Abstract
This study aimed to evaluate the activity of cholinesterases and adenosine deaminase (ADA) in blood and serum of rats infected with Trypanosoma cruzi. Twelve adult rats were used in the experiment divided into two uniform groups. Rodents from group A (control group) were non-infected and animals from group B served as infected, receiving intraperitoneally 3.3×107 trypomastigotes/each. Blood collection was performed at days 60 and 120 post-infection (PI) in order to evaluate the hemogram, blood activity of acetylcholinesterase, and serum butyrylcholinesterase and ADA activities. Hematological parameters did not differ between groups. A significant increase (P<0.05) of acetylcholinesterase activity was observed in blood while butyrylcholinesterase had a significant reduction (P<0.01) in serum of infected rats at days 60 and 120 PI. ADA activity in serum showed an inhibition in infected animals when compared to non-infected at day 120 PI. Based on these results, it is possible to conclude that the activity of cholinesterases and ADA were changed in animals infected with T. cruzi. The possible causes of these alterations will be discussed in this paper.
INTRODUCTION
The etiological agent of Chagas disease, the flagellate protozoan of Kinetoplastida order, Trypanosoma cruzi, causes a frequent anthropozoonosis in Latin America which affects approximately 10 million individuals, with an additional of 40 million people at risk of infection (Lana and Tafuri, 2000; Schofield et al., 2006). The parasite’s life cycle alternates between vertebrates and insects (triatomines), with different developmental stages in each host. It is an obligatory intracellular parasite, which replicates the amastigote form followed by eventual dissemination throughout the host by the morphologically distinct trypomastigote (Caetano et al., 2009).
The infection consists basically of an acute phase, in which parasites are relatively plentiful both in peripheral blood and tissues, followed by a chronic phase characterized by presence of parasites mainly in tissues such as heart, esophagus, colon and peripheral nervous system (Higuchi et al., 2003), causing various clinical conditions, including megacolon, megaesophagus and chagasic cardiopathy (Santos et al., 2005; Teixeira et al., 2006; Barbosa et al., 2009).
The innate immune response to T. cruzi involves production of cytokines with capacity of regulate natural killer cell activity. Some of these, such as IFN-a, IFN-b (type 1 interferons), IL-12 and nitric oxide have also been implied in protection against the parasite (Vespa et al., 1994; Michailowsky et al., 2001; Caetano et al., 2009). The immune response of host in Chagas disease is widely studied and seems to be elucidated. However, the cholinergic and purinergic systems, which play an important role in inflammatory processes of many diseases, are still poorly studied in infections by T. cruzi.
Acetylcholine is a classical neurotransmitter in the central nervous system and peripheral nervous system (Tucek, 1988) and is involved in the regulation of immune and other physiological functions (Kawashima and Fujii, 2003). Cholinesterases are enzymes present in cholinergic and non-cholinergic tissues as well as blood and other body fluids. They are divided into two classes according to their catalytic properties and specificity for substrates, sensitivity to inhibitors and tissue distribution (Chatonnet and Lockridge, 1989). Acetylcholinesterase (AChE: EC 3.1.1.7) is a membrane-bound enzyme mainly found in the brain, muscles, erythrocytes, lymphocytes and cholinergic neurons (Schetinger et al., 2000; Kawashima and Fujii, 2003) that preferentially hydrolyses esters with acetyl group. Butyrylcholinesterase (BChE: EC 3.1.1.8) is found in the intestine, liver, kidney, heart, lung and serum (Ecobicon and Corneau, 1973) and hydrolyzes other esters like butyrylcholine (Taylor and Brown, 1999). However, BChE can also take the place of AChE in ACh degradation when AChE is inhibited or absent (Li et al., 2000).
Adenosine acts as a central nervous system modulator in mammals, regulating cell metabolism, triggers a variety of physiological effects and participates in apoptosis, necrosis and cell proliferation. It also acts as an endogenous regulator of innate immunity, protecting the host from excessive tissue injury associated with strong inflammation (Burnstock, 2006; Desrosiers et al., 2007). Adenosine deaminase (ADA: EC 3.5.4.4) is considered a key enzyme in purine metabolism, catalyzing the irreversible deamination of adenosine to inosine (Franco et al., 1997). ADA is present in all cell types, but it can be found with high activity in the thymus, lymphoid tissues and peripheric lymphocytes. It has been demonstrated that this enzyme plays an important role in lymphocyte function and it is essential for the normal growth, differentiation and proliferation of T lymphocytes (Franco et al., 1997; Codero et al., 2001).
As mentioned previously, acetylcholine and adenosine are important transmitters and modulators of vital functions in the host, among them the regulation of immune response. Consequently, this study aimed to evaluate the activity of cholinesterase and ADA in blood and serum of rats experimentally infected with T. cruzi.
MATERIALS AND METHODS
Animals
Twelve adult male rats (Rattus norvegicus), weighing an average of 284(±23) g were used in the experiment. The animals were kept in cages, six animals each, in an experimental room with temperature and humidity controlled (25°C; 70%), fed with commercial ration and water ad libitum. A period of 30 days of adaptation was performed prior to the experimental study. The whole procedure was approved by the Animal Welfare Committee of Federal University of Santa Maria (UFSM), number 10/2011.
Groups, T. cruzi Isolated and Estimation of Parasitemia
Rats were divided into two groups with six animals each group. Group A was used as negative control and group B was infected with T. cruzi. Inoculation procedure was performed intraperitoneally with 3.3×107 trypomastigote forms of a strain of T. cruzi maintained in cell culture (Trypanosomatid culture collection of the University of São Paulo, number 874 isolated of Didelphis marsupialis), whereas the control group received a physiological solution. Parasitemia was estimated daily by microscopic examination of smears (Da Silva et al., 2006).
Samples Collection
After 60 and 120 days of infection, all survivor rats were anesthetized with isoflurane inside an anesthetic chamber for blood collection (1.5 ml by intracardic puncture). Approximately 10 minutes after collection, the animals showed signs of recovery. A volume of 0.5 ml of blood (with anticoagulant) was used to perform the hemogram and analyze AChE activity in blood. A volume of 40 μl of blood was diluted 1:100 (v/v) in lysis solution (0.1 mmol/l potassium/sodium phosphate buffer containing 0.03% Triton X-100) to determine AChE activity in blood. The remaining blood (1 ml) was utilized to obtain serum to measurement of enzymes activities (BChE and ADA). At the 120-day post-infection (PI), all survivor rats were anesthetized following the same procedures prior cited, and then decapitated, as recommended by the Ethics Committee. Heart was removed for histological analysis and slides were stained with hematoxylin and eosin. A small fragment of this organ was placed in tubes filled with ethanol (70%) to conservation, and further to perform the polymerase chain reaction for confirmation of infection.
Hematological Parameters
Hematological parameters were performed for monitoring the disease. Erythrocytes count, hematocrit, hemoglobin concentration and total leukocytes count were evaluated during the study. Smears were mounted and stained by the panoptic method to leukogram differential. Erythrocytes and leukocytes count and hemoglobin concentration were determined using an electronic counter. The determination of hematocrit was performed according to the technique described by Feldman et al. (2000).
Enzymatic Activity
The AChE enzymatic assay in the total blood was performed by the method of Ellman et al. (1961) modified by Worek et al. (1999). The specific activity of total blood AChE was calculated from the quotient between AChE activity and hemoglobin content and the results were expressed in mU/l mol Hb. The same method was used for the determination of BChE activity in the serum, except when the acetylcholine substrate was replaced by butyrylthiocholine and the results were expressed as μmol BcSCh/h/mg of protein. ADA activity was spectrophotometrically measured in serum according to Giusti and Gakis (1971). The reaction was initialized by addition of substrate (adenosine), and the specific activity is reported in U/l.
Statistical Analysis
The data were analyzed statistically by the Student’s t-test for independent samples. Differences were considered significant when P<0.05.
RESULTS
Parasitemia and Infection Curse in Rats
Trypomastigotes forms were observed in blood smears at days 4 and 5 PI, but it represented low parasitemia (one to three parasites/slide). After 6 days of infection, no parasites were observed in the blood smears. Clinical changes were not observed along the 120 days of experiment. Hematologic parameters (erythrogram and leukogram) had no differences between groups during the two analyzed periods.
Enzymatic Activity
A significant increase (P<0.05) of AChE activity was observed in blood of infected rats at days 60 and 120 PI (Fig. 1a). In contrast, BChE activity was significantly reduced (P<0.01) in serum of infected rats in the same period (Fig. 1b). ADA activity in serum had an inhibition in infected animals, when compared to non-infected at day 120 PI (Fig. 2).
Fig. 1.
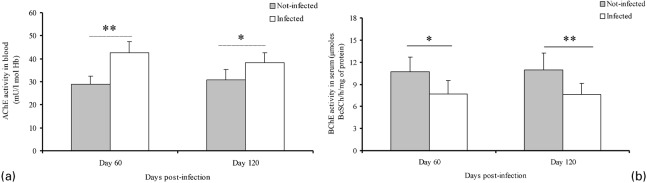
Acetylcholinesterase activity in blood (a) and butyrylcholinesterase activity in serum (b) of Trypanosoma cruzi-infected rats. Asterisk indicates statistical difference between infected and not-infected groups (*P<0.05, **P<0.01).
Fig. 2.
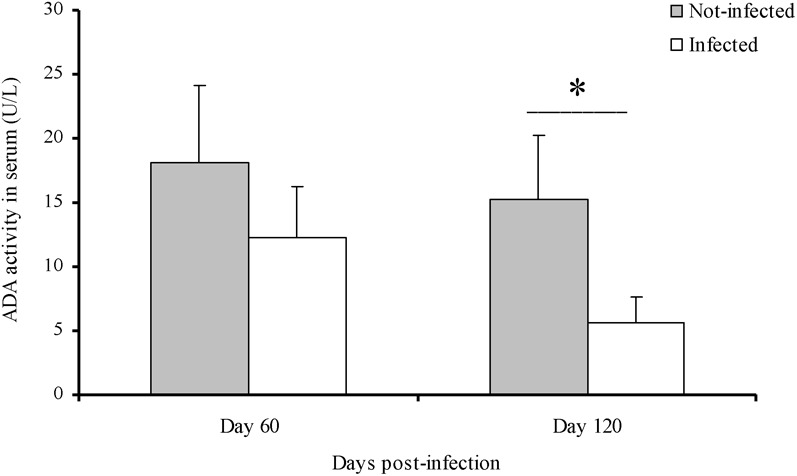
Adenosine deaminase activity in serum of Trypanosoma cruzi-infected rats. Asterisk indicates statistical difference between infected and non-infected groups (*P<0.01).
Histology and Molecular Analysis in Heart
Histology of heart tissue showed mononuclear inflammatory infiltrate between muscle fibers in rats infected with T. cruzi (Fig. 3). Amastigotes forms were not observed in histology of heart. However, polymerase chain reaction of cardiac muscle had positive reaction for T. cruzi.
Fig. 3.
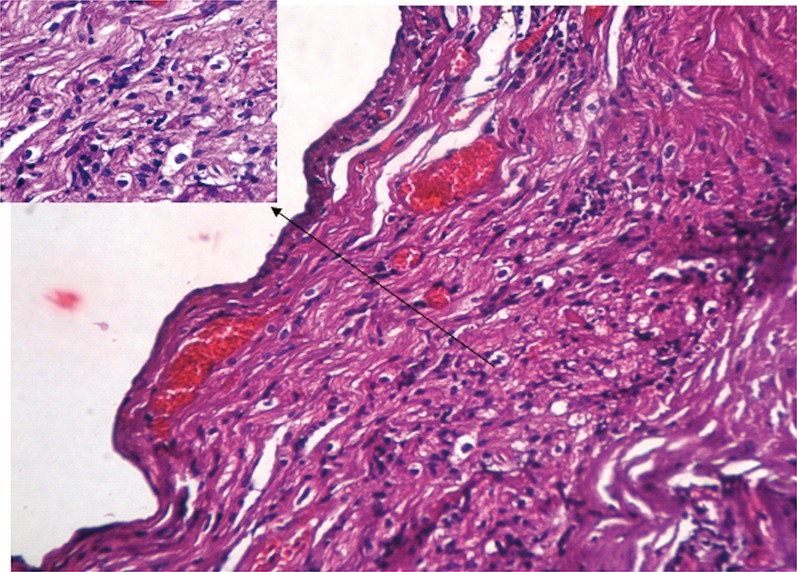
Histology of heart tissue showed mononuclear inflammatory infiltrate between muscle fibers in rats infected with Trypanosoma cruzi. Slides were stained with hematoxylin and eosin, objective, ×100.
DISCUSSION
Reductions in acetyltransferase and AChE activities in skeletal muscle are described in mice infected by T. cruzi (Brennessel et al., 1985). Alternatively, changes in activity of AChE and BChE in blood, serum, plasma and brain of rats and cats infected with Trypanosoma evansi were observed (Wolkmer et al., 2010; Da Silva et al., 2010; Da Silva et al., 2011b). ADA activity was not measured in mammals infected with T. cruzi yet, but recently our research group found alteration in the activity of ADA in serum, erythrocytes and lymphocytes of rats infected with T. evansi (Da Silva et al., 2011a), as well as in patients with visceral leishmaniasis (Tripathi et al., 2008).
In this study, activity of AChE in blood increased significantly in rats infected with T. cruzi. Similar results were observed in lymphocytes and brain of rats infected with T. evansi, which had activation of the enzyme AChE (Da Silva et al., 2011b; Da Silva et al., 2011c). This increase in the activity of AChE in total blood may be explained as an inflammatory response caused by the parasite as described in the literature (Vespa et al., 1994; Michailowsky et al., 2001). When the AChE increases, a rapid degradation of ACh occurs, which is considered as a protein with anti-inflammatory action, since it binds to nicotinic receptors in leukocytes surfaces (as lymphocytes). Thus, it inhibits the proliferation of cytokines, serotonin, histamine, nitric oxide, lysosomal enzymes, prostaglandins and leukotrienes which are mediators of inflammatory processes (Kawashima and Fujii, 2003; Nizri et al., 2006; Das, 2007). As shown by researchers, the inflammatory mediators are produced during infection with T. cruzi (Vespa et al., 1994; Michailowsky et al., 2001). Therefore, it is possible to suggest that the inflammatory reaction triggered by the T. cruzi infection might lead to this increase in AChE activity and, as an outcome, a decrease in ACh, which possess anti-inflammatory characteristics, similar as T. evansi infection (Da Silva et al., 2011c).
A reduction in BChE activity was observed in serum of rats infected with T. cruzi. Similar findings of this study were described in cats and rats infected with T. evansi (Da Silva et al., 2010; Wolkmer et al., 2010). Since BChE is synthesized in the liver (Kutty, 1980), alterations in hepatic function (by lesion) would probably result in reduced amounts of this enzyme in blood (Singh, 1976; Lemberg and Macchi, 1981). Several studies in T. cruzi-infected animals demonstrated hepatic damage caused by this disease (Souza et al., 2006); however, hepatic enzymes were not evaluated in the present study, which could prove the prior suspicion. Thus, the decreased activity reduction of BChE in infection caused by T. cruzi could be related to hepatic lesions, but the possibility that this inhibition may be related to inflammatory processes cannot be ignored, since this enzyme may participate in the regulation of immune response (Das, 2007).
The reduction in ADA activity observed in serum of rats infected with T. cruzi has also been observed in serum, erythrocytes, lymphocytes (Da Silva et al., 2011a) and platelets (Oliveira et al., 2011) of rodents infected with T. evansi. Reported cases of leishmaniasis and theileriasis described an increase in ADA activity in serum of infected mammals (Khambu et al., 2007; Altug et al., 2008). The reduction in ADA activity could cause an increase in extracellular concentrations of adenosine, which would be converted to inosine. Adenosine acts as a sensor and provides information to the immune system regarding tissue damage or acute inflammatory changes occurring in the vicinity of the immune system (Kumar and Sharma, 2009). Reduction in the activity of ADA would lead to interaction of adenosine with adenosine receptors that exist in many cell types, with possible anti-inflammatory effects, including the inhibition of the Th1 immune response. In acute infection caused by T. cruzi, there is a predominance of Th1 and cellular response with production of interferon-gamma (Kumar and Tarleton, 2001). However, inhibition of this response by the action of extracellular adenosine in purinergic receptors could be a compensatory effect, attenuating inflammation and tissue damage. Thus, the anti-inflammatory action of adenosine as a manner to preserve cells and tissues is a probable hypothesis in T. cruzi infection.
Based on these results, it is possible to conclude that T. cruzi infection in rats induces changes in activity of AChE in blood and BChE and ADA in serum. As discussed above, these changes are probably related to inflammatory host responses against the parasite. Further investigations of these enzymes in inflammatory cells and heart tissue of experimentally infected rodents may help in the conclusions obtained here.
REFERENCES
- Altug N, Yüksek N, Agaoglu ZT, Keles I.(2008)Determination of adenosine deaminase activity in cattle naturally infected with Theileria annulata. Tropical Animal Health Production 40449–456. [DOI] [PubMed] [Google Scholar]
- Barbosa VSA, Holanda CMCX, Câmera ACJ, Silva RP, Oliveira DP, Moreira JA, Medeiros AC.(2009)Trypanosoma cruzi: biodistribution of technetium-99 m pertechnetate in infected rats. Experimental Parasitology 123309–312. [DOI] [PubMed] [Google Scholar]
- Brennessel DJ, Wittner M, Braunstein V, Herbert B.(1985)Acetylcholinesterase levels in skeletal muscle of mice infected with Trypanosoma cruzi. American of Society Tropical Medicine and Hygiene 34460–464. [DOI] [PubMed] [Google Scholar]
- Burnstock G.(2006)Purinergic signaling — an overview. Novartis Found Symposium 27626–48. [PubMed] [Google Scholar]
- Caetano LC, Santello FH, Filipin MDV, Brazão V, Caetano LN, Toldo MPA, Caldeira JC, Prado-Junior JC.(2009)Trypanosoma cruzi: dehydroepiandrosterone (DHEA) and immune response during the chronic phase of the experimental Chagas’ disease. Experimental Parasitology 16327–32. [DOI] [PubMed] [Google Scholar]
- Chatonnet A, Lockridge O.(1989)Comparision of butyrylcholinesterase and acetylcholinesterase. Biochemistry Journal 260625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codero O, Salgado F, Fernández-Alonso C, Herrera C, Lluis C, Franco R, Nogueira M.(2001)Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. Journal of Leukocyte Biology 70920–930. [PubMed] [Google Scholar]
- Das UN.(2007)Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Medical Science Monitor 13214–221. [PubMed] [Google Scholar]
- Da Silva AS, Doyle RL, Monteiro SG.(2006)Método de contenção e confecção de esfregaço sanguíneo para pesquisa de hemoparasitas em ratos em camundongos. Faculdade de Zootecnia, Veterinária e Agronomia 13153–157. [Google Scholar]
- Da Silva AS, Spanevello R, Stefanello N, Wolkmer P, Costa MM, Zanette RA, Lopes STA, Santurio JM, Schetinger MRC, Monteiro SG.(2010)Influence of Trypanosoma evansi in blood, plasma, and brain cholinesterase of experimentally infected cats. Research Veterinary Science 88281–284. [DOI] [PubMed] [Google Scholar]
- Da Silva AS, Bellé LP, Bitencourt PER, Souza VCG, Costa MM, Oliveira CB, Jaque JA, Leal DBR, Moretto MB, Mazzanti CM, Lopes STA, Monteiro SG.(2011a)Activity of the enzyme adenosine deaminase in serum, erythrocytes and lymphocytes of rats infected with Trypanosoma evansi. Parasitology 138201–208. [DOI] [PubMed] [Google Scholar]
- Da Silva AS, Monteiro SG, Gonçalves JF, Spanevello R, Oliveira CB, Costa MM, Jaques JA, Morsch VM, Schetinger MRC, Mazzanti CM, Lopes STA.(2011b)Acetylcholinesterase activity and lipid peroxidation in the brain and spinal cord of rats infected with Trypanosoma evansi. Veterinary Parasitology 175237–244. [DOI] [PubMed] [Google Scholar]
- Da Silva AS, Monteiro SG, Gonçalves JF, Spanevello R, Oliveira CB, Costa MM, Jaques JA, Schetinger MRC, Mazzanti CM, Lopes STA.(2011c)Trypanosoma evansi: Immune response and acetylcholinesterase activity in lymphocytes from infected rats. Experimental Parasitology 127475–480. [DOI] [PubMed] [Google Scholar]
- Desrosiers MD, Katherine MC, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y.(2007)Adenosine deamination sustains dendritic cell activation in inflammation. Journal of Immunology 1791884–1892. [DOI] [PubMed] [Google Scholar]
- Ecobicon DJ, Corneau AM.(1973)Pseudocholinesterase of mammalian plasma: physiochemical properties and organophosphate inhibition in eleven species. Toxicology Applied Pharmacology 2492–100. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Coutney KO, Andres V, Featherstone RM.(1961)A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 788–95. [DOI] [PubMed] [Google Scholar]
- Feldman BV, Zinkl JG, Jain NC.(2000)Schalm’s Veterinary Hematology Philadelphia, PA: Lippincott Williams & Wilkins; 1344 [Google Scholar]
- Franco R, Casado V, Ciruela F, Saura C, Mallol J, Canela EI, Llouis C.(1997)Cell surface adenosine deaminase: much more than an ectoenzyme. Progress of Neurobiology 52283–294. [DOI] [PubMed] [Google Scholar]
- Giusti G, Gakis C.(1971)Temperature conversion factors, activation energy, relative substrate specificity and optimum pH of adenosine deaminase from human serum and tissues. Enzyme 12417–425. [DOI] [PubMed] [Google Scholar]
- Higuchi MDL, Benvenuti LA, Martins Reis M, Metzger M.(2003)Pathophysiology of the heart in Chagas disease: current status and new developments. Cardiovascular Research 6096–107. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T.(2003)The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Science 74675–696. [DOI] [PubMed] [Google Scholar]
- Khambu B, Mehta KD, Rijal S, Lamsal M, Majhi S, Baral N.(2007)Serum nitrate level and adenosine deaminase activitiy is altered in visceral Leishmaniasis. Nepal Medical College Journal 940–44. [PubMed] [Google Scholar]
- Kumar S, Tarleton RL.(2001)Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. Journal of Immunology 1664596–4603. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sharma A.(2009)Adenosine: an endogenous modulator of innate immune system with therapeutic potential. European Journal of Pharmacology 6167–15. [DOI] [PubMed] [Google Scholar]
- Kutty KM.(1980)Biological function of cholinesterase. Clinical Biochemistry 13239–243. [DOI] [PubMed] [Google Scholar]
- Lana M, Tafuri WL.(2000)Trypanosoma cruzi e doença de Chagas In Parasitologia humana, org. Neves, D. P.73–96.São Paulo: Editora Atheneu [Google Scholar]
- Lemberg A, Macchi MC.(1981) Usefulness of serum pseudocholinesterase isoenzymes in acute and chronic liver diseases and neoplasms (experimental and clinical studies). Acta Gastroenterologica Latinoamericana 11125–132. [PubMed] [Google Scholar]
- Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O.(2000)Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. Journal of Neurochemistry 751320–1331. [DOI] [PubMed] [Google Scholar]
- Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT.(2001)Pivotal role of interleukin-12 and interferon-g axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. American Journal Pathology 1591723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T.(2006)Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 50540–547. [DOI] [PubMed] [Google Scholar]
- Oliveira CB, Da Silva AS, Vargas LB, Bitencourt PER, Souza VCG, Costa MM, Leal CAM, Moretto MB, Leal DBR, Lopes STA, Monteiro SG.(2011)Activities of adenine nucleotide and nucleoside degradation enzymes in platelets of rats infected by Trypanosoma evansi. Veterinary Parasitology 1789–14. [DOI] [PubMed] [Google Scholar]
- Santos CD, Caldeira JC, Toldo MPA, Prado JC.(2005)Trypanosoma cruzi: effects of repetitive stress during the development of experimental infection. Experimental Parasitology 11096–101. [DOI] [PubMed] [Google Scholar]
- Schetinger MRC, Porto NM, Moretto MB, Morsch VM, Rocha JBT, Vieira V, Moro F, Neis RT, Bittencourt S, Bonacorso HG, Zanatta N.(2000)New benzodiazepines alter acetylcholinesterase and ATPase activities. Neurochemistry Research 25949–955. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R.(2006)The future of Chagas disease control. Trends Parasitology 22583–588. [DOI] [PubMed] [Google Scholar]
- Singh DS.(1976)Serum cholinesterase in hepatic disorders. Journal of the Indian Medical Association 6649–51. [PubMed] [Google Scholar]
- Souza EM, Oliveira GM, Boykin DW, Kumar A, Hu Q, Soeiro MNC.(2006)Trypanocidal activity of the phenyl-substituted analogue of furamidine DB569 against Trypanosoma cruzi infection in vivo. Journal of Antimicrobial Chemotherapy 58610–614. [DOI] [PubMed] [Google Scholar]
- Taylor P, Brown JH.(1999)Acetylcholine Basic Neurochemistry: Molecular, cellular and Medical Aspects Siegel G J.214–242.Philadelphia, PA: Lippincott-Raven Publishers [Google Scholar]
- Teixeira ARL, Nascimento RJ, Sturm NR.(2006)Evolution and pathology in Chagas disease — a review. Memórias do Instituto Oswaldo Cruz 101463–491. [DOI] [PubMed] [Google Scholar]
- Tripathi K, Kumar R, Bharti K, Kumar P, Shrivastav R, Sundar S, Pai K.(2008)Adenosine deaminase activity in sera of patients with visceral leishmaniasis in India. Clinica Chimica Acta 388135–138. [DOI] [PubMed] [Google Scholar]
- Tucek S.(1988)Choline acetyltransferase and the synthesis of acetylcholine Handbook of Experimental Pharmacology: The Cholinergic Synapse Whittaker V P.125–165.Berlin: Springer Verlag [Google Scholar]
- Vespa GNR, Cunha FQ, Silva JS.(1994)Nitric oxide is involved in the control of Trypanosoma cruzi-induced parasitemia and directly kills parasite in vitro. Infection and Immunology 625177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkmer P, Traesel CK, Franscicato C, Da Silva AS, Lopes STA, Monteiro SG, Mazantti CM.(2010)Trypanosoma evansi: cholinesterase activity in acutely infected Wistar rats. Experimental Parasitology 125151–255. [DOI] [PubMed] [Google Scholar]
- Worek F, Mast U, Kiderlen D.(1999)Improved determination of acetylcholinestrase activity in human whole blood. Clinica Chimica Acta 28873–90. [DOI] [PubMed] [Google Scholar]


