Chapter 3.1: Hemodynamic monitoring and support for prevention and management of AKI
As discussed in Chapters 2.3 and Appendix D, patients with AKI and at increased risk for AKI require careful attention to be paid to their hemodynamic status. This is first because hypotension results in decreased renal perfusion and, if severe or sustained, may result in kidney injury. Second, the injured kidney loses autoregulation of blood flow, a mechanism that maintains relatively constant flow despite changes in pressure above a certain point (roughly, a mean of 65 mm Hg).
Management of blood pressure and cardiac output require careful titration of fluids and vasoactive medication. Vasopressors can further reduce blood flow to the tissues if there is insufficient circulating blood volume. Conversely, patients with AKI are also at increased risk for fluid overload (see Chapter 3.2) and continued fluid resuscitation despite increased intravascular volume can cause harm. Fluids and vasoactive medications should be managed carefully and in concert with hemodynamic monitoring. Hemodynamic evaluation and monitoring are discussed in Appendix D.
In this chapter therapies aimed at correcting hemodynamic instability will be discussed. Available therapies to manage hypotension include fluids, vasopressors and protocols which integrate these therapies with hemodynamic goals. There is an extensive body of literature in this field and for a broader as well as more in depth review the reader is directed to the various reviews and textbooks devoted to critical care and nephrology.70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81
FLUIDS
3.1.1: In the absence of hemorrhagic shock, we suggest using isotonic crystalloids rather than colloids (albumin or starches) as initial management for expansion of intravascular volume in patients at risk for AKI or with AKI. (2B)
RATIONALE
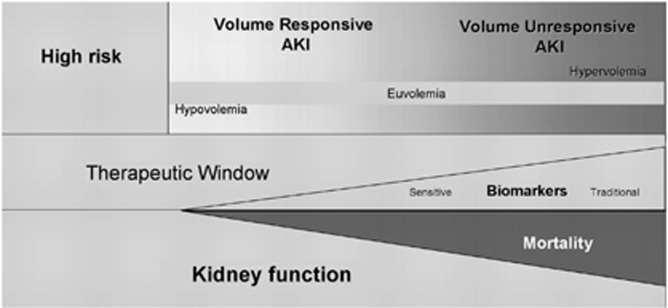
Despite the recognition of volume depletion as an important risk factor for AKI, there are no randomized controlled trials (RCTs) that have directly evaluated the role of fluids vs. placebo in the prevention of AKI, except in the field of contrast-induced acute kidney injury (CI-AKI) (see Chapter 4.4). It is accepted that optimization of the hemodynamic status and correction of any volume deficit will have a salutary effect on kidney function, will help minimize further extension of the kidney injury, and will potentially facilitate recovery from AKI with minimization of any residual functional impairment. AKI is characterized by a continuum of volume responsiveness through unresponsiveness (Figure 8),78, 82 and large multicenter studies have shown that a positive fluid balance is an important factor associated with increased 60-day mortality.78, 83, 84
Figure 8.
Conceptual model for development and clinical course of AKI. The concept of AKI includes both volume-responsive and volume-unresponsive conditions. These conditions are not mutually exclusive, and a given patient may progress from one to the other. Time runs along the x-axis, and the figure depicts a closing “therapeutic window” as injury evolves and kidney function worsens. Biomarkers of injury and function will begin to manifest as the condition worsens, but traditional markers of function (e.g., urea nitrogen and creatinine) will lag behind hypothetical “sensitive” markers of kidney injury. Mortality increases as kidney function declines. AKI, acute kidney injury. Reproduced from Himmelfarb J, Joannidis M, Molitoris B, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol 2008; 3: 962–967 with permission from American Society of Nephrology82 conveyed through Copyright Clearance Center, Inc; accessed http://cjasn.asnjournals.org/content/3/4/962.long
The amount and selection of the type of fluid that should be used in the resuscitation of critically ill patients is still controversial. This guideline focuses on the selection of the fluid (colloid vs. crystalloid fluid in the prevention and early management of AKI). The three main end-points of the studies explored were the effects on mortality, need for RRT, and—if possible—the incidence of AKI. Although many trials have been conducted to compare fluid types for resuscitation, studies without AKI outcomes were not systematically reviewed for this Guideline. Suppl Table 1 summarizes the RCTs examining the effect of starch for the prevention of AKI.
Albumin vs. Saline
The role of albumin physiology in critically ill patients, and the pros and cons for administering albumin to hypoalbuminemic patients, have recently been discussed.85 Results of the Saline vs. Albumin Fluid Evaluation (SAFE) study, a RCT comparing 4% human albumin in 0.9% saline with isotonic saline in ICU patients, seem to indicate that albumin is safe, albeit no more effective than isotonic saline (the standard of care choice of isotonic sodium chloride in most centers) for fluid resuscitation. SAFE demonstrated further no difference in renal outcomes, at least based on the need for and duration of RRT.86 The SAFE study was a double-blind study and it was noted that patients in the albumin arm received 27% less study fluid compared to the saline arm (2247 vs. 3096 ml) and were approximately 1 l less positive in overall fluid balance.86 Furthermore, very few patients in the trial received large volume fluid resuscitation (>5 l) and thus the results may not be applicable to all patients. The Work Group noted that while isotonic crystalloids may be appropriate for initial management of intravascular fluid deficits, colloids may still have a role in patients requiring additional fluid.
Hydroxyethylstarch vs. Saline
Hydroxyethylstarch (HES) is a widely used, relatively inexpensive alternative to human albumin for correcting hypovolemia. Different HES preparations are available that vary with regard to concentration, mean molecular weight (MW), molar substitution, and substitution of hydroxyethyl for hydroxyl groups. The mean MW of the different HES preparations ranges between 70 000 and 670 000 Da. The colloid osmotic pressure effect is strongly dependent upon the concentration of colloid in the solution; e.g., 6% HES is iso-oncotic, whereas 10% HES is hyperoncotic. The number of hydroxyethyl groups per glucose molecule is specified by the molar substitution, ranging between 0.4 (tetrastarch) and 0.7 (heptastarch). Accordingly, HES solutions with a molar substitution of 0.5 or 0.6 are referred to as “pentastarch” or “hexastarch”, respectively. More recently, tetrastarches (HES 130/0.4 and HES 130/0.42) have also been introduced.87 High molecular substitution starch may impair coagulation by reducing the concentration of factor VIII: VIIIc and von Willebrand factor. Platelet activity may also be affected by blockade of the platelet fibrinogen receptor glycoprotein IIb/IIIa. Smaller starch molecules and those with less molecular substitution produce negligible coagulation defects.88
Aside from these negative effects on coagulation, development of renal dysfunction has been a concern associated with the use of mainly hypertonic HES. Hypertonic HES may induce a pathological entity known as “osmotic nephrosis” with potential impairment of renal function.89 It has even been recommended that “HES should be avoided in ICUs and during the perioperative period” (for a summary of this controversy, see de Saint-Aurin et al.90 and Vincent91).
The first major randomized trial in patients with sepsis compared HES 200/0.60 to 0.66 with gelatin and showed a greater incidence of AKI in the HES group, but no effect on survival.92 Criticisms of this study include a higher baseline SCr level in the HES group, small sample size, and short follow-up duration of 34 days. In the Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study,93 patients with severe sepsis were randomly assigned to receive a hypertonic (10%) solution of low MW HES (HES 200/0.5), or an isotonic modified Ringer's lactate solution. Patients in the HES group received a median cumulative dose of 70.4 ml per kilogram of body weight. The mortality was not significantly different, although showing a trend toward greater mortality at 90 days. However, the hypertonic HES group had a significantly higher rate of AKI (34.9% vs. 22.8%) and more days on which RRT was required (Suppl Table 1). Also, this study has been criticized for: i) using a hyperoncotic colloid solution with potentially harmful renal effects as shown in experimental research;94 ii) markedly exceeding the pharmaceutically recommended daily dose limit for 10% HES 200/0.5 by more than 10% in >38% of patients; and iii) pre-existing renal dysfunction in 10% of study patients, which represents a contra-indication for infusion of 10% HES 200/0.5.95 Posthoc analyses of the VISEP study showed the cumulative dose of HES to be a significant independent predictor for both mortality and RRT at 90 days. The median cumulative dose of HES in the VISEP Study was 70 ml/kg compared to 31 ml/kg in the study by Schortgen et al.92
A systematic review of RCTs on the use of HES for fluid management in patients with sepsis totaling 1062 patients, including 537 patients from the VISEP study, showed an almost two-fold increased risk of AKI with HES compared to crystalloids.96 Given these limitations, the results of these studies should be interpreted with caution. Furthermore, a large, prospective observational study found that HES infusion of any type (median volume 555 ml/d; intraquartile range 500–1000) did not represent an independent risk factor for renal impairment.97; however, recently in a large cohort of critically ill patients (approximately 8000 subjects), infusion of 10% HES 200/0.5 instead of HES 130/0.4 appeared to be an independent risk factor for RRT.87 Finally, a recent comprehensive Cochrane review98 concluded that there is no evidence from RCTs that resuscitation with colloids, instead of crystalloids, reduces the risk of death in patients with trauma, burns, or following surgery.
The mechanisms of colloid-induced renal injury are incompletely understood, but may involve both direct molecular effects and effects of elevated oncotic pressure.99 These concerns have led to the widespread adoption of lower MW starches as iso-oncotic solution, as resuscitation fluids. Theoretically, such solutions may have lower nephrotoxicity; however, as yet, no appropriately powered prospective randomized studies have reported the clinical benefit and safety of such solutions in comparison with crystalloids. A recent study by Magder et al. compared 10% 250/0.45 HES to isotonic saline in 262 patients who underwent cardiac surgery.100 These investigators tested whether fewer patients required catecholamines the morning after cardiac surgery (a chief determinant of ICU discharge) with HES compared to saline, and found indeed this was the case (10.9% vs. 28.8% P=0.001). Importantly, the study found no evidence of nephrotoxicity: no difference in the daily creatinine, development of AKI by RIFLE criteria during hospital stay (16% in both groups), or need for RRT (1% in each group). Importantly, patients in the saline group received nearly 60% more volume for fluid resuscitation in the ICU compared to HES (887 vs. 1397 ml; P<0.0001). While overall volumes were small, advocates for colloid resuscitation will note that this is exactly the reason colloids are preferred for patients requiring large-volume resuscitation.
The tonicity of colloid preparations may also vary by agent. A recent meta-analysis101 described 11 randomized trials with a total of 1220 patients: seven evaluating hyperoncotic albumin and four evaluating hyperoncotic starch. Hyperoncotic albumin decreased the odds of AKI by 76% while hyperoncotic starch increased those odds by 92% (odds ratio [OR] 1.92; CI 1.31–2.81; P=0.0008). Parallel effects on mortality were observed. This meta-analysis concluded that the renal effects of hyperoncotic colloid solutions appear to be colloid-specific, with albumin displaying renoprotection and hyperoncotic starch showing nephrotoxicity. A 7000-patient study comparing 6% HES 130/0.4 in saline with saline alone was scheduled to begin in Australia and New Zealand in 2010. This study will provide further high-quality data to help guide clinical practice.102
Thus, the use of isotonic saline as the standard of care for intravascular volume expansion to prevent or treat AKI is based upon the lack of clear evidence that colloids are superior for this purpose, along with some evidence that specific colloids may cause AKI, in addition to higher costs. It is acknowledged that colloids may be chosen in some patients to aid in reaching resuscitation goals, or to avoid excessive fluid administration in patients requiring large volume resuscitation, or in specific patient subsets (e.g., a cirrhotic patient with spontaneous peritonitis, or in burns). Similarly, although hypotonic or hypertonic crystalloids may be used in specific clinical scenarios, the choice of crystalloid with altered tonicity is generally dictated by goals other than intravascular volume expansion (e.g., hypernatremia or hyponatremia). One of the concerns with isotonic saline is that this solution contains 154 mmol/l chloride and that administration in large volumes will result in relative or absolute hyperchloremia (for a review, see Kaplan et al.103). Although direct proof of harm arising from saline-induced hyperchloremia is lacking, buffered salt solutions approximate physiological chloride concentrations and their administration is less likely to cause acid-base disturbances. Whether use of buffered solutions results in better outcomes is, however, uncertain.
VASOPRESSORS
3.1.2: We recommend the use of vasopressors in conjunction with fluids in patients with vasomotor shock with, or at risk for, AKI. (1C)
RATIONALE
Sepsis and septic shock are major contributing factors to AKI7 and vasopressor requirement appears to be highly associated with AKI in this population. Despite the high prevalence of AKI during critical illness in general, and severe sepsis specifically, success has been limited in improving the outcome of this complication.104 Septic shock is the prototype of a high output–low resistance condition, although severe pancreatitis, anaphylaxis, burns, and liver failure share similar physiologic alterations. Persistent hypotension, despite ongoing aggressive fluid resuscitation or after optimization of intravascular volume in patients with shock, places patients at risk for development of AKI. In the setting of vasomotor paralysis, preservation or improvement of renal perfusion can only be achieved through use of systemic vasopressors once intravascular volume has been restored.105
It is not known which vasopressor agent is most effective for prevention or treatment of patients with AKI and septic shock. Most studies have focused on norepinephrine, dopamine, or vasopressin. Small open-label studies have shown improvement in creatinine clearance (CrCl) following a 6- to 8-hour infusion of norepinephrine106 or terlipressin,107 while vasopressin reduced the need for norepinephrine and increased urine output and CrCl.108 A large RCT109 comparing dopamine to norepinephrine as initial vasopressor in patients with shock showed no significant differences between groups with regard to renal function or mortality. However, there were more arrhythmic events among the patients treated with dopamine than among those treated with norepinephrine, and a subgroup analysis showed that dopamine was associated with an increased rate of death at 28 days among the patients with cardiogenic shock, but not among the patients with septic shock or those with hypovolemic shock. Thus, although there was no difference in primary outcome with dopamine as the first-line vasopressor agent and those who were treated with norepinephrine, the use of dopamine was associated with a greater number of adverse events.109
Vasopressin is gaining popularity in the treatment of shock refractory to norepinephrine.110 Compared to norepinephrine, it increases blood pressure and enhances dieresis, but has not as yet been proven to enhance survival nor to reduce the need for RRT.111 A recent posthoc analysis of the above mentioned RCT used the RIFLE criteria for AKI to compare the effects of vasopressin vs. norepinephrine.112 In patients in the RIFLE-R category, vasopressin as compared to norepinephrine was associated with a trend to a lower rate of progression to F or L categories respectively, and a lower rate of use of RRT. Mortality rates in the R category patients treated with vasopressin compared to norepinephrine were 30.8 vs. 54.7%, P=0.01, but this did not reach significance in a multiple logistic regression analysis. This study suggests thus that vasopressin may reduce progression to renal failure and mortality in patients at risk of kidney injury who have septic shock. The Work Group concluded that current clinical data are insufficient to conclude that one vasoactive agent is superior to another in preventing AKI, but emphasized that vasoactive agents should not be withheld from patients with vasomotor shock over concern for kidney perfusion. Indeed, appropriate use of vasoactive agents can improve kidney perfusion in volume-resuscitated patients with vasomotor shock.
PROTOCOLIZED HEMODYNAMIC MANAGEMENT
3.1.3: We suggest using protocol-based management of hemodynamic and oxygenation parameters to prevent development or worsening of AKI in high-risk patients in the perioperative setting (2C) or in patients with septic shock (2C).
RATIONALE
A resuscitation strategy devised for patients with hypotension from septic shock that is based upon achieving specific physiologic end-points within 6 hours of hospital admission has been termed Early Goal-Directed Therapy (EGDT). This approach has been endorsed by the “Surviving Sepsis Campaign”113 and has gained considerable acceptance despite only one, single-center, RCT evaluating its effectiveness. This protocolized strategy, consisting of fluids, vasoactive medication, and blood transfusions targeting physiological parameters, is recommended by many experts for the prevention of organ injury in septic-shock patients.
Similarly, protocolized care strategies in surgical patients at high risk for postoperative AKI have been extensively studied in an effort to provide optimal oxygen delivery to tissues in the perioperative period. In these patients, goal-directed therapy is defined as hemodynamic monitoring with defined target values and with a time limit to reach these stated goals. Together these protocols with bundled, hemodynamic, and tissue-support measures have the potential to reduce the risk of AKI following major surgical procedures in high-risk patients (e.g., age >60 years, emergent surgery, elevated American Society of Anesthesiologists score, preoperative comorbid illnesses).
Protocolized hemodynamic management strategies in septic shock
Early fluid resuscitation in the management of hypotensive patients with septic shock has been a standard treatment paradigm for decades.93, 113, 114 What has not been clear, however, is how much fluid to give, for how long, or what type of fluid therapy is optimal in the physiologic support of septic shock.93, 113, 114 In 2001, Rivers et al.115 published the results of a small (n=263), open-label, single-center study that compared a treatment protocol that the authors referred to as EGDT in the emergency management of septic shock. EGDT is predicated upon the premise that an early, protocolized resuscitation program with predefined physiologic end-points will prevent organ failure and improve the outcome of patients presenting with septic shock.
Hypotensive patients with severe infection are rapidly assessed for evidence of tissue hypoperfusion and microcirculatory dysfunction by mean arterial blood pressure measurement and plasma lactate levels.115 Blood lactate levels are neither sensitive nor specific but are readily available measures of tissue hypoperfusion and do correlate with adverse outcomes in sepsis.116, 117 Early recognition of septic shock then initiates a protocol of resuscitation with the goal of reestablishing tissue perfusion in patients within 6 hours of diagnosis. The physiologic goals are: i) return of mean arterial blood pressure to ⩾65 mm Hg; ii) central venous pressure between 8–12 mm Hg; iii) improvement in blood lactate levels; iv) central venous oxygen saturation (ScvO2) >70% and v) a urine output of ⩾0.5 ml/kg/h.
In the study by Rivers et al. the protocol-driven process resulted in more rapid use of fluids, more blood transfusions, and in a small number of patients, earlier use of dobutamine over the 6-hour time period than standard emergency care. The in-hospital mortality rate in the control group was 46.5% vs. 30.5% in the EGDT group (P<0.01).115 Follow-up, predominantly observational studies, have found less dramatic but generally similar effects,118, 119, 120, 121, 122 though not without exception.123
The Rivers study did not specifically look at AKI outcomes, but multiple-organ function-scoring systems (i.e., APACHE II and SAPS 2) both showed significant improvements with EGDT. In a subsequent study, prevention of AKI was significantly improved in patients randomized to a modified EGDT strategy (without measurement of ScvO2) compared to a standard-care group.119 Criticisms of the Rivers study include: i) a complex, multistep protocol for which individual interventions have not been validated; ii) the use of a treatment team in the active-therapy arm, thus risking a Hawthorn effect; iii) high mortality in the standard-care arm; and iv) the study was a small single-center study. Three large multicenter clinical trials in the USA, UK, and Australia are currently underway to definitively evaluate this promising therapy.
Goal-directed therapy for hemodynamic support during the perioperative period in high-risk surgical patients
Efforts to improve tissue oxygen delivery by optimizing hemodynamic support in high-risk surgical patients to prevent AKI and other adverse patient outcomes have been investigated for many years.124, 125, 126 A recent meta-analysis of these studies by Brienza et al.127 concluded that protocolized therapies (regardless of the protocol) with specific physiological goals can significantly reduce postoperative AKI. A major problem in interpreting these studies is the lack of standardized hemodynamic and tissue oxygenation targets and management strategies used to verify the efficacy of these measures over standard perioperative care. A heterogeneous collection of study populations, types of surgical procedures, monitoring methods, and treatment strategies comprise this recent meta-analysis.127 The basic strategy of goal-directed therapy to prevent AKI in the perioperative period is based on protocols that avoid hypotension, optimize oxygen delivery, and include careful fluid management, vasopressors when indicated, and inotropic agents and blood products if needed.127
The relative merits and risk:benefit ratio of each discrete element of EGDT in the successful resuscitation of patients with septic shock requires further study. Given the limitations of the current studies and lack of comparative effectiveness studies comparing individual protocols, we can only conclude that protocols for resuscitation in the setting of septic shock and high-risk surgery appear to be superior to no protocol.
RESEARCH RECOMMENDATIONS
Randomized trials of isotonic crystalloid vs. colloid therapy for intravascular volume expansion to prevent or treat AKI should be conducted in a variety of settings (critical illness, high-risk surgery, sepsis), including patient subsets. In particular, colloids may improve efficiency of fluid resuscitation but some (starch) also carry some concerns regarding effects on the kidneys. If colloid results in less volume overload, it may lead to improved outcomes.
Comparisons of specific solutions, with specific electrolyte composition or colloid type, for effectiveness in preventing AKI should be conducted. Specifically, there is a need to examine physiologic electrolyte solutions vs. saline.
Studies are needed that compare different types of vasopressors for prevention and treatment of AKI in hemodynamically unstable patients. Some evidence suggests that certain vasopressors may preserve renal function better than others (e.g., vasopressin analogues vs. catecholamines) and studies are needed to compare them in this setting.
The choice of a target mean arterial perfusion pressure range of 65–90 mm Hg as a component of resuscitation (perhaps in the context of age, chronic blood pressure, or other comorbidities) also needs further study.
The specific components of goal-directed therapy that accrue benefits for patients at risk for AKI need to be determined. Is it the timing of protocolized hemodynamic management that is beneficial: prophylactically in high-risk surgical patients, or early in the course of severe sepsis? In contrast to the benefits of prophylactic or EGDT, protocolized use of inotropes to normalize mixed venous oxygen saturation or supranormalize oxygen delivery in “late” critical illness did not result in decreased AKI128 or improved outcomes.128, 129 Alternatively, is it attention to hemodynamic monitoring, the protocol itself that standardizes supportive care to achieve the stated goals, or a single or combination of the multiple possible interventions that improves outcome? Thus, further research is required to determine the specific components of goal-directed therapy that accrue benefits for patients at risk for AKI, if such benefits actually occur.
Chapter 3.2: General supportive management of patients with AKI, including management of complications
Supportive management to prevent AKI was discussed in the previous chapter and, for many patients, many of the supportive therapies will continue even if AKI develops. Furthermore, an important goal of early management of AKI is to prevent further injury and to facilitate recovery of renal function. These goals can often best be achieved by strict attention to supportive therapy. However, as renal function deteriorates, complications arise that require different management. Some of these issues have been discussed in Chapter 2.3 and several books have been devoted, in large part, to management of the many complications that arise from AKI130, 131, 132, 133; the reader is referred to these sources. Particular attention should be given to the assessment of the circulating volume and fluid administration, the prevention and/or treatment of hyperkalemia and metabolic acidosis, the knowledge of the changes in pharmacokinetics of many drugs with discontinuation of all potentially nephrotoxic drugs, and dose adaptation of drugs excreted by the kidneys to the patient's renal function. Finally, many of the other chapters in this section of the guideline deal with supportive measures (e.g., diuretics for fluid management).
Chapter 3.3: Glycemic control and nutritional support
GLYCEMIC CONTROL IN CRITICAL ILLNESS: RENAL EFFECTS AND OUTCOMES
3.3.1: In critically ill patients, we suggest insulin therapy targeting plasma glucose 110–149 mg/dl (6.1–8.3 mmol/l). (2C)
RATIONALE
As outlined in a recent review,134 stress hyperglycemia is a distinctive clinical feature of critical illness. Stress mediators, and central and peripheral insulin resistance appears pivotal to the occurrence of stress hyperglycemia. Inflammatory mediators and counter-regulatory hormones have been shown to impede crucial elements of the insulin-signaling pathway. Still, exogenous insulin administration normalizes blood glucose levels in this setting. Insulin treatment may counteract hepatic insulin resistance during acute critical illness. Extensive observational data have shown a consistent, almost linear, relationship between blood glucose levels in patients hospitalized with MI and adverse clinical outcomes, even in patients without established diabetes.135, 136
It has never been entirely clear, however, whether glycemia serves as a mediator of these outcomes or merely as a marker of the sickest patients, who present with the well-known counter-regulatory stress response to illness.137 Interestingly, Kosiborod et al.135 recently showed, in a population with MI, that while hypoglycemia was associated with increased mortality, this risk was confined to patients who developed spontaneous hypoglycemia. In contrast, iatrogenic hypoglycemia after insulin therapy was not associated with higher mortality risk.
Tight glycemic control is frequently used in patients at risk of AKI, and in the management of those who develop AKI. It has been proposed that tight glycemic control can reduce the incidence and severity of AKI. Since the landmark trial of Van den Berghe et al.,138 additional studies provided initial confirmation of the benefits (reduced morbidity and mortality), and some additional mechanistic insights of tight glycemic control in critically ill patients.139 Further secondary analysis of the original trial, which was conducted in 1548 mechanically ventilated surgical ICU patients, found that intensive insulin therapy (IIT) target plasma glucose 80–110 mg/dl (4.44–6.11 mmol/l) was associated with substantial cost savings compared to conventional insulin therapy (CIT) target plasma glucose 180–200 mg/dl (9.99–11.1 mmol/l).140 However, when Van den Berghe et al. repeated their original study in a different population of critically ill patients (medical rather than surgical ICU patients), the primary end-point of in-hospital mortality did not differ between groups (40% CIT group vs. 37.3% IIT group; P=0.33).141 As in the original surgical ICU study, a variety of secondary end-points were improved in this study, including a lower incidence of AKI and need for RRT. In the original surgical ICU study, severe AKI (peak SCr >2.5 mg/dl [>221 μmol/l]) developed in 7.2% of the IIT group, compared to 11.2% of the CIT group (P=0.04); the incidence of RRT was also lower in the IIT group than the CIT group (4.8% vs. 8.2%, respectively; P=0.007).138 In the medical ICU study, the IIT group similarly had a significantly lower rate of AKI (doubling of SCr, 5.4%) than the CIT group (8.9%, P=0.04), although RRT incidence was not decreased.141 In a recent analysis, Schetz et al.142 combined the renal end-points of both of these trials and used a modified version of the RIFLE classification of AKI to demonstrate that tight glycemic control reduced the incidence of severe AKI (peak SCr increments two- or three-fold increased from baseline) from 7.6% to 4.5% (P=0.0006) in a combined patient population of 2707. The need for RRT was not decreased in the overall population or the medical ICU population, but was significantly lower in the surgical ICU patients managed with IIT (4% vs. 7.4%, P=0.008).
Several newer studies have provided additional insight concerning the efficacy and safety of tight glycemic control in critically ill patients.93, 95, 143, 144, 145, 146 Thomas et al.145 conducted a systematic review of randomized trials of tight glycemic control in 2864 critically ill patients, and found a 38% risk reduction of AKI with IIT, and a nonsignificant trend towards less acute dialysis requirement. However, IIT was also associated with a greater than four-fold increase in the risk of hypoglycemia. A body of literature demonstrating that uncontrolled hyperglycemia was associated with increased AKI following cardiac surgery led to the conduct of a 400-patient, single-center RCT of tight vs. conventional intraoperative glucose control.143, 144 The investigators found that this approach did not decrease perioperative morbidity or mortality (included in a composite end-point that included AKI within 30 days of surgery): the composite end-point occurred in 44% of the IIT group vs. 46% of the CIT group. Although the incidence of hypoglycemia was similar in the groups, there was a significantly higher incidence of stroke in the IIT group (4.3%) compared to the CIT group (0.54%), as well as trends towards higher mortality and more postoperative heart block in the IIT group, raising concerns about the safety of this approach.
Further prospective comparison of IIT vs. CIT in critically ill septic patients was provided in the VISEP trial, which also incorporated a comparison on crystalloid vs. colloid infusions in a 2 × 2 factorial design.93 Patients with severe sepsis or septic shock in 18 ICUs were randomized to IIT (target glycemia 80–110 mg/dl [4.44–6.11 mmol/l] n=247) or CIT (target glycemia 180–200 mg/dl [9.99–11.1 mmol/l] n=290) (Suppl Tables 2 and 3). There were no significant differences in 28-day or 90-day mortality, Sequential Organ Failure Assessment scores, or AKI rates between the groups. However, hypoglycemia (blood glucose level <40 mg/dl [<2.22 mmol/l]) was more frequent in the IIT group (12% vs. 2% P<0.001) and led to early termination of the IIT study arm. Following publication of this study, Thomas et al., updated the meta-analysis (discussed above) to include these data, and reported that, with the addition of the VISEP data, the analysis of a 3397-patient group found a 36% risk reduction of AKI with IIT, but this pooled estimate was no longer statistically significant (relative risk [RR] 0.74; 95% CI 0.47–1.17).95 In a detailed review of the VISEP trial, Thomas et al., also noted that another multicenter mixed ICU trial of intensive insulin therapy (the GLUCOCONTROL Study: Comparing the effects of two glucose control regimens by insulin in intensive care unit patients; available at: http://www.clinicaltrials.gov/ct/show/NCT00107601) was stopped after 1101 patients were enrolled because of greater rates of hypoglycemia with IIT.95 Such data have raised significant concerns regarding the effectiveness and safety of using IIT with tight glycemic control to prevent or ameliorate morbidity and mortality in patients at high risk of AKI and other forms of organ injury.
The recent meta-analysis of IIT vs. CIT by Wiener et al.146 continued to find a greater incidence of hypoglycemia with IIT, but the balance of evidence now suggests no improvement in survival with this approach. Twenty-nine RCTs totaling 8432 patients contributed data for this meta-analysis. Twenty-seven studies reported no difference in hospital mortality (21.6% in IIT vs 23.3% in CIT) with a pooled RR of 0.93 (95% CI 0.85–1.03; P=NS). Nine studies reported no difference in incidence of new RRT. There was a significant benefit of tight glycemic control in reducing the incidence of septicemia but this was associated with a significantly increased risk of hypoglycemia (blood glucose <40 mg/dl [<2.22 mmol/l]) in patients randomized to IIT with a pooled RR of 5.13 (95% CI 4.09–6.43; P<0.05).
In summary, pooled analysis of early multicenter studies has failed to confirm the early observations of beneficial effects of IIT on renal function; the risk of hypoglycemia with this approach is significant, and even the survival benefits of IIT are in doubt. More recently, the international Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study, with a targeted enrolment of 6100 patients, set out to definitively determine the risk-benefit comparison of tight glycemic control in critically ill patients (Suppl Table 3).147, 148 In this trial, adult patients were randomized within 24 hours after admission to an ICU to receive either intensive glucose control (target blood glucose range of 81–108 mg/dl [4.50–5.99 mmol/l]), or conventional glucose control (target of ⩽180 mg/dl [⩽9.99 mmol/l]).148 The primary outcome was mortality from any cause within 90 days after randomization. The two groups had similar characteristics at baseline. A total of 829 patients (27.5%) in the intensive-control group and 751 (24.9%) in the conventional-control group died (OR for intensive control, 1.14; 95% CI 1.02–1.28; P=0.02). The treatment effect did not differ significantly between surgical patients and medical patients. There was no significant difference between the two treatment groups in incidence of new RRT (15.4% vs. 14.5%), respectively. Severe hypoglycemia (blood glucose level ⩽40 mg/dl [⩽2.22 mmol/l]) was reported in 6.8% in the intensive-control group and in 0.5% in the conventional-control group (P<0.001). In summary, the largest randomized trial of intensive vs. conventional insulin therapy found that intensive glucose control actually increased mortality among adults in the ICU: a blood glucose target of ⩽180 mg/dl (⩽9.99 mmol/l) resulted in lower mortality than did a target of 81–108 mg/dl (4.50–5.99 mmol/l). Furthermore, this trial confirmed the consistent finding of an increased incidence of hypoglycemia associated with IIT, without any proven benefit in reducing mortality, organ dysfunction, or bacteremia.
There were some methodological differences between the Leuven and NICE-SUGAR studies, possibly explaining the different outcomes.149 These comprised different target ranges for blood glucose in control and intervention groups, different routes for insulin administration and types of infusion pumps, different sampling sites, and different accuracies of glucometers, as well as different nutritional strategies and varying levels of expertise. Finally, Griesdale et al.150 performed a meta-analysis of trials of intensive vs. conventional glycemic control that included most of the studies in the Wiener meta-analysis, in addition to some newer studies, including data supplied by the NICE-SUGAR investigators. All 26 trials that reported mortality found a pooled RR of death with IIT compared to CIT of 0.93 (95% CI 0.83–1.04). Among the 14 trials reporting hypoglycemia, the pooled RR with IIT was 6.0 (95% CI 4.5–8.0). However, in subset analysis, patients in surgical ICUs appeared to benefit from IIT while patients in the other ICU settings (medical or mixed) did not. Although results from the early trials were better in studies that included surgical138 rather than purely medical ICU patients141, and this latest meta-analysis appears to confirm that trend, it should be noted that no such phenomenon was noted in the NICE-SUGAR trial. Overall, the data do not support the use of IIT aiming to control plasma glucose below 110 mg/dl (6.11 mmol/l) in critically ill patients, although subset analyses suggest that further trials may disclose benefits in perioperative patients, and perhaps through the use of less-intensive glucose control targets.
Considering the balance between potential benefits and harm (see Suppl Table 2), the Work Group suggests using insulin for preventing severe hyperglycemia in critically ill patients, but in view of the danger of potentially serious hypoglycemia, we recommend that the average blood glucose should not exceed 150 mg/dl (8.33 mmol/l), but that insulin therapy should not be used to lower blood glucose to less than 110 mg/dl (6.11 mmol/l). The Work Group recognizes that these proposed thresholds have never directly been examined in RCTs but are interpolated from the comparisons tested in the trials so far.
NUTRITIONAL ASPECTS IN THE PREVENTION AND TREATMENT OF CRITICALLY ILL PATIENTS WITH AKI
Protein-calorie malnutrition is an important independent predictor of in-hospital mortality in patients with AKI. In a prospective study of 300 AKI patients, 42% presented with signs of severe malnutrition on admission.151
The nutritional management of AKI patients must consider the metabolic derangements and proinflammatory state associated with renal failure, the underlying disease process and comorbidities, as well as the derangements in nutrient balance caused by RRT. Very few systematic studies have assessed the impact of nutrition on clinical end-points used in these guidelines (i.e., mortality, need for RRT, and incidence of AKI). Recommendations are therefore largely based on expert opinion. Several expert panels have developed clinical practice guidelines for the nutritional management of patients with AKI, whether treated with or without RRT.152, 153, 154, 155, 156 A recent narrative review has also provided updated information on this topic.157
3.3.2: We suggest achieving a total energy intake of 20–30 kcal/kg/d in patients with any stage of AKI. (2C)
RATIONALE
Carbohydrate metabolism in AKI is characterized by hyperglycemia due to peripheral insulin resistance158, 159 and accelerated hepatic gluconeogenesis, mainly from conversion of amino acids released during protein catabolism that cannot be suppressed by exogenous glucose infusions.160 In addition, hypertriglyceridemia commonly occurs due to inhibition of lipolysis. The clearance of exogenously administered lipids can be reduced.161 The modifications of energy metabolism are usually not caused by AKI per se but related to acute comorbidities and complications.162 Energy consumption is not increased by AKI. Even in multiple-organ failure, the energy expenditure of critically ill patients amounts to not more than 130% of resting energy expenditure. The optimal energy-to-nitrogen ratio during AKI has not been clearly determined. In a retrospective study of AKI patients undergoing continuous venovenous hemofiltration (CVVH), less negative or weakly positive nitrogen balance was associated with an energy intake of approximately 25 kcal/kg/d.163 In a randomized trial in AKI patients comparing 30 and 40 kcal/kg/d energy provision, the higher energy prescription did not induce a more positive nitrogen balance but was associated with a higher incidence of hyperglycemia and hypertriglyceridemia and a more positive fluid balance.164 These observations provide a rationale to maintain a total energy intake of at least 20, but not more than 25–30 kcal/kg/d, equivalent to 100–130% of resting energy expenditure. Energy provision should be composed of 3–5 (maximum 7) g per kilogram body weight carbohydrates and 0.8–1.0 g per kilogram body weight fat.
3.3.3: We suggest to avoid restriction of protein intake with the aim of preventing or delaying initiation of RRT. (2D)
3.3.4: We suggest administering 0.8–1.0 g/kg/d of protein in noncatabolic AKI patients without need for dialysis (2D), 1.0–1.5 g/kg/d in patients with AKI on RRT (2D), and up to a maximum of 1.7 g/kg/d in patients on continuous renal replacement therapy (CRRT) and in hypercatabolic patients. (2D)
RATIONALE
Protein hypercatabolism driven by inflammation, stress, and acidosis is a common finding in critically ill patients.157, 165, 166 The optimal amount of protein supplementation in AKI patients is unknown. Patients with AKI are at high risk of malnutrition. Since malnutrition is associated with increased mortality in critically ill patients, nutritional management should aim at supplying sufficient protein to maintain metabolic balance. Hence, nutritional protein administration should not be restricted as a means to attenuate the rise in BUN associated with declining GFR. On the other hand, there is little evidence that hypercatabolism can be overcome simply by increasing protein intake to supraphysiologic levels. While, in a crossover study of AKI patients, nitrogen balance was related to protein intake and was more likely to be positive with intakes larger than 2 g/kg/d,167 only 35% of patients achieved a positive nitrogen balance in a study applying a nutrient intake as high as 2.5 g/kg/d protein.168 No outcome data are currently available concerning the clinical efficacy and the safety of such high protein intakes, which may contribute to acidosis and azotemia, and increase dialysis dose requirements.
Due to their continuous nature and the high filtration rates, CRRT techniques can better control azotemia and fluid overload associated with nutritional support but may also result in additional losses of water-soluble, low-molecular-weight substances, including nutrients.169 Normalized protein catabolic rates of 1.4 to 1.8 g/kg/d have been reported in patients with AKI receiving CRRT.170, 171, 172 In a recent study in critically ill cancer patients with AKI and treated with sustained low-efficiency dialysis (SLED), those with higher BUN and serum albumin levels, which were associated with infusion of higher amount of total parenteral nutrition, had a lower mortality risk.173
In CRRT, about 0.2 g amino acids are lost per liter of filtrate, amounting to a total daily loss of 10–15 g amino acids. In addition, 5–10 g of protein are lost per day, depending on the type of therapy and dialyzer membrane. Similar amounts of protein and amino acids are typically lost by peritoneal dialysis (PD). Nutritional support should account for the losses related to CRRT, including PD, by providing a maximum of 1.7 g amino acids/kg/d.
3.3.5: We suggest providing nutrition preferentially via the enteral route in patients with AKI. (2C)
RATIONALE
Enteral feeding may be more difficult in patients with AKI because of impaired gastrointestinal motility and decreased absorption of nutrients secondary to bowel edema.174 Moreover, multiple factors negatively affect gastrointestinal function in critically ill patients, e.g., medications (sedatives, opiates, catecholamines, etc.), glucose and electrolyte disorders, diabetes, or mechanical ventilation. However, the provision of nutrients via the gut lumen helps maintain gut integrity, decreases gut atrophy, and decreases bacterial and endotoxin translocation. Furthermore, AKI is a major risk factor for gastrointestinal hemorrhage.175 Enteral nutrition should exert protective effects on the risk of stress ulcers or bleeding. Clinical studies have suggested that enteral feeding is associated with improved outcome/survival in ICU patients.176, 177 Hence, enteral nutrition is the recommended form of nutritional support for patients with AKI. If oral feeding is not possible, then enteral feeding (tube feeding) should be initiated within 24 hours, and has been shown to be safe and effective.178
Pediatric considerations
In children with AKI, physiological macronutrient requirements are age-dependent, reflecting the developmental dynamics of growth and metabolism. Research exploring nutritional requirements in children with critical illness and AKI is limited to observational studies. With respect to calorie provision, it is generally agreed that critically ill children, like adults, should receive 100–130% of the basal energy expenditure, which can be estimated with acceptable precision and accuracy by the Caldwell-Kennedy equation179: (resting energy expenditure [kcal/kg/d]=22+31.05 × weight [kg]+1.16 × age [years]).
In a recent survey of the nutritional management of 195 children with AKI on CRRT, the maximal calorie prescription in the course of treatment averaged 53, 31, and 21 kcal/kg/d, and that for protein intake 2.4, 1.9, and 1.3 g/kg/d in children aged <1, 1–13, and >13 years, respectively.180 Although not validated by outcome studies, these figures provide an orientation for the macronutrient supply typically achieved in and tolerated by children with AKI receiving CRRT.
RESEARCH RECOMMENDATIONS
The risk-benefit ratio of diets with low, medium, and high protein contents in different stages of AKI should be addressed in RCTs.
Given gastrointestinal tract dysfunction in AKI, the possible benefit of enteral vs. parenteral feeding in AKI patients should be further evaluated in prospective RCTs.
Chapter 3.4: The use of diuretics in AKI
Diuretics are frequently used in patients at risk of AKI, and in the management of those who develop AKI. Since fluid overload is one of the major symptoms of AKI, diuretics are often used for patients with AKI to facilitate fluid management. Recent observational studies showed that 59–70% of patients with AKI were given diuretics at the time of nephrology consultation or before the start of RRT.181, 182 In addition, oliguric AKI has a worse prognosis than nonoliguric AKI and physicians often prescribe diuretics to convert oliguric to nonoliguric AKI.183 Diuretics are also used to control fluid balance and permit administration of nutrition and medications. Furthermore, several diuretics have potentially renoprotective effects that might prevent development of AKI and hasten its recovery. However, diuretics can also be harmful, by reducing the circulating volume excessively and adding a prerenal insult, worsening established AKI. Therefore, it is essential to evaluate usefulness of diuretics to improve outcome of patients with AKI, not just for fluid management.
3.4.1: We recommend not using diuretics to prevent AKI. (1B)
3.4.2: We suggest not using diuretics to treat AKI, except in the management of volume overload. (2C)
RATIONALE
Loop diuretics have several effects that may protect against AKI. They may decrease oxygen consumption in the loop of Henle by inhibiting sodium transport, thus potentially lessening ischemic injury. Loop diuretics act at the luminal surface of the thick ascending limb of the loop of Henle and inhibit the Na-K-2Cl cotransporter,184, 185 resulting in a loss of the high medullary osmolality and decreased ability to reabsorb water. Inhibition of active sodium transport also reduces renal tubular oxygen consumption, potentially decreasing ischemic damage of the most vulnerable outer medullary tubular segments;183 therefore, furosemide might protect kidneys against ischemic injury.186 Furosemide also might hasten recovery of AKI by washing out necrotic debris blocking tubules, and by inhibiting prostaglandin dehydrogenase, which reduces renovascular resistance and increases renal blood flow.186, 187 Based on these properties, loop diuretics might be expected to prevent or ameliorate AKI. However, there are only minimal data to support this theory, and there is some evidence of harm associated with loop diuretic use to prevent or treat AKI.188, 189, 190, 191 Furosemide is the most commonly prescribed diuretic in the acute-care setting,183, 184, 185 and a number of RCTs have tested whether furosemide is beneficial for prevention or treatment of AKI. Specifically, prophylactic furosemide was found to be ineffective or harmful when used to prevent AKI after cardiac surgery,189, 190 and to increase the risk of AKI when given to prevent CI-AKI.191 Epidemiologic data have suggested that the use of loop diuretics may increase mortality in patients with critical illness and AKI,181 along with conflicting data that suggest no harm in AKI.182 Finally, furosemide therapy was also ineffective and possibly harmful when used to treat AKI.188, 192
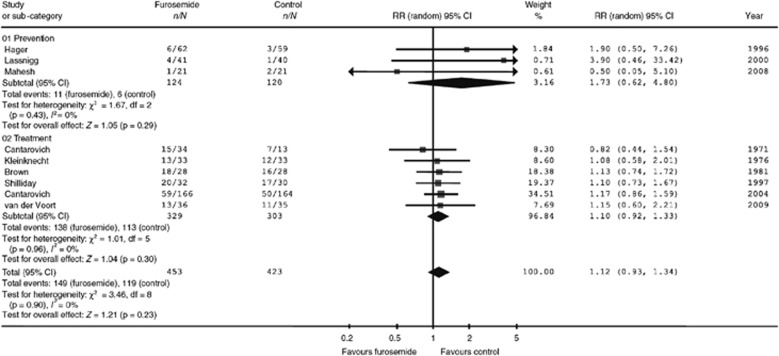
There is no evidence that the use of diuretics reduces the incidence or severity of AKI. Ho et al.192, 193 conducted two comprehensive systematic reviews on the use of the loop diuretic frusemide (furosemide) to prevent or treat AKI. Furosemide had no significant effect on in-hospital mortality, risk for requiring RRT, number of dialysis sessions, or even the proportion of patients with persistent oliguria. Results from the most recent review193 are shown in Figure 9 and Figure 10. The primary prevention studies included patients who underwent cardiac surgery,189 coronary angiography,191 and major general or vascular surgery.194 In two of these studies, all participants had mild pre-existing renal impairment. Two of the three studies reported mortality in patients randomized to furosemide (n=103) vs. placebo (n=99), with a pooled RR of 2.67 (95% CI 0.75–7.25; P=0.15). All three studies reported RRT incidence in patients randomized to furosemide (n=128) vs. placebo (n=127), with a pooled RR of 4.08 (95% CI 0.46–35.96; P=0.21). Thus, subanalysis to separate primary and secondary prevention trials did not alter the conclusion that, within the sample size limitations of this study, furosemide is not effective for the prevention of AKI.
Figure 9.
Effect of furosemide vs. control on all-cause mortality. Reprinted from Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010; 65: 283–293 with permission from John Wiley and Sons193; accessed http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2044.2009.06228.x/full
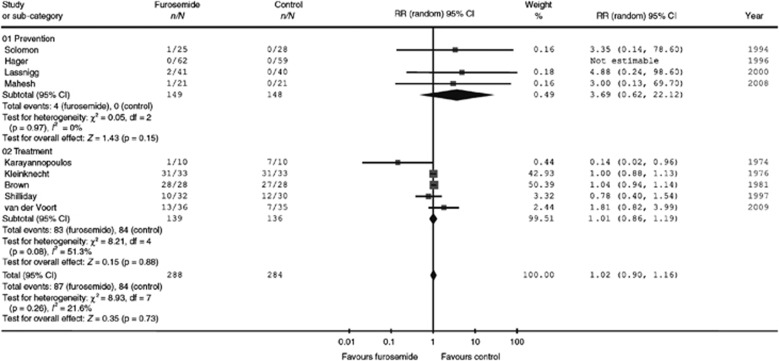
Figure 10.
Effect of furosemide vs. control on need for RRT. Reprinted from Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010; 65: 283–293 with permission from John Wiley and Sons193; accessed http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2044.2009.06228.x/full
The systematic review and meta-analysis by Ho and Power193 also included six studies that used furosemide to treat AKI, with doses ranging from 600 to 3400 mg/d (Figure 9 and Figure 10).192 No significant reduction was found for in-hospital mortality or for RRT requirement. The largest single study of furosemide for treating AKI was conducted by Cantarovich et al.,188 which included 338 patients with AKI requiring dialysis. Patients were randomly assigned to the administration of either furosemide (25 mg/kg/d i.v. or 35 mg/kg/d orally) or placebo. Although time to reach 2 l/d of diuresis was shorter with furosemide (5.7 days) than placebo (7.8 days, P=0.004), there was no difference in survival and number of dialysis sessions. At present, the current evidence does not suggest that furosemide can reduce mortality in patients with AKI.
Furosemide may, however, be useful in achieving fluid balance to facilitate mechanical ventilation according to the lung-protective ventilation strategy in hemodynamically stable patients with acute lung injury. On the other hand, the literature also suggests that high-dose furosemide (>1 g/d) may cause ototoxicity. In the first meta-analysis by Ho and Sheridan,192 high doses of furosemide (range 1–3.4 g/d) caused deafness or tinnitus more frequently than the control (RR 3.97; 95% CI 1.00–15.78; P=0.05). When administered as continuous infusion a dose of 0.5 mg/kg/hour was not associated with ototoxicity.195 Taken together with several small studies showing that the prophylactic use of diuretics to prevent AKI actually increased AKI incidence, these data raise significant concerns regarding use of loop diuretics to prevent or treat AKI in any setting. We similarly conclude that there is no evidence that the use of loop diuretics reduces the severity of AKI, or improves outcomes in this syndrome. Although the use of loop diuretics in early or established AKI facilitates management of fluid balance, hyperkalemia, and hypercalcemia, and is indicated for these clinical purposes, any putative role in the prevention or amelioration of AKI course is unproven.
Two recent studies have investigated whether the administration of furosemide to patients treated with CVVH could be associated with a more rapid discontinuation of the dialysis therapy. van der Voort et al., observed, as expected, an increased urinary volume and sodium excretion, but this intervention did not lead to a shorter duration of renal failure or more frequent renal recovery.195 The second study by Uchino et al.,196 analyzed data from the B.E.S.T. kidney and found that, from a total of 529 critically ill patients who survived during CRRT, 313 patients were removed successfully from CRRT while 216 patients needed “repeat RRT” after temporary discontinuation. Urine output (during the 24 hours before stopping CRRT) was identified as a significant predictor of successful cessation, but the predictive ability of urine output was negatively affected by the use of diuretics. Thus, a beneficial role for loop diuretics in facilitating discontinuation of RRT in AKI is not evident.
Mannitol
Mannitol has been frequently used in the past for prevention of AKI; however, most of the studies are retrospective, underpowered, and, overall, the studies did not meet the criteria of the Work Group to be included in formulation of recommendations. Prophylactic mannitol has been promoted in patients undergoing surgery. While in most of these instances mannitol increases urine flow, it is highly probable that mannitol does not convey additional beneficial effects beyond adequate hydration on the incidence of AKI.
In radiocontrast-induced nephropathy, loop diuretics and mannitol in one study have been shown to exacerbate ARF.191 Weisberg et al.,197 randomized patients undergoing contrast-medium investigations to receive saline or one of three renal vasodilator/diuretic drugs (dopamine [2 μg/kg/min], mannitol [15 g/dl in a one-half isotonic saline solution given at 100 ml/h] or atrial natriuretic peptide). Dopamine, mannitol, and atrial natriuretic peptide were associated with a much higher incidence of renal dysfunction in diabetic subjects compared to patients receiving saline alone.
Mannitol is often added to the priming fluid of the cardiopulmonary bypass system to reduce the incidence of renal dysfunction, but the results of these studies are not very convincing.198 Two small randomized trials—one in patients with pre-existing normal renal function,199 the second in patients with established renal dysfunction200—did not find differences for any measured variable of renal function. More convincing are the results obtained with the preventive administration of mannitol, just before clamp release, during renal transplantation.201, 202 The sparse controlled data available have shown that 250 ml of mannitol 20% given immediately before vessel clamp removal reduces the incidence of post-transplant AKI, as indicated by a lower requirement of post-transplant dialysis. However, 3 months after transplantation, no difference is found in kidney function compared to patients who did not receive mannitol.203
It has also been suggested that mannitol is beneficial in rhabdomyolysis by stimulating osmotic diuresis and by lowering the intracompartmental pressure in the affected crushed limbs204, 205, 206; again, these studies were either not randomized or underpowered. A separate guideline on crush injury associated with disasters, mainly earthquake victims, is under preparation by the ISN Renal Disaster Relief Task Force.
In summary, despite experimental animal data and the anecdotal human evidence for the beneficial effects of mannitol, there are no adequately powered prospective RCTs comparing mannitol vs. other strategies. Based on these considerations, the Work Group concludes that mannitol is not scientifically justified in the prevention of AKI.
RESEARCH RECOMMENDATION
Given the potential to mitigate fluid overload but also to worsen renal function and possibly cause kidney injury, further study is required to clarify the safety of loop diuretics in the management of patients with AKI.
Chapter 3.5: Vasodilator therapy: dopamine, fenoldopam, and natriuretic peptides
DOPAMINE FOR THE PREVENTION OR TREATMENT OF AKI
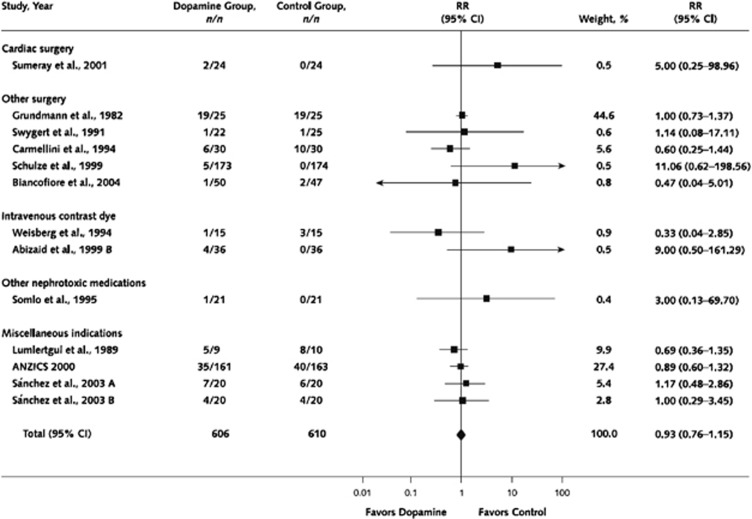
Dopamine was once commonly used for renal protection in the critically ill. However, with multiple negative studies, including a randomized, double-blind, placebo-controlled trial of adequate size and power,207 its use has been abandoned by most. Low-dose dopamine administration (1–3 μg/kg/min) to healthy individuals causes renal vasodilation, natriuresis, and increased GFR; because of these effects, it has been given as prophylaxis for AKI associated with radiocontrast administration, repair of aortic aneurysms, orthotopic liver transplantation, unilateral nephrectomy, renal transplantation, and chemotherapy with interferon.208 The majority of prevention trials with low-dose dopamine have been small, inadequately randomized, of limited statistical power, and with end-points of questionable clinical significance. Furthermore, recent data suggest that the renal vasodilatory effect of dopamine found in healthy populations is not preserved in patients with AKI. Using Doppler ultrasound, Lauschke et al.209 found that dopamine significantly increased renal vascular resistance in AKI patients. Kellum and Decker210 found no benefit of dopamine for prevention or therapy of AKI in an adequately-powered meta-analysis, and Marik211 found no benefit in a systematic review.
There is also limited evidence that the use of dopamine to prevent or treat AKI causes harm. Although the meta-analysis by Friedrich et al.,212 found no significant increase in adverse events or evidence of harm from low-dose dopamine, there is significant literature demonstrating adverse effects of dopamine, even at low doses. It can trigger tachyarrhythmias and myocardial ischemia, decrease intestinal blood flow, cause hypopituitarism, and suppress T-cell function.208 Taken together with the lack of positive trials to support the use of dopamine for AKI prevention or therapy, the aforementioned potential deleterious effects of this drug provide additional arguments for abandoning its use entirely for the prevention and therapy of AKI.
3.5.1: We recommend not using low-dose dopamine to prevent or treat AKI. (1A)
RATIONALE
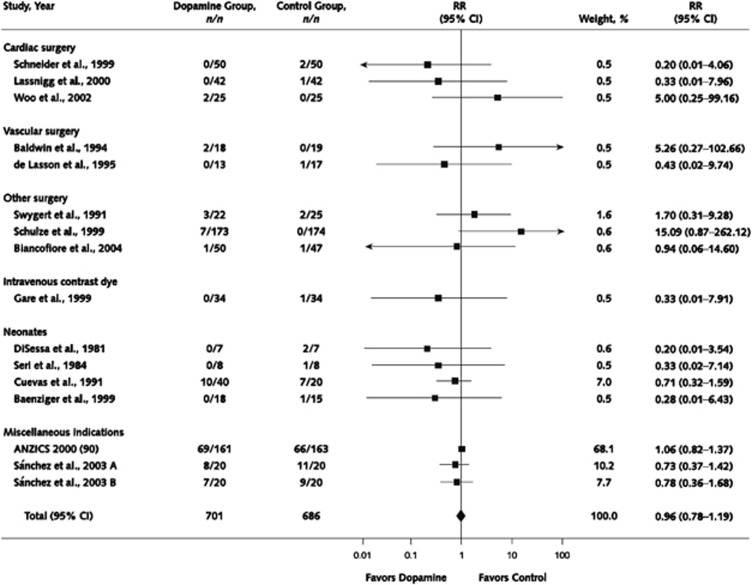
In their meta-analysis, Friedrich et al.,212 did not specifically separate prophylactic trials from trials where dopamine was used therapeutically in patients with established AKI, because many of the original trials failed to do so.210 The authors analyzed 61 randomized or quasi-randomized controlled trials of low-dose dopamine, and found no improvement of survival (Figure 11), no decrease in dialysis requirement (Figure 12), no improvement in renal function, and improved urine output only on the first day of dopamine therapy.212 Similarly, although there were trends towards transiently greater urine output, lower SCr, and higher GFR in dopamine-treated patients on day 1 of therapy (but not days 2 and 3), there was no evidence of a sustained beneficial effect on renal function. In an earlier systematic review, Kellum et al.,210 performed an analysis of studies that reported incidence of AKI as an outcome, which developed in 15.3% in the dopamine arms and 19.5% in the control arms (RR 0.79 [0.54–1.13]). Similar to the earlier analysis by Kellum et al., restriction of the Work Group's analysis to prevention trials did not disclose any benefit of dopamine vs. placebo therapy. Similarly, analysis of adequate trials restricted to patients treated for AKI does not suggest a benefit of dopamine therapy. Specifically, a relatively large randomized, placebo-controlled trial in 328 critically ill patients with early AKI sufficiently powered to detect a small benefit was reported.207 There was no effect of low-dose dopamine on renal function, need for dialysis, ICU or hospital length of stay (LOS), or mortality (Suppl Table 4). Taken together, these analyses found no evidence that dopamine therapy is effective in the prevention or treatment of AKI.
Figure 11.
Effect of low-dose dopamine on mortality. Reprinted from Friedrich JO, Adhikari N, Herridge MS et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 2005; 142: 510–524 with permission from American College of Physicians212; accessed http://www.annals.org/content/142/7/510.full
Figure 12.
Effect of low-dose dopamine on need for RRT. Reprinted from Friedrich JO, Adhikari N, Herridge MS et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 2005; 142: 510–524 with permission from American College of Physicians212; accessed http://www.annals.org/content/142/7/510.full
FENOLDOPAM FOR THE PREVENTION OR TREATMENT OF AKI
Fenoldopam mesylate is a pure dopamine type-1 receptor agonist that has similar hemodynamic renal effects as low-dose dopamine, without systemic α- or β-adrenergic stimulation.213
3.5.2: We suggest not using fenoldopam to prevent or treat AKI. (2C)
RATIONALE
The results of animal experiments and small human studies measuring perioperative GFR in patients undergoing coronary artery bypass graft and aortic cross-clamp surgery suggested that fenoldopam might prevent or ameliorate the course of AKI.139 Cogliati et al.,214 conducted a double-blind, randomized trial of fenoldopam infusion for renal protection in 193 high-risk cardiac surgery patients, who were randomized to receive a continuous infusion of fenoldopam, 0.1 μg/kg/min (95 patients) or placebo (98 patients) for 24 hours. AKI was defined as a postoperative SCr level of ⩾2 mg/dl (⩾177 μmol/l) with an increase in SCr level of ⩾0.7 mg/dl (⩾61.9 μmol/l) from preoperative to maximum postoperative values. AKI developed in 12 of 95 (12.6%) patients receiving fenoldopam and in 27 of 98 (27.6%) patients receiving placebo (P=0.02), and RRT was started in 0 of 95 and 8 of 98 (8.2%) patients, respectively (P=0.004). These results suggested that a 24-hour infusion of 0.1 μg/kg/min of fenoldopam prevented AKI in a high-risk population undergoing cardiac surgery. A meta-analysis of 1059 patients in 13 studies that included this trial found that fenoldopam reduces the need for RRT and in-hospital death in cardiovascular surgery patients.215 However, the pooled studies included both prophylactic and early therapeutic studies, as well as propensity-adjusted case-matched studies (rather than purely randomized trials). A 1000-patient RCT of fenoldopam to prevent the need for RRT after cardiac surgery is currently underway (ClinicalTrials.gov identifier: NCT00621790); meanwhile, this remains an unproven indication for fenoldopam therapy.
Finally, Morelli et al.,216 in a prospective, double-blind trial, randomized 300 septic patients without renal dysfunction to receive infusions of fenoldopam (0.09 μg/kg/min) and compared these individuals to a placebo group; the treatment continued as long as the patient was in the ICU. The fenoldopam group had a significantly lower rate of AKI (29 vs. 51 patients, P=0.006; OR of 0.47, P=0.005), and shorter ICU stays, without any increase in complications. The incidence of severe AKI, dialysis, and death were not different between the groups. This study requires a larger confirmatory trial, which should be powered to test effectiveness in improving dialysis-free survival.
Emerging data from experimental AKI models suggest that fenoldopam may have multiple protective effects in AKI, including anti-inflammatory effects independent of any vasodilatory action.217, 218 Further large studies will be required to determine if fenoldopam is an effective renoprotective agent.213, 219 As discussed elsewhere in this guideline (Section 4), despite promising pilot study findings, fenoldopam was ultimately found to be ineffective for the prevention of CI-AKI,220 and as a potent antihypertensive (the only approved indication for the drug), fenoldapam carries a significant risk of hypotension.
Fenoldopam mesylate has also been studied for early treatment of AKI. Tumlin et al.,221 conducted a randomized, placebo-controlled pilot trial of low-dose fenoldopam mesylate in ICU patients with early AKI and found no benefit, though they did show a trend towards lower 21-day mortality and decreased need for dialysis in fenoldopam-treated patients (11% difference in dialysis-free survival). In secondary analyses, fenoldopam tended to reduce the primary end-point in patients without diabetes and postoperative cardiothoracic surgery patients with early ATN.
Brienza et al.,222 conducted a prospective, multicenter, RCT of fenoldopam therapy for early AKI in critically ill patients. The study included hemodynamically stable adults with renal dysfunction. This 100- subject study compared 4-day infusions of fenoldopam (0.1 μg/kg/min) or dopamine (2 μg/kg/min); there was no placebo arm. The primary end-point of the study was a between-group comparison of the maximum change of SCr over time during the 4-day study period. The peak SCr values and maximum increments during the study did not differ between the fenoldopam and dopamine groups; however, in the fenoldopam group at the end of infusion, SCr had decreased by 0.29 ± 0.77 mg/dl (25.6 ± 68.1 μmol/l), a value significantly different from the dopamine group (0.09 ± 0.94 mg/dl [7.96 ± 83.1 μmol/l] P=0.05). Also, the maximum decreases of SCr levels from study entry were significantly larger in the fenoldopam group. There was no difference in heart rate, blood pressure, incidence of hypotension, or urinary output (apart from a transiently higher value within the first study day in the dopamine group). The authors concluded that, for critically ill patients with impaired renal function, a continuous infusion of fenoldopam 0.1 μg/kg/min improves renal function when compared to renal-dose dopamine, without significant adverse effects. The study has, however, a number of deficiencies, including the lack of a true control, unblinding of the investigators, and an unorthodox AKI definition, among other limitations, but taken together with other positive trends in the literature, these results add to the discourse around fenoldopam's use to treat early AKI in critically ill patients. Similarly, Landoni et al.,223 in a recently published meta-analysis found that fenoldopam decreased the risk of requiring acute RRT and resulted in a lower all-cause, in-hospital mortality (15.1%) compared to controls (18.9% OR 0.64; 95% CI 0.4–0.91), along with a nonsignificant trend towards more hypotension or pressor use in the fenoldopam group.
Our analysis revealed three suitable prophylactic studies of adequate size and study design (Suppl Tables 5 and 6) that reported AKI incidence in patients randomized to fenoldopam (n=1790) vs. placebo (n=1839). The pooled RR and 95% CI was 0.96 (0.76–1.2), P=NS. Only one study reported mortality (8-day) in sepsis patients randomized to fenoldopam (35%, n=150) vs. placebo (44%, n=150), with a RR of 0.79 (95% CI 0.59–1.05; P=0.1).
In our analysis of the two suitable studies of fenoldopam therapy for AKI, only one study221 reported (21-day) mortality in critically ill patients with early AKI randomized to fenoldopam (11/80; 13.8%) vs. placebo (n=19/75, 25.3% P=0.068) (Suppl Tables 7 and 8). The other study222 reported the change in renal function in AKI patients randomized to fenoldopam (n=50) vs. dopamine (n=50), defined by the absolute SCr change between the beginning and end of the study drug infusion and maximum decrease from study entry, which were significantly larger in the fenoldopam group with a pooled RR of 0.96 (95% CI 0.76–1.2; P=NS). These two studies reported new RRT incidence in patients with AKI randomized to fenoldopam (n=130) vs. placebo (n=125). In the study by Tumlin et al., no difference in requirement of RRT was found (with fenoldopam, 13 of 80 patients; 16.25%); with placebo (19 of 75 patients; 25.3% P=0.163). Requirement of RRT was very rare in the study of Brienza et al., and was prescribed in a total of only five patients; three in the dopamine group and two in fenoldopam group (P=NS). Overall, no data from adequately powered multicenter trials with clinically significant end-points and adequate safety are available to recommend fenoldopam to either prevent or treat AKI. The guideline recommendation against using fenoldopam places a high value on avoiding potential hypotension and harm associated with the use of this vasodilator in high-risk perioperative and ICU patients, and a low value on potential benefit, which is currently only suggested by relatively low-quality single-center trials.
RESEARCH RECOMMENDATION
While randomized trials of fenoldopam to treat AKI in a variety of settings (critical illness, high-risk surgery— particular cardiac, sepsis) may be considered, the pharmacologic strategy of renal vasodilatation has not been successful to date and different approaches are likely needed.
NATRIURETIC PEPTIDES FOR THE PREVENTION OR TREATMENT OF AKI
Several natriuretic peptides are in clinical use or in development for treatment of congestive heart failure (CHF) or renal dysfunction, and could potentially be useful to prevent or treat AKI.
Atrial natriuretic peptide (ANP) is a 28-amino-acid peptide with diuretic, natriuretic, and vasodilatory activity.224 ANP is mainly produced in atrial myocytes, and the rate of release from the atrium increases in response to atrial stretch.225 Early animal studies showed that ANP decreases preglomerular vascular resistance and increases postglomerular vascular resistance, leading to increased GFR.226 It also inhibits renal tubular sodium reabsorption. Increases in GFR and diuresis have also been confirmed in clinical studies.227 It could thus be expected that ANP might be useful for treatment of AKI, and several RCTs have been conducted to test this hypothesis.
3.5.3: We suggest not using atrial natriuretic peptide (ANP) to prevent (2C) or treat (2B) AKI.
RATIONALE
There have been several negative studies of prophylactic ANP therapy; for example, ANP failed in two studies to prevent primary renal transplant dysfunction228, 229 and ANP prophylaxis also failed to prevent CI-AKI.230 Based on the positive results of small clinical studies using ANP to treat AKI, a randomized placebo-controlled trial in 504 critically ill patients with AKI was conducted.231 Patients received 24-hour i.v. infusion of either ANP (0.2 μg/kg/min) or placebo. The primary outcome was dialysis-free survival for 21 days after treatment. Despite the large size of the trial, ANP administration had no effect on 21-day dialysis-free survival, mortality, or change in plasma creatinine concentration. Of note, the mean SCr at enrollment (anaritide group: 4.4 mg/dl [389 μmol/l] placebo group: 5.0 mg/dl [442 μmol/l]) in this study confirms that intervention in this trial was extremely late in the course of AKI. In subgroup analysis, dialysis-free survival was higher in the treatment group for patients with oliguria (<400 ml/d; ANP 27%, placebo 7%, P=0.008). A subsequent trial in 222 patients with oliguric renal failure, however, failed to demonstrate any benefit of ANP.232 The dose and duration of ANP treatment and primary outcome were the same as the previous study. The dose of ANP might have been too high (0.2 μg/kg/min) in both studies: hypotension (systolic blood pressure <90 mm Hg) occurred more frequently in the ANP groups of both trials (in the first study, 46% vs. 18%, P<0.001; and in the second study, 97% vs. 58%, P<0.001), and this may have negated any potential benefit of renal vasodilation in these patients. In addition to an excessive dose, the failure of these large studies has also been attributed in subsequent analyses to the late initiation of the drug to patients with severe AKI and an inadequate duration of infusion (only 24 hours).
A promising, but underpowered, study of ANP to treat AKI immediately following cardiac surgery showed a decreased rate of postoperative RRT compared to placebo-treated patients.233 In this study, Sward et al. randomized 61 patients with AKI following cardiac surgery (defined as a SCr increase ⩾50% from a baseline <1.8 mg/dl [<159 μmol/l]) to receive infusion of ANP or placebo until the SCr decreased below the baseline value at enrollment, the patient died, or one of four prespecified dialysis criteria was reached. Of note, all patients received infusions of furosemide (20–40 mg/h) and oliguria, defined as a urine output ⩽0.5 ml/kg/h for 3 hours, was an exclusion criterion and an automatic dialysis indication. The primary end-point was the rate of dialysis within 21 days of enrollment. CrCl was significantly higher on the third study day in ANP-treated subjects (P=0.04). Using prespecified dialysis criteria, 21% of patients in the ANP group and 47% in the placebo group were dialyzed within 21 days (hazard ratio [HR] 0.28; 95% CI 0.10–0.73; P=0.009). The combined secondary end-point of death-or-dialysis was similarly improved in the ANP group (28%) compared to placebo (57% HR 0.35; 95% CI 0.14–0.82; P=0.017). The incidence of hypotension during the first 24 hours was 59% in the ANP group and 52% in controls (P=NS).
It is intriguing to speculate on the potential reasons for the positive outcome of this trial, compared to larger prior studies of ANP for AKI prevention and therapy. Apart from the possibility that this is a false-positive, underpowered study, possible explanations include the use of ANP earlier in the course of AKI (the mean SCr in the prior ANP studies was much higher), and at lower doses (50 ng/kg/min vs. 200 ng/kg/min) that avoided the significant rate of hypotension observed in prior trials. The use of prespecified dialysis criteria was another strength of this trial. More recently, Sward et al.,234 compared the renal hemodynamic effects of ANP and furosemide in 19 mechanically ventilated post–cardiac surgery patients with normal renal function, measuring renal blood flow, GFR, and renal oxygen extraction. ANP infusion (25–50 ng/kg/min) increased GFR, filtration fraction, fractional excretion of sodium, and urine output, accompanied by a 9% increase in tubular sodium absorption and a 26% increase in renal oxygen consumption. Furosemide infusion (0.5 mg/kg/h) increased urine output 10-fold and fractional excretion of sodium 15-fold, while decreasing tubular sodium absorption by 28% and lowering renal oxygen consumption by 23%. Furosemide also lowered GFR by 12% and filtration fraction by 7%. Thus, although the balance of renal hemodynamic and tubular effects of the two drugs appears to favor furosemide for improving renal oxygen delivery-consumption balance, ANP is more likely to acutely improve GFR. One might speculate that the use of furosemide infusion in all of the subjects in the successful ANP trial may have provided an important protection against renal ischemia by reducing tubular sodium absorption and associated oxygen consumption, despite an increase in GFR in the ANP group. A larger prospective trial of ANP to improve dialysis-free survival in this setting is required, perhaps with and without furosemide infusion.
Pooled analysis of 11 studies involving 818 participants in the prevention cohort showed a trend toward reduction in the need for RRT in the ANP group (OR 0.45; 95% CI 0.21–0.99; P=0.05). Restricting the analysis to studies that used low-dose ANP preparations did not change the overall effect for this outcome. There was no significant difference noted between the ANP and control groups for mortality in the prevention category (OR 0.67; 95% CI 0.19–2.35; P=0.53), and this effect was unchanged by restricting the analysis to studies that used low-dose ANP preparations. However, these studies were generally of poor quality, several without reported baseline SCr values or clear definitions of AKI or RRT indications (Suppl Tables 10 and 11), and only one was of adequate quality.
Nigwekar et al., recently conducted a systematic review and meta-analysis of ANP for management of AKI.235 They found 19 relevant studies, among which 11 studies were for prevention and eight were for treatment of AKI. Pooled analysis of the eight treatment studies, involving 1043 participants, did not show significant difference for RRT requirement between the ANP and control groups (OR 0.59; 95% CI 0.32–1.08; P=0.12). There was also no significant difference for mortality (OR 1.01; 95% CI 0.72–1.43; P=0.89). However, low-dose ANP preparations were associated with significant reduction in RRT requirement (OR 0.34; 95% CI 0.12–0.96; P=0.04). The incidence of hypotension was not different between the ANP and control groups for low-dose studies (OR 1.55; 95% CI 0.84–2.87), whereas it was significantly higher in the ANP group in the high-dose ANP studies (OR 4.13; 95% CI 1.38–12.41). Finally, a pooled analysis of studies that examined oliguric AKI did not show any significant benefit from ANP for RRT requirement (OR 0.46; 95% CI 0.19–1.12; P=0.09) or mortality (OR 0.94; 95% CI 0.62–1.43; P=0.79). Only two of the treatment studies included in the Nigwekar analysis231, 232 were of adequate size and quality to meet the criteria for our systematic review (Suppl Tables 12 and 13), which found no significant inconsistencies in the findings of both trials that (combined) included 720 subjects (351 treated with ANP) (Suppl Table 12). Thus, although subset analyses separating low-dose from high-dose ANP trials suggest potential benefits, the preponderance of the literature suggests no benefit of ANP therapy for AKI. Therefore, the Work Group suggests that these agents not be used to prevent or treat AKI. This conclusion is based on placing a high value on avoiding potential hypotension and harm associated with the use of a vasodilator in high-risk perioperative and ICU patients, and a low value on potential benefit which is supported by relatively low-quality evidence from retrospective subset analyses from negative multicenter trials.
Urodilatin is another natriuretic peptide that is produced by renal tubular cells, and was found to have the same renal hemodynamic effect as ANP without systemic hypotensive effects.236 Limited data suggest that urodilatin improves the course of established postoperative AKI.237 Fifty-one patients who received orthotopic heart transplants received urodilatin (6–20 ng/kg/min) up to 96 hours postoperatively. AKI occurred in 6% of these patients, compared to 20% in a historical control group that did not receive urodilatin.237 However, in another small, placebo-controlled study of 24 patients who underwent orthotopic heart transplants, the incidence of AKI was unchanged,238 although duration of hemofiltration (HF) was significantly shorter and the frequency of intermittent hemodialysis (IHD) less in those who received urodilatin. Taken together, these data suggest that natriuretic peptides may have a role in the therapy of early AKI following cardiac surgery, but further prospective trials are needed to confirm this potential indication.
Nesiritide (brain natriuretic peptide) is the latest natriuretic peptide introduced for clinical use, and is approved by the Food and Drug Administration (FDA) only for the therapy of acute, decompensated CHF. Meta-analysis of outcome data from these and some other nesiritide CHF trials has generated some controversy.239, 240, 241 Sackner-Bernstein et al.,239 analyzed mortality data from 12 randomized trials; in three trials that provided 30-day mortality data, they found a trend towards an increased risk of death in nesiritide-treated subjects. In another meta-analysis of five randomized trials that included 1269 subjects,240 the same investigators also found that there was a relationship between nesiritide use and worsening renal function, defined as a SCr increase >0.5 mg/dl (>44.2 μmol/l). Nesiritide doses ⩽0.03 μg/kg/min significantly increased the risk of renal dysfunction compared to non–inotrope-based controls or compared to all control groups (including inotropes). Even at doses ⩽0.015 μg/kg/min, nesiritide was associated with increased renal dysfunction compared to controls. There was no difference in dialysis rates between the groups. Another retrospective study determined independent risk factors for 60-day mortality by multivariate analysis in a cohort of 682 elderly heart-failure patients treated with nesiritide vs. those who were not.242 When patients were stratified according to nesiritide usage, AKI emerged as an independent risk factor for mortality only among patients who received the drug. Strikingly, among these heart-failure patients who developed AKI, nesiritide usage emerged as the only independent predictor of mortality.
The manufacturers of nesiritide convened an expert panel, which concluded that further trial data are needed to discern the effects of nesiritide therapy on renal function and survival in patients with decompensated CHF. The panel also re-emphasized that the indication for nesiritide therapy is acute decompensated CHF, not chronic intermittent therapy or other uses, and in particular noted that the drug should not be used to improve renal function or in place of diuretic therapy in CHF patients, as there is no proof of the utility of the drug for these purposes. A 7000-patient multicenter RCT in acute decompensated heart failure is currently in progress to determine the clinical effectiveness of nesiritide therapy for acute decompensated heart failure (the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure; Clinicaltrials.gov identifier NCT00475852). Meanwhile, nesiritide is approved for treatment of symptomatic acute decompensated heart failure.
Uncontrolled studies using nesiritide for cardiovascular support of patients with CHF undergoing cardiac surgery have suggested beneficial effects on renal function. Mentzer et al.,243 conducted a 303-patient, multicenter, randomized, double-blind trial of a 24- to 96-hour infusion of 0.01 μg/kg/min of nesiritide vs. placebo in patients with chronic left ventricular dysfunction (ejection fraction ⩽40%) undergoing cardiac surgery using cardiopulmonary bypass. The Nesiritide Administered Peri-Anesthesia in Patients Undergoing Cardiac Surgery trial was an exploratory, safety-oriented study with five primary end-points, including three renal end-points and two hemodynamic end-points. There were no significant differences between the groups in baseline patient characteristics; SCr values were ∼1.1 mg/dl (97.2 μmol/l), with eGFR ∼80 ml/min per 1.73 m2. The mean duration of study drug infusion was ∼40 hours in both groups. Perioperative renal function quantified by the three renal primary end-points was better in the nesiritide group (peak SCr increase of 0.15 mg/dl [13.3 μmol/l] vs. placebo group 0.34 mg/dl [30.1 μmol/l] P<0.001; eGFR decrease of −10.2 ml/min per 1.73 m2 vs. placebo −17.8 ml/min per 1.73 m2, P=0.001; initial 24-hour urinary output 2.9 ± 1.2 l vs. placebo 2.3 ± 1 l; P<0.001). The RR of AKI in the nesiritide group compared to placebo was 0.58 (0.27–1.21); the 180-day mortality was also reduced in the nesiritide group (RR 0.48 [0.22–1.05] P=0.046) (Suppl Table 9). These trends were more pronounced in the small, 62-patient subset with preoperative SCr values >1.2 mg/dl (>106 μmol/l). Although SCr increased postoperatively in both groups, it returned to baseline within 12 hours in the nesiritide group, and remained elevated throughout hospitalization in the placebo group. Use of vasoactive drugs and hemodynamic parameters did not differ significantly between the groups. Adverse events also were similar between the groups, as was 30-day and 180-day mortality (although capture of mortality data was incomplete). Thus, it appears that administration of nesiritide infusion during and after cardiac surgery with cardiopulmonary bypass in patients with preoperative left ventricular dysfunction has favorable short-term effects on renal function, with short-term adverse effects comparable to placebo infusion; however, as mentioned earlier, this is not an FDA-approved indication for this drug. It is interesting to speculate that, based upon these results, any renoprotective effect of this vasoactive drug during and after cardiopulmonary bypass is not mediated by effects on systemic perfusion (similar in both groups), but rather suggesting an effect on regional perfusion or a pleiotropic phenomenon. Unfortunately, these promising pilot study findings have not been followed up with a confirmatory prospective clinical trial.
A prospective, randomized clinical trial (the Nesiritide Study), found no benefit of nesiritide for 21-day dialysis and/or death in patients undergoing high-risk cardiovascular surgery.244 However, the study did demonstrate that the prophylactic use of nesiritide was associated with reduced incidence of AKI, the latter defined by the AKIN Group, in the immediate postoperative period (nesiritide 6.6% vs. placebo 28.5%, P=0.004). Recently, Lingegowda et al.245 investigated whether the observed renal benefits of nesiritide had any long-term impact on cumulative patient survival and renal outcomes. Data on all 94 patients from the Nesiritide Study were obtained with a mean follow-up period of 20.8 ± 10.4 months. No differences in cumulative survival between the groups were noted, but patients with in-hospital incidence of AKI had a higher rate of mortality than those with no AKI (41.4% vs. 10.7% P=0.002). It seemed, thus, that the possible renoprotection provided by nesiritide in the immediate postoperative period was not associated with improved long-term survival in patients undergoing high-risk cardiovascular surgery.
In summary, although evidence from a variety of small studies suggests the potential for therapy with natriuretic peptides to be useful for the prevention or treatment of AKI in a variety of settings, there are no definitive trials to support the use of ANP, BNP, or nesiritide for these purposes. Thus, the Work Group suggests that these agents not be used for prevention or treatment of AKI.
RESEARCH RECOMMENDATION
We recommend further trials of ANP at doses below 0.1 μg/kg/min, for the prevention or treatment of AKI. There is a possibility that ANP might be effective if it is given at a lower dose (0.01–0.05 μg/kg/min) in patients prophylactically or with early AKI, and during a longer period than in previous large studies.
Chapter 3.6: Growth factor intervention
Recovery from AKI involves increased expression of various growth factors acting via autocrine, paracrine, and endocrine mechanisms. The advent of recombinant growth factors has stimulated research exploring their therapeutic potential in AKI. Experimental studies have yielded promising results with individual growth factors246 including insulin-like growth factor-1 (IGF-1), hepatic growth factor, and, more recently, erythropoietin. The physiological basis for the use of erythropoietin in the prevention of AKI has recently been described.247
3.6.1: We recommend not using recombinant human (rh)IGF-1 to prevent or treat AKI. (1B)
RATIONALE
IGF-1 is a peptide with renal vasodilatory, mitogenic and anabolic properties. rhIGF-1 has been demonstrated to accelerate the recovery of renal function in several animal models of AKI.248, 249, 250, 251 Three double-blind, placebo-controlled RCTs have addressed the usefulness of IGF-1 in adults with imminent or established AKI.252, 253, 254 Franklin et al.,252 administered rhIGF-1 every 12 hours for 3 days postoperatively to 54 patients undergoing abdominal aortic surgery. While no patient developed ARF, a smaller proportion of IGF-1–treated patients showed a decline in GFR as compared to the placebo group (22% vs. 33%). Hladunewich et al.,254 administered rhIGF-1 or placebo in 43 patients undergoing cadaveric renal transplantation at high risk of delayed graft function. Treatment was started within 5 hours of transplantation and continued for 6 days. On day 7, neither inulin clearance, nor urine flow or fractional sodium excretion differed between the treatment arms, nor did the nadir SCr after 6 weeks or the proportion of patients require post-transplantation dialysis. Hirschberg et al.,253 treated 72 patients suffering from AKI mainly due to sepsis or hemodynamic shock with either rhIGF-1 or placebo for a mean of 10.6 days. No differences were observed with respect to changes in GFR, urine output, need for RRT, and mortality. Hence, despite its therapeutic efficacy in various animal models of ARF, rhIGF-1 largely failed to prevent or accelerate recovery from established AKI in humans. In addition, the high cost of this treatment should be mentioned.
Based on an analysis of the three RCTs with rhIGF-1 that are currently available and which were overall negative or at least equivocal, and considering that there is no benefit and the concern over potential harm and cost associated with this drug, the Work Group recommends against its use in patients with AKI.
Erythropoietin
A small pilot trial evaluated the effectiveness of erythropoietin in the prevention of AKI after elective coronary artery bypass graft.255 Patients received either 300 U/kg of erythropoietin or saline i.v. before surgery. AKI was defined as a 50% increase in SCr levels over baseline within the first five postoperative days. Of 71 patients, 13 developed postoperative AKI: three of the 36 patients in the erythropoietin group (8%) and 10 of the 35 patients in the placebo group (29% P=0.035). The increase in postoperative SCr concentration and the decline in postoperative eGFR were significantly lower in the erythropoietin group than in the placebo group.
More recently, Endre et al.,256 performed a prospective randomized trial with erythropoietin in the primary prevention of AKI in ICU patients at risk for AKI (Suppl Table 14). As a guide for choosing the patients for treatment the urinary levels of two biomarkers, the proximal tubular brush border enzymes c-glutamyl transpeptidase and alkaline phosphatase were measured. Randomization to either placebo or two doses of erythropoietin was triggered by an increase in the biomarker concentration product to levels above 46.3. The primary outcome was the relative average SCr increase from baseline over 4–7 days. The triggering biomarker concentration product selected patients with more severe illness and at greater risk of AKI, dialysis, or death; however, the urinary marker elevations were transient. The use of the biomarkers allowed randomization within an average of 3.5 hours of a positive sample. There was no difference in the incidence of erythropoietin-specific adverse events; however, there was also no difference in the primary outcome between the placebo and treatment groups.
RESEARCH RECOMMENDATION
Recent animal studies suggest a potential clinical benefit of erythropoietin in AKI. In various rodent models of AKI, erythropoietin consistently improved functional recovery. The renoprotective action of erythropoietin may be related to pleomorphic properties including antiapoptotic and antioxidative effects, stimulation of cell proliferation, and stem-cell mobilization.247 Although one recent RCT in the prevention of human AKI was negative, the usefulness of erythropoietin in human AKI should be further tested in RCTs.
Chapter 3.7: Adenosine receptor antagonists
The activation of tubuloglomerular feedback in response to elevated luminal chloride concentrations in the distal renal tubules is an early event in ischemic AKI. Adenosine released as part of the tubuloglomerular feedback loop binds to glomerular adenosine A1 receptor, causing vasoconstriction of the afferent arteriole, decreased renal blood flow and GFR, and sodium and water retention. This well-known role of adenosine in this phenomenon has stimulated a body of research seeking to prevent or treat AKI with adenosine receptor antagonists, primarily in three clinical syndromes with increased risk of AKI: perinatal asphyxia, radiocontrast exposure, and cardiorenal syndrome. Theophylline is a nonselective adenosine receptor antagonist.
3.7.1: We suggest that a single dose of theophylline may be given in neonates with severe perinatal asphyxia, who are at high risk of AKI. (2B)
RATIONALE
AKI occurs in 60% of neonates suffering from perinatal asphyxia.257 Experimental studies indicated an important role of adenosine-mediated vasoconstriction in neonatal kidneys exposed to normocapnic hypoxemia.258 A potential renoprotective effect of theophylline in perinatal asphyxia has been assessed in three randomized, placebo-controlled clinical trials,259, 260, 261 including a total of 171 term neonates. Theophylline was uniformly administered in the first hour of life as a single i.v. bolus at a dose of 5 mg/kg259, 261 or 8 mg/kg.260 The three studies all observed significantly higher GFR, higher urine output with more negative fluid balance, and lower urinary β2-microglobulin excretion, with theophylline as compared to placebo during the first 3–5 days of life. In each study, theophylline treatment was associated with a significantly reduced risk of severe renal dysfunction (17–25% vs. 55–60% in placebo group, RR 0.3–0.41). The beneficial effect was selective for kidney function, whereas the incidence of central nervous system, cardiac, pulmonary, and gastrointestinal complications was unaltered. Patient survival was not affected by treatment. In line with these studies in mature neonates, a similar improvement of GFR and urine output was observed during the first 2 days of life by administration of 1 mg/kg theophylline vs. placebo in 50 very preterm neonates with respiratory distress syndrome.262 The further evolution of renal function was followed throughout the first year of life by Bhat et al.,260 who found equally normal glomerular and tubular function in both groups from 6 weeks of age onward. Hence, while theophylline clearly improves renal function in the first week of life in postasphyctic neonates, the overall benefit from this intervention in neonatal intensive care is less evident in view of the complete long-term recovery of renal function in the placebo-treated controls and the absence of an effect on patient survival.
In recent years, the advent of selective adenosine A1 receptor antagonists has prompted the conduct of some interesting clinical trials, which to date have focused on the prevention and treatment of cardiorenal syndrome. In a double-blind placebo-controlled trial of 63 patients with CHF, single doses of the adenosine A1 antagonist BG9719 had a marked stimulatory effect on diuresis and increased GFR.263 When coadministered with furosemide, BG9719 showed a synergistic diuretic effect and prevented the decrease in GFR associated with the loop diuretic.
Rolofylline, another adenosine A1 receptor antagonist, was tested in two double-blind placebo-controlled RCTs in patients with acute decompensated heart failure. In the first study, rolofylline or placebo was administered either concomitantly with furosemide for 3 days (146 patients), or as a single infusion in 35 diuretic resistant patients.264 In both substudies, rolofylline improved urine output and CrCl compared to placebo. The second trial involved 301 patients hospitalized for acute heart failure with renal impairment who received either placebo or one of three doses of rolofylline for 3 days.265 Rolofylline administration dose-dependently attenuated the rise in SCr observed in the placebo group within 14 days, and tended to reduce 60-day mortality or readmission for cardiovascular or renal causes.
Three pivotal phase III trials in a total of 2500 patients were recently completed, aiming to corroborate the renoprotective effects of rolofylline in patients with cardiorenal syndrome, and to establish drug safety. The final results of the PROTECT trial have recently been published.266 Rolofylline, as compared to placebo, did not provide a benefit with respect to the three primary end-points: survival, heart-failure status, and changes in renal function. Persistent renal impairment developed in 15.0% of patients in the rolofylline group and in 13.7% of patients in the placebo group (P=0.44). By 60 days, death or readmission for cardiovascular or renal causes had occurred in similar proportions of patients assigned to rolofylline and placebo (30.7% and 31.9%, respectively; P=0.86). Adverse-event rates were similar overall; however, only patients in the rolofylline group had seizures, a known potential adverse effect of A1-receptor antagonists. Thus, rolofylline does not appear to be effective for treatment of cardiorenal AKI.
RESEARCH RECOMMENDATION
It appears that if there are benefits of using adenosine receptor antagonists to decrease tubuloglomerular feedback-mediated vasoconstriction and increase renal blood flow and GFR in AKI, they may be limited to very specific populations (e. g., asphyctic neonates). These benefits must be balanced against potential adverse drug effects: both renal (increased renal blood flow and distal salt delivery might harmfully increase tubular oxygen consumption in the presence of ATN), and nonrenal (lower seizure threshold). Thus, further studies are still needed to clarify the role for theophylline in neonates.
Chapter 3.8: Prevention of aminoglycoside- and amphotericin-related AKI
AMINOGLYCOSIDE NEPHROTOXICITY
Aminoglycoside antimicrobial agents are highly potent, bactericidal antibiotics effective against multiple Gram-negative, and selected Gram-positive bacterial pathogens when administered with beta-lactams and other cell-wall active antimicrobial agents.267, 268, 269 Progressive antimicrobial resistance to other antimicrobial agents and lack of new alternatives to aminoglycoside antibiotics have caused a recent increase in their use. Aminoglycosides have many favorable attributes, including their remarkable stability, predictable pharmacokinetics, low incidence of immunologically mediated side-effects, and lack of hematologic or hepatic toxicity. Nephrotoxicity, and to a lesser degree ototoxicity and neuromuscular blockade, continue to be the major dose-limiting toxicities of the aminoglycosides. Careful dosing and therapeutic drug monitoring of aminoglycosides using pharmacokinetic and pharmacodynamic principles can mitigate the risk of AKI with these clinically useful, yet nephrotoxic antibiotics.270 A number of meta-analyses and treatment guidelines have been published recently indicating that the risk of AKI attributable to aminoglycosides is sufficiently frequent that they should no longer be added to other standard antimicrobial agents for the empirical or directed treatment of a number of severe Gram-positive or Gram-negative bacterial infections.271, 272, 273, 274, 275, 276 The intrinsic risk of AKI with the administration of aminoglycosides has led some authors to call for elimination of aminoglycosides as a therapeutic option in current clinical management of infectious diseases.277 The anticipated demise of aminoglycosides from our therapeutic armamentarium has not occurred, however, in light of recent developments with progressive antimicrobial resistance to beta-lactams, quinolones, and a number of other classes of antimicrobial agents.
3.8.1: We suggest not using aminoglycosides for the treatment of infections unless no suitable, less nephrotoxic, therapeutic alternatives are available. (2A)
RATIONALE
Aminoglycosides exhibit a number of favorable pharmacokinetic and pharmacodynamic advantages, but a major dose-limiting toxicity of the aminoglycosides remains the risk of drug-induced AKI.270 The risk of AKI attributable to aminoglycosides is sufficiently high (up to 25% in some series, depending upon the definition of AKI used and the population studied)271, 272, 273, 274, 275, 276, 278 that they should no longer be used for standard empirical or directed treatment, unless no other suitable alternatives exist. The intrinsic risk of AKI with the administration of aminoglycosides has led some authors to recommend the elimination of aminoglycosides as a clinical treatment option.277 Certainly their use should be restricted to treat severe infections where aminoglycosides are the best, or only, therapeutic option.
Aminoglycosides should be used for as short a period of time as possible. Repeated administration of aminoglycosides over several days or weeks can result in accumulation of aminoglycosides within the renal interstitium and within the tubular epithelial cells.279 This can result in a higher incidence of nephrotoxicity with repeated exposure to aminoglycosides over time. Older patients (>65 years), patients with pre-existing renal dysfunction, and septic patients with intravascular volume depletion and rapid alterations in fluid dynamics may be at greater risk for aminoglycoside nephrotoxicity. Other risk factors for aminoglycoside-induced AKI are diabetes mellitus, concomitant use of other nephrotoxic drugs, prolonged use, excessive blood levels, or repeated exposure to separate courses of aminoglycoside therapy over a short time interval.267, 268, 269, 270, 271, 272, 273, 274, 275, 276, 277, 278, 279
3.8.2: We suggest that, in patients with normal kidney function in steady state, aminoglycosides are administered as a single dose daily rather than multiple-dose daily treatment regimens. (2B)
RATIONALE
Aminoglycoside demonstrates concentration-dependent bactericidal activity, with a prolonged “postantibiotic effect”, thereby permitting extended interval dosing in an effort to optimize efficacy and minimize toxicity. This dosing strategy and a number of other measures to limit aminoglycoside uptake in renal tubular cells, prevent apoptosis, limit oxygen injury, and protect mitochondrial function have all been recommended to minimize the risk of AKI and preserve the therapeutic value of these important antimicrobial agents.280, 281, 282, 283, 284, 285, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 296 Single-dose daily or extended-interval dosing of aminoglycosides offer a number of theoretical and practical advantages to maintain antimicrobial activity while limiting possible nephrotoxicity. This convenient and inexpensive aminoglycoside dosing strategy has been widely adopted at many centers when using this potentially toxic, yet highly effective, class of antibiotics.
When feasible in patients with normal and stable kidney function, once-daily (often referred to as extended-interval) dosing of aminoglycosides should be used to limit aminoglycoside nephrotoxicity. The pharmacokinetic and pharmacodynamic properties of aminoglycosides favor high dosing strategies with extended intervals between doses. The key therapeutic parameter for efficacy is peak blood level divided by minimum inhibitory concentration (MIC) of the infecting organism (Cmax/MIC) in an effort to obtain >10-fold Cmax/MIC. Aminoglycosides induce a prolonged postantibiotic effect (inhibition of bacterial growth after blood levels have fallen below the MIC of the organism). The length of the postantibiotic effect is directly related to the peak blood levels. These pharmacokinetic/pharmacodynamic parameters make single-dose daily strategies an attractive option when using aminoglycosides.
The nephrotoxicity of aminoglycosides has been very well studied280, 281, 282, 284, 285, 286, 287, 288, 289, 290, 291, 292, 293, 295, 296 and is primarily related to uptake of aminoglycosides through a receptor known as megalin, expressed on epithelial cells along the proximal convoluted tubule.293 Aminoglycosides are concentrated in the proximal convoluted tubules, where they bind avidly to polyanionic, phospholipid-containing membranes. Aminoglycosides induce myeloid body formation, impair protein synthesis, degrade mitochondrial function, and culminate in apoptosis and eventual necrosis of renal tubular epithelial cells. Direct glomerular injury can occur288 but is usually a secondary consequence of aminoglycoside-induced tubular impairment. As the receptor uptake of aminoglycosides is saturable, high-level intermittent doses of aminoglycosides actually reduced the daily uptake and accumulation of aminoglycosides when compared to multiple-daily dosing strategies. This should limit the risk of nephrotoxicity, at least in principle.
The potential efficacy of single-dose daily regimens (or other extended dosing treatment programs) of aminoglycosides vs. multiple-daily dosing strategies has been extensively studied in numerous controlled and uncontrolled clinical studies over many years297, 298, 299, 300, 301, 302, 303, 304, 305, 306, 307, 308, and the subject has been the focus of a number of formal meta-analyses.309, 310, 311, 312, 313, 314 These investigations include pediatric populations, elderly populations, empirical therapy, targeted therapy, treatment directed towards Gram-negative bacterial pathogens and Gram-positive bacterial pathogens.
The cumulative results of this evidence-based review and numerous meta-analyses indicate that once-daily dosing strategies generally tend to result in less AKI when compared to multiple-dose dosing strategies, although the benefit accrued by the single-daily dose strategy is modest and inconsistent across a number of these studies. For this reason, a level 2 recommendation is suggested in support of the use of single-daily dose strategies over multiple-dose daily strategies. It should be noted that multiple-daily dosing strategies continue to be the standard of care for enterococcal endocarditis; no detailed, randomized trials have been reported comparing single-daily vs. multiple-daily regimens for enterococcal endocarditis.272, 315, 316, 317
The use of single-daily dosing of aminoglycosides is generally well-tolerated but bolus infusions of aminoglycosides should be avoided. The high-dose, once-daily aminoglycoside regimens should be administered over 60 minutes to avoid untoward events such as neuromuscular blockade. This recommendation is particularly important when patients are receiving other potential neuromuscular blocking agents, or have underlying disorders affecting neuromuscular transmission (e.g., myasthenia gravis).
3.8.3: We recommend monitoring aminoglycoside drug levels when treatment with multiple daily dosing is used for more than 24 hours. (1A)
RATIONALE
Therapeutic drug monitoring has been the standard of care when administering aminoglycosides for many years. Aminoglycoside levels are variable among individuals, and subtle changes in the volume distribution, renal blood flow, and filtration rate can affect renal handling of aminoglycosides and alter the risk of nephrotoxicity. For these reasons, therapeutic drug monitoring, in combination with or independent from, single-dose daily treatment regimens is recommended.318, 319, 320, 321 When using therapeutic drug monitoring in single-dose or extended-dose treatment strategies, the Cmax should be at least 10-fold greater than the MIC of the infecting microorganism. This Cmin (trough level) should be undetectable by 18–24 hours to limit accumulation of aminoglycosides in renal tubular cells and to minimize the risk of AKI. The usual dosing strategy for once-daily aminoglycosides is 5 mg/kg/d for gentamicin and tobramycin (with normal renal function); 6 mg/kg/d for netilmicin; and 15 mg/kg/d for amikacin. The multiple-dose daily regimen for gentamicin and tobramycin is usually 1.7 mg/kg every 8 hours with peak blood levels at 8 ± 2 μg/ml (17±4 μmol/l) and trough of 1–2 μg/ml (2–4 μmol/l). Amikacin levels with the multiple-dose daily dosing strategy should be a peak of 20±5 μg/ml (34±9 μmol/l) and a trough of 5–8 μg/ml (9–14 μmol/l). We recommend therapeutic drug monitoring when using prolonged courses of aminoglycosides to limit the risk of nephrotoxicity when using multiple-daily dosing, and suggest therapeutic drug monitoring when using single-daily dosing strategies.
3.8.4: We suggest monitoring aminoglycoside drug levels when treatment with single-daily dosing is used for more than 48 hours. (2C)
RATIONALE
The timing of measurement of peak doses of aminoglycosides with single-daily dosing strategies is not standardized and remains somewhat controversial. Some investigators do not measure therapeutic drug levels at all in patients receiving this dosing strategy. Others recommend at least a single peak measurement to ensure that the blood levels are 10-fold greater than the MIC of the infecting organism. Many investigators recommend at least one or at least a weekly Cmin level obtained at either 12, 18, or 24 hours after the aminoglycoside dose.267, 268, 269, 270 The Cmin level should be below the limits of detection of the assay (<1 μg/ml) at these time intervals.
Measuring aminoglycoside levels with multiple-daily dosing strategies have been standardized for Cmax to be obtained 30 minutes after a 30-minute infusion, and Cmin right before the next dose for trough levels. The aminoglycosides should be administered in patients who are volume-replete; volume depletion increases the risk of nephrotoxicity in experimental studies and is suggested in clinical studies. Additionally, potassium repletion has been shown experimentally and clinically to diminish the risk of AKI related to aminoglycoside administration.
Single-dose daily regimens are difficult to apply in patients with pre-existing kidney disease, and patients with vacillating eGFR and hemodynamics, such as critically ill patients in the ICU setting. The changing pharmacokinetics and pharmacodynamics of antibiotics in general and aminoglycosides in particular, in the critically ill patient, are such that the avoidance of single-daily dosing and application of frequent therapeutic drug monitoring is indicated.322
3.8.5: We suggest using topical or local applications of aminoglycosides (e.g., respiratory aerosols, instilled antibiotic beads), rather than i.v. application, when feasible and suitable. (2B)
RATIONALE
Local instillation of aminoglycosides for a variety of indications is gaining more widespread use in a selected set of clinical situations where aminoglycoside levels can be concentrated at specific tissue sites. The use of aminoglycoside-loaded beads for the prevention and treatment of bone and joint infections have become commonplace as a strategy to limit nephrotoxicity, while providing antimicrobial activity of aminoglycosides at the tissue level.323 Local concentrations of aminoglycoside are achieved for prolonged periods when administered by this route. Aminoglycoside aerosol delivery systems are now in use to provide high intrapulmonary antibiotic levels with minimal systemic and kidney concentrations of the antibiotic. This strategy has been used successfully in cystic fibrosis patients for the management of difficult-to-treat Gram-negative bacillary pneumonia.324, 325 However, significant nephrotoxicity with the use of inhaled tobramycin has been described in at least two cases.326, 327
RESEARCH RECOMMENDATIONS
No standard method exists for therapeutic drug monitoring of aminoglycosides by single daily dosing. Uniform guidance, based upon carefully performed pharmacokinetic/pharmacodynamic studies on the optimal timing and method of therapeutic drug monitoring with single-daily dosing regimens, would be of great assistance.319
It is generally recommended that patients receiving extended-dosing interval aminoglycosides should have aminoglycosides administered at even greater dosing intervals if mild or moderate degrees of underlying renal impairment exist. Optimal therapeutic monitoring in the setting of infrequent dosing intervals for patients with underlying CKD needs to be standardized and uniform recommendations need to be provided by careful pharmacokinetic/pharmacodynamic observational studies.
The impact of IHD and high-flux CRRT upon the efficacy and toxicity of extended-duration dosing of aminoglycosides needs further study. As membranes with greater sieving coefficients come into greater use, the impact on aminoglycoside elimination needs to be carefully considered. This could be investigated by RCTs using standard dosing intervals vs. individualized dosing regimens, with frequent drug-level monitoring and the use of efficacy measures and kidney injury markers as outcomes.
The interaction between aminoglycosides and other antimicrobial agents, and other therapeutic agents with nephrotoxic potential needs to be more carefully quantified. The degree of aminoglycoside-induced nephrotoxicity alone vs. combination effects with such drugs as vancomycin, amphotericin B, cephalosporins, extended-spectrum penicillins, colistin, loop diuretics, clindamycin, cisplatin, and nonsteroidal anti-inflammatory agents needs to be more carefully examined in observational studies.
AMPHOTERICIN B NEPHROTOXICITY
Amphotericin B has been the standard of treatment for life-threatening systemic mycoses for over 50 years. This polyene antifungal agent is insoluble in water and needs to be solubilized with deoxycholate and given i.v. in the absence of electrolyte solutions to maintain solubility. Despite its broad-spectrum fungicidal activity against a large number of invasive systemic mycoses, drug-induced nephrotoxicity is common and remains the principal dose-limiting toxicity of amphotericin B.328, 329, 330 Amphotericin B has numerous other significant toxicities, including thrombophlebitis, electrolyte disturbances, hypoplastic anemia, and systemic toxicity associated with fever, chills, hypotension, and cytokine release.331, 332 AKI related to amphotericin B is clinically significant and is associated with higher mortality rates, increased LOS, and increased total costs of health care when managing patients with systemic fungal infection.328, 330
Over the past two decades, three major advances in antifungal therapy have become clinically available: i) the lipid formulations of amphotericin B; ii) the introduction of the echinocandin class of antifungal agents; and iii) an expanding number of azoles with extended activity against a variety of fungal pathogens. Therapeutic alternatives to amphotericin B have been a welcome addition in the management of systemic mycoses and selected, protozoan, parasitic infections, but their incremental costs and tradeoffs in spectrum of activity against fungal pathogens need to be considered, in addition to their favorable toxicity profiles and reduced potential for nephrotoxicity. A number of therapeutic options are now available to the clinician when deciding upon the choice for empiric or directed antifungal therapy. Avoidance of risk of nephrotoxicity is one of the major, but not the only, determinants when selecting antifungal therapy at present.
3.8.6: We suggest using lipid formulations of amphotericin B rather than conventional formulations of amphotericin B. (2A)
RATIONALE
The broad-spectrum, polyene, antifungal agent amphotericin B deoxycholate has been the mainstay of treatment for systemic mycoses for decades. Despite its well-known toxicity profile, the potent antifungal activity of amphotericin B, in addition to its activity against certain protozoan parasites (Plasmodium spp., Leishmania spp., Naegleria spp.), indicates that this therapy will remain a standard agent in clinical medicine for the foreseeable future.
Amphotericin B–induced nephrotoxicity is related to multiple mechanisms, including ischemic injury and direct tubular- and glomerular-cell membrane toxicity. Amphotericin causes vasoconstriction of the afferent renal arteriole along with a systemic inflammatory response that may reduce renal blood flow. Amphotericin B also directly inserts into human cellular membranes, where it disrupts membrane permeability and physiology.331, 332 Tubular epithelial cells residing in the deep medullary regions of the kidney are particularly susceptible to injury where considerable osmotic stress exists across cell membranes even under physiologic conditions. The end result is enzymuria, loss of renal tubular concentrating ability, renal tubular acidosis, increasing urinary losses of potassium and magnesium, and decreased glomerular function, resulting in azotemia and decreased synthesis of erythropoietin. Amphotericin B–induced nephrotoxicity is often accompanied by concomitant administration of other potentially nephrotoxic agents such as cyclosporine A, aminoglycosides, chemotherapeutic agents, and a number of other potentially nephrotoxic agents.328, 329, 333
Considerable efforts have been undertaken to try to limit nephrotoxicity and permit the continued use of amphotericin B deoxycholate for the management of systemic mycoses. Simple maneuvers, such as salt repletion and provision of adequate amounts of potassium, are beneficial in animal models in the prevention of amphotericin B nephrotoxicity. These measures have a mixed record in clinical practice, and their capacity to prevent AKI when treating severe fungal infections remain unclear. The relative ease and simple logic of volume repletion and potassium supplementation during amphotericin B therapy supports their routine use, despite the relative lack of compelling clinical evidence to recommend these maneuvers.
Various dosing strategies have also been instituted in an attempt to limit amphotericin B–induced nephrotoxicity. One strategy is to give amphotericin B as a continuous infusion rather than a 2- to 4-hour infusion to limit nephrotoxicity.329, 334 While there is some suggestion that a continuous infusion may limit nephrotoxicity, enthusiasm for this strategy is tempered by the potential loss of some antifungal activity. Amphotericin B exhibits concentration-dependent antifungal activity, and continuous infusion of low-doses of amphotericin B could result in suboptimal protection for some patients with invasive fungal infections.334
Another common strategy is the administration of alternate-day doses of amphotericin B, rather than daily doses.335, 336 This strategy is better tolerated and might reduce nephrotoxicity without sacrificing efficacy in stable patients. However, clear evidence that this strategy reduces nephrotoxicity is not supported by large, adequately controlled clinical trials as yet.
One of the major innovations in amphotericin B therapy over the last 15 years has been the introduction of lipid formulations of amphotericin to limit the problem of nephrotoxicity associated with conventional amphotericin B deoxycholate. Three lipid formulations are available including: amphotericin B colloidal dispersion, amphotericin B lipid complex, and liposomal amphotericin B. Amphotericin B colloidal dispersion is formulated by amphotericin B complexed with cholesteryl sulfate. Amphotericin B lipid complex is composed of amphotericin B complexed with dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol. Liposomal amphotericin consists of amphotericin B complexed with hydrogenated soy phosphatidylcholine, distearoylphosphatidylcholine, and cholesterol.337, 338, 339, 340 Other formulations that might further reduce the risk of AKI from amphotericin B include nanoparticle packaging in micelles with polyaspartic acid.340
The safety and efficacy (in incidence of nephrotoxicity) of lipid formulations of amphotericin have been studied in numerous experimental and clinical trials with conventional amphotericin B deoxycholate as the comparator.337, 338, 339, 341, 342, 343, 344, 345, 346, 347, 348, 349, 350 A detailed analysis of these various trials, and a number of meta-analyses that have analyzed this clinical question, concluded that the lipid formulations are less nephrotoxic than amphotericin B deoxycholate.344, 346 When feasible, we recommend that lipid formulations supplant the use of conventional amphotericin B deoxycholate to reduce the risk of nephrotoxicity.
The incremental costs associated with the lipid formulations and their relative efficacy for systemic mycoses remains the subject of considerable debate. The existing evidence would suggest that the overall risk-benefit ratio and cost-effectiveness with these lipid formulations is essentially cost-neutral with amphotericin B deoxycholate.337, 339 Attempts to increase the doses of lipid formulations of amphotericin further to improve efficacy have resulted in mixed results and are not recommended at present.342, 343
Lipid formulations of amphotericin are less nephrotoxic but require different dosing strategies (three- to five-fold higher doses than deoxycholate formulations of amphotericin B). Some of these agents continue to induce general systemic toxicity reactions similar to those observed with the deoxycholate formulation (e.g., amphotericin B colloidal dispersion).
3.8.7: In the treatment of systemic mycoses or parasitic infections, we recommend using azole antifungal agents and/or the echinocandins rather than conventional amphotericin B, if equal therapeutic efficacy can be assumed. (1A)
RATIONALE
Another approach to prevent amphotericin B nephrotoxicity is to avoid polyene antifungal agents entirely and use alternative agents, such as the azoles and echinocandins.351, 352, 353, 354, 355 Azole antifungal agents inhibit sterol synthesis in fungal cell membranes by blocking the activity of the 14-demethylase enzyme essential for ergosterol synthesis. Nephrotoxicity is an unusual event following the use of azole compounds. The echinocandins are beta-glucan inhibitors that interfere with cell-wall synthesis of fungal elements, and have an entirely different mechanism of action from that of amphotericin B. Both the azole compounds (voriconazole, fluconazole, itraconazole, and posaconazole) and the echinocandins (caspofungin, anidulafungin, and micafungin) compare favorably to amphotericin B with respect to their efficacy against a variety of the systemic mycoses. Both classes of antifungal agents have the advantage of lacking the intrinsic nephrotoxicity associated with amphotericin B deoxycholate. Both the azole compounds and echinocandins have proven to be less nephrotoxic than conventional amphotericin B deoxycholate in observational studies, historical control studies, and in small comparative trials.355
An important consideration in using these antifungal agents is their relative efficacy with respect to the likely pathogen that is targeted for treatment. Candida krusei is intrinsically resistant to the azoles and Candida parapsilosis is frequently resistant to the echinocandins. Amphotericin B-resistant strains of selected Aspergillus spp. and Pseudallescheria boydii are well described and require alternative therapies.
There is currently insufficient evidence as to whether the echinocandins, the azoles, or the lipid formulations of amphotericin B differ significantly from each other with respect to the risk of nephrotoxicity. No adequately controlled, large, randomized studies have been reported to date comparing the relative risk of nephrotoxicity amphotericin B lipid formulations with either azole or echinocandin antifungal agents. Such studies face the difficulty of recruiting sufficient numbers of patients with similar baseline risk for drug-induced AKI, and with a balance of exposure to other potentially nephrotoxic agents. Until such time as these studies are completed, no evidence-based recommendations can be given about the relative risk of AKI attributable directly to these antifungal agents.
RESEARCH RECOMMENDATIONS
Some studies indicate that the liposomal form of amphotericin B is less nephrotoxic than amphotericin B lipid complex or amphotericin B colloidal dispersion. RCTs in patients with systemic mycosis, with the rate of AKI as a primary or secondary end-point, should be conducted to answer this question.
Innovative strategies to formulate amphotericin B in microvesicles, nanoparticles, or micelles should be undertaken to limit nephrotoxicity in treating fungal infections. Clinical trials should compare existing formulations to these novel formulations, and could generate cost-effective, yet non-nephrotoxic derivatives of amphotericin B.
Carefully selected combinations of antifungal therapies to enhance efficacy and shorten duration of therapy may limit toxicity and reduce costs in the treatment of fungal infections. Investigations need to be carried out in the laboratory and in clinical studies to improve the care of patients with severe fungal infections. The costs and complication rates of AKI, and other toxicities of short-course combination treatment, should be compared to standard dosing regimens of antifungal therapy.
Markers of early nephrotoxicity and mechanisms to avoid nephrotoxicity with amphotericin B formulations need to be studied further in clinical investigations. These antifungal agents are given for prolonged periods, and should allow ample opportunity to test the validity of novel biomarkers of drug-induced nephrotoxicity. A group monitored with novel AKI biomarkers should be compared to conventional monitoring of AKI, to determine if one or more early biomarkers of kidney injury add to standard clinical care in the prevention of drug-induced AKI.
Chapter 3.9: Other methods of prevention of AKI in the critically ill
ON-PUMP VS. OFF-PUMP CORONARY ARTERY BYPASS SURGERY
The type of cardiac surgery is important in the discussion on risk for kidney problems associated with this surgery. Valvular procedures or aorta surgery are associated with a higher risk. One of the most controversial risk factors is on-pump vs. off-pump coronary artery bypass surgery. Off-pump coronary artery bypass obviously removes the bypass circuit but can be associated with greater hemodynamic instability secondary to ventricular compression as the heart is manipulated to access the coronary arteries.356 It is possible, with standard operative techniques, to perform coronary artery bypass surgery (but not valve surgery) without using cardiopulmonary bypass. This technique is known as “off-pump” coronary artery bypass surgery.
It has been hypothesized that preservation of physiologic renal perfusion by avoidance of cardiopulmonary bypass would partially nullify the risk of AKI in patients receiving coronary artery bypass surgery. Potential benefits that have been posited for off-pump coronary artery bypass (compared to on-pump procedures) are reduced mortality, reduction of AKI risk (and in particular, acute dialysis, which is associated with a perioperative mortality of 42% in the Society of Thoracic Surgeons database), reduced risk of cerebral dysfunction (due to stroke and neurocognitive dysfunction, the latter sometimes referred to as “pump head”), reduction in ICU stay and days in hospital, and reduction in atrial fibrillation. As in other areas covered by these guidelines only mortality, risk for RRT, and AKI risk are addressed as end-point measures. It must, however, be remembered that the potential benefits of off-pump coronary artery bypass might be predominantly outside these areas of focus.
3.9.1: We suggest that off-pump coronary artery bypass graft surgery not be selected solely for the purpose of reducing perioperative AKI or need for RRT. (2C)
RATIONALE
As detailed in Suppl Tables 15 and 16, which summarize RCTs, the balance of the potential benefit and harms is uncertain and the quality of the evidence is weak, that off-pump surgery is associated with better outcomes of the three end-points used in these guidelines: incidence of AKI, need for RRT, or mortality.
A recent good-quality RCT357 was performed in 2203 patients (only ∼8% of patients with SCr >1.5 mg/dl [>133 μmol/l]) (Suppl Table 16). There was no significant difference between off-pump and on-pump coronary artery bypass graft in the rate of the 30-day composite outcome. The rate of the 1-year composite outcome was higher for off-pump than for on-pump coronary artery bypass graft. Follow-up angiograms in the majority of the patients revealed that the overall rate of graft patency was lower in the off-pump group than in the on-pump group (82.6% vs. 87.8%, P<0.01).
A comprehensive meta-analysis including RCTs, and abstracts from the proceedings of scientific meetings through February 2010, was recently published.358 AKI was defined by a mixture of criteria, including biochemical parameter, urine output, and dialysis requirement. Mortality was evaluated among the studies that reported kidney-related outcomes. This analysis compared off-pump with the more traditional on-pump technique. Off-pump coronary artery bypass graft was associated with a statistically significant 40% lower odds of postoperative AKI and a nonsignificant 33% lower odds for dialysis requirement. Within the selected trials, off-pump coronary artery bypass graft surgery was not associated with a significant decrease in mortality. It is apparent from this meta-analysis that the trials were clinically heterogeneous, particularly in regards to their definitions of kidney outcomes, and mostly were of poor to fair quality (based on the Jadad score). The very low event rates (often 0–1 patients) make the estimates suspect and highly imprecise. There is also a question of publication bias. There are several large trials in progress that are likely to generate more definitive data. In chronic dialysis patients, there are observational US Renal Data Systems data to weakly support the use of off-pump technique (slightly lower mortality). However, with any technical advance that is introduced in certain centers, institutional familiarity with the technique, operator experience, and characteristics of the population referred to the center are likely to be important modulators of outcomes. In conclusion, based on the analysis of the RCTs and the recent meta-analysis, the Work Group found that there was not enough evidence to recommend off-pump coronary artery bypass for reducing AKI or the need for RRT.
RESEARCH RECOMMENDATION
Further studies are needed to clarify the role of off-pump coronary artery bypass in patients with increased risk for AKI.
N-ACETYLCYSTEINE (NAC)
3.9.2: We suggest not using NAC to prevent AKI in critically ill patients with hypotension. (2D)
RATIONALE
NAC has been most frequently applied in the prevention of CI-AKI, and this topic is discussed in more detail in Chapter 4.4.
NAC is a modified form of L-cysteine, an amino acid that is a precursor to reduced glutathione that can regenerate glutathione stores. It is known to be a potent antioxidant that scavenges oxygen-free radicals in the body. It also has vasodilatory properties derived from enhanced nitric oxide availability.359 NAC has been shown to attenuate ischemic and nephrotoxic ARF in a number of animal studies,360, 361, 362, 363 and the pharmacological characteristics of NAC that could play a role in the prevention of AKI have recently been summarized.364 NAC undergoes extensive first-pass metabolization in the gastric mucosa and liver. This results in a very low oral bioavailability, with substantial intrapatient variability (3–20%), as well as inconsistency between available oral products. The plasma half-life of acetylcysteine after i.v. injection is approximately 6–40 minutes, and there is extensive binding to plasma and tissue proteins through the sulfhydryl group. Virtually no acetylcysteine can be detected in the systemic circulation after i.v. or oral administration, suggesting that any potential therapeutic benefit must be due to secondary effects such as the induction of glutathione synthesis, rather than due to direct effects. As these secondary effects are not directly measurable, the determination of the optimal dosage schedule has been necessarily empirical.365
A particularly important problem with NAC is whether it can alter SCr independent of a change in GFR. NAC has been reported to decrease SCr levels in subjects with normal kidney function. This reduction in SCr was not accompanied by a change in serum cystatin C levels. This suggests an effect independent of a change in GFR, such as an increase in tubular secretion of creatinine or a decrease in creatinine production.366 By contrast, in vitro analysis on the effect of NAC on SCr367 showed no analytical interference with the measurement of SCr by any of the commonly used analytical methods. Haase et al.,368 studied 30 patients with normal kidney function who received i.v. NAC for 24 hours in association with cardiac surgery. No change in the ratio of SCr to cystatin C, compared to baseline values, was observed at the end of the 24-hour infusion or 48 hours after the cessation of the infusion. In addition, there was no effect on urinary creatinine excretion during the infusion. However, in clinical practice, NAC is generally recommended for patients with CKD, with an eGFR <60 ml/min per 1.73 m2. Mainra et al.,369 observed no change in SCr or cystatin C at 4, 24, or 48 hours after administration of a single 600-mg dose of NAC to 30 patients with CKD Stage 3. Finally, Rehman et al.,370 tested the potentially confounding effect of NAC in a CKD population (Stages 3–5) following doses of NAC currently recommended for prophylaxis of AKI. There was no effect of NAC on either SCr or cystatin C levels.
It is thus safe to conclude that NAC, in doses currently recommended for prophylaxis of AKI, has—by itself—no effect on SCr or cystatin C levels. In addition, NAC is inexpensive and appears to be safe, although it may have some detrimental effects on myocardial and coagulation function.371, 372, 373 The “safety” of NAC should further be amended, particularly when high i.v. doses are used, as in some of the RCTs in CI-AKI. When prospectively studied in acetaminophen poisoning, i.v. NAC produced anaphylactoid reactions in up to 48% of participants.374 Although most of these reactions were mild, at least one death has been reported in a patient with asthma.375 It should also be noted that the doses of acetaminophen used are still much higher than in the “high doses” used, particularly in AKI trials. Besides the prevention of CI-AKI, NAC has also been tested in the setting of cardiothoracic surgery and liver transplantation, and in hypotensive critically ill patients.
NAC IN CRITICALLY ILL PATIENTS
3.9.3: We recommend not using oral or i.v. NAC for prevention of postsurgical AKI. (1A)
RATIONALE
The above recommendation is based on an evaluation of the available literature on prevention studies with NAC in cardiovascular and abdominal vascular surgery, and liver transplantation.
The tables summarize the RCTs where either oral or i.v. NAC was compared to placebo; only studies containing a minimum of 50 patients in each study arm have been included. In addition, a recent meta-analysis is available,376 containing 10 studies involving a total of 1193 adult patients undergoing major surgery. Seven studies (1003 patients) evaluated the effects of NAC in patients undergoing cardiac surgery, and three of these (508 patients) exclusively studied patients with pre-existing renal impairment. Two studies (111 patients) evaluated the effects of NAC on patients undergoing abdominal aneurysm repair surgery and one study (79 patients) was of patients undergoing major abdominal cancer surgery. End-points in most of the studies were mortality, need for RRT, or varying increases in postoperative SCr concentrations compared to preoperative SCr values.
Suppl Tables 17 and 18 summarize the five studies where NAC was compared to placebo in patients undergoing cardiac surgery and who were not exposed to radiocontrast media.377, 378, 379, 380, 381 All five studies analyzed the effects of NAC in patients with moderate, pre-existing renal functional impairment. Surgery included elective or emergency coronary artery bypass graft operations or heart valve surgery. NAC was given i.v. in most of the studies; mortality was evaluated at different follow-up times; either in-hospital or at 30 or 90 days. Only one study found a significantly lower mortality at 30 days.377 None of the studies found either a difference in need for RRT, or in AKI defined as variable changes in SCr after surgery. All studies were of A-level quality. Two relatively small studies evaluated the effects of NAC on patients undergoing abdominal aneurysm repair surgery382, 383 and did not find any protective effect on renal function.
Further, one meta-analysis376 did not find evidence that NAC used perioperatively can alter mortality or renal outcomes after major cardiovascular or abdominal cancer surgery when radiocontrast agents are not used. In none of the studies were significant treatment-related adverse effects of NAC reported. These reports suggest that NAC, in the context of cardiovascular surgery, is not associated with increased risk of mortality, surgical re-exploration, or allogeneic transfusion.
Only one single study has compared NAC to placebo in critically ill patients (Suppl Table 18).384 One hundred and forty-two ICU patients with new-onset (within 12 hours) of at least ⩾30 consecutive minutes of hypotension and/or vasopressor requirement were randomized to receive either oral NAC or placebo for 7 days, in addition to standard supportive therapy. AKI was defined as ⩾0.5 mg/dl (⩾44 μmol/l) increase in SCr. Patients who received NAC had an incidence of AKI of 15.5%, compared to 16.9% in those receiving placebo (NS). There were no significant differences between treatment arms in any of the secondary outcomes examined, including incidence of a 50% increase in SCr, maximal rise in creatinine, recovery of renal function, length of ICU and hospital stay, and requirement for RRT. Mortality in both arms was 10%. Based on this single study, which is underpowered but did not show any beneficial effect on incidence of AKI, need for RRT, or patient mortality, we suggest not using NAC to prevent AKI in critically ill patients with hypotension.
SPONSORSHIP
KDIGO gratefully acknowledges the following sponsors that make our initiatives possible: Abbott, Amgen, Belo Foundation, Coca-Cola Company, Dole Food Company, Genzyme, Hoffmann-LaRoche, JC Penney, NATCO—The Organization for Transplant Professionals, NKF—Board of Directors, Novartis, Robert and Jane Cizik Foundation, Shire, Transwestern Commercial Services, and Wyeth. KDIGO is supported by a consortium of sponsors and no funding is accepted for the development of specific guidelines.
DISCLAIMER
While every effort is made by the publishers, editorial board, and ISN to see that no inaccurate or misleading data, opinion or statement appears in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor, copyright holder, or advertiser concerned. Accordingly, the publishers and the ISN, the editorial board and their respective employers, office and agents accept no liability whatsoever for the consequences of any such inaccurate or misleading data, opinion or statement. While every effort is made to ensure that drug doses and other quantities are presented accurately, readers are advised that new methods and techniques involving drug usage, and described within this Journal, should only be followed in conjunction with the drug manufacturer's own published literature.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Table 1: Summary table of RCTs examining the effect of starch for the prevention of AKI.
Appendix D: Evaluation and General Management Guidelines for Patients with AKI.
SUPPLEMENTARY MATERIAL
Supplementary Table 2: Evidence profile of RCTs examining insulin vs. conventional glucose therapy for the prevention of AKI.
Supplementary Table 3: Summary table of RCTs examining the effect of insulin for the prevention of AKI.
SUPPLEMENTARY MATERIAL
Supplementary Table 4: Summary table of RCTs examining the effect of dopamine vs. placebo for the treatment of AKI.
Supplementary Table 5: Evidence profile of RCTs examining fenoldopam vs. control for the prevention of AKI.
Supplementary Table 6: Summary table of RCTs examining the effect of fenoldopam for the prevention of AKI.
Supplementary Table 7: Evidence profile of RCTs of fenoldopam vs. placebo for the treatment of AKI.
Supplementary Table 8: Summary table of RCTs of examining the effect of fenoldopam for the treatment of AKI.
Supplementary Table 9: Summary table of RCTs of nesiritide vs. control for the prevention of AKI.
Supplementary Table 10: Evidence profile of RCTs examining anaritide vs. control for the prevention of AKI.
Supplementary Table 11: Summary table of RCTs examining the effect of anaritide vs. control for the prevention of AKI.
Supplementary Table 12: Evidence profile of RCTs examining anaritide vs. placebo for the treatment of AKI.
Supplementary Table 13: Summary table of RCTs examining the effect of ANP vs. placebo for the treatment of AKI.
SUPPLEMENTARY MATERIAL
Supplementary Table 14: Summary table of RCTs examining the effect of erythropoietin vs. placebo for the prevention of AKI.
SUPPLEMENTARY MATERIAL
Supplementary Table 15: Evidence profile of RCT examining on vs. off pump cardiothoracic surgery.
Supplementary Table 16: Summary table of RCTs examining the effect of on vs. off pump CABG for the prevention of AKI.
Supplementary Table 17: Evidence profile of RCTs examining NAC vs. placebo in the prevention of AKI.
Supplementary Table 18: Summary table of RCTs examining the effect of NAC vs. placebo in the prevention of AKI.
Supplementary material is linked to the online version of the paper at http://www.kdigo.org/clinical_practice_guidelines/AKI.php
References
- Bellomo R, Wan L, May CN.Vasoactive drugs and acute renal failureIn: Ronco C, Bellomo R, Kellum J (eds).Critical Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 2009416–419. [Google Scholar]
- Bouchard J, Mehta RL.Fluid balance issues in the critically ill patientIn: Ronco C, Costanzo MR, Bellomo R, Maisel A (eds).Fluid Overload: Diagnosis and Management S Karger AG: Basel, Switzerland; 201069–78. [DOI] [PubMed] [Google Scholar]
- Finfer S, Jones DA.Crystalloids and colloidsIn: Ronco C, Bellomo R, Kellum J (eds).Critical Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 2009571–575. [Google Scholar]
- Holmes CL, Walley KR.ShockIn: Murray PT, Brady HR, Hall JB (eds).Intensive Care in Nephrology Taylor & Francis: New York, NY; 20061–18. [Google Scholar]
- Levine JS, Iglesias JI.Diuretic use and fluid managementIn: Murray PT, Brady HR, Hall JB (eds).Intensive Care in Nephrology Taylor & Francis: New York, NY; 2006315–337. [Google Scholar]
- McDermott G, Neligan PJ.What vasopressor agent should be used in the septic patientIn: Deutschman CS, Neligan PJ (eds).Evidence-Based Practice of Critical Care Saunders: Philadelphia, PA; 2010206–211. [Google Scholar]
- Neligan PJ, Fanning N.What is the best way to fluid-resuscitate a patient with sepsisIn: Deutschman CS, Neligan PJ (eds).Evidence-Based Practice of Critical Care Saunders: Philadelphia, PA; 2010198–205. [Google Scholar]
- Polanco PM, Pinsky MR.Hemodynamic monitoring in the intensive care unitIn: Ronco C, Bellomo R, Kellum J (eds).Criti cal Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 200937–45. [Google Scholar]
- Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol. 2010;6:521 –529. doi: 10.1038/nrneph.2010.100. [DOI] [PubMed] [Google Scholar]
- Schetz M.Assessment of volume statusIn: Ronco C, Bellomo R, Kellum J (eds).Critical Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 2009499–504. [Google Scholar]
- Venkataraman R, Kellum JA.Principles of fluid therapyIn: Ronco C, Bellomo R, Kellum J (eds).Critical Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 2009568–571. [Google Scholar]
- Wajanaponsan N, Pinsky MR.Monitoring and management of systemic hemodyamicsIn: Jorres A, Ronco C, Kellum JA (eds).Management of Acute Kidney Problems1st Edn.Springer: New York, NY; 2010147–154. [Google Scholar]
- Himmelfarb J, Joannidis M, Molitoris B, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962 –967. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422 –427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74 . doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL. Relevance of albumin in modern critical care medicine. Best Pract Res Clin Anaesthesiol. 2009;23:183 –191. doi: 10.1016/j.bpa.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247 –2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- Ertmer C, Rehberg S, Van Aken H, et al. Relevance of non-albumin colloids in intensive care medicine. Best Pract Res Clin Anaesthesiol. 2009;23:193 –212. doi: 10.1016/j.bpa.2008.11.001. [DOI] [PubMed] [Google Scholar]
- McMahon BA, Murray PT. Urinary liver fatty acid-binding protein: another novel biomarker of acute kidney injury. Kidney Int. 2010;77:657 –659. doi: 10.1038/ki.2010.5. [DOI] [PubMed] [Google Scholar]
- Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491 –503. doi: 10.1053/j.ajkd.2007.10.044. [DOI] [PubMed] [Google Scholar]
- de Saint-Aurin RG, Kloeckner M, Annane D. Crystalloids versus colloids for fluid resuscitation in critically-ill patients. Acta Clin Belg Suppl. 2007. pp. 412–416. [DOI] [PubMed]
- Vincent JL. Fluid resuscitation: colloids vs crystalloids. Acta Clin Belg Suppl. 2007. pp. 408–411. [PubMed]
- Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicen tre randomised study. Lancet. 2001;357:911 –916. doi: 10.1016/S0140-6736(00)04211-2. [DOI] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125 –139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- Eisenbach C, Schonfeld AH, Vogt N, et al. Pharmacodynamics and organ storage of hydroxyethyl starch in acute hemodilution in pigs: influence of molecular weight and degree of substitution. Intensive Care Med. 2007;33:1637 –1644. doi: 10.1007/s00134-007-0716-x. [DOI] [PubMed] [Google Scholar]
- Thomas G, Balk EM, Jaber BL. Effect of intensive insulin therapy and pentastarch resuscitation on acute kidney injury in severe sepsis. Am J Kidney Dis. 2008;52:13 –17. doi: 10.1053/j.ajkd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ. Systematic review of randomized clinical trials on the use of hydroxyethyl starch for fluid management in sepsis. BMC Emerg Med. 2008;8:1 . doi: 10.1186/1471-227X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr Y, Payen D, Reinhart K, et al. Effects of hydroxyethyl starch administration on renal function in critically ill patients. Br J Anaesth. 2007;98:216 –224. doi: 10.1093/bja/ael333. [DOI] [PubMed] [Google Scholar]
- Perel P, Roberts I, Pearson M. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2007;4:CD000567 . doi: 10.1002/14651858.CD000567.pub3. [DOI] [PubMed] [Google Scholar]
- Schortgen F, Brochard L. Colloid-induced kidney injury: experimental evidence may help to understand mechanisms. Crit Care. 2009;13:130 . doi: 10.1186/cc7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magder S, Potter BJ, Varennes BD, et al. Fluids after cardiac surgery: a pilot study of the use of colloids versus crystalloids. Crit Care Med. 2010;38:2117 –2124. doi: 10.1097/CCM.0b013e3181f3e08c. [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ, Dunzendorfer S, Gaioni LU, et al. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care. 2010;14:R191 . doi: 10.1186/cc9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle JR, Bellomo R. Fluid administration and the kidney. Curr Opin Crit Care. 2010;16:332 –336. doi: 10.1097/MCC.0b013e32833be90b. [DOI] [PubMed] [Google Scholar]
- Kaplan LJ, Kellum JA. Fluids, pH, ions and electrolytes. Curr Opin Crit Care. 2010;16:323 –331. doi: 10.1097/MCC.0b013e32833c0957. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Varpula M, Ruokonen E, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med. 2007;33:435 –443. doi: 10.1007/s00134-006-0504-z. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008;36:S179 –S186. doi: 10.1097/CCM.0b013e318169167f. [DOI] [PubMed] [Google Scholar]
- Redl-Wenzl EM, Armbruster C, Edelmann G, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med. 1993;19:151 –154. doi: 10.1007/BF01720530. [DOI] [PubMed] [Google Scholar]
- Albanese J, Leone M, Delmas A, et al. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med. 2005;33:1897 –1902. doi: 10.1097/01.ccm.0000178182.37639.d6. [DOI] [PubMed] [Google Scholar]
- Lauzier F, Levy B, Lamarre P, et al. Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Med. 2006;32:1782 –1789. doi: 10.1007/s00134-006-0378-0. [DOI] [PubMed] [Google Scholar]
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779 –789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- Delmas A, Leone M, Rousseau S, et al. Clinical review: Vasopressin and terlipressin in septic shock patients. Crit Care. 2005;9:212 –222. doi: 10.1186/cc2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877 –887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- Gordon AC, Russell JA, Walley KR, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36:83 –91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: internati onal guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296 –327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858 –873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368 –1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670 –1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- Phua J, Koay ES, Lee KH. Lactate, procalcitonin, and amino-terminal pro-B-type natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock. 2008;29:328 –333. doi: 10.1097/SHK.0b013e318150716b. [DOI] [PubMed] [Google Scholar]
- Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425 –432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SM, Huang CD, Lin HC, et al. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26:551 –557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- Nguyen HB, Corbett SW, Menes K, et al. Early goal-directed therapy, corticosteroid, and recombinant human activated protein C for the treatment of severe sepsis and septic shock in the emergency department. Acad Emerg Med. 2006;13:109 –113. doi: 10.1197/j.aem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Rhodes A, Bennett ED. Early goal-directed therapy: an evidence-based review. Crit Care Med. 2004;32:S448 –S450. doi: 10.1097/01.ccm.0000145945.39002.8d. [DOI] [PubMed] [Google Scholar]
- Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21:128 –140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- Ho BC, Bellomo R, McGain F, et al. The incidence and outcome of septic shock patients in the absence of early-goal directed therapy. Crit Care. 2006;10:R80 . doi: 10.1186/cc4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- donati A, Loggi S, Preiser JC, et al. Goal-directed intraoperati ve therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132:1817 –1824. doi: 10.1378/chest.07-0621. [DOI] [PubMed] [Google Scholar]
- Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med. 2000;28:3396 –3404. doi: 10.1097/00003246-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC, Appel PL, Kram HB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176 –1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- Brienza N, Giglio MT, Marucci M, et al. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079 –2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025 –1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717 –1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- Section VII. Acute renal failureIn: Schrier RW (ed).Diseases of the Kidney and Urinary Tract8th Edn, vol. 2.Lippincott Williams & Wilkins: Philadelphia, PA; 2007930–1207. [Google Scholar]
- Part VI. Diagnosi s and management of specific disordersIn: Jorres A, Ronco C, Kellum JA (eds).Management of Acute Kidney Problems1st Edn.Springer: New York, NY; 2010269–467. [Google Scholar]
- Murugan R, Kellum JA. Acute kidney injury: what's the prognosis. Nat Rev Nephrol. 2011;7:209 –217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siew ED, Himmelfarb J.Metabolic and nutritional complications of acute kidney injuryIn: Himmelfarb J, Sayegh MH (eds).Chronic Kidney Disease, Dialysis, and Transplantation. A Companion to Brenner and Rector's The Kidney3rd Edn:London, UK; 2011654–667. [Google Scholar]
- Van Cromphaut SJ. Hyperglycaemia as part of the stress response: the underlying mechanisms. Best Pract Res Clin Anaesthesiol. 2009;23:375 –386. doi: 10.1016/j.bpa.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301:1556 –1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078 –3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Siegel MD. Glucose control in the ICU--how tight is too tight. N Engl J Med. 2009;360:1346 –1349. doi: 10.1056/NEJMe0901507. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359 –1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Palevsky PM, Murray PT. Acute kidney injury and critical care nephrology. NephSAP. 2006;5 (2:72 –120. [Google Scholar]
- Van den Berghe G, Wouters PJ, Kesteloot K, et al. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med. 2006;34:612 –616. doi: 10.1097/01.ccm.0000201408.15502.24. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449 –461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Schetz M, Vanhorebeek I, Wouters PJ, et al. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol. 2008;19:571 –578. doi: 10.1681/ASN.2006101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233 –243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194 –203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- Thomas G, Rojas MC, Epstein SK, et al. Insulin therapy and acute kidney injury in critically ill patients a systemat ic review. Nephrol Dial Transplant. 2007;22:2849 –2855. doi: 10.1093/ndt/gfm401. [DOI] [PubMed] [Google Scholar]
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933 –944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- Bellomo R. Does intensive insulin therapy protect renal function in critically ill patients. Nat Clin Pract Nephrol. 2008;4:412 –413. doi: 10.1038/ncpneph0855. [DOI] [PubMed] [Google Scholar]
- Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283 –1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Schetz M, Vlasselaers D, et al. Clinical review: Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target. J Clin Endocrinol Metab. 2009;94:3163 –3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821 –827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaccadori E, Lombardi M, Leonardi S, et al. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10:581 –593. doi: 10.1681/ASN.V103581. [DOI] [PubMed] [Google Scholar]
- Btaiche IF, Mohammad RA, Alaniz C, et al. Amino Acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008;28:600 –613. doi: 10.1592/phco.28.5.600. [DOI] [PubMed] [Google Scholar]
- Cano N, Fiaccadori E, Tesinsky P, et al. ESPEN Guidelines on Enteral Nutrition: Adult renal failure. Clin Nutr. 2006;25:295 –310. doi: 10.1016/j.clnu.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Druml W. Nutritional management of acute renal failure. J Ren Nutr. 2005;15:63 –70. doi: 10.1053/j.jrn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McClave SA, Hurt RT.Clinical guidelines and nutrition therapy: better understanding and greater application to patient care Crit Care Clin 201026451 –466.viii. [DOI] [PubMed] [Google Scholar]
- McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adu lt Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277 –316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- Fiaccadori E, Regolisti G, Cabassi A. Specific nutritional problems in acute kidney injury, treated with non-dialysis and dialytic modalities. NDT Plus. 2010;3:1 –7. doi: 10.1093/ndtplus/sfp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi S, Pupim LB, Simmons EM, et al. Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol. 2005;289:F259 –F264. doi: 10.1152/ajprenal.00002.2005. [DOI] [PubMed] [Google Scholar]
- May RC, Clark AS, Goheer MA, et al. Specific defects in insulin-mediated muscle metabolism in acute uremia. Kidney Int. 1985;28:490 –497. doi: 10.1038/ki.1985.155. [DOI] [PubMed] [Google Scholar]
- Cianciaruso B, Bellizzi V, Napoli R, et al. Hepatic uptake and release of glucose, lactate, and amino acids in acutely uremic dogs. Metabolism. 1991;40:261 –269. doi: 10.1016/0026-0495(91)90107-8. [DOI] [PubMed] [Google Scholar]
- Druml W, Mitch WE. Metabolic abnormalities in acute renal failure. Semin Dial. 1996;9:484 –490. [Google Scholar]
- Schneeweiss B, Graninger W, Stockenhuber F, et al. Energy metabolism in acute and chronic renal failure. Am J Clin Nutr. 1990;52:596 –601. doi: 10.1093/ajcn/52.4.596. [DOI] [PubMed] [Google Scholar]
- Macias WL, Alaka KJ, Murphy MH, et al. Impact of the nutritional regimen on protein catabolism and nitrogen balance in patients with acute renal failure. JPEN J Parenter Enteral Nutr. 1996;20:56 –62. doi: 10.1177/014860719602000156. [DOI] [PubMed] [Google Scholar]
- Fiaccadori E, Maggiore U, Rotelli C, et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005;20:1976 –1980. doi: 10.1093/ndt/gfh956. [DOI] [PubMed] [Google Scholar]
- Fiaccadori E, Cremaschi E. Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care. 2009;15:474 –480. doi: 10.1097/MCC.0b013e328332f6b2. [DOI] [PubMed] [Google Scholar]
- Powell-Tuck J. Nutritional interventions in critical illness. Proc Nutr Soc. 2007;66:16 –24. doi: 10.1017/S0029665107005253. [DOI] [PubMed] [Google Scholar]
- Scheinkestel CD, Adams F, Mahony L, et al. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition. 2003;19:733 –740. doi: 10.1016/s0899-9007(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Tan HK, Bhonagiri S, et al. High protein intake during continuous hemodiafiltration: impact on amino acids and nitrogen balance. Int J Artif Organs. 2002;25:261 –268. doi: 10.1177/039139880202500403. [DOI] [PubMed] [Google Scholar]
- Druml W. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl. 1999. pp. S56–S61. [PubMed]
- Chima CS, Meyer L, Hummell AC, et al. Protein catabolic rate in patients with acute renal failure on continuous arteriovenous hemofiltration and total parenteral nutrition. J Am Soc Nephrol. 1993;3:1516 –1521. doi: 10.1681/ASN.V381516. [DOI] [PubMed] [Google Scholar]
- Leblanc M, Garred LJ, Cardinal J, et al. Catabolism in critical illness: estimation from urea nitrogen appearance and creatinine production during continuous renal replacement therapy. Am J Kidney Dis. 1998;32:444 –453. doi: 10.1053/ajkd.1998.v32.pm9740161. [DOI] [PubMed] [Google Scholar]
- Marshall MR, Golper TA, Shaver MJ, et al. Urea kinetics during sustained low-efficiency dialysis in critically ill patients requiring renal replacement therapy. Am J Kidney Dis. 2002;39:556 –570. doi: 10.1053/ajkd.2002.31406. [DOI] [PubMed] [Google Scholar]
- Salahudeen AK, Kumar V, Madan N, et al. Sustained low efficiency dialysis in the continuous mode (C-SLED): dialysis efficacy, clinical outcomes, and survival predictors in critically ill cancer patients. Clin J Am Soc Nephrol. 2009;4:1338 –1346. doi: 10.2215/CJN.02130309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnert J, Dumitrascu D, Neeser G, et al. Gastric emptying of a liquid meal in intensive care unit patients (abstr) Gastroenterology. 1998;114:A865 . [Google Scholar]
- Fiaccadori E, Maggiore U, Clima B, et al. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int. 2001;59:1510 –1519. doi: 10.1046/j.1523-1755.2001.0590041510.x. [DOI] [PubMed] [Google Scholar]
- Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051 –2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Scheinkestel CD, Kar L, Marshall K, et al. Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition. 2003;19:909 –916. doi: 10.1016/s0899-9007(03)00175-8. [DOI] [PubMed] [Google Scholar]
- Fiaccadori E, Maggiore U, Giacosa R, et al. Enteral nutrition in patients with acute renal failure. Kidney Int. 2004;65:999 –1008. doi: 10.1111/j.1523-1755.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- Caldwell MD, Kennedy-Caldwell C. Normal nutritional requirements. Surg Clin North Am. 1981;61:489 –507. doi: 10.1016/s0039-6109(16)42432-1. [DOI] [PubMed] [Google Scholar]
- Zappitelli M, Goldstein SL, Symons JM, et al. Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med. 2008;36:3239 –3245. doi: 10.1097/CCM.0b013e31818f3f40. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547 –2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- Uchino S, Doig GS, Bellomo R, et al. Diuretics and mortality in acute renal failure. Crit Care Med. 2004;32:1669 –1677. doi: 10.1097/01.ccm.0000132892.51063.2f. [DOI] [PubMed] [Google Scholar]
- Karajala V, Mansour W, Kellum JA. Diuretics in acute kidney injury. Minerva Anestesiol. 2009;75:251 –257. [PubMed] [Google Scholar]
- Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part II) Clin Pharmacokinet. 1990;18:460 –471. doi: 10.2165/00003088-199018060-00003. [DOI] [PubMed] [Google Scholar]
- Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I) Clin Pharmacokinet. 1990;18:381 –408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- Ludens JH, Hook JB, Brody MJ, et al. Enhancement of renal blood flow by furosemide. J Pharmacol Exp Ther. 1968;163:456 –460. [PubMed] [Google Scholar]
- Ludens JH, Williamson HE. Effect of furosemide on renal blood flow in the conscious dog. Proc Soc Exp Biol Med. 1970;133:513 –515. doi: 10.3181/00379727-133-34508. [DOI] [PubMed] [Google Scholar]
- Cantarovich F, Rangoonwala B, Lorenz H, et al. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44:402 –409. [PubMed] [Google Scholar]
- Lassnigg A, Donner E, Grubhofer G, et al. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97 –104. doi: 10.1681/ASN.V11197. [DOI] [PubMed] [Google Scholar]
- Lombardi R, Ferreiro A, Servetto C. Renal function after cardiac surgery: adverse effect of furosemide. Ren Fail. 2003;25:775 –786. doi: 10.1081/jdi-120024293. [DOI] [PubMed] [Google Scholar]
- Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416 –1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420 . doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65:283 –293. doi: 10.1111/j.1365-2044.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- Hager B, Betschart M, Krapf R. Effect of postoperat ive intravenous loop diuretic on renal function after major surgery. Schweiz Med Wochenschr. 1996;126:666 –673. [PubMed] [Google Scholar]
- van der Voort PH, Boerma EC, Koopmans M, et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med. 2009;37:533 –538. doi: 10.1097/CCM.0b013e318195424d. [DOI] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Morimatsu H, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med. 2009;37:2576 –2582. doi: 10.1097/CCM.0b013e3181a38241. [DOI] [PubMed] [Google Scholar]
- Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259 –265. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- Schetz M. Should we use diuretics in acute renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:75 –89. doi: 10.1016/j.bpa.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Yallop KG, Sheppard SV, Smith DC. The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine. Anaesthesia. 2008;63:576 –582. doi: 10.1111/j.1365-2044.2008.05540.x. [DOI] [PubMed] [Google Scholar]
- Smith MN, Best D, Sheppard SV, et al. The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction. Anaesthesia. 2008;63:701 –704. doi: 10.1111/j.1365-2044.2007.05408.x. [DOI] [PubMed] [Google Scholar]
- Schnuelle P, Johannes van der Woude F. Perioperative fluid management in renal transplantation: a narrative review of the literature. Transpl Int. 2006;19:947 –959. doi: 10.1111/j.1432-2277.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- van Valenberg PL, Hoitsma AJ, Tiggeler RG, et al. Mannitol as an indispensable constituent of an intraoperative hydration protocol for the prevention of acute renal failure after renal cadaveric transplantation. Transplantation. 1987;44:784 –788. doi: 10.1097/00007890-198712000-00012. [DOI] [PubMed] [Google Scholar]
- Weimar W, Geerlings W, Bijnen AB, et al. A controlled study on the effect of mannitol on immediate renal function after cadaver donor kidney transplantation. Transplantation. 1983;35:99 –101. [PubMed] [Google Scholar]
- Better OS, Rubinstein I, Winaver JM, et al. Mannitol therapy revisited (1940–1997) Kidney Int. 1997;52:886 –894. doi: 10.1038/ki.1997.409. [DOI] [PubMed] [Google Scholar]
- Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med. 2006;354:1052 –1063. doi: 10.1056/NEJMra054329. [DOI] [PubMed] [Google Scholar]
- Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553 –1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139 –2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- Murray PT. Yearbook of Intensive Care and Emergency Medicine. Springer-Verlag: Berlin, Germany; 2003. Use of dopaminergic agents for renoprotection in the ICU; pp. 637–648. [Google Scholar]
- Lauschke A, Teichgraber UK, Frei U.et al.lsquo;Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure Kidney Int 2006691669 –1674. [DOI] [PubMed] [Google Scholar]
- Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526 –1531. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Marik PE. Low-dose dopamine: a systematic review. Intensive Care Med. 2002;28:877 –883. doi: 10.1007/s00134-002-1346-y. [DOI] [PubMed] [Google Scholar]
- Friedrich JO, Adhikari N, Herridge MS, et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510 –524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- Murray PT. Fenoldopam: renal-dose dopamine redux. Crit Care Med. 2006;34:910 –911. doi: 10.1097/01.CCM.0000202437.86001.44. [DOI] [PubMed] [Google Scholar]
- Cogliati AA, Vellutini R, Nardini A, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: a randomized clinical study. J Cardiothorac Vasc Anesth. 2007;21:847 –850. doi: 10.1053/j.jvca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai GG, Marino G, et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27 –33. doi: 10.1053/j.jvca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Morelli A, Ricci Z, Bellomo R, et al. Prophylactic fenoldopam for renal protection in sepsis: a randomized, double-blind, placebo-controlled pilot trial. Crit Care Med. 2005;33:2451 –2456. doi: 10.1097/01.ccm.0000186413.04875.ef. [DOI] [PubMed] [Google Scholar]
- Aravindan N, Natarajan M, Shaw AD. Fenoldopam inhibits nuclear translocation of nuclear factor kappa B in a rat model of surgical ischemic acute renal failure. J Cardiothorac Vasc Anesth. 2006;20:179 –186. doi: 10.1053/j.jvca.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Aravindan N, Samuels J, Riedel B, et al. Fenoldopam improves cortico-medullary oxygen delivery and attenuates angiogenesis gene expression in acute ischemic renal injury. Kidney Blood Press Res. 2006;29:165 –174. doi: 10.1159/000095350. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Prophylactic fenoldopam for renal protection? No, thank you, not for me--not yet at least. Crit Care Med. 2005;33:2681 –2683. doi: 10.1097/01.ccm.0000186743.30595.aa. [DOI] [PubMed] [Google Scholar]
- Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropat hy: a randomized controlled trial. JAMA. 2003;290:2284 –2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- Tumlin JA, Finkel KW, Murray PT, et al. Fenoldopam mesylate in early acute tubular necrosis: a randomized, double-blind, placebo-controlled clinical trial. Am J Kidney Dis. 2005;46:26 –34. doi: 10.1053/j.ajkd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Brienza N, Malcangi V, Dalfino L, et al. A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Crit Care Med. 2006;34:707 –714. doi: 10.1097/01.CCM.0000201884.08872.A2. [DOI] [PubMed] [Google Scholar]
- Landoni G, Biondi-Zoccai GG, Tumlin JA, et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56 –68. doi: 10.1053/j.ajkd.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Ricksten SE, Sward K.Atrial natriuretic peptide in acute renal failureIn: Ronco C, Bellomo R, Kellum J (eds).Critical Care Nephrology2nd Edn.Saunders Elsevier: Philadelphia, PA; 2009429–433. [Google Scholar]
- Vesely DL. Natriuretic peptides and acute renal failure. Am J Physiol Renal Physiol. 2003;285:F167 –F177. doi: 10.1152/ajprenal.00259.2002. [DOI] [PubMed] [Google Scholar]
- Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986;324:473 –476. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- Valsson F, Ricksten SE, Hedner T, et al. Effects of atrial natriuretic peptide on renal function after cardiac surgery and in cyclosporine-treated heart transplant recipients. J Cardiothorac Vasc Anesth. 1994;8:425 –430. doi: 10.1016/1053-0770(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, Richardson AJ, Kirby JE, et al. Effect of intravenous infusion of atriopeptin 3 on immediate renal allograft function. Kidney Int. 1991;39:164 –168. doi: 10.1038/ki.1991.21. [DOI] [PubMed] [Google Scholar]
- Sands JM, Neylan JF, Olson RA, et al. Atrial natriuretic factor does not improve the outcome of cadaveric renal transplantation. J Am Soc Nephrol. 1991;1:1081 –1086. doi: 10.1681/ASN.V191081. [DOI] [PubMed] [Google Scholar]
- Kurnik BR, Allgren RL, Genter FC, et al. Prospective study of atrial natriuretic peptide for the prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 1998;31:674 –680. doi: 10.1053/ajkd.1998.v31.pm9531185. [DOI] [PubMed] [Google Scholar]
- Allgren RL, Marbury TC, Rahman SN, et al. Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med. 1997;336:828 –834. doi: 10.1056/NEJM199703203361203. [DOI] [PubMed] [Google Scholar]
- Lewis J, Salem MM, Chertow GM, et al. Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. Am J Kidney Dis. 2000;36:767 –774. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- Sward K, Valsson F, Odencrants P, et al. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med. 2004;32:1310 –1315. doi: 10.1097/01.ccm.0000128560.57111.cd. [DOI] [PubMed] [Google Scholar]
- Sward K, Valsson F, Sellgren J, et al. Differential effects of human atrial natriuretic peptide and furosemide on glomerular filtration rate and renal oxygen consumption in humans. Intensive Care Med. 2005;31:79 –85. doi: 10.1007/s00134-004-2490-3. [DOI] [PubMed] [Google Scholar]
- Nigwekar SU, Navaneethan SD, Parikh CR, et al. Atrial natriuretic peptide for management of acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:261 –272. doi: 10.2215/CJN.03780808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssmann W, Meyer M, Forssmann K. The renal urodilatin system: clinical implications. Cardiovasc Res. 2001;51:450 –462. doi: 10.1016/s0008-6363(01)00331-5. [DOI] [PubMed] [Google Scholar]
- Hummel M, Kuhn M, Bub A, et al. Urodilatin: a new peptide with beneficial effects in the postoperative therapy of cardiac transplant recipients. Clin Investig. 1992;70:674 –682. doi: 10.1007/BF00180284. [DOI] [PubMed] [Google Scholar]
- Brenner P, Meyer M, Reichenspurner H, et al. Significance of prophylactic urodilatin (INN: ularitide) infusion for the prevention of acute renal failure in patients after heart transplantation. Eur J Med Res. 1995;1:137 –143. [PubMed] [Google Scholar]
- Sackner-Bernstein JD, Kowalski M, Fox M, et al. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900 –1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487 –1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- Topol EJ. Nesiritide - not verified. N Engl J Med. 2005;353:113 –116. doi: 10.1056/NEJMp058139. [DOI] [PubMed] [Google Scholar]
- Iglesias JI, DePalma L, Hom D, et al. Predictors of mortality in adult patients with congestive heart failure receiving nesiritide--retrospect ive analysis showing a potential adverse interaction between nesiritide and acute renal dysfunction. Nephrol Dial Transplant. 2008;23:144 –153. doi: 10.1093/ndt/gfm565. [DOI] [PubMed] [Google Scholar]
- Mentzer RM, Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49:716 –726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Ejaz AA, Martin TD, Johnson RJ, et al. Prophylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgery. J Thorac Cardiovasc Surg. 2009;138:959 –964. doi: 10.1016/j.jtcvs.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Lingegowda V, Van QC, Shimada M, et al. Long-term outcome of patients treated with prophylactic nesiritide for the prevention of acute kidney injury following cardiovascular surgery. Clin Cardiol. 2010;33:217 –221. doi: 10.1002/clc.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman MR. Potential role of growth factors in the prophylaxis and treatment of acute renal failure. Kidney Int Suppl. 1998;64:S19 –S22. [PubMed] [Google Scholar]
- Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care. 2008;14:621 –626. doi: 10.1097/MCC.0b013e328317ee82. [DOI] [PubMed] [Google Scholar]
- Ding H, Kopple JD, Cohen A, et al. Recombinant human insulin-like growth factor-i accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest. 1993;91:2281 –2287. doi: 10.1172/JCI116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender M, Popovtzer MM, Weiss O, et al. Insulin-like growth factor-1 (IGF-1) enhances recovery from HgCl2-in duced acute renal failure: the effects on renal IGF-1, IGF-1 receptor, and IGF-binding protein-1 mRNA. J Am Soc Nephrol. 1995;5:1782 –1791. doi: 10.1681/ASN.V5101782. [DOI] [PubMed] [Google Scholar]
- Miller SB, Martin DR, Kissane J, et al. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci U S A. 1992;89:11876 –11880. doi: 10.1073/pnas.89.24.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinec D, Reilly JM, Sicard GA, et al. Insulin-like growth factor-I attenuates delayed graft function in a canine renal autotransplantation model Surgery 1996120221 –225.discussion 225–226. [DOI] [PubMed] [Google Scholar]
- Franklin SC, Moulton M, Sicard GA, et al. Insulin-like growth factor I preserves renal function postoperatively. Am J Physiol. 1997;272:F257 –F259. doi: 10.1152/ajprenal.1997.272.2.F257. [DOI] [PubMed] [Google Scholar]
- Hirschberg R, Kopple J, Lipsett P, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423 –2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Hladunewich MA, Corrigan G, Derby GC, et al. A randomized, placebo-controlled trial of IGF-1 for delayed graft function: a human model to study postischemic ARF. Kidney Int. 2003;64:593 –602. doi: 10.1046/j.1523-1755.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30:253 –260. doi: 10.1159/000223229. [DOI] [PubMed] [Google Scholar]
- Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020 –1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- Karlowicz MG, Adelman RD. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol. 1995;9:718 –722. doi: 10.1007/BF00868721. [DOI] [PubMed] [Google Scholar]
- Gouyon JB, Guignard JP. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 1988;33:1078 –1083. doi: 10.1038/ki.1988.114. [DOI] [PubMed] [Google Scholar]
- Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia--a study in a developing country. Pediatr Nephrol. 2005;20:1249 –1252. doi: 10.1007/s00467-005-1980-z. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Shah ZA, Makhdoomi MS, et al. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. 2006;149:180 –184. doi: 10.1016/j.jpeds.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Jenik AG, Ceriani Cernadas JM, Gorenstein A, et al. A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics. 2000;105:E45 . doi: 10.1542/peds.105.4.e45. [DOI] [PubMed] [Google Scholar]
- Cattarelli D, Spandrio M, Gasparoni A, et al. A randomised, double blind, placebo controlled trial of the effect of theophylline in prevention of vasomotor nephropathy in very preterm neonates with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2006;91:F80 –F84. doi: 10.1136/adc.2005.073650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb SS, Brater DC, Thomas I, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348 –1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- Givertz MM, Massie BM, Fields TK, et al. The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551 –1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Cotter G, Dittrich HC, Weatherley BD, et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. 2008;14:631 –640. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Massie BM, O’Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419 –1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Kopterides P. Old antibiotics for infections in critically ill patients. Curr Opin Crit Care. 2007;13:592 –597. doi: 10.1097/MCC.0b013e32827851d7. [DOI] [PubMed] [Google Scholar]
- Rea RS, Capitano B. Optimizing use of aminoglycosides in the critically ill. Semin Respir Crit Care Med. 2007;28:596 –603. doi: 10.1055/s-2007-996406. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840 –851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- Zahar JR, Rioux C, Girou E, et al. Inappropriate prescribing of aminoglycosides: risk factors and impact of an antibiotic control team. J Antimicrob Chemother. 2006;58:651 –656. doi: 10.1093/jac/dkl288. [DOI] [PubMed] [Google Scholar]
- Bliziotis IA, Michalopoulos A, Kasiakou SK, et al. Ciprofloxacin vs an aminoglycoside in combination with a beta-lactam for the treatment of febrile neutropenia: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2005;80:1146 –1156. doi: 10.4065/80.9.1146. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Matthaiou DK, Bliziotis IA. The role of aminoglycosides in combination with a beta-lactam for the treatment of bacterial endocarditis: a meta-analysis of comparative trials. J Antimicrob Chemother. 2006;57:639 –647. doi: 10.1093/jac/dkl044. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Matthaiou DK, Karveli EA, et al. Meta-analysis: randomized controlled trials of clindamycin/aminoglycoside vs. beta-lactam monotherapy for the treatment of intra-abdominal infections. Aliment Pharmacol Ther. 2007;25:537 –556. doi: 10.1111/j.1365-2036.2006.03240.x. [DOI] [PubMed] [Google Scholar]
- Glasmacher A, von Lilienfeld-Toal M, Schulte S, et al. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clin Microbiol Infect. 2005;11 (Suppl 5:17 –23. doi: 10.1111/j.1469-0691.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- Paul M, Benuri-Silbiger I, Soares-Weiser K, et al. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668 . doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Silbiger I, Grozinsky S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2006. p. CD003344. [DOI] [PubMed]
- English WP, Williams MD.Should aminoglycoside antibiotics be abandoned Am J Surg 2000180512 –515.discussion 515–516. [DOI] [PubMed] [Google Scholar]
- Cosgrove SE, Vigliani GA, Fowler VG, Jr, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48:713 –721. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- Olaison L, Schadewitz K. Enterococcal endocarditis in Sweden, 1995–1999: can shorter therapy with aminoglycosides be used. Clin Infect Dis. 2002;34:159 –166. doi: 10.1086/338233. [DOI] [PubMed] [Google Scholar]
- Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem Toxicol. 2003;41:1447 –1452. doi: 10.1016/s0278-6915(03)00186-8. [DOI] [PubMed] [Google Scholar]
- Bledsoe G, Crickman S, Mao J, et al. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21:624 –633. doi: 10.1093/ndt/gfi225. [DOI] [PubMed] [Google Scholar]
- Bledsoe G, Shen B, Yao YY, et al. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol Sci. 2008;102:433 –443. doi: 10.1093/toxsci/kfn008. [DOI] [PubMed] [Google Scholar]
- Ekor M, Farombi EO, Emerole GO. Modulation of gentamicin-induced renal dysfunction and injury by the phenolic extract of soybean (Glycine max) Fundam Clin Pharmacol. 2006;20:263 –271. doi: 10.1111/j.1472-8206.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Feldman L, Efrati S, Eviatar E, et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72:359 –363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- Girton RA, Sundin DP, Rosenberg ME. Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am J Physiol Renal Physiol. 2002;282:F703 –F709. doi: 10.1152/ajprenal.00060.2001. [DOI] [PubMed] [Google Scholar]
- Horibe T, Matsui H, Tanaka M, et al. Gentamicin binds to the lectin site of calreticulin and inhibits its chaperone activity. Biochem Biophys Res Commun. 2004;323:281 –287. doi: 10.1016/j.bbrc.2004.08.099. [DOI] [PubMed] [Google Scholar]
- Kaynar K, Gul S, Ersoz S, et al. Amikacin-induced nephropathy: is there any protective way. Ren Fail. 2007;29:23 –27. doi: 10.1080/08860220601039072. [DOI] [PubMed] [Google Scholar]
- Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86 –98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Montagut C, Bosch F, Villela L, et al. Aminoglycoside-associated severe renal failure in patients with multiple myeloma treated with thalidomi de. Leuk Lymphoma. 2004;45:1711 –1712. doi: 10.1080/10428190310001638841. [DOI] [PubMed] [Google Scholar]
- Morales AI, Rodriguez-Barbero A, Vicente-Sanchez C, et al. Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sci. 2006;78:2373 –2377. doi: 10.1016/j.lfs.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Parlakpinar H, Koc M, Polat A, et al. Protective effect of aminoguanidine against nephrotoxicity induced by amikacin in rats. Urol Res. 2004;32:278 –282. doi: 10.1007/s00240-004-0399-5. [DOI] [PubMed] [Google Scholar]
- Rougier F, Claude D, Maurin M, et al. Aminoglycoside nephrotoxicity. Curr Drug Targets Infect Disord. 2004;4:153 –162. doi: 10.2174/1568005043340858. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hilpert J, Jacobsen C, et al. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem. 2002;277:618 –622. doi: 10.1074/jbc.M109959200. [DOI] [PubMed] [Google Scholar]
- Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21:433 –442. doi: 10.3109/08860229909085109. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Nagai J, Adachi Y, et al. Targeted preventi on of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists. J Control Release. 2004;95:423 –433. doi: 10.1016/j.jconrel.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Yanagida C, Ito K, Komiya I, et al. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem Biol Interact. 2004;148:139 –147. doi: 10.1016/j.cbi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Baciewicz AM, Sokos DR, Cowan RI. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003;37:182 –186. doi: 10.1177/106002800303700203. [DOI] [PubMed] [Google Scholar]
- Barclay ML, Kirkpatrick CM, Begg EJ. Once daily aminoglycoside therapy. Is it less toxic than multiple daily doses and how should it be monitored. Clin Pharmacokinet. 1999;36:89 –98. doi: 10.2165/00003088-199936020-00001. [DOI] [PubMed] [Google Scholar]
- Graham AC, Mercier RC, Achusim LE, et al. Extended-interval aminoglycoside dosing for treatment of enterococcal and staphylococcal osteomyelitis. Ann Pharmacother. 2004;38:936 –941. doi: 10.1345/aph.1D514. [DOI] [PubMed] [Google Scholar]
- Kiel PJ, Lo M, Stockwell D, et al. An evaluation of amikacin nephrotoxicity in the hematology/oncology population. Am J Ther. 2008;15:131 –136. doi: 10.1097/MJT.0b013e31815adfde. [DOI] [PubMed] [Google Scholar]
- Kraus DM, Pai MP, Rodvold KA. Efficacy and tolerability of extended-interval aminoglycoside administration in pediatric patients. Paediatr Drugs. 2002;4:469 –484. doi: 10.2165/00128072-200204070-00005. [DOI] [PubMed] [Google Scholar]
- Nestaas E, Bangstad HJ, Sandvik L, et al. Aminoglycoside extended interval dosing in neonates is safe and effective: a meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2005;90:F294 –F300. doi: 10.1136/adc.2004.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloquin CA, Berning SE, Nitta AT, et al. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38:1538 –1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- Peters-Volleberg GW, Dortant PM, Speijers GJ. Comparison of tobramycin nephrotoxicity in young adult and aged female rats. Pharmacol Toxicol. 1999;84:147 –153. doi: 10.1111/j.1600-0773.1999.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Rougier F, Claude D, Maurin M, et al. Aminoglycoside nephrotoxicity: modeling, simulation, and control. Antimicrob Agents Chemother. 2003;47:1010 –1016. doi: 10.1128/AAC.47.3.1010-1016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier F, Ducher M, Maurin M, et al. Aminoglycoside dosages and nephrotoxicity: quantitative relationships. Clin Pharmacokinet. 2003;42:493 –500. doi: 10.2165/00003088-200342050-00007. [DOI] [PubMed] [Google Scholar]
- Rybak MJ, Abate BJ, Kang SL, et al. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549 –1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth AR, Tan KH. Once-daily versus multiple-daily dosing with intra-venous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2006;3:CD002009 . doi: 10.1002/14651858.CD002009.pub2. [DOI] [PubMed] [Google Scholar]
- Ali MZ, Goetz MB. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796 –809. doi: 10.1093/clinids/24.5.796. [DOI] [PubMed] [Google Scholar]
- Bailey TC, Little JR, Littenberg B, et al. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:786 –795. doi: 10.1093/clinids/24.5.786. [DOI] [PubMed] [Google Scholar]
- Barza M, Ioannidis JP, Cappelleri JC, et al. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;312:338 –345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri ols-Lisart R, Alos-Alminana M. Effectiveness and safety of once-daily aminoglycosides: a meta-analysis. Am J Health Syst Pharm. 1996;53:1141 –1150. doi: 10.1093/ajhp/53.10.1141. [DOI] [PubMed] [Google Scholar]
- Hatala R, Dinh T, Cook DJ. Once-daily aminoglycoside dosing in immuno-competent adults: a meta-analysis. Ann Intern Med. 1996;124:717 –725. doi: 10.7326/0003-4819-124-8-199604150-00003. [DOI] [PubMed] [Google Scholar]
- Munckhof WJ, Grayson ML, Turnidge JD. A meta-analysis of studies on the safety and efficacy of aminoglycosides given either once daily or as divided doses. J Antimicrob Chemother. 1996;37:645 –663. doi: 10.1093/jac/37.4.645. [DOI] [PubMed] [Google Scholar]
- Gavalda J, Onrubia PL, Gomez MT, et al. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J Antimicrob Chemother. 2003;52:514 –517. doi: 10.1093/jac/dkg360. [DOI] [PubMed] [Google Scholar]
- Le T, Bayer AS. Combination antibiotic therapy for infective endocarditis. Clin Infect Dis. 2003;36:615 –621. doi: 10.1086/367661. [DOI] [PubMed] [Google Scholar]
- Tam VH, McKinnon PS, Levine DP, et al. Once-daily aminoglycoside in the treatment of Enterococcus faecalis endocarditis: case report and review. Pharmacotherapy. 2000;20:1116 –1119. doi: 10.1592/phco.20.13.1116.35029. [DOI] [PubMed] [Google Scholar]
- Beauchamp D, Labrecque G. Aminoglycoside nephrotoxicity: do time and frequency of administration matter. Curr Opin Crit Care. 2001;7:401 –408. doi: 10.1097/00075198-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Bertino JS, Jr, Erb TA, et al. Application of Bayes theorem to aminoglycoside-associated nephrotoxicity: comparison of extended-interval dosing, individualized pharmacokinetic monitoring, and multiple-daily dosing. J Clin Pharmacol. 2004;44:696 –707. doi: 10.1177/0091270004266633. [DOI] [PubMed] [Google Scholar]
- Murry KR, McKinnon PS, Mitrzyk B, et al. Pharmacodynamic characterization of nephrotoxicity associated with once-daily aminoglycoside. Pharmacotherapy. 1999;19:1252 –1260. doi: 10.1592/phco.19.16.1252.30876. [DOI] [PubMed] [Google Scholar]
- Streetman DS, Nafziger AN, Destache CJ, et al. Individualized pharmacokinetic monitoring results in less aminoglycoside-associated nephrotoxicity and fewer associated costs. Pharmacotherapy. 2001;21:443 –451. doi: 10.1592/phco.21.5.443.34490. [DOI] [PubMed] [Google Scholar]
- Heintz BH, Matzke GR, Dager WE. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermit tent hemodialysis. Pharmacotherapy. 2009;29:562 –577. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol. 2008;69:207 –212. doi: 10.5414/cnp69207. [DOI] [PubMed] [Google Scholar]
- Boyle MP. Adult cystic fibrosis. JAMA. 2007;298:1787 –1793. doi: 10.1001/jama.298.15.1787. [DOI] [PubMed] [Google Scholar]
- Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23 –30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- Cannella CA, Wilkinson ST. Acute renal failure associated with inhaled tobramycin. Am J Health Syst Pharm. 2006;63:1858 –1861. doi: 10.2146/ajhp060196. [DOI] [PubMed] [Google Scholar]
- Izquierdo MJ, Gomez-Alami llo C, Ortiz F, et al. Acute renal failure associated with use of inhaled tobramycin for treatment of chronic airway colonization with Pseudomonas aeruginosa. Clin Nephrol. 2006;66:464 –467. doi: 10.5414/cnp66464. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Burke JP, Lloyd JF, et al. Clinical and economic outcomes of conventional amphoteri cin B-associated nephrotoxicity. Clin Infect Dis. 2002;35:e120 –e127. doi: 10.1086/344468. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ. Nephrotoxicity in the setting of invasive fungal diseases. Mycoses. 2008;51 (Suppl 1:25 –30. doi: 10.1111/j.1439-0507.2008.01525.x. [DOI] [PubMed] [Google Scholar]
- Wingard JR, Kubilis P, Lee L, et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis. 1999;29:1402 –1407. doi: 10.1086/313498. [DOI] [PubMed] [Google Scholar]
- Pai MP, Norenberg JP, Telepak RA, et al. Assessment of effective renal plasma flow, enzymuria, and cytokine release in healthy volunteers receiving a single dose of amphotericin B desoxycholate. Antimicrob Agents Chemother. 2005;49:3784 –3788. doi: 10.1128/AAC.49.9.3784-3788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlam DE, Siddiq MM, Parton LA, et al. Apoptosis contributes to amphotericin B-induced nephrotoxicity. Antimicrob Agents Chemother. 2001;45:679 –685. doi: 10.1128/AAC.45.3.679-685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer K, Schaffner A, Vavricka SR, et al. Nephrotoxicity of cyclosporine a and amphotericin B-deoxycholate as continuous infusion in allogenic stem cell transplantation. Swiss Med Wkly. 2002;132:316 –320. doi: 10.4414/smw.2002.10004. [DOI] [PubMed] [Google Scholar]
- de Rosa FG, Bargiacchi O, Audagnotto S, et al. Continuous infusion of amphotericin B deoxycholate: does decreased nephrotoxicity couple with time-dependent pharmacodynamics. Leuk Lymphoma. 2006;47:1964 –1966. doi: 10.1080/10428190600687133. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J, Rai VK, et al. Amphotericin B treatment for Indian visceral leishmaniasis: response to 15 daily versus alternate-day infusions. Clin Infect Dis. 2007;45:556 –561. doi: 10.1086/520665. [DOI] [PubMed] [Google Scholar]
- Techapornroong M, Suankratay C. Alternate-day versus once-daily administration of amphotericin B in the treatment of cryptococcal meningitis: a randomized controlled trial. Scand J Infect Dis. 2007;39:896 –901. doi: 10.1080/00365540701383147. [DOI] [PubMed] [Google Scholar]
- Kleinberg M. What is the current and future status of conventional amphotericin B. Int J Antimicrob Agents. 2006;27 (Suppl 1:12 –16. doi: 10.1016/j.ijantimicag.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Saliba F, Dupont B. Renal impairment and amphotericin B formulation s in patients with invasive fungal infections. Med Mycol. 2008;46:97 –112. doi: 10.1080/13693780701730469. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ, Sanz MA, Tramarin A, et al. Prospective study of amphotericin B formulations in immunocompromised patients in 4 European countries. Clin Infect Dis. 2006;43:e29 –e38. doi: 10.1086/505969. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Jalil Miah MA, Lee ES, et al. Reduced renal toxicity of nanoparticular amphotericin B micelles prepared with partially benzylated poly-L-aspartic acid. Biol Pharm Bull. 2006;29:1700 –1705. doi: 10.1248/bpb.29.1700. [DOI] [PubMed] [Google Scholar]
- Alexander BD, Wingard JR. Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis. 2005;40 (Suppl 6:S414 –S421. doi: 10.1086/429335. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289 –1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- Garbino J, Adam A. Use of high-dose liposomal amphotericin B: efficacy and tolerance. Acta Biomed. 2006;77 (Suppl 4:19 –22. [PubMed] [Google Scholar]
- Girois SB, Chapuis F, Decullier E, et al. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2005;24:119 –130. doi: 10.1007/s10096-005-1281-2. [DOI] [PubMed] [Google Scholar]
- Hachem RY, Boktour MR, Hanna HA, et al. Amphotericin B lipid complex versus liposomal amphotericin B monotherapy for invasive aspergillosis in patients with hematologic malignancy. Cancer. 2008;112:1282 –1287. doi: 10.1002/cncr.23311. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Gotzsche PC. Amphotericin B lipid soluble formulations vs. amphotericin B in cancer patients with neutropenia. Cochrane Database Syst Rev. 2000;3:CD000969 . doi: 10.1002/14651858.CD000969. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Wheat LJ, Cloud GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137:105 –109. doi: 10.7326/0003-4819-137-2-200207160-00008. [DOI] [PubMed] [Google Scholar]
- Olson JA, Adler-Moore JP, Schwartz J, et al. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob Agents Chemother. 2006;50:2122 –2131. doi: 10.1128/AAC.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerareddy PR, Vobalaboina V. Lipid-based formulations of amphotericin B. Drugs Today (Barc) 2004;40:133 –145. doi: 10.1358/dot.2004.40.2.799425. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Alle rgy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764 –771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- Boogaerts M, Winston DJ, Bow EJ, et al. Intravenous and oral itraconazole versus intrave nous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacteri al therapy. A randomized, controlled trial. Ann Intern Med. 2001;135:412 –422. doi: 10.7326/0003-4819-135-6-200109180-00010. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Gotzsche PC. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database Syst Rev. 2002;2:CD000239 . doi: 10.1002/14651858.CD000239. [DOI] [PubMed] [Google Scholar]
- Park SH, Choi SM, Lee DG, et al. Intravenous itraconazole vs. amphotericin B deoxycholate for empirical antifungal therapy in patients with persistent neutropenic fever. Korean J Intern Med. 2006;21:165 –172. doi: 10.3904/kjim.2006.21.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad II, Hanna HA, Boktour M, et al. Novel antifungal agents as salvage therapy for invasive aspergillosis in patients with hematologic malignancies: posaconaz ole compared with high-dose lipid formulations of amphotericin B alone or in combination with caspofungin. Leukemia. 2008;22:496 –503. doi: 10.1038/sj.leu.2405065. [DOI] [PubMed] [Google Scholar]
- Wegner B, Baer P, Gauer S, et al. Caspofungin is less nephrotoxic than amphotericin B in vitro and predominantly damages distal renal tubular cells. Nephrol Dial Transplant. 2005;20:2071 –2079. doi: 10.1093/ndt/gfh948. [DOI] [PubMed] [Google Scholar]
- Schwann NM, Horrow JC, Strong MD, III, et al. Does off-pump coronary artery bypass reduce the incidence of clinically evident renal dysfunction after multivessel myocardial revascularization Anesth Analg 200499959 –964.table of contents. [DOI] [PubMed] [Google Scholar]
- Shroyer AL, Grover FL, Hattler B, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361:1827 –1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- Seabra VF, Alobaidi S, Balk EM, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2010;5:1734 –1744. doi: 10.2215/CJN.02800310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrati S, Dishy V, Averbukh M, et al. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int. 2003;64:2182 –2187. doi: 10.1046/j.1523-1755.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- Conesa EL, Valero F, Nadal JC, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R730 –R737. doi: 10.1152/ajpregu.2001.281.3.R730. [DOI] [PubMed] [Google Scholar]
- DiMari J, Megyesi J, Udvarhelyi N, et al. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:F292 –F298. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- Jiang B, Haverty M, Brecher P. N-acetyl-L-cysteine enhances interleukin-1beta-induced nitric oxide synthase expression. Hypertension. 1999;34:574 –579. doi: 10.1161/01.hyp.34.4.574. [DOI] [PubMed] [Google Scholar]
- Nitescu N, Ricksten SE, Marcussen N, et al. N-acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusion. Nephrol Dial Transplant. 2006;21:1240 –1247. doi: 10.1093/ndt/gfk032. [DOI] [PubMed] [Google Scholar]
- Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3:281 –287. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- Van Praet JT, De Vriese AS. Prevention of contrast-induced nephropathy: a critical review. Curr Opin Nephrol Hypertens. 2007;16:336 –347. doi: 10.1097/MNH.0b013e3281ca6fe5. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Fischereder M, Kruger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407 –410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Guerin V, Launay-Vacher V, et al. Effect of N-acetylcysteine on serum creatinine level. Nephrol Dial Transplant. 2001;16:1514 –1151. doi: 10.1093/ndt/16.7.1514. [DOI] [PubMed] [Google Scholar]
- Haase M, Haase-Fielitz A, Ratnaike S, et al. N-Acetylcysteine does not artifactually lower plasma creatinine concentration. Nephrol Dial Transplant. 2008;23:1581 –1587. doi: 10.1093/ndt/gfm818. [DOI] [PubMed] [Google Scholar]
- Mainra R, Gallo K, Moist L. Effect of N-acetylcysteine on renal function in patients with chronic kidney disease. Nephrology (Carlton) 2007;12:510 –513. doi: 10.1111/j.1440-1797.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- Rehman T, Fought J, Solomon R. N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD patients. Clin J Am Soc Nephrol. 2008;3:1610 –1614. doi: 10.2215/CJN.01560408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Szakmany T, Koszegi T. Prophylactic N-acetylcysteine decreases serum CRP but not PCT levels and microalbuminuria following major abdominal surgery. A prospective, randomised, double-blinded, placebo-controlled clinical trial. Intensive Care Med. 2003;29:749 –755. doi: 10.1007/s00134-003-1723-1. [DOI] [PubMed] [Google Scholar]
- Niemi TT, Munsterhjelm E, Poyhia R, et al. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagul Fibrinolysis. 2006;17:29 –34. doi: 10.1097/01.mbc.0000195922.26950.89. [DOI] [PubMed] [Google Scholar]
- Peake SL, Moran JL, Leppard PI. N-acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit Care Med. 1996;24:1302 –1310. doi: 10.1097/00003246-199608000-00006. [DOI] [PubMed] [Google Scholar]
- Lynch RM, Robertson R. Anaphylactoid reactions to intravenous N-acetylcysteine: a prospective case controlled study. Accid Emerg Nurs. 2004;12:10 –15. doi: 10.1016/j.aaen.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19:594 –595. doi: 10.1136/emj.19.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KM, Morgan DJ. Meta-analysis of N-acetylcysteine to prevent acute renal failure after major surgery. Am J Kidney Dis. 2009;53:33 –40. doi: 10.1053/j.ajkd.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Adabag AS, Ishani A, Koneswaran S, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. 2008;155:1143 –1149. doi: 10.1016/j.ahj.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Burns KE, Chu MW, Novick RJ, et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoin g CABG surgery: a randomized controlled trial. JAMA. 2005;294:342 –350. doi: 10.1001/jama.294.3.342. [DOI] [PubMed] [Google Scholar]
- El-Hamamsy I, Stevens LM, Carrier M, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7 –12. doi: 10.1016/j.jtcvs.2006.05.070. [DOI] [PubMed] [Google Scholar]
- Si sillo E, Ceriani R, Bortone F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. 2008;36:81 –86. doi: 10.1097/01.CCM.0000295305.22281.1D. [DOI] [PubMed] [Google Scholar]
- Wijeysundera DN, Beattie WS, Rao V, et al. N-acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth. 2007;54:872 –881. doi: 10.1007/BF03026790. [DOI] [PubMed] [Google Scholar]
- Hynninen MS, Niemi TT, Poyhia R, et al. N-acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: a randomized, double-blind, placebo-controlled trial. Anesth Analg. 2006;102:1638 –1645. doi: 10.1213/01.ANE.0000219590.79796.66. [DOI] [PubMed] [Google Scholar]
- Macedo E, Abdulkader R, Castro I, et al. Lack of protection of N-acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair-a randomized controlled trial. Nephrol Dial Transplant. 2006;21:1863 –1869. doi: 10.1093/ndt/gfl079. [DOI] [PubMed] [Google Scholar]
- Komisarof JA, Gilkey GM, Peters DM, et al. N-acetylcysteine for patients with prolonged hypotension as prophylaxis for acute renal failure (NEPHRON) Crit Care Med. 2007;35:435 –441. doi: 10.1097/01.CCM.0000253816.83011.DB. [DOI] [PubMed] [Google Scholar]