Abstract
Dogs are the main domestic reservoirs of L. (L.) chagasi. Once in the vertebrate host, the parasite can cause visceral leishmaniasis, which can also be transmitted to humans. Cytokines are key elements of the host immune response against Leishmania spp. To investigate whether tumor necrosis factor (TNF)-α, interleukin (IL)-4 and IL-10 are associated with pattern infection in dogs, these cytokines were quantified in the spleen and liver of dogs naturally infected with L. (L.) chagasi, with or without clinical manifestations, and their levels were correlated with the parasite load verified in these organs. A total of 40 adult dogs naturally infected with L. (L.) chagasi were assessed, together with 12 uninfected control dogs. Samples from spleen and liver were used to determine the cytokine levels by capture ELISA and for quantifying parasite load by real-time PCR. Statistical analysis was performed using the minimum Chi square method and group means were compared using the Tukey test. TNF-α, IL-4 and IL-10 levels in infected dogs were higher than in control groups; the liver was the main cytokine-producing organ during infection. The level of splenic TNF-α showed correlation with parasite load and may represent an important marker for infection process evolution, with the participation of IL-10. These results may contribute to a clearer understanding of the immune response in dogs infected with L. (L.) chagasi, which may lead to the development of prophylactic or preventive measures for these animals.
INTRODUCTION
Leishmaniasis is caused by a protozoan of the Trypanosomatidae family, genus Leishmania. It occurs in 88 countries, 65 of which also present the visceral form. Most cases (90%) of visceral leishmaniasis (VL) in humans occur in the rural or suburban areas of five countries, including Brazil (Desjeux, 2004). In Brazil, as in other countries in South America, migration into urban areas contributed to the expansion of VL (Desjeux, 2004) and this expansion occurred especially in the northeastern (Dantas–Torres, 2006) and southeastern regions (Santiago et al, 2007). Besides the high incidence and broader distribution that the expansion itself represents, the spread into new areas also carries the threat that severe and lethal forms of the disease may emerge when associated with malnutrition (Gontijo and Melo, 2004) and HIV/AIDS infection (Ashford, 2000).
Dogs are considered to be the main domestic reservoirs of L. (L.) chagasi (Moreno and Alvar, 2002). The parasite is transmitted from one infected dog to another through the phlebotomine bites and possibly through other arthropod vectors, such as fleas and ticks (Coutinho et al., 2005; Ferreira et al., 2009; Dantas–Torres et al., 2010) and through blood transfusions (Owens et al., 2001). After the phlebotomine feed on the dog’s blood, the parasites rapidly spread into the lymph nodes and spleen through the lymph and blood and eventually reach the kidneys and liver. They may also reach the reproductive organs, skin, digestive and respiratory systems and the bladder (Molyneux and Ashford, 1983; Ashford, 2000).
Once in the vertebrate host, the parasite can cause lesions and symptoms that are characteristic of canine visceral leishmaniasis (CVL), although some infected dogs may be oligosymptomatic or asymptomatic (Mancianti and Meciani, 1988), and some can evolve to spontaneous cure (Fisa et al., 1999).
The most frequent signs of VL are lymphadenopathy, onychogryphosis, cutaneous lesions, weight loss, cachexia and locomotor abnormalities (Semião–Santos et al., 1995). The asymptomatic form represents 20 to 40% of the serum-positive population, of which 80% actually develops the disease (Noli, 1999).
Cytokines have been recognized as key elements of the host immune response against Leishmania spp. Parasitized macrophages, lymphocytes, dendritic cells and natural killers produce cytokines that are involved with both the inflammatory and adapted responses to the parasite (Sacks and Sher, 2002; Brodskyn et al., 2003).
It is known that cell-mediated immunity is able to control infection, leaving some dogs asymptomatic (Santos–Gomes et al., 2002). The occurrence of dogs asymptomatic for VL has been associated with the activation of Th1 cells that produce interferon (IFN)-γ, interleukin (IL)-2 and tumor necrosis factor (TNF)-α (Pinelli et al., 1994; Pinelli et al., 1995). Leishmania parasites are killed by macrophages activated by IFN-γ producing lymphocytes via a nitric oxide dependent mechanism (Vouldoukis et al., 1996; Holzmuller et al., 2005). However, other more recent studies have verified that both asymptomatic and symptomatic dogs produce cytokines belonging to Th1 and Th2 profiles, and that these coexist in CVL (Corrêa et al., 2007), but cell mediated immunity observed in L. infantum infected asymptomatic dogs depended on the preferential expression of Th1 cytokines (Chamizo et al., 2005).
The few studies that evaluated the effecter role of TNF-α have used infected dogs as models. In symptomatic dogs infected with L. (L.) chagasi, a correlation between the level of TNF-α in the serum and the active disease has not been identified (Lima et al., 2007).
Expression of IL-4 has been detected in the bone marrow of dogs naturally infected with L. (L.) chagasi, specially in those more severely affected, which suggests an association between IL-4 and the disease (Quinnell et al., 2001). Another study conducted with dogs infected with L. (L.) infantum, showed an increase in IL-4 expression in mononuclear cells of the peripheral blood after six months infection. This expression was quantitatively similar in both asymptomatic and symptomatic dogs (Manna et al., 2006).
IL-10 has been indicated as one of the mainsuppressing cytokines of the protective immune response, both in murine models and in humans with VL (Barral et al., 1993; Bacellar et al., 2000). However, more recently, studies have indicated that the anti-inflammatory effects of IL-10 may limit the side effect caused by an excessive inflammatory response, while also interfering with the process of parasite elimination (Trinchieri, 2007).
To investigate whether TNF-α, IL-4 and IL-10 is associated with pattern infection in dog, these cytokines were quantified in the spleen and liver of dogs naturally infected with L. (L.) chagasi, with or without clinical manifestations, and their levels were correlated with the parasite density load verified in these organs.
ANIMALS AND METHODS
The Study Area
The county of Araçatuba (21°12′32″S; 50°25′38″W), with an area of 1 167 311 km2 (450 701 mi2), is located in the state of São Paulo. It is a region known to be endemic for CVL (Nunes et al., 2008).
Animals
A total of 40 adult dogs, aged between 2 and 4 years old, males and females, of undefined breed and of different weights, from the Zoonosis Control Center of Araçatuba, were serum positive for L. (L.) chagasi by indirect ELISA (Lima et al., 2003).
The canines were divided in two groups: one group with 20 asymptomatic dogs; the other group with 20 symptomatic dogs showing at least three clinical signs of CVL. These could include fever, dermatitis, lymphadenopathy, onychogryphosis, weight loss, cachexia, locomotor abnormalities, conjunctivitis, epistaxis, hepatosplenomegaly, edema and apathy.
A group of 12 healthy dogs, also aged between 2 and 4 years old, both males and females, of undefined breed and of different weights, from a non-endemic area (Londrina, Paraná State, Brazil) were included in the study as negative controls. These dogs were serum negative for L. (L.) chagasi by indirect ELISA, according to the protocol described by Lima et al. (2003), and were PCR negative for this protozoan species.
The present research received approval from Research Ethics Committee for the use of Experimental Animals of the Faculdade de Odontologia de Araçatuba, Universidade Estadual Paulista (Brazil) protocol no. 57/06.
Sample Collection
Dogs were euthanized as follows: initially they were administered a tranquilizer with 0.05 mg/kg acepromazine maleate i.v., followed by a 15-minute rest period and fast injection (20 seconds) of 15 mg/kg of sodium thiopental i.v., followed by 10 ml of 19.1% potassium chloride through the same route. Total blood samples were collected for serological analysis, together with samples of spleen and liver, which were used to determine cytokine levels and for quantifying the parasite load.
Spleen and Liver Extracts
Extracts from spleen and liver were collected for quantitative analysis of TNF-α, IL-4 and IL-10 cytokines by capture-ELISA. To achieve this, 1 g of either spleen or liver and 2 ml of RPMI-1640 media (Sigma, St. Louis, MO, USA), pH 7.2, were used. The samples were refrigerated and grounded in a tissue homogenizer (Ultra Turrax T 8, Staufen, Germany) for about 5 minutes. The resulting homogenate was centrifuged at 10 000×g for 15 minutes at 4°C (Eppendorf 5810 R, Hamburg, Germany) and the supernatant was immediately stored at −80°C (Revco, Golden Valley, MN, USA).
TNF-α, IL-4 and IL-10 Quantitative Analysis
The TNF-α, IL-4 and IL-10 cytokines from the spleen and liver extracts were quantified using anti-canine monoclonal antibody (mAb) and polyclonal antibody biotinylated (R&D System, Minneapolis, MN, USA). Canine recombinant TNF-α, IL-4 and IL-10 (R&D System, Minneapolis, MN, USA) were used to produce a standard curve. The ELISA assay was performed in accordance with the manufacturer’s instructions. The test was developed with 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma, St. Louis, MO, USA). Plates were read with a Spectra Count apparatus (Pachard Bio Science Company, Piscataway, NJ, USA) using a 450 nm filter. The minimal sensitivity for detecting TNF-α was 3.12 pg/50 μl, for IL-4 was 78 pg/50 μl, and for IL-10 was 90.65 pg/50 μl.
Parasite Load
Parasite density was determined using real-time PCR following the protocol described by Rolão et al. (2004). To achieve this, 35 mg of spleen or liver from each dog were used for DNA extraction with a commercial kit (PCR template Preparation Kit, Roche Diagnostics GmbH, Mannheim, Germany), in accordance with the manufacturer’s instructions.
The promastigote form of L. (L.) chagasi was used to create the standard curve. Quantitative analysis of parasites was performed by counts with a Neubauer hemocytometer chamber (average of four counts). Parasite suspension was diluted in series of 10, varying from 1×106 to 10−1 parasites. These dilutions were then used for DNA extraction, as described above. Primers and internal probes were designed according to regions of the kinetoplast minicircle of L. (L.) chagasi (GenBank accession number AF169140), in accordance with Rolão et al. (2004). DNA samples were analyzed with the following (Applied Byosystems, Foster City, CA, USA): direct primer LshNRf (forward, 5′-GGTTAGCCGATGGTGGTCTT-3′), reverse LshNRr (5′-GCTATATCATATGTCCAAGCACTTACCT-3′), and internal probe LshNRp TaqMan® (5′-ACCACCTAAGGTCAACCC-3′). PCR was performed inan Icycler iQ thermocycler (Bio-Rad Laboratories, CA, USA). An aliquot of 2 μl of each DNA sample was added to a mix containing 10 μl of TaqMan Universal Master Mix, No AmpErase® UNG (Applied Biosystems, Foster City, CA, USA) and 2 μl of primers (900 nM) and TaqMan MGB probe (FAM™ fluorophore) (200nM) in a 20 μl final volume. Samples were assayed in duplicate and a negative control was included in each assay. PCR conditions were: 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds. The detection threshold was determined as the mean of the base line plus 10 x the standard deviation, representing the background level calculated from cycles 2 to 10.
Statistical Analysis
The variables studied (TNF-α, IL-4, and IL-10 levels and parasite density in the spleen and liver) were analyzed with the minimum Chi square method which considers the group effect. Group means were compared using the Tukey test, in which the data are tested for normality and homogeneity of variance, since these are prerequisites for this type of analysis. Correlation analysis was performed between the values for TNF-α, IL-4, IL-10 and the values corresponding to the parasite load in each group. Regression analysis was performed for parasite load in the spleen and liver, in each group. The results were considered significant when P<0.05. SAS (SAS 9.1 SAS Institute, Cary, NC, USA) was used for all statistical analysis performed in this study.
RESULTS
Production of Anti-L. (L.) chagasi Antibodies
The production of anti-Leishmania IgG antibodies was evaluated in 20 asymptomatic and 20 symptomatic dogs naturally infected by L. (L.) chagasi. The mean titers of anti-Leishmania IgG antibodies in asymptomatic (OD = 1.191±SD 0.431) and symptomatic (OD = 1.414±SD 0.484) were similar (Fig. 1), and no statistical difference was observed in anti-Leishmania antibody production. In contrast, no serum reactivity occurred against this total antigen in 12 dogs from the non-endemic area (OD = 0.138±SD 0.033) Student t test, P<0.0001) (Fig. 1).
Fig. 1.
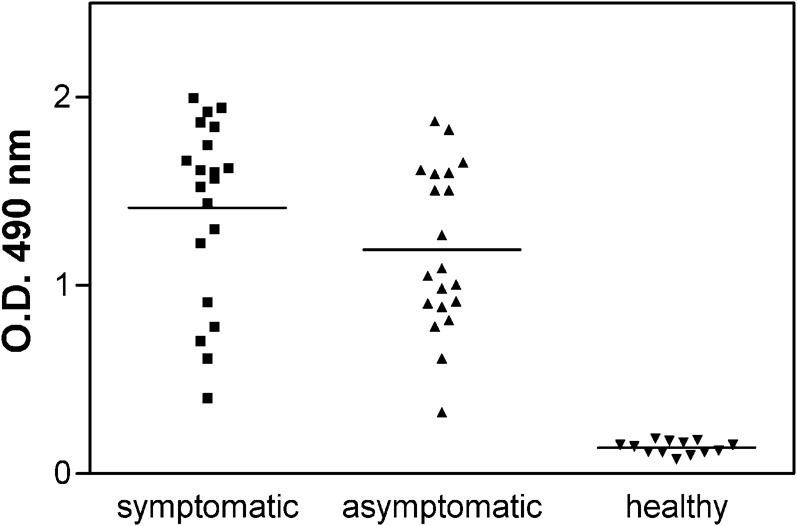
Optical density of serum samples tested by the enzyme immunoassays using crude protein derived from promastigotes of Leishmania (L.) chagasi, in the diagnosis of canine leishmaniasis in Araçatuba, SP, Brazil. The straight line represents mean of each group.
Quantitative Analysis of Cytokines
The mean TNF-α production was higher in the liver extracts, in both the asymptomatic and symptomatic groups compared to that from the spleen (P<0.05). Similar results were observed for IL-4 and IL-10 (Table 1).
Table 1. Means  and standard deviation (s) of TNF-α, IL-4 and IL-10 in spleen and liver extracts compared to the parasite load verified in these organs, in both asymptomatic (n = 20) and symptomatic (n = 20) dogs naturally infected with L. (L.) chagasi compared to healthy dogs (n = 12).
and standard deviation (s) of TNF-α, IL-4 and IL-10 in spleen and liver extracts compared to the parasite load verified in these organs, in both asymptomatic (n = 20) and symptomatic (n = 20) dogs naturally infected with L. (L.) chagasi compared to healthy dogs (n = 12).
| Variable | Group | Organ ( ±s) ±s) |
|
| Spleen | Liver | ||
| TNF-α (pg/ml) | Asymptomatic | 0.174±0.073 aB | 0.599±0.237 bA |
| Symptomatic | 0.183±0.060 aB | 0.763±0.214 aA | |
| Control | 0.041±0.013 bA | 0.146±0.040 cA | |
| IL-4 (pg/ml) | Asymptomatic | 21.014±3.679 aB | 69.769±16.894 aA |
| Symptomatic | 22.319±6.009 aB | 75.474±23.283 aA | |
| Control | 8.811±2.637 bB | 23.851±7.472 bA | |
| IL-10 (pg/ml) | Asymptomatic | 7.178±5.897 aB | 99.774±39.336 aA |
| Symptomatic | 9.584±8.789 aB | 107.532±35.742 aA | |
| Control | 0.723±0.488 bB | 26.059±9.152 bA | |
| Parasite load (log) | Asymptomatic | 2.633±1.138 aA | 1.950±1.150 bB |
| Symptomatic | 2.644±1.242 aA | 2.383±1.288 aB | |
Means followed by distinct letters, with small caps representing comparisons between groups in the same organs and large caps between organs in the same group, are those that differed according to the Tukey (p<0,05).
a; b - difference between asymptomatic and symptomatic group.
c - difference between asymptomatic, symptomatic and control group.
Observation also verified that the mean value of TNF-α in liver extracts from symptomatic dogs was significantly higher compared to that of asymptomatic dogs (P<0.05); however, this difference was not observed in the spleen extract from dogs in the two groups. The mean value of IL-4 in the spleen and liver of asymptomatic dogs revealed no significant difference compared to the group of symptomatic dogs. Similar observations were obtained for IL-10 levels. The mean value determined in infected dogs, asymptomatic or not, both in the spleen and in the liver, was higher for the three cytokines tested, than those from the liver and spleen of control dogs (P<0.05) (Table 1).
Quantitative Analysis of Parasite Load
The mean value of the parasite load from the spleen of asymptomatic and symptomatic dogs was higher than the parasite load from the liver of dogs in these two groups (P<0.05). The mean value of the parasite load in asymptomatic dogs showed no difference compared to that from the spleen of symptomatic dogs, although a significant increase in the liver of symptomatic dogs was observed compared to that from the liver of asymptomatic dogs (P<0.05) (Table 1).
Correlation Between Cytokine Levels (TNF-α, IL-4 and IL-10) and the Parasite Load by Pearson’s Linear Correlation Coefficient
The results showed that a weak positive linear correlation existed between the levels of TNF-α in the spleen and the parasite load observed in this organ, in the group of symptomatic dogs. A weak positive linear correlation was also determined between the IL-10 level in the liver and the parasite load in the same organ, in asymptomatic dogs (Table 2).
Table 2. Correlation (r) between each cytokine and parasite load from spleen and liver of asymptomatic (N = 20) and symptomatic (N = 20) dogs naturally infected with L. (L.) chagasi. Presence of correlation is represented by *.
| Cytokine | Group | Organ (r) | |
| Spleen | Liver | ||
| TNF-α | Asymptomatic | −0.361 | −0.075 |
| Symptomatic | 0,353 ** | −0.231 | |
| IL-4 | Asymptomatic | −0.188 | 0.098 |
| Symptomatic | −0.090 | −0.100 | |
| IL-10 | Asymptomatic | 0.204 | 0.448* |
* p<0.05 significant correlation between parasite load in organ and cytokine.
** p<0.1 significant correlation between parasite load in organ and cytokine.
Correlation Between Parasite Load in the Spleen and Liver by Pearson’s Linear Correlation Coefficient
A strong positive linear correlation was observed between parasite load in the spleen and liver in both groups studied; in asymptomatic dogs, r was 0.93553 and in symptomatic dogs r was 0.94686 (Fig. 2).
Fig. 2.
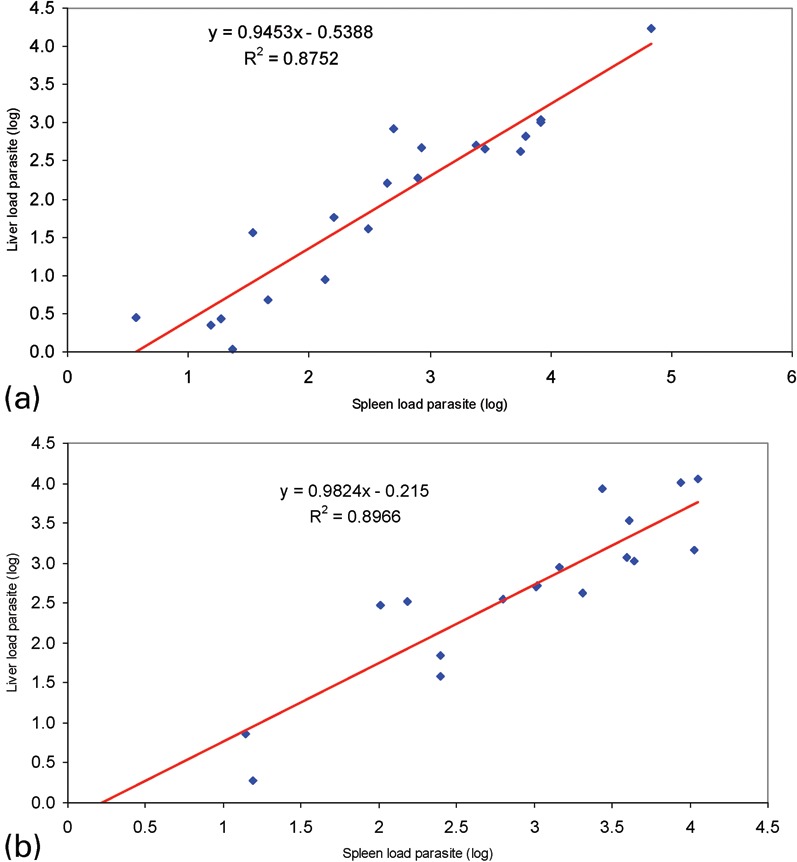
Linear positive correlation between parasite load from DNA samples from spleen and liver from asymptomatic (a) and symptomatic (b) dogs naturally infected with L. (L.) chagasi (P<0.0001). (a) r = 0.93553; (b) r = 0.94686.
DISCUSSION
In this study, the cytokines of spleen and liver extracts from VL dogs from an endemic area were analyzed to investigate the role of these mediators in the infectious process of the disease. Analysis of the results verified that the TNF-α levels in the spleen and liver were higher in the two groups of infected dogs compared to those of healthy dogs. The higher levels verified in infected dogs indicate that the presence of L. (L.) chagasi induces an immune response with relevant expression of TNF-α. Confirming that TNF-α is produced when the protozoan is present, Carrillo et al. (2007) showed that in mononuclear leucocytes of peripheral blood of asymptomatic dogs infected with L. (L.) chagasi and stimulated with a soluble Leishmania antigen, an increase in the gene expression of TNF-α occurs.
In infected dogs, asymptomatic or not, the level of liver TNF-α was higher compared to the spleen from the same animals. These results are similar to those obtained with BALB/c mice infected with L. (L.) donovani, in which the level of liver TNF-α was higher than that observed in the spleen (Mukherjee et al., 2003). In this study, observation verified that the level of TNF-α was higher in the liver of the symptomatic dogs than in the asymptomatic. Similarly, Panaro et al. (2010) showed that TNF-α mRNA levels in peripheral blood mononuclear cell from dogs with natural Leishmania infantum infection were significantly increased in symptomatic versus asymptomatic. This increase in hepatic TNF-α was associated with an increase in the parasite load in this organ; in fact, histological and biochemical changes that take place in the liver during the course of the disease increase the parasite load in this organ, in dogs naturally infected by L. (L.) chagasi (Giunchetti et al., 2008).
No difference in the level of TNF-α was observed when comparing the spleen of asymptomatic and symptomatic dogs. This can be explained by the ability of the spleen to express high levels of this cytokine, as TNF-α mediation is required for spleen tissue remolding when the parasite persists in this organ, as seen in mice infected with L. (L.) donovani (Engwerda et al., 1998). These results are similar to those reported by Lage et al. (2007) in which no difference in the level of TNF-α in spleen cells was observed between symptomatic and asymptomatic dogs naturally infected with L. (L.) chagasi.
Similar to the results obtained for TNF-α, a significant statistical difference was also verified when values of IL-4 from spleen and liver from infected dogs were compared to those from non-infected canines. Similar results were observed in a study involving oligosymptomatic dogs experimentally infected with L. (L.) infantum, in which the level of IL-4 in the spleen was higher than that from non-infected dogs (Strauss–Ayali et al., 2007). Additionally, levels of IL-4 in dogs naturally infected with L. (L.) chagasi were shown to be higher than those of non-infected dogs (Lage et al., 2007). Compared to the levels verified in healthy canines, the increased level of IL-4 observed in the spleen of infected dogs indicates that this cytokine plays a key role in this infectious process. The persistence of the parasite in the spleen of dogs infected with L. (L.) infantum may be associated with the high levels of IL-4 (Strauss–Ayali et al., 2007). A recent study conducted on mononuclear cells from the peripheral blood of dogs experimentally infected with L. (L.) infantum suggests that the lack of IL-4 expression, combined with a lack of IL-13 at the onset of the infectious process, may delay parasite multiplication (Sanchez–Robert et al., 2008). The level of IL-4 was higher in the liver compared to the level verified in the spleen in the two groups of infected dogs. This result is different than that from other reports. In studies conducted on BALB/c mice infected with L. (L.) donovani (Mukherjee et al., 2003; Rolão et al., 2007), the level of IL-4 in the spleen was higher than that in the liver, during the course of infection. The difference between the two studies can be explained by the animal model used in each study and by the fact that the dogs in this study were naturally infected and therefore no information was available regarding when infection started or what moment the examination represented during the course of the disease.
No difference was detected between the levels of IL-4 in the spleen and liver of infected dogs in both groups. Similar results, regarding the levels observed in the spleen, were also reported by Lage et al. (2007). Lack of difference for this cytokine in dogs naturally infected with (L.) infantum, whether asymptomatic or not, was also observed in the peripheral blood by other authors (Manna et al., 2006).
The lack of association between the level of IL-4 in the spleen and the clinical status of the dogs suggests that the cytokine may not be relevant to the evolution of the infectious process, although it may still play an important role during establishment of the infection (Strauss–Ayali et al., 2007).
No linear correlation was detected between parasite load in the spleen and liver and the level of IL-4 in these organs. The high level of IL-4 observed may not enough to explain the progression from asymptomatic dogs to the symptomatic condition. However, it could contribute to polyclonal B cell activation and hypergammaglobulinemia, typical of the disease. Lack of correlation could be attributed to the fact that the level of resistance or susceptibility for infection is preferentially related to the capacity to produce IFN-γ, rather than the presence of IL-4, as observed by Lehmann et al. (2000) in mice infected with L. (L.) donovani. This result differs from that obtained with BALB/c mice experimentally infected with L. (L.) infantum, in which a weak correlation between IL-4 and the parasite load in the spleen and liver was detected (Rolão et al., 2007).
Similar to that verified for TNF-α and IL-4, in this study, a statistically significant difference was observed when comparing IL-10 levels in the spleen and liver from infected dogs with levels from non-infected dogs. These results are similar to those observed by Alves et al. (2008) in a study involving dogs naturally infected with L. (L.) chagasi. Studies on humans and mice have shown association between infection susceptibility and elevated levels of IL-10 and TGF-β (Kane and Moser, 2001; Trinchieri, 2007).
The level of IL-10 was higher in the liver compared to that in the spleen in both groups of infected dogs. Studies conducted on BALB/c mice infected with L. (L.) donovani (Mukherjee et al., 2003; Rolão et al., 2007) showed that the levels of this cytokine in the liver were higher than those verified in the spleen during the course of infection. The production of IL-10 and TGF-β was also observed in the liver and spleen of asymptomatic and symptomatic dogs infected with L. (L.) chagasi (Corrêa et al., 2007; Lage et al., 2007),
In addition, the level of IL-10 in the liver of asymptomatic dogs showed a positive linear correlation with the level of parasite load in this organ. This suggests that IL-10 in the liver may be participating in the regulatory process that increases the parasite load and thus interferes with the evolution of the infection.
Using real-time PCR, a powerful tool for evaluating Leishmania (L.) chagasi loads (Da Silva et al., 2010), a strong positive correlation between parasite load in the spleen and in the liver was observed in the two groups of infected dogs in this study. These results suggest that the immune response to control the spread of the parasite in both organs is regulated by similar mechanisms, possibly without IL-4 participation. Similar results were observed in mice experimentally infected with L. (L.) donovani (Mukherjee et al., 2003) or with L. (L.) infantum (Rolão et al., 2007).
The liver of symptomatic dogs showed a higher parasite load compared to that in asymptomatic dogs. This difference could be related to physiopathological characteristics of this organ. An occurrence of intralobular liver fibrosis in symptomatic animals may contribute to an increase in parasite load (Melo et al., 2008). In addition, the spleen is more susceptible to L. (L.) chagasi: a higher parasite load was detected compared to the liver. This difference may be associated with the reduction in CD5+lymphocytes. Guerra et al. (2009) demonstrated that dogs with high splenic parasitism presented a significant decrease in absolute counts in this population of T lymphocytes in comparison with dogs presenting medium splenic parasitism.
Analysis of the results suggests that TNF-α plays an important role in the pathological process of VL, especially in the liver of symptomatic dogs. The association between the level of TNF-α and an increase in the parasite load observed in this group is an important indicator of the process of the evolution of VL infection. Moreover, the increased levels of TNF-α, IL-4 and IL-10 in the liver from infected dogs, compared to healthy canines, attribute to these cytokines an important role in the pathogenesis of VL.
These results may contribute to a clearer understanding of the immune response in dogs infected with L. (L.) chagasi, which could help in the development of vaccines or drugs.
Considering that in domestic areas dogs are the main hosts of the parasite and that they can transmit VL to humans, any new measure or treatment that benefits the canine population will most likely benefit humans.
Additional studies may add to current understanding regarding the role of cytokines and other immune mediators in dogs naturally infected with L. (L.) chagasi.
REFERENCES
- Alves CF, de Amorim IFG, Moura EP, Ribeiro RR, Alves CF, Michalick MS, Kalapothakis E, Bruna-Romero O, Tafuri WL, Teixeira MM, Melo MN.(2008)Expression of IFN-γ, TNF-α, IL-10 and TGF-β in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Veterinary Immunology and Immunopathology 127332–339. [DOI] [PubMed] [Google Scholar]
- Ashford RW.(2000)The leishmaniases as emerging and reemerging zoonoses. International Journal of Parasitology 301269–1281. [DOI] [PubMed] [Google Scholar]
- Bacellar O, D’Oliveira A, Jr, Jerônimo S, Carvalho EM.(2000)IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine 121228–1231. [DOI] [PubMed] [Google Scholar]
- Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG.(1993)Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proceedings of the National Academy Sciences of USA 903442–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodskyn C, de Oliveira CI, Barral A, Barral-Netto M.(2003)Vaccines in leishmaniasis: advances in the last five years. Expert Reviews Vaccines 2705–717. [DOI] [PubMed] [Google Scholar]
- Carrillo E, Ahmed S, Goldsmith-Pestana K, Nieto J, Osorio Y, Travi B, Moreno J, McMahon-Pratt D.(2007)Immunogenicity of the P-8 amastigote antigen in the experimental model of canine visceral leishmaniasis. Vaccine 251534–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamizo C, Moreno J, Alvar J.(2005)Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Veterinary Immunology and Immunopathology 10367–75. [DOI] [PubMed] [Google Scholar]
- Corrêa APFL, Dossi ACS, Vasconcelos RO, Munari DP, Lima VMF.(2007)Evaluation of transformation growth factor β1, interleukin-10, and interferon-γ in male symptomatic and asymptomatic dogs naturally infected by Leishmania (Leishmania) chagasi. Veterinary Parasitology 143267–274. [DOI] [PubMed] [Google Scholar]
- Coutinho MT, Bueno LL, Sterzik A, Fujiwara RT, Botelho JR, De Maria M, Genaro O, Linardi PM.(2005)Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Veterinary Parasitology 128149–155. [DOI] [PubMed] [Google Scholar]
- Da Silva RN, Amorim AC, Brandão RMSS, De Andrade HM, Yokoo M, Ribeiro ML, Bartchewsky W, Socorro-Silva A, De Castro JAF, Do Monte SJH.(2010)Real-time PCR in clinical practice: a powerful tool for evaluating Leishmania chagasi loads in naturally infected dogs. Annals of Tropical Medicine and Parasitology 104137–143. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F.(2006)Current epidemiological status of visceral leishmaniasis in Northeastern Brazil. Revista de Saúde Pública 40537–541. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Lorusso V, Testini G, Paiva-Cavalcanti M, Figueredo LA, Stanneck D, Mencke N, Filho BrandãoSP, Alves LC, Otranto D.(2010)Detection of Leishmania infantum in Rhicephalus sanguineus tichs from Brazil and Italy. Parasitology Research 106857–860. [DOI] [PubMed] [Google Scholar]
- Desjeux P.(2004)Leishmaniasis: current situation 448 and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases 27305–318. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Murphy ML, Cotterell SEJ, Smelt SC, Kaye PM.(1998)Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. European Journal of Immunology 28669–680. [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Fattori KR, Souza F, Lima VM.(2009)Potential role for dog fleas in the cycle of Leishmania spp. Veterinary Parasitology 165150–154. [DOI] [PubMed] [Google Scholar]
- Fisa R, Gállego M, Castillejo S, Aisa MJ, Serra T, Riera C, Carrió J, Gállego J, Portús M.(1999)Epidemiology of canine leishmaniosis in Catalonia (Spain) the example the Priorat focus. Veterinary Parasitology 8387–97. [DOI] [PubMed] [Google Scholar]
- Giunchetti RC, Mayrink W, Carneiro CM, Corrêa-Oliveira R, Martins-Filho OA, Marques MJ, Tafuri WL, Reis AB.(2008)Histopathological and immunohistochemical investigations of the hepatic compartment associated with parasitism and serum biochemical changes in canine visceral leishmaniasis. Research in Veterinary Science 84269–277. [DOI] [PubMed] [Google Scholar]
- Gontijo CMF, Melo MN.(2004)Leishmaniose Visceral no Brasil: quadro atual, desafios e perspectivas. Revista Brasileira de Epidemiologia 7338–349. [Google Scholar]
- Guerra LL, Teixeira-Carvalho A, Giunchetti RC, Martins-Filho AO, Reis AB, Corrêa-Oliveira R.(2009)Evaluation of the influence of tissue parasite density on hematological and phenotypic cellular parameters of circulating leukocytes and splenocytes during ongoing canine visceral leishmaniasis. Parasitology Research 104611–622. [DOI] [PubMed] [Google Scholar]
- Holzmuller P, Cavaleyra M, Moreaux J, Kovacic R, Vincendeau P, Papierok G, Lemesre JL.(2005)Lymphocytes of dogs immunised with purified excreted-secreted antigens of Leishmania infantum co-incubated with Leishmania infected macrophages produce IFN gamma resulting in nitric oxide-mediated amastigote apoptosis. Veterinary Immunology and Immunophatology 106247–257. [DOI] [PubMed] [Google Scholar]
- Kane MM, Mosser DM.(2001)The role of IL-10 in promoting disease progression in leishmaniasis. The Journal of Immunology 1661141–1147. [DOI] [PubMed] [Google Scholar]
- Lage RS, Oliveira GC, Busek SU, Guerra LL, Giunchetti RC, Corrêa-Oliveira R, Reis AB.(2007)Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Veterinary Immunology and Immunophatology 115135–145. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Enssle K.-H, Lehmann I, Emmendörfer A, Lohmann-Matthes M.-L.(2000)The capacity to produce IFN-γ rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. Journal of Interferon & Cytokine Research 2063–77. [DOI] [PubMed] [Google Scholar]
- Lima VMF, Gonçalves ME, Ikeda FA, Luvizotto MCR, Feitosa MM.(2003)Anti-leishmania antibodies in cerebrospinal fluid from dogs with visceral leishmaniasis. Brazilian Journal of Medicine and Biological Research 36485–489. [DOI] [PubMed] [Google Scholar]
- Lima VMF, Peiro JR, Vaconcelos RO.(2007)IL-6 and TNF-α production during active canine visceral leishmaniasis. Veterinary Immunology and Immunophatology 115189–193. [DOI] [PubMed] [Google Scholar]
- Mancianti F, Meciani N.(1988)Specific serodiagnosis of canine leishmaniasis by indirect immunofluorescence, indirect hemagglutination, and counterimmunoelectrophoresis. American Journal Veterinary Research 491409–1411. [PubMed] [Google Scholar]
- Manna L, Reale S, Viola E, Vitale F, Manzillo VF, Michele PL, Caracappa S, Gravino AE.(2006)Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Veterinary Parasitology 142271–280. [DOI] [PubMed] [Google Scholar]
- Melo F, Amaral M, Oliveira P, Lima W, Andrade M, Michalick M, Raso P, Tafuri W, Tafuri W.(2008)Diffuse intralobular liver fibrosis in dogs naturally infected with Leishmania (Leishmania) chagasi. American Journal of Tropical Medicine and Hygiene 79198–204. [PubMed] [Google Scholar]
- Molyneux D H, Ashford R W.(eds)(1983)The Biology of Trypanosoma and Leishmania, Parasites of Man and Domestic Animals 87.London: Taylor & Francis, Cambridge University Press [Google Scholar]
- Moreno J, Alvar J.(2002)Canine leishmaniasis: epidemiological risk and the experimental model. TRENDS in Parasitology 18399–405. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Ghosh AK, Ghose AC.(2003)Infection pattern and immune response in the spleen and liver of BALB/c mice intracardially infected with Leishmania donovani amastigotes. Immunology Letters 86131–138. [DOI] [PubMed] [Google Scholar]
- Noli C.(1999)Canine leishmaniosis. Waltham Focus 916–24. [Google Scholar]
- Nunes CM, Lima VMF, Paula HB, Perri SHV, Andade AM, Dias FEF, Burattini MN.(2008)Dog culling and replacement in area endemic for visceral leishmaniasis in Brazil. Veterinary Parasitology 15319–23. [DOI] [PubMed] [Google Scholar]
- Owens SD, Oakley DA, Marryott K, Hatchett W, Walton R, Nolan TJ, Newton A, Steurer F, Schantz P, Giger U.(2001)Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. Journal of the American Veterinary Medical Association 2191076–1083. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Brandonisio O, Cianciulli A, Cavallo P, Lacasella V, Paradies P, Testini G, De Caprariis D, Mitolo V, Otranto D.(2010)Cytokine expression in dogs with natural Leishmania infantum infection. Parasitology 136823–831. [DOI] [PubMed] [Google Scholar]
- Pinelli E, Gonzalo RM, Boog CJ, Rutten VP, Gebhard D, Del Real G, Ruitenberg EJ.(1995)Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. European Journal of Immunology 251594–1600. [DOI] [PubMed] [Google Scholar]
- Pinelli E, Killick-Kendrick R, Wagenaar J, Bernardina W, Del Real G, Ruitenberg J.(1994)Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infection Immunology 62229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnell RJ, Courtenay O, Shaw MA, Day MJ, Garcez LM, Dye C, Kaye PM.(2001)Tissue cytokine responses in canine visceral leishmaniasis. The Journal of Infectious Diseases 1831421–1424. [DOI] [PubMed] [Google Scholar]
- Rolão N, Cortes S, Rodrigues OR, Campino L.(2004)Quantification of Leishmania infantum parasites in tissue biopsies by real time polymerase chain reaction and polymerase chain reaction-enzyme linked immunosorbent assay. The Journal Parasitology 901150–1154. [DOI] [PubMed] [Google Scholar]
- Rolão N, Cortes S, Gomes-Pereira S, Campino L.(2007)Leishmania infatum: mixed T-helper-1/T-helper-2 immune response in experimentally infected BALB/c mice. Experimental Parasitology 115270–276. [DOI] [PubMed] [Google Scholar]
- Sacks D, Sher A.(2002)Evasion of innate immunity by parasitic protozoa. Nature Immunology 31041–1047. [DOI] [PubMed] [Google Scholar]
- Sanchez-Robert E, Altet L, Alberola J, Rodriguez-Cortés A, Ojeda A, López-Fuertes L, Timon M, Sanchez A, Francino O.(2008)Longitudinal analysis of cytokine gene expression and parasite load in PBMC in Leishmania infantum experimentally infected dogs. Veterinary Immunology and Immunophatology 125168–175. [DOI] [PubMed] [Google Scholar]
- Santiago MEB, Vasconcelos RO, Fattori KR, Munari DP, Michelin AF, Lima VMF.(2007)An investigation of Leishmania spp. in Didelphis spp. from urban and peri-urban areas in Bauru (São Paulo, Brazil). Veterinary Parasitology 150283–290. [DOI] [PubMed] [Google Scholar]
- Santos-Gomes GM, Rosa R, Leandro C, Cortes S, Romao P, Silveira H(2002)Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. Veterinary Immunology and Immunopathology 8821–30. [DOI] [PubMed] [Google Scholar]
- Semião-Santos SJ, el Harith A, Ferreira E, Pires CA, Sousa C, Gusmão R.(1995)Évora district as a new focus for canine leishmaniasis in Portugal. Parasitology Research 81235–239. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D, Baneth G, Jaffe CL.(2007)Splenic immune responses during canine visceral leishmaniasis. Veterinary Research 38547–564. [DOI] [PubMed] [Google Scholar]
- Trinchieri G.(2007)Interleukin-10 production by effector T cells: Th1 cells show self control. The Journal of Experimental Medicine 204239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouldoukis I, Drapier JC, Nüssler AK, Tselentis Y, Silva OA, Gentilini M, Mossalayi DM, Monjour L, Dugas B.(1996)Canine visceral leishmaniasis: successful chemotherapy induces macrophage antileishmanial activity via the L-arginine nitric oxide pathway. Antimicrobian Agents Chemotheraphy 40253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


