Abstract
Although, when applied under controlled conditions in India and Nepal, indoor residual spraying (IRS) has been found to reduce sandfly densities significantly, it is not known if IRS will be as effective when applied generally in these countries, via the national programmes for the elimination of visceral leishmaniasis. The potential benefits and limitations of national IRS programmes for the control of sandflies were therefore evaluated in the districts of Vaishali (in the Indian state of Bihar), Sarlahi (in Nepal) and Sunsari (also in Nepal). The use of technical guidelines, levels of knowledge and skills related to spraying operations, insecticide bio-availability on the sprayed surfaces, concentrations of the insecticide on the walls of sprayed houses, insecticide resistance, and the effectiveness of spraying, in terms of reducing sandfly densities within sprayed houses (compared with those found in unsprayed sentinel houses or control villages) were all explored. It was observed that IRS programme managers, at district and subdistrict levels in India and Nepal, used the relevant technical guidelines and were familiar with the procedures for IRS operation. The performance of the spraying activities, however, showed important deficiencies. The results of bio-assays and the chemical analysis of samples from sprayed walls indicated substandard spraying and suboptimal concentrations of insecticide on sprayed surfaces. This was particularly obvious at one of the Nepali study sites (Sunsari district), where no significant vector reduction was achieved. Sandfly resistance to the insecticide used in India (DDT) was widespread but the potential vectors in Nepal remained very susceptible towards a pyrethroid similar to the one used there. The overall short-term effectiveness of IRS was found to be satisfactory in two of the three study sites (in terms of reduction in the densities of the sandfly vectors). Unfortunately, the medium-term evaluation, conducted 5 months after spraying, was probably made invalid by flooding or lime plastering in the study areas. Preparation for, and the monitoring of, the IRS operations against sandfly populations in India and Nepal need to be improved.
Human visceral leishmaniasis (VL) or kala-azar, an often fatal disease if untreated, is usually caused by the parasite Leishmania donovani. On the Indian subcontinent, L. donovani is transmitted by the blood-feeding females of the sandfly Phlebotomus argentipes and mostly affects the poorest communities in rural villages (Alvar et al., 2006; WHO, 2008; Boelaert et al., 2009; Mondal et al., 2009). In Bangladesh, India and Nepal about 200 million people, in 109 districts, are considered ‘at risk’ of VL (Joshi et al., 2009). In 2005 a programme for the elimination of VL from the Indian subcontinent was initiated by the World Health Organization (WHO) and launched by the governments of Bangladesh, India and Nepal. In support of this elimination programme, researchers from the three countries involved, in collaboration with the WHO’s Special Programme for Research and Training in Tropical Diseases (TDR), have carried out operational research in order to inform policy makers about the various options available for the control of P. argentipes. Under carefully controlled conditions, the indoor spraying of a residual insecticide — most commonly known as ‘indoor residual spraying’ or IRS — was found to be particularly efficacious, causing a 72.4% reduction in sandfly densities (Joshi et al., 2009). The use of long-lasting insecticide-treated bednets (LLIN) also appears effective, with the 43.7% reduction in sandfly densities seen by Joshi et al. (2009) when such nets were deployed confirming the encouraging results from other VL-endemic areas (Ostyn et al., 2008). In addition, in their recent study, Picado et al. (2010) observed how, in India and Nepal, the use of LLIN led to a 25% reduction in sandfly densities (when they compared ‘intervention’ houses with control, sentinel households that had been given untreated nets on the night of sandfly collection). Although Joshi et al. (2009) also found that the lime plastering of walls and floor areas could lead to a similar reduction in sandflies (by 42%) to that seen when LLIN were deployed, such plastering is relatively expensive (Das et al., 2008) and the cheaper mud plastering appears to have no significant effects on sandfly populations (Joshi et al., 2009).
Although several trials of sandfly-control methods have therefore given encouraging result, a vector-control method may not be as effective when delivered under the routine operating conditions of a national control programme as when evaluated in a carefully controlled trial. The main aims of the present study were to evaluate the effectiveness of IRS, against P. argentipes populations, when performed within the national sandfly-control programmes in India and Nepal, and to identify the main factors associated with the success or failure of these programmes.
MATERIALS AND METHODS
Study Sites and Dates
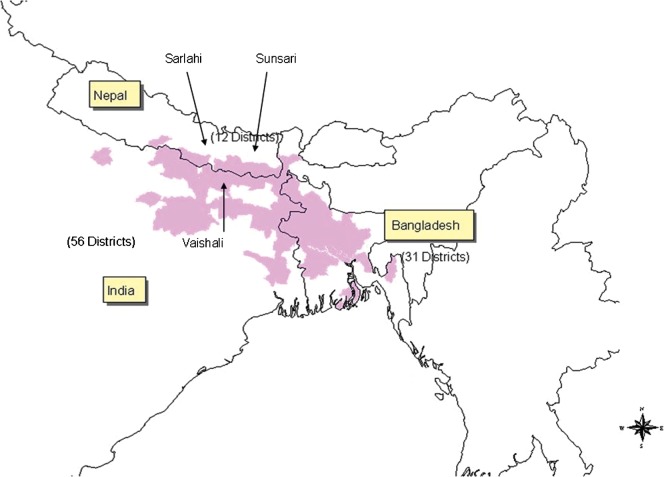
The three study areas (see Figure 1) were selected because each has been badly affected by VL, with recent annual incidence exceeding 20 cases/10,000 residents (Mondal et al., 2009), and, in consequence, each has been prioritized for sandfly-control activities by the relevant national government. One of the study areas, formed by the neighbouring primary-healthcare (PHC) areas of Hazipur and Vaishali, was in the Vaishali district of Bihar state, in eastern India, and at the beginning of the present study held 116,056 households and 580,282 people. The other two study areas were in Nepal, with one (formed by the subdistricts of Babargunj, Gamharia, Atrauli and Hariwan, together holding 111,076 households and 635,701 people) in the Sarlahi district and the other (the subdistrict of Koshi, holding 5153 households and 25,767 people) in the Sunsari district.
Figure 1.
Map showing the districts of India, Nepal and Bangladesh with endemic visceral leishmaniasis (kala-azar;  ) and the locations of the three study areas.
) and the locations of the three study areas.
The study ran from March 2008 to March 2009, the study villages being sprayed, by the national VL-control programmes, in April–June 2008.
Performance and Output/outcome Measurements Related to Spraying Operations
Six methodological tools were used to measure the performance and results of the IRS: (1) formal interviews with the district officers responsible for the VL-control programme; (2) structured observations of the spraying teams, with a standardized checklist used; (3) the monitoring of P. argentipes densities at different points in time, to assess ‘effectiveness’; (4) the bio-assay-based monitoring of the bio-availability of insecticides on sprayed surfaces; (5) the quantification of insecticide concentrations on sprayed walls; and (6) the assessment, in tube bio-assays with standard treated papers, of the susceptibility of local P. argentipes to the insecticide used in the national IRS in India [i.e. 1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane (DDT)] or to a pyrethroid (deltamethrin) similar to the lambda-cyhalothrin used in Nepal.
interviews with district officers about vector management and the analysis of the availability and use of guidelines at district level
The researchers visited the relevant programme managers — those at the National Vector Borne Disease Control Programme (NVBDCP) and State Health Society (Bihar) in India, and the District Public Health Officers (DPHO), Vector Control Officer (VCO) and Malaria Inspector in Nepal. Using a structured questionnaire, these managers were asked about the organization of their vector-control programmes and their programmes’ prospects and limitations. Additionally, they were asked about the existence, availability and use of technical guidelines for spraying activities. Any relevant guidelines that were available were also reviewed during the visits.
observation of spraying activities
A standardized checklist was developed for documenting the IRS procedures in each study area. Trained observers from the research teams then followed national spray teams in each study area, using the checklist to record each team’s activities. A sample-size calculation for analysing spraying activities — based on the assumption that 80% of the observed spraying activities would be of an acceptable standard (with 15% precision and 90% confidence level) — resulted in a target of at least 19 different spray teams to be observed in each study area. In fact, this minimum was exceeded, since 22 spray teams (randomly selected from a list provided by the district office) were observed in each of the three areas.
programme coverage
Formal, questionnaire-based interviews with district-level programme managers as well as document analysis of reports provided by the relevant spraying teams were used to assess the ‘reported coverage’ of target houses (as a percentage of the target houses that were sprayed).
assessment of the bio-availability of insecticides on sprayed surfaces
In the Indian study site and Sarlahi, field bio-assays were conducted in 10 houses in the sprayed villages and 10 (control) houses in the unsprayed villages. In each house used, 10 female P. argentipes (caught in unsprayed villages in the same study area) were exposed for 30 min on each of four walls, using standard WHO plastic cones (WHO, 1998). Mortality of the test sandflies was recorded 24 h post-exposure (WHO, 2005a). Corrected mortality was calculated, using the formula of Abbot (1925), as 100(Pi-C)/(100-C), where Pi and C are the percentage mortalities observed in the sprayed and unsprayed houses, respectively. The bio-assays were performed three times in each test house: 2 weeks, 4 weeks and 5–6 months after the IRS.
chemical analysis of insecticide quality and the insecticide concentrations on filter papers exposed to IRS
In each study area, six houses to be sprayed were randomly selected for the determination, using a filter-paper method (WHO, 2005a), of the post-spraying insecticide concentrations on their walls. The minimum sample size was calculated to be 34 filter papers/study area, based on the assumption that at least 85% of the collected filter papers (with a precision of 10% at a 90% confidence interval) would have adequate concentrations of insecticide. Before spraying, and without the knowledge of the sprayers, four squares of filter paper [No. 1 (Whatman, Maidstone, U.K.) in Nepal and No. 2 (Whatman) in India], each measuring 5×5 cm, were placed at different heights (from about 30 to 180 cm above ground level), together with nine different kinds of ‘fake’ papers (to mislead the sprayers, in case they noticed the filters as something unusual) on the four walls of each room in each house. Filter papers were collected within a week of the insecticide spraying and taken to a research laboratory where they were air-dried, coded, sealed individually in aluminum foil and stored at 4°C until they could be chemically analysed.
The filter-paper samples, along with external standards (DDT and lambda-cyhalothrin, each of >95% purity), were analysed at the Indian Institute of Chemical Technology in Hyderabad) using standard quantitative gas chromatography. Each test paper was extracted with hexane, which was concentrated, under reduced pressure, to a known volume and then injected into a high-resolution gas chromatograph (in triplicate). The mean concentration of active ingredient (i.e. DDT or lambda-cyhalothrin) on each filter-paper sample was then determined by comparison with the external standards.
The concentrations of active ingredient in samples of the wettable-powder (WP) formulations of insecticide used by the spraying teams [which, according to the insecticide manufacturers, contained 50% (w/w) DDT or 10% (w/w) lambda-cyhalothrin] and in random samples of field-collected filter-paper samples from India (N = 5) and Nepal (N = 6) were evaluated by the Centre Wallon de Recherches Agronomiques in Gembloux, Belgium — a collaborating centre of the World Health Organization Pesticide Evaluation Scheme (WHOPES).
evaluating sandfly susceptibility to the insecticide used
Insecticide susceptibility was tested in tube bio-assays, using the WHO’s standard chamber method (WHO, 1998) and test papers, impregnated with 4% DDT (for the tests on Indian sandflies) or 0.05% deltamethrin (for the tests on Nepali sandflies), provided by the WHOPES collaborating centre at the School of Biological Sciences, University Sains, Malaysia. In each assay, 15–20 unfed, non-gravid female P. argentipes (caught in the study area of interest) were introduced into a WHO susceptibility chamber lined with the relevant insecticide-impregnated paper and left for 1 h. In each study area, five replicates (with 15–20 flies each) and one control, with unimpregnated paper and 20 P. argentipes, were run. After the 1-h exposure, percentage knock-down was recorded before the P. argentipes were taken out of the test chamber, placed in a 150-ml paper cup that was covered with netting, and maintained for 24 h at 27±2°C and 80%±10% relative humidity, with a small cotton-wool swab soaked in 10% (w/v) sucrose solution placed on the netting top. Percentage mortality was recorded 24 h post-exposure.
monitoring vector densities in sprayed and unsprayed houses
The minimum sample required (in each of the three study areas) for estimating the reduction of vector densities was determined to be 35 sprayed (‘intervention’) households and 35 unsprayed (‘control’) households (in ‘sprayed’ or unsprayed villages). This was based on a power of 90%, a significant P-value of ⩽0.05 and the assumption that spraying would be followed by a 60% reduction in P. argentipes densities in the intervention area and a 20% reduction in the control area.
In each study area, the densities of sandflies in 10 sprayed households (with similar physical structure) in each of the four ‘sprayed’ villages and 10 unsprayed households — which were either in ‘unsprayed’ villages adjacent to the villages where IRS was carried out (Sarlahi district, Nepal) or also in the ‘sprayed’ villages but left unsprayed, as sentinel houses (the two other study areas) — were investigated pre-intervention and 2 weeks, 4 weeks and 5–6 months after IRS. At each time-point, in each study household, one CDC light trap (Dinesh et al., 2008) was run, in the corner of the main room, 2.5–5.0 cm from the wall and 15.0 cm from the floor, for one night, from 18.00 hours to 06.00 hours. The sandflies caught in each trap were collected in test tubes and killed, either by freezing at −20°C for 20 min or by adding chloroform-soaked cotton-wool to the test tubes. They were then separated by gender and identified, to species (if Phlebotomus) or just to genus (if Sergentomyia), from their external morphological characteristics (Lewis, 1982).
Statistical Analysis
All the field and laboratory data were checked, cleaned of obvious errors, and entered into databases produced using version 3.2.2 of the Epi Info software package (Centers for Disease Control and Prevention, Atlanta, GA). The data analysis was performed using version 13 of the SPSS software package (SPSS Inc, Chicago, IL) and version 10 of the Stata package (StataCorp, College Station, TX). Normal or Poisson distributions, as appropriate, were assumed for the calculation of 95% confidence intervals (CI).
For the calculation of the mean concentration of insecticide in the sprayed samples of filter paper, two excessive values (the results of massive ‘over-spraying’) were removed. The normality of the remaining data was checked non-parametrically, using the Kolmogorov–Smirnov test, and box plots were constructed to show the distributions of the concentration values graphically. Finally, the mean percentages of the target concentrations actually achieved were calculated.
In each study area, for each post-spraying assessment, the percentage reduction in sandfly counts attributable to IRS was calculated as 100[(mean post-intervention value for the intervention group-mean baseline value for the intervention group)-(mean post-intervention value for the control/sentinel group-mean baseline value for the control/sentinel group)]/(mean baseline value for the intervention group).
Ethical Approval
Written informed consent was obtained from the head of each study household. The study protocol was approved by the ethical review committees of the Rajendra Memorial Research Institute of Medical Sciences (Patna, India), the Institute of Medicine at Tribhuvan University (Kathmandu, Nepal), the B. P. Koirala Institute of Health Sciences (Dharan, Nepal) and the WHO (Geneva).
RESULTS
National and District IRS Programmes in India and Nepal
organization of IRS programmes in India and Nepal
In India, the National Vector Borne Disease Control Programme (at central level) and the State Health Society (Bihar) (at state level) are responsible for organizing IRS. The IRS in Nepal is the responsibility of the Epidemiology and Disease Control Division (EDCD) of the Ministry of Health (at central level) and the District Public Health Offices (at district level). In Bangladesh, no IRS for VL control is currently conducted (Mondal et al., 2008) but it is planned (WHO, 2005b).
Table 1 shows how the spraying teams in India and Nepal are organized and supervised. Both countries use temporary workers. In India these workers tend to have considerable experience and have received occasional on-the-job training. In Nepal, however, the spraymen are often newly hired and inexperienced, although they do attend a 3-day training course before they are involved in routine IRS. A few of the spraymen encountered in Nepal had at least 5 years’ experience in spraying and were capable of repairing spray pumps, if needed. The training of the newly hired sprayers in Nepal appeared to be too short and too theoretical, particularly when (in order to allow for rotation every 10 or 15 days) more spraymen were employed than would otherwise be needed.
Table 1. Characteristics of the national programmes of indoor residual spraying (IRS) against Phlebotomus argentipes in India and Nepal.
| IRS in India | IRS in Nepal |
| Daily waged sprayers, frequently with experience from previous national IRS programmes. Each spray team has six members (two pairs of sprayers, one person preparing insecticide and one supervisor) and two stirrup pumps. In each day of spraying, each team is expected to spray 60–80 houses, together holding 300–400 people. | Daily waged sprayers, usually newly trained and with no previous IRS experience. Each spray team consists of four sprayers (with four, 8- or 11-litre hand-compression pumps) and one foreman. For every three spray teams (i.e. a spray ‘group’) there is one insecticide distributor and one government-level supervisor. In each day of spraying, using its 12 spray pumps, each spray group of 17 people is expected to spray 120 houses, together holding about 600 people |
| Uses DDT | Uses synthetic pyrethroid (lambda-cyhalothrin) |
| 1 kg DDT-based wettable powder (50%) is mixed with 10 litres of water and sprayed to give 1 g DDT/m2 | 50 g of lambda-cyhalothrin-based wettable powder (10%) is mixed with 8 litres of water and sprayed to give 0.025 g lambda-cyhalothrin/m2 |
| In 2008, each sprayer received a daily wage equivalent to U.S.$1.43 while each supervisor got the equivalent of U.S.$1.46/day | In 2008, each sprayer received a daily wage equivalent to U.S.$1.28 while each supervisor got the equivalent of U.S.$1.54 |
| For a population of 1 million, 75 tonnes of 50% DDT are required annually, for two IRS cycles | For a population of 1 million, 15 tonnes of 10% lambda-cyhalothrin is required annually, for two IRS cycles |
| Spray months are February/March and May/June (for Bihar and Jharkhand) or April/May and July/August (for West Bengal and Uttar Pradesh) | Spray months are normally March/April and September/October (in each cycle of 2008, spraying was delayed by about 8 weeks because of elections) |
| Target villages for IRS are those with reported cases of visceral leishmaniasis in the previous 5 years (including those with new cases of the disease reported in the current year) | Target villages for IRS are those with reported cases of visceral leishmaniasis in the previous 1 year |
availability of IRS guidelines at district level
National guidelines about IRS procedures were available in the study districts and, according to the respondents interviewed, were followed (Table 2). In-depth interviews with programme managers showed that monitoring of spray operations was, however, rarely carried out.
Table 2. Organization of the national programmes of indoor residual spraying (IRS) against Phlebotomus argentipes in India and Nepal, as reported by the (vector-control) programme managers at district level in the three study areas.
| Response in: | |||
| Subject | India (Vaishali) | Nepal (Sarlahi) | Nepal (Sunsari) |
| staff knowledge and guidelines | |||
| Any training of vector-control management and, if so, where? | Yes, in the country | Yes, some senior officers trained outside the country | Yes, in the country (but malaria inspectors have no training) |
| Are vector-control guidelines available? | Yes | Yes | Yes |
| Knowledge about vector control guidelines | Adequate | Adequate | Adequate |
| Are vector-control guidelines followed during spraying? | Yes | Yes | Yes |
| insecticides and spraying | |||
| Selection criterion for district/villages considered in need of IRS | All endemic districts (2007) or according to recent incidence of visceral leishmaniasis in the district (2008) | According to recent incidence of visceral leishmaniasis in the village | According to recent incidence of visceral leishmaniasis in the village |
| How many houses should be covered by each spray team? | 60–80 (according to national guidelines) | 36 (not mentioned in the guidelines) | 36 (not mentioned in the guidelines) |
| How much insecticidal formulation (as supplied by manufacturer) should be used per house and per person in a target village? | 175–233 g/house and 37.5 g/person | 37.5 g/house and 7.5 g/person | 37.5 g/house and 7.5 g/person |
| When should spraying occur (ideally before and at peak of vector season)? | February/March and May/June | April/May and August/September* | March/April and September/October* |
| How many spray teams were used in the last cycle? | 110 | 27 | 6 (i.e. two spray groups) |
| How many spray pumps in the district are working/available? | 177/237 (74.7%) | 35/37 (94.6%) | 24/84 (28.6%) |
| Do you have enough pumps and accessories for vector control? | No | No | No |
| Do you have a dedicated storeroom in which to keep insecticides? | Only some primary-healthcare units have separate stores | Separate store in the capital but not at district level | Separate store in the capital but not at district level |
| Do you recommend and provide protection for those handling insecticides, such as: | |||
| Masks? | No | Yes | No |
| Gloves? | No | Yes | No |
| Coats/aprons? | No | No | No |
| Caps? | No | Yes | No |
| Boots? | No | No | No |
| Eye protection? | No | No | No |
| How do you dispose of any unwanted insecticide or empty packaging contaminated with insecticide? | Empty sachets are buried | Sacks are burned and insecticides are buried | Pouches and sacks are burned or buried |
| achievements (direct results) | |||
| Are record forms available? | Yes | Yes | Yes |
| Are records complete? | Yes | Yes | Yes |
| Are reporting deadlines met or exceeded by <1 week? | Yes | Yes | Yes |
| What was the target population for the first cycle in 2008 (and the percentage covered, as reported by spraying teams)? | 580,282 (99.6%) | 22,509 (20.26%)† | 32,945 (100%) |
*Constituency elections in Nepal led to the spraying cycles in 2008 being conducted later than usual, in May/June and November.
†In Sarlahi, much of the spraying was focal, with the focus on villages where cases of visceral leishmaniasis had recently occurred.
knowledge, skills and performance of vector-control managers
District programme managers reported themselves to be familiar with IRS guidelines (Table 2). They were knowledgeable about the selection criteria for IRS villages, the most suitable months for spraying, the number of houses to be sprayed per spray team per day, the target number of houses per cycle, and the relevant safety issues (although the use of protective clothing by spray teams was rare; see below). They complained about insufficiencies in spraying equipment (including accessories), inadequate storage facilities at the operational level and insufficient quantities of purchased insecticide. They were aware of issues delaying IRS cycles, such as poor weather (in India) and political instability (in Nepal). The respondents stated that their spraying teams used record forms adequately and reported in a timely manner, but that insufficient transport facilities for supervisors meant that supervision was insufficient.
IRS Delivery at Local Level
equipment and sprayers’ performance
The condition of the spraying pumps used in India was generally better than that of the pumps used in Nepal (Table 3). The mixing of insecticides was improper in two of the three study areas (in India and in Sunsari, Nepal). This is of particular concern in India, as DDT-based sprays require thorough mixing before use. The correct distance between nozzle and the surface being sprayed was not maintained, and the spray swath was not uniform. In India the spraying-cycle number, team number and date of spraying were marked on sprayed houses using stencils; in Nepal, however, only the date of spraying was recorded on a sprayed house. The use of protective clothing was inadequate in all three study areas.
Table 3. Observations on the delivery of indoor residual spraying (IRS), at community level. in India and Nepal (based on the observation of 22 spray teams in each of the three study areas).
| Observation/response in: | |||
| Subject | India (Vaishali) | Nepal (Sarlahi) | Nepal (Sunsari) |
| No. of pumps observed | 36 | 12 | 19 |
| general condition of the pumps | |||
| Leakage | No leakage | Leakage in two | Leakage in several |
| Nozzle | Good | Working | Two not working |
| Pressure gauge | Good | Good | None functional |
| Holding capacity (litres) | 15 (stirrup pump) | 8 (Hudson pump) | 11 (B&G pump) |
| Method of mixing insecticide | Incorrect | Correct | Incorrect in some houses |
| Shaking of the pump | Inadequate | Inadequate | Inadequate |
| Distance of nozzle from the surface (ideally 45 cm) | Not maintained at 45 cm | Adequate | Not maintained at 45 cm |
| Spray swath | Not uniform (40–65 cm) | Not uniform (25–70 cm) | Not uniform (38–90 cm) |
| marks stencilled on sprayed houses | |||
| Number of spray cycle? | Yes | Yes | No |
| Number of spray group? | No | Yes | No |
| Number of spray team? | Yes | Yes | No |
| Sprayer number? | No | Yes | No |
| Date of spray? | Yes | Yes | Yes |
| Use of protection (gloves, coat, eye glasses, boots, caps, masks etc.) | No | No | No |
Outputs of the IRS Programmes
IRS coverage
Although, in the first spraying cycle of 2008, the reported coverage of target households was excellent in all the study areas (Table 2), there was no external evaluation of the reliability of coverage data reported by the spraymen.
insecticide bio-availability on sprayed surfaces
In the Indian study site and one of the Nepali study areas (Sarlahi), the mortality of P. argentipes that had been exposed to sprayed walls for 1 h decreased with time post-spraying, to Abbott-corrected values of <25% after 5 months (Fig. 2).
Figure 2.
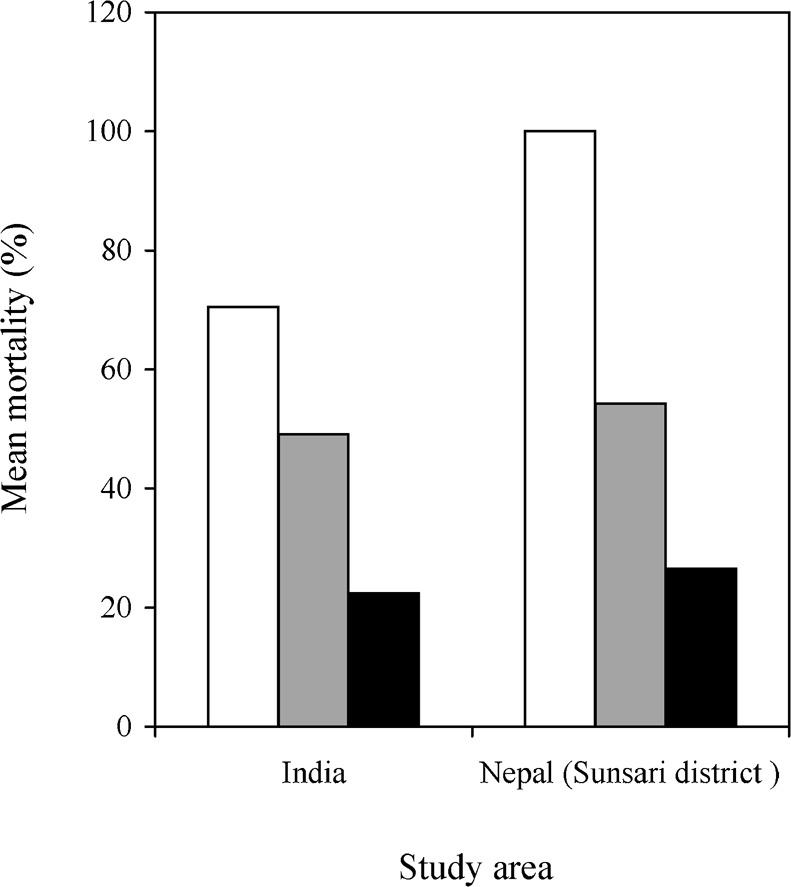
The Abbot-corrected sandfly mortalities recorded in bio-assays on DDT-sprayed surfaces in India (Vaishali) and lambda-cyhalothrin-sprayed surfaces in the Sunsari district of Nepal, 2 weeks (□), 4 weeks ( ) and 5–6 months (▪) after spraying.
) and 5–6 months (▪) after spraying.
quality of the insecticide available
Analysis of three samples of DDT-based wettable powder supplied by the manufacturer indicated that the samples contained a mean of 376.5 g DDT/kg (CI = 375.8–377.2 g/kg) or only 75.3% of the expected value of 500 g/kg. The analysis of three samples of the lambda-cyhalothrin-based wettable powder revealed that the sample contained a mean of 100.8 g lambda-cyhalothrin/kg (CI = 99.8–101.8 g/kg) or about 100% of the expected value of 100 g/kg.
insecticide concentrations achieved by IRS
In India, the national IRS programme aims to suspend the DDT-based wettable powder in water, to give a suspension containing, in theory, 50 g DDT/litre, and then to use stirrup pumps to spray this suspension to give about 1 g DDT/m2. In the present study, however, the residual DDT concentrations in the field-collected filter-paper samples showed much variation, with village-level means varying between 66% and 90% of the target concentration and individual concentrations varying from 9.1% to 330% of the target value (Fig. 3). After two extremely high values (the result of massive ‘over-spraying’) were excluded, the overall mean concentration achieved in the Indian study area was just 0.73 g/m2 or 73.0% (CI = 50.4%–94.8%) of the target value.
Figure 3.
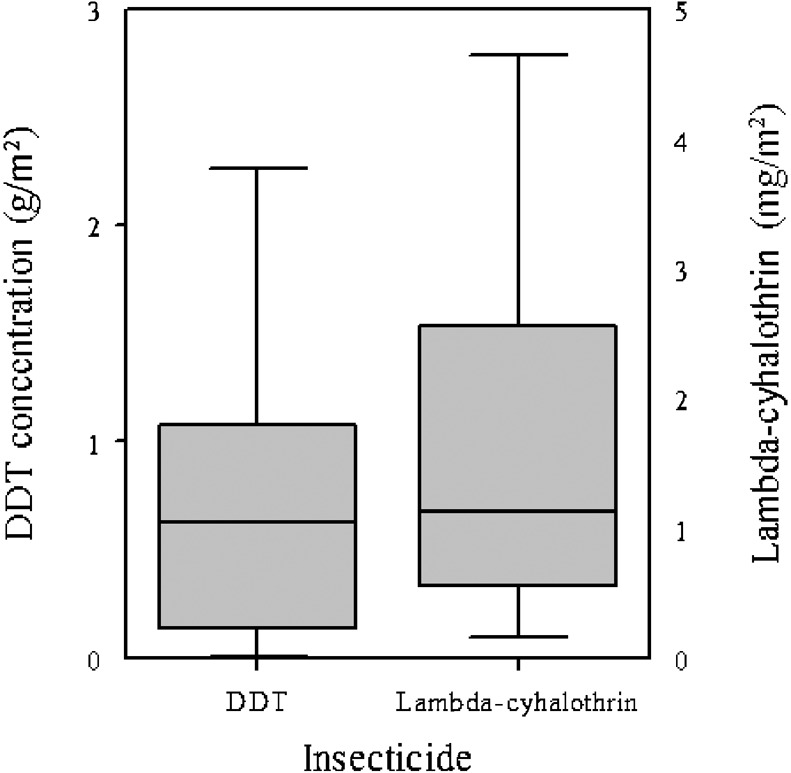
Box plots showing the DDT and lambda-cyhalothrin concentrations on filter-paper samples that had been sprayed by spray teams involved in indoor residual spraying against Phlebotomus argentipes. The results in each plot come from 40 filter-paper samples (from six houses in four villages). The concentrations were approximately normally distributed, with P-values of 0.52 for DDT and 0.98 for lambda-cyhalothrin.
In Nepal, the national IRS programme aims to spray lambda-cyhalothrin to achieve a target concentration of 25 mg/m2. In the present study in the Sunsari district, however, the mean concentration found in filter papers from sprayed walls was only 1.74 mg/m2 or just 6.92% (CI = 4.9%–9.0%) of the target, with the inter-sample variation ranging from below the detection limit to 70.4% of the target concentration (Fig. 3). At village level, the highest mean value (still only 14.5% of the target) was recorded in Dharan-17, probably because the performance of the spraymen in this village was the best observed in Nepal. When an experienced sprayman was informed about the testing, he achieved, through correct spraying, a value of 29.5 mg/m2 (slightly above the target concentration).
The results of the chemical analysis of filter-paper subsamples at the WHOPES collaborating centre in Belgium confirmed the values obtained by the Indian Institute of Chemical Technology in Hyderabad (data not shown).
insecticide susceptibility assessment
In the Indian study area, the mean corrected mortality of female P. argentipes from three districts (Vaishali, Muzaffarpur, Samastipur) exposed to DDT in 44 tests was only 54.2% (CI = 48.8%–59.6%). The mean corrected mortality of female P. argentipes from the study villages in the Sunsari district of Nepal when exposed to deltamethrin was much higher, at 97.0% (CI = 95.0%–99.0%).
Impact of IRS on Vector Populations
vector densities before and after IRS
In two of the three study areas (Vaishali in India and Sarlahi in Nepal), IRS led to significant reductions in the numbers of P. argentipes within sprayed houses for at least 4 weeks after the spraying (Table 4). In unsprayed sentinel houses within Indian ‘sprayed’ villages, the reduction in vector densities was less pronounced but not significantly lower than that in the sprayed houses, indicating a general, community-wide reduction in P. argentipes.
Table 4. The mean numbers of sandflies and Phlebotomus argentipes caught (per house per trap-night) in CDC light traps set in unsprayed houses [that were either in unsprayed villages (Sarlahi district, Nepal) or left unsprayed in ‘sprayed’ villages] or sprayed houses.
| Mean number and (95% confidence interval) (insects per house per trap-night) in: | ||||||
| Sprayed houses | Unsprayed houses | Spraying-attributable reduction (%) in: | ||||
| Study area and time-point | Sandflies | P. argentipes | Sandflies | P. argentipes | Sandflies | P. argentipes |
| India (Vaishali district) | ||||||
| 2 weeks before spraying | 57.0(49.8–64.9) | 41.0(35.0–47.8) | 16.0(12.3–20.4) | 12.0(8.8–15.9) | – | – |
| 2 weeks after spraying | 7.0(4.7–10.1) | 3.3(1.7–5.6) | 16.0(12.3–20.4) | 13.5(10.1–17.6) | −48.49 | −122.26 |
| 4 weeks after spraying | 13.8(10.4–17.9) | 7.5(5.1–10.7) | 20.0(15.9–24.9) | 14.3(10.8–18.5) | −42.63 | −75.36 |
| 6 months after spraying* | 3.3(1.6–5.3) | 2.3(1.0–4.3) | 1.8(0.7–3.6) | 1.25(0.4–2.9) | −23.44 | −62.96 |
| Nepal (Sunsari district)† | ||||||
| 2 weeks before spraying | 77.0(68.4–86.1) | 7.3(4.9–10.4) | 97.5(88.1–107.7) | 7.5(5.1–10.7) | – | – |
| 2 weeks after spraying | 5.5(2.8–9.8) | 0.5(0.0–2.8) | 11.0(6.9–16.7) | 3.0(1.1–6.5) | 19.48 | −31.03 |
| 4 weeks after spraying | 215.0(200.9–229.9) | 5.0(3.1–7.7) | 32.8(27.4–38.9) | 4.0(2.3–6.5) | 263.25 | 17.24 |
| 5 months after spraying | 15.3(11.7–19.6) | 2.8(1.4–4.9) | 11.5(8.4–15.3) | 2.5(1.2–4.6) | 27.60 | 6.90 |
| Nepal (Sarlahi district) | ||||||
| 2 weeks before spraying | 384.0(365.5–404.2) | 54.8(47.7–62.5) | 202.8(189.0–217.2) | 20.3(16.1–25.2) | – | – |
| 2 weeks after spraying | 205.0(191.2–219.5) | 0.0(–) | 210.0(196.3–225.0) | 32.5(27.2–38.6) | −87.72 | −95.73 |
| 4 weeks after spraying | 128.0(117.2–139.6) | 0.0(–) | 110.5(100.4–121.3) | 6.8(4.4–9.8) | −82.81 | −87.32 |
| 5 months after spraying | 215.0(200.9–229.9) | 5.0(3.1–7.7) | 123.8(113.1–135.1) | 5.0(3.1–7.7) | −69.21 | −68.29 |
*Severe flooding in the Indian study villages 5 months after spraying prevented any research in the villages for another month and almost certainly markedly reduced the densities of adult sandflies.
†Not estimated in Dharan-15 or Dharan-17.
In Nepal’s Sunsari district (where only low pyrethroid concentrations were achieved on the walls; see above) only a short-lived vector reduction was observed, 2 weeks after spraying (Table 4). When comparing the IRS impact on vector abundance in sprayed houses with houses in unsprayed control villages (in Sarlahi district, Nepal), the IRS appeared to have a beneficial impact for at least 4 weeks.
Unfortunately the medium-term effects of the IRS, which were planned to be assessed 5 months after spraying, could not be accurately determined because of severe monsoon-related flooding in the study area in India (which reduced the numbers of adult sandflies to very low levels even in the unsprayed villages, and delayed the last follow-up by a month) and the lime plastering of houses in the study areas in Nepal, in preparation for annual religious festivities (which also reduced the numbers of adult sandflies to very low levels even in the unsprayed villages).
DISCUSSION
Considerable effort and resources (including, in 2008, about U.S.$4 million in India and U.S.$1.2 million in Nepal; unpubl. obs.) are going into the organization of national programmes of IRS for the control of VL in India and Nepal. Unfortunately, if the present results are typical of these national programmes, the reductions in sandfly densities being achieved via routine IRS will be much lower than those seen in strictly controlled efficacy trials (Joshi et al., 2009). Unless the current IRS programmes in India and Nepal are much improved, the substantial and sustainable reduction of vector abundance, to interrupt the transmission of L. donovani, seems unlikely.
There are significant operational challenges and this was particularly obvious in Nepal’s Sunsari district, where the management of pressure pumps and spraying performance was poor, only a small fraction (a mean of 6.9%) of the target insecticide concentration was found on sprayed surfaces, and, in consequence, only a small and short-lived reduction in P. argentipes densities was measured. In Nepal’s Sarlahi district, where the performance of the spraymen was better and the initial bio-availability of the pyrethroid used on the sprayed surfaces was satisfactory, vector reduction was much more pronounced, at least in the first month after IRS. Unfortunately, most families in this study area plastered the walls of their houses with lime 4–5 months after the IRS cycle and this probably reduced sandfly densities for a short time (Joshi et al., 2009), confounding the 5-month follow-up for the present study.
In the Indian study area (Vaishali district), the bio-availability of DDT was substandard and the local sandfly population showed reduced levels of susceptibility towards the insecticide (probably as the result of a long history of irregular DDT spraying in Bihar state). Furthermore, the concentration of DDT on sprayed surfaces was highly variable, and often suboptimal, because of the poor performance of many of the spray teams and, probably, because of the substandard DDT product delivered by the manufacturer (although this has to be further tested on a larger sample). Despite these limitations, however, the mean concentration of DDT on the sprayed walls was found to be 73% of the target value, and a considerable reduction in the numbers of adult P. argentipes was observed in the first month after spraying. The medium-term effects of the IRS in Vaishali could not be measured well because of flooding in the study villages 5 months after the spraying.
The main limitations to IRS delivery detected at national level, in both India and Nepal, during the course of the present study were delays in IRS cycles because of parliamentary elections and/or the late delivery of funds (in both 2008 and 2009). At district level, the main limitations were the inadequate training of sprayers (particularly in Nepal), insufficient and/or inadequate spray pumps (particularly in Sunsari, Nepal), and inadequate tools for monitoring and evaluation. There were also clear problems at operational level (i.e. among the spraying teams), with poor insecticide-storage facilities at PHC level, the non-use or insufficient use of protective clothing (in all three study areas), and the poor performance of many spray teams (with insufficient mixing or insufficient shaking of insecticides, irregular spraying, and the incomplete marking of sprayed houses).
Despite the problems with the IRS detected in the present study, several important strengths were also made apparent. An enormous know-how about the organization of IRS services at the national level (particularly in India) was noted (but not documented in detail). There was a well established logistics system for bringing insecticides from the manufacturer to the districts where they were to be used (although some logistical problems remained at the district/subdistrict level). Operational guidelines were available in all of the VL-endemic study districts. In India, experienced spraying teams were being used and some capacity building and re-training were being done. In all three study areas, reported household coverage with IRS was high (but this needs to be checked against actual coverage).
In conclusion, in the national programmes of IRS against VL, substandard spraying is a matter of concern (particularly in Nepal) and may well contribute to the development of insecticide resistance (Mukhopadhyay et al., 1987; Dhiman et al., 2003) and to the insufficient long-term depletion in the numbers of P. argentipes. Major lessons to be learned from this study include the need for (1) better monitoring and (2) the timely identification of shortcomings, with adequate response mechanisms in place. Rapid tests for detecting insecticide concentrations on sprayed surfaces (Morou et al., 2008) would be of an enormous value in monitoring performance and, hopefully, such tests will become available in the near future and at an acceptable cost for national vector-control programmes.
Acknowledgments
The study was supported by the Special Programme for Research and Training in Tropical Diseases (WHO/TDR) in Geneva, Switzerland. The authors thank Drs S. N. Sharma (National Vector Borne Disease Control Programme, New Delhi), F. H. M. Nurunnabi Chowdhury (Directorate General of Health Services, Dhaka) and G. D. Thakur (Epidemiology and Disease Control Division, Kathmandu, Nepal) for their continuous support and advice. They are also very grateful to Dr N. Rao (Indian Institute of Chemical Technology, Hyderabad) for analysis of the filter-paper samples, and the staff of the Centre Wallon de Recherches Agronomiques (Gembloux, Belgium) for the validation of chemical tests. Important co-workers in the study teams at the Rajendra Memorial Research Institute of Medical Sciences (N. Kumar Sinha, M. Prasad, S. A. Khan and S. Kumar), Tribhuvan University’s Institute of Medicine (Professor C. K. Gurung, K. Raj Pant and S. Raj Adhikari), B. P. Koirala Institute of Health Sciences (Professor S. Rijal and L. Roy), the Bangladeshi National Institute of Preventive and Social Medicine (N. Maheswary and S. Faria), and the International Centre for Diarrhoeal Disease Research (S. Alam) are also thanked. Finally, the authors are grateful to the populations in the study villages, for their patience and support in many research activities.
REFERENCES
- 1.Abbott WS.(1925)A method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18265–267. [Google Scholar]
- 2.Alvar J, Yacato S, Bern C.(2006)Leishmaniasis and poverty. Trends in Parasitology 22552–557. [DOI] [PubMed] [Google Scholar]
- 3.Boelaert M, Meheus F, Sanchez A, Singh SP, Vanlerberghe V, Picado A, Meessen B, Sundar S.(2009)The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Tropical Medicine and International Health 14639–644. [DOI] [PubMed] [Google Scholar]
- 4.Das M, Banjara M, Chowdhury R, Kumar V, Rijal S, Joshi A, Akhter S, Das P, Kroeger A.(2008)Visceral leishmaniasis on the Indian subcontinent: a multi-centre study of the cost of three interventions for the control of the sandfly vector, Phlebotomus argentipes. Annals of Tropical Medicine and Parasitology 102729–741. [DOI] [PubMed] [Google Scholar]
- 5.Dhiman RC, Raghavendra K, Kumar V, Kesari S, Kishore K.(2003)Susceptibility status of Phlebotomus argentipes to insecticides in districts Vaishali and Patna. Journal of Communicable Diseases 3549–51. [PubMed] [Google Scholar]
- 6.Dinesh DS, Das P, Picado A, Davis C, Spybroek N, Boelaert M, Coosemans M.(2008)The efficacy of indoor CDC light traps for collecting the sandfly Phlebotomus argentipes, vector of Leishmania donovani. Medical and Veterinary Entomology 22120–123. [DOI] [PubMed] [Google Scholar]
- 7.Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, Das P, Boelaert M, Coosemans M.(2010). Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Neglected Tropical Diseases 4e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi A, Das M, Akhter S, Chowdhury R, Mondal D, Kumar V, Das P, Kroeger A, Boelaert M, Petzold M.(2009)Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: cluster randomized trials in Bangladesh, India and Nepal. BMC Medicine 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis DJ.(1982)A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bulletin of the British Museum (Natural History), Entomology Series 45121–209. [Google Scholar]
- 10.Mondal D, Alam MS, Karim Z, Haque R, Boelaert M, Kroeger A.(2008)Present situation of vector-control management in Bangladesh: a wake up call. Health Policy 87369–376. [DOI] [PubMed] [Google Scholar]
- 11.Mondal D, Singh SP, Kumar N, Joshi AB, Sundar S, Das P, Siddhivinayak H, Kroeger A, Boelaert M.(2009)Visceral leishmaniasis elimination programme in India, Bangladesh, and Nepal: reshaping the case finding/case management strategy. PLoS Neglected Tropical Diseases 3355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morou E, Ismail HM, Dowd AJ, Hemingway J, Labrou N, Paine M, Vontas J.(2008)A dehydrochlorinase-based pH change assay for determination of DDT in sprayed surfaces. Analytical Biochemistry 37860–64. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay AK, Chakravarty AK, Kureel VR, Shivraj(1987)Resurgence of Phlebotomus argentipes & Ph. papatasi in parts of Bihar (India) after DDT spraying. Indian Journal of Medical Research 85158–160. [PubMed] [Google Scholar]
- 14.Ostyn B, Vanlerberghe V, Picado A, Dinesh DS, Sundar S, Chappuis F, Rijal S, Dujardin JC, Coosemans M, Boelaert M, Davies C.(2008)Vector control by insecticide-treated nets in the fight against visceral leishmaniasis in the Indian subcontinent, what is the evidence? Tropical Medicine and International Health 131073–1085. [DOI] [PubMed] [Google Scholar]
- 15.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, Rijal S, Das P, Rowland M, Sundar S, Coosemans M, Boelaert M, Davies CR.(2010)Effect of village-wide use of long-lasting insecticidal nets on visceral leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Neglected Tropical Diseases 4e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (1998)Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-efficacy and Persistence of Insecticides on Treated Surfaces: Report of the WHO Informal Consultation Document WHO/CDS/MAL/98.12Geneva: WHO [Google Scholar]
- 17.World Health Organization(2005a)Guidelines for Laboratory and Field Testing of Long Lasting Insecticidal Mosquito Nets. Document WHO/CDS/WHO-PES/GCDPP/2005.11Geneva: WHO [Google Scholar]
- 18.World Health Organization(2005b)Regional Strategic Framework for Elimination of Kala-azar from South East Asia Region (2005–2015) Document SEA-VBC-85-REV-1New Delhi: WHO [Google Scholar]
- 19.World Health Organization(2008)Kala-azar Elimination in South–east Asia Region: Training Module Document SEA-CD-182New Delhi: WHO [Google Scholar]