Abstract
Various models of experimental hypertension and clinical examples of increased renin formation from a stenotic kidney or a juxtaglomerular cell tumor have shown that increased circulating angiotensin II (Ang II) stimulates the intrarenal/intratubular renin–angiotensin system (RAS) that elicits renal vasoconstriction, enhanced tubular sodium reabsorption, and progressive development of hypertension and renal injury. The enhanced intrarenal Ang II activity is due to both receptor-mediated Ang II uptake and Ang II type 1 (AT1) receptor–mediated stimulation of renal angiotensinogen (AGT) mRNA and protein by proximal tubule cells. The increased AGT secretion leads to local formation of Ang II and spillover of AGT into the distal nephron segments as reflected by increased AGT excretion in the urine, which provides an index of intrarenal RAS activity. In clinical studies, increased urinary excretion of AGT has been demonstrated in hypertension, type 1 and type 2 diabetes mellitus, and several types of chronic kidney diseases. In addition, renin secretion from principal cells of the collecting ducts is increased by AT1 receptor activation and acts on AGT from the proximal tubule to form more Ang I. Renin and/or (pro)renin activity is enhanced by binding to the (pro)renin receptor (PRR) on intercalated cells or secreted as soluble PRR contributing further to AGT cleavage, thus making more substrate available for Ang II conversion by local angiotensin-converting enzyme. The augmented intratubular Ang II concentrations together with elevated renal interstitial Ang II concentrations contribute to sustained stimulation of sodium reabsorption, vasoconstriction, development of hypertension, and progressive renal injury and fibrosis.
Keywords: angiotensin-converting enzyme, chronic kidney disease, diabetes mellitus, prorenin receptors, sodium reabsorption, urinary angiotensinogen
Many studies have demonstrated the cardinal importance of the intrarenal renin–angiotensin system (RAS) in the maintenance of sodium homeostasis. Furthermore, when inappropriately activated, the RAS contributes to the development of hypertension, which ultimately may lead to renal injury and chronic kidney disease.1, 2, 3, 4 Although there are various ways that derangements in renal function or kidney diseases can contribute to hypertension, inappropriate overactivation of the intrarenal RAS is of critical importance not only because of the overall renal and peripheral vasoconstriction produced by angiotensin II (Ang II) but also because of the powerful effects that Ang II exerts on tubular transport in all segments of the nephron.5, 6, 7 Importantly, once there is overactivation of the RAS leading to increased Ang II activity, an insidious stimulatory mechanism leading to sustained activation of the intratubular RAS develops, which can lead to inappropriate activation of the intratubular RAS in the entire nephron population.2 There are numerous experimental examples that can be used to support this concept such as 2-kidney 1-clip hypertensive animal models; however, a case study published a few years ago also exemplifies this point.
CASE STUDY
A paper by Beevers et al.8 described an interesting case involving a 22-year-old male student who went to the hospital following an ankle injury while playing rugby. A routine blood pressure measurement revealed hypertension at 170/110 mm Hg. The electrocardiogram and echocardiography results indicated left ventricular hypertrophy, suggesting that the hypertension was not of acute onset. Plasma electrolytes showed hypokalemia (3.0 mEq/l) and the estimated glomerular filtration rate was 94 ml/min. Plasma renin activity was elevated at 26 ng AI/ml/h (angiotensin I per ml/h) and plasma aldosterone was also markedly elevated at 101 ng/dl. Ultrasound examination revealed a well-defined 4-cm mass in the interpolar cortex of the left kidney. Catheterization of the renal veins indicated renin levels of 20 ng AI/ml/h from the right kidney and 23 ng AI/ml/h from the left kidney. The patient had an open left total nephrectomy. Histological examination confirmed the presence of a juxtaglomerular cell tumor. Following nephrectomy, the blood pressure fell to 144/68 mm Hg without the need for any antihypertensive medication. The following day, his blood pressure was 100/60 mm Hg, and after a few days, the blood pressure was stabilized at 130/70 mm Hg with no antihypertensive medication. Follow-up measurements at 6 weeks showed that plasma renin activity had returned to normal levels of 2.3 ng AI/ml/h and blood pressure was 104/69 mm Hg.
ANG II–INDUCED AUGMENTATION OF INTRARENAL AGT AND ANG II
This case raises an important question regarding the inability of the contralateral kidney to respond to the elevated arterial pressure with appropriate increases in sodium excretion, thus minimizing the extent of the hypertension. Apparently, the normal contralateral kidney was also affected by the increased renin produced by the tumor leading to increased concentration of Ang I with conversion to Ang II. Were these effects just due to the increased circulating Ang II causing marked renal vasoconstriction and enhanced tubular sodium reabsorption? This appears not to be the case as experimental studies have failed to demonstrate a direct correlation and that there is a dissociation between the increases in circulating Ang II concentrations and the increases in blood pressure in different models of Ang II–dependent hypertension including 2-kidney 1-clip Goldblatt hypertension, Ang II–induced hypertension, and TGR(Ren2) hypertension.9 Furthermore, animal studies involving infusions of Ang II in rats and mice have demonstrated progressive increases in intrarenal Ang II levels that are greater than can be explained from the circulating Ang II levels alone and are closely associated with the progressive increases in arterial pressure.2 This augmentation of Ang II is associated with increased angiotensinogen (AGT) mRNA and protein in proximal tubules and marked increases in the urinary excretion of AGT.10 It is intriguing that the stimulation of tubular AGT production and increased urinary excretion of AGT and Ang II does not occur in response to a physiological stimulus, such as a reduction in overall salt consumption. Under those conditions, there is increased renin activity in the juxtoglomerular apparatus and collecting ducts, which leads to increases in intrarenal Ang II, but this appears to occur primarily in the renal interstitium.11
In contrast to what happens in response to a low-salt diet, Ang II–dependent hypertension is characterized by a marked increase in the expression of kidney AGT localized primarily to the proximal tubular cells and associated increases in urinary excretion of AGT, Ang II, and renin.12, 13, 14 The subsequent increases in intratubular as well as interstitial Ang II lead to the activation of Ang II type 1 (AT1) receptors, in the tubules as well as the renal microcirculation, that exert pleiotropic stimulatory effects leading to vasoconstriction of the afferent and efferent arterioles, mesangial cell constriction, increased sensitivity of the tubuloglomerular feedback mechanism, and upregulation of tubular sodium transport in several nephron segments.5, 9, 15, 16 Collectively, AT1 receptor activation is the essential stimulus for the development of hypertension in the various experimental models of hypertension. The finding that AT1A knockout mice do not develop hypertension following unilateral arterial constriction provides further confirmation of the importance of AT1 receptor activation in the pathophysiology of hypertension.17 Pharmacological studies have also provided further support for the role of AT1 receptors in mediating the development of hypertension. AT1 receptor blockade completely prevents the development of Ang II–induced hypertension and also prevents the increases in interstitial and intracellular Ang II that occur progressively over a period of 14 days of Ang II infusion.10
URINARY AGT
The sources of the augmented urinary AGT and Ang II levels have remained a controversial issue because it is known that liver-derived AGT in the systemic circulation contributes to intrarenal Ang II levels and may gain access to the tubules by filtering across the glomerular capillary membrane.18, 19 However, AGT has a molecular weight of 62 kDa, which would limit its free filtration across intact healthy glomerular capillary membranes.20 Thus, most of the urinary AGT originates from the tubules rather than from filtration when the glomerular filtration barrier is intact. The secretion of AGT by proximal tubules is illustrated in Figure 1. Nevertheless, the increased glomerular permeability that occurs with long-term hypertension, diabetes, chronic kidney diseases, and other associated conditions may be associated with increased filtration of AGT into the proximal tubules where some of it is internalized into proximal tubule cells via megalin-dependent endocytosis.18 Regardless of whether the bulk of the increased tubular load of angiotensinogen is due to filtration or increased secretion of intrarenally formed AGT, this means that greater amounts of substrate are available for the formation of Ang I and conversion to Ang II in the tubules, which can then act on the luminal AT1 receptors to stimulate sodium transport.
Figure 1.
Cascade of intratubular renin–angiotensin system (RAS) in angiotensin II (Ang II)–dependent hypertension. In Ang II–dependent hypertension, the kidney maintains de novo intrarenal Ang II formation–enhanced proximal tubule angiotensinogen (AGT) formation and spillover into distal nephron segments coupled with enhancement of CD renin and stimulation of tubular angiotensin converting enzyme (ACE) (refer to text for relevant references). AA, afferent arteriole; CD, collecting duct; CNT, connecting tubule; DT, distal tubule; EA, efferent arteriole; IC, intercalated cell; JGA, juxtaglomerular apparatus; PC, principal cell; PRR, prorenin receptor; PT, proximal tubule; U, urine.
Studies on transgenic mice harboring the human AGT gene linked to the kidney androgen promoter in the proximal tubules demonstrated that increased intrarenal Ang II formation following androgen-induced stimulation of the human AGT gene subsequently leads to increases in mRNA and protein expression of endogenous mouse AGT in the proximal tubules.21 These data demonstrate that the augmentation of endogenous kidney AGT can be elicited by local increases in Ang II levels. Another study utilized val5-Ang II as the peptide being infused to determine if there was an enhanced formation of endogenous Ang II in the tubules. Urinary levels of the endogenous Ang II, ileu5-Ang II, were increased progressively over a 2-week period, indicating that chronic Ang II infusion leads to increased intratubular formation of native endogenous Ang II.12 This conclusion has been supported further by studies showing that chronic angiotensin-converting enzyme (ACE) inhibition markedly attenuates the progressive hypertension elicited by chronic Ang II infusions.22 The unique role of ACE in the kidney has been demonstrated further by studies showing that chronic Ang I infusions into mice with ACE expression only in the kidneys will lead to sufficient amounts of Ang II formation to cause development of the same degree of hypertension as in wild-type mice.23 In contrast, mice that lack kidney ACE but have ACE present in the rest of the body have an attenuated hypertensive response to chronic Ang II infusions.6 Collectively, these studies support an important role for Ang II–mediated augmentation of intrarenal AGT, which is then secreted into the tubules. Intrarenal ACE appears to be essential for the augmentation of intratubular formation of Ang II.
The findings of increased urinary AGT in rats and mice subjected to chronic Ang II infusion indicate that increased secretion of AGT into the proximal tubule lumen then spills over and traverses the distal nephron segments and collecting ducts before being excreted in the urine. Thus, the increased urinary AGT may be considered to be a reflection of intrarenal RAS activation, and this has been found in several hypertensive models including spontaneously hypertensive rats, Dahl rats on high salt, Cyp1a1-Ren2 TG rats, and also in diabetic models resulting from streptozotocin induction or genetic modification.4, 24
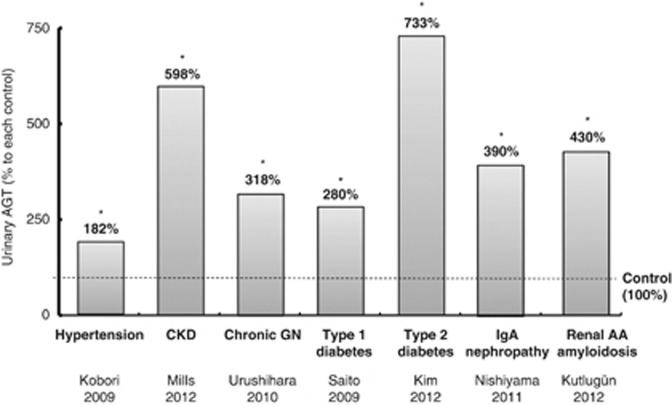
The above findings suggest that urinary AGT can be used as an index of intrarenal RAS activation and thus may be a useful biomarker. In efforts to translate the findings from the animal studies to human subjects, various clinical studies have demonstrated increased urinary AGT levels not only in hypertensive subjects not under treatment with RAS blockers or inhibitors25 but also in patients with chronic kidney disease,26, 27 chronic glomerulonephritis,28 type 1 and type 2 diabetes,29, 30 immunoglobulin A nephropathy,31 and renal amyloidosis.32 These results are presented in Figure 2 and provide compelling data that the intrarenal RAS is activated in many kidney diseases and thus provide a mechanistic rationale for treating patients with either AT1 receptor blockers or ACE inhibitors to prevent progressive deterioration of renal function as a consequence of an inappropriately elevated intrarenal RAS. The animal studies have shown that treatment with AT1 receptor blockers not only prevents the development of hypertension but also prevents the increases in intrarenal AGT mRNA and protein expression, intrarenal Ang II, and urinary AGT and urinary Ang II excretion rates.10, 12 Larger clinical studies are needed in human subjects to determine if treatment of hypertensive and diabetic subjects with AT1 receptor blockers or ACE inhibitors can reduce urinary AGT levels, thus indicating successful reduction in intrarenal RAS activity and hopefully can halt the progression of renal injury.
Figure 2.
Urinary angiotensinogen (AGT) in clinical studies of hypertension, diabetes, and kidney disease. As explained in the text, emerging data show that hypertension, diabetes, and several kidney diseases are associated with increased urinary angiotensinogen excretion rates. Data are from refs. 25,27–32.
AUGMENTATION OF RENIN IN COLLECTING DUCTS
As already mentioned, the increased urinary excretion of AGT in hypertensive models indicates that some of the tubular AGT spills over into the distal nephron segments and eventually into the urine. These findings are significant because of the presence of renin in the principal cells of the connecting tubule and collecting ducts and the prorenin receptor (PRR) in the intercalated cells.33, 34, 35, 36 Both prorenin mRNA and the PRR mRNA are upregulated in Ang II–dependent hypertension leading to increased secretion of renin and prorenin in distal nephron segments, where renin is then available to catalyze the cleavage of Ang I from the AGT spilling into the distal nephron from the proximal tubule.34, 36 As shown in Figure 1, the increased availability of renin along with the upregulation of PRR by the intercalated cells leads to enhanced overall renin activity.2, 37 In addition, the soluble form of PRR is secreted into the lumen contributing further to renin-driven formation of Ang I, which can be converted to Ang II by locally available ACE.36 The increase in collecting duct renin is caused directly by Ang II–mediated stimulation of AT1 receptors on the principal cells of collecting duct and is prevented by AT1 receptor blockers. Also, this receptor stimulation is independent of the arterial pressure because it occurs in both kidneys of 2-kidney 1-clip Goldblatt hypertensive rats.38
CONCLUSION
Collectively, the findings are consistent with the interpretation that elevated circulating and/or intrarenal Ang II levels exert an augmentation effect to increase proximal tubule production and secretion of AGT, part of which spills over into the distal nephron segments, and is then acted upon by the enhanced renin/prorenin secreted by the principal cells and facilitated by PRR and soluble PRR formed in the intercalated cells. When inappropriately stimulated by increased circulating Ang II levels, elevated arterial pressure, oxidative stress, increased cytokine production, and pathologic effects of diabetes, there is a robust activation of an intratubular RAS that, if left unabated, contributes not only to excess sodium retention and progressive development of hypertension but also to an insidious process of kidney injury which can progress to chronic kidney disease and eventual end-stage renal failure.4
Acknowledgments
I thank the many colleagues, postdoctoral fellows, and students who have participated in this long-term project evaluating the role of the intrarenal RAS in the pathophysiology of hypertension. I also thank Debbie Olavarrieta for preparing the manuscript and figures and Ryosuke Sato for preparation of Figure 2. Research support to the author includes grants from NIH (RO1HL-26371, P20RR-017659, P30HL101285, and P30GM103337). Publication costs for this article were supported by the Turkish Society of Hypertension and Renal Diseases, a nonprofit national organization in Turkey.
The author declared no competing interests.
References
- Navar LG, Hamm LL.The kidney in blood pressure regulationIn: Wilcox CS (ed).Atlas of Diseases of the Kidney. Hypertension and the Kidney Current Medicine: Philadelphia, PA; 19991.1–1.22. [Google Scholar]
- Navar LG, Kobori H, Prieto MC, et al. Intratubular renin–angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar LG, Prieto MC, Satou R, et al. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM, Imig JD, et al. Renal actions of angiotensin II and AT1 receptor blockersIn: Epstein M, Brunner HR (eds).Angiotensin II Receptor Antagonists Hanley & Belfus: Philadelphia, PA; 2000189–214. [Google Scholar]
- Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- Beevers DG, Maheshwari MB, Ryan PG, et al. Hypertension due to a renin-secreting juxtaglomerular cell tumor. Am J Hypertens. 2008;21:1359–1361. doi: 10.1038/ajh.2008.281. [DOI] [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM, Nishiyama A, et al. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Prieto-Carrasquero MC, Ozawa Y, et al. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Seth DM, Prieto MC, et al. Activation of the renin–angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am J Physiol Renal Physiol. 2013;304:F505–F514. doi: 10.1152/ajprenal.00587.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Liu L, Lara LS, et al. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo JL, Li XC, Garvin JL, et al. Intracellular angiotensin II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2005;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka L, Vaneckova I, Huskova Z, et al. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J Hypertens. 2008;26:1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl M, Kaminski H, Castrop H, et al. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Niimura F, Shimizu A, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano D, Kobori H, Burford JL, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of II ANG stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Villalobos RA, Satou R, Ohashi N, et al. Intrarenal mouse rennin–angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Villalobos RA, Billet S, Kim C, et al. Intrarenal Angiotensin-converting enzyme induces hypertension in response to Angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–459. doi: 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Kamiyama M, Harrison-Bernard LM, et al. Cardinal role of the intrarenal renin–angiotensin system in the pathogenesis of diabetic nephropathy. J Invest Med. 2013;61:256–264. doi: 10.231/JIM.0b013e31827c28bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Alper AB, Jr, Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin–angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Ohashi N, Katsurada A, et al. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KT, Kobori H, Hamm LL, et al. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012;27:3176–3181. doi: 10.1093/ndt/gfs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara M, Kondo S, Kagami S, et al. Urinary angiotensinogen accurately reflects intrarenal renin–angiotensin system activity. Am J Nephrol. 2010;31:318–325. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Urushihara M, Kotani Y, et al. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251–257. doi: 10.1016/j.diabres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Konishi Y, Ohashi N, et al. Urinary angiotensinogen reflects the activity of intrarenal renin–angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlugun AA, Altun B, Aktan U, et al. The relation between urinary angiotensinogen and proteinuria in renal AA amyloidosis patients. Amyloid. 2012;19:28–32. doi: 10.3109/13506129.2012.654530. [DOI] [PubMed] [Google Scholar]
- Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin–angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- Prieto-Carrasquero MC, Kobori H, Ozawa Y, et al. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani A, Kelly DJ, Cox AJ, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Lara LS, Luffman C, et al. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto MC, Navar LG.Collecting duct renin: a critical link in angiotensin II-dependent hypertensionIn: Frohlich ED, Re RN (eds).The Local Cardiac Renin–Angiotensin Aldosterone System2nd edn.Springer Science+Business Media, LLC: New York, NY; 2009133–141. [Google Scholar]
- Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]