Am Soc Nephrol. 2006;1:1167–1172. doi: 10.2215/CJN.02300606. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr, Larson TS, Bergstralh EJ, et al. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001;12:791–799. doi: 10.1681/ASN.V124791. [DOI] [PubMed] [Google Scholar]
- Taji Y, Kuwahara T, Shikata S, et al. Meta-analysis of antiplatelet therapy for IgA nephropathy. Clin Exp Nephrol. 2006;10:268–273. doi: 10.1007/s10157-006-0433-8. [DOI] [PubMed] [Google Scholar]
- Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- Xie Y, Nishi S, Ueno M, et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63:1861–1867. doi: 10.1046/j.1523-1755.2003.00935.x. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Fujimoto S, Hara S, et al. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: a controlled study. Clin J Am Soc Nephrol. 2008;3:1301–1307. doi: 10.2215/CJN.00310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche FM, Schwarz A, Keller F. Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol. 1999;51:147–152. [PubMed] [Google Scholar]
- Kim SM, Moon KC, Oh KH, et al. Clinicopathologic characteristics of IgA nephropathy with steroid-responsive nephrotic syndrome. J Korean Med Sci. 2009;24 (Suppl:S44–S49. doi: 10.3346/jkms.2009.24.S1.S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KN, Lai FM, Chan KW, et al. An overlapping syndrome of IgA nephropathy and lipoid nephrosis. Am J Clin Pathol. 1986;86:716–723. doi: 10.1093/ajcp/86.6.716. [DOI] [PubMed] [Google Scholar]
- Lai KN, Lai FM, Ho CP, et al. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long-term controlled trial. Clin Nephrol. 1986;26:174–180. [PubMed] [Google Scholar]
- Gutierrez E, Gonzalez E, Hernandez E, et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007;2:51–57. doi: 10.2215/CJN.02670706. [DOI] [PubMed] [Google Scholar]
- Praga M, Gutierrez-Millet V, Navas JJ, et al. Acute worsening of renal function during episodes of macroscopic hematuria in IgA nephropathy. Kidney Int. 1985;28:69–74. doi: 10.1038/ki.1985.120. [DOI] [PubMed] [Google Scholar]
- Bennett WM, Kincaid-Smith P. Macroscopic hematuria in mesangial IgA nephropathy: correlation with glomerular crescents and renal dysfunction. Kidney Int. 1983;23:393–400. doi: 10.1038/ki.1983.32. [DOI] [PubMed] [Google Scholar]
- Abe T, Kida H, Yoshimura M, et al. Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol. 1986;25:37–41. [PubMed] [Google Scholar]
- Tang Z, Wu Y, Wang QW, et al. Idiopathic IgA nephropathy with diffuse crescent formation. Am J Nephrol. 2002;22:480–486. doi: 10.1159/000065281. [DOI] [PubMed] [Google Scholar]
- Pankhurst T, Lepenies J, Nightingale P, et al. Vasculitic IgA nephropathy: prognosis and outcome. Nephron Clin Pract. 2009;112:c16–c24. doi: 10.1159/000210570. [DOI] [PubMed] [Google Scholar]
- Coppo R, Basolo B, Roccatello D, et al. Plasma exchange in progressive primary IgA nephropathy. Int J Artif Organs. 1985;8 (Suppl 2:55–58. [PubMed] [Google Scholar]