Human cystic echinococcosis (CE) is a worldwide parasitic infection in which the larval cysts of Echinococcus granulosus develop in some tissue or organ, very often in the liver and/or the lungs. On very rare occasions, a cyst may be found inside the pulmonary artery, the result of the rupture of an intracardiac cyst or, more rarely, dissemination from a hepatic infection (Bulman et al., 2007). Most cysts in the pulmonary artery have only been detected at autopsy (Gilsanz et al., 1977). Surgery is usually the therapeutic approach of choice.
Two Tunisian patients with Echinococcus-attributable pulmonary embolism, as a rare complication after the surgical removal of a hepatic cyst (Case 1) or a cardiac cyst (Case 2) are described below. In both cases, who presented at the Fattouma Bourguiba University Hospital in Monastir, diagnosis was based on medical history and imaging features and chemotherapy led to a good outcome.
CASE REPORTS
Case 1
In April 2007, a 45-year-old woman presented with gradually worsening dyspnoea. The patient reported that she had had surgery for CE twice, having had a cyst removed from her right lung 16 years previously and a hepatic cyst excised 3 years previously. Her inferior vena cava had been accidentally injured during the removal of the liver cyst.
On physical examination, including auscultation, the patient was found to have no obvious lung abnormalities but to be tachycardic (100 beats/min). The results of routine laboratory investigations were all within normal limits. Arterial-blood partial pressures of oxygen (pO2) and carbon dioxide (pCO2) were 78.8 and 46 mmHg, respectively. An electrocardiogram showed a right-axis deviation and sinus tachycardia. A chest X-ray revealed multiple, small, rounded parenchymal masses while angiography based on contrast-enhanced computerized tomography (CT) showed an oval, hypo-dense mass, measuring about 3 cm in width, within the pulmonary artery of the left lower lobe and its segmentar branches [Fig. 1 (a) and (b)]. Several bilateral parenchymal cysts were also seen. Abdominal CT revealed a cystic mass within the retrohepatic vena cava (but no parenchymal hepatic cyst) and two intraperitoneal cysts. No cardiac cyst or dilatation of the right ventricle were visible by transthoracic echocardiography. Pressure in the pulmonary artery (systolic = 27 mmHg) appeared normal. A T2-weighted scan by magnetic-resonance imaging (MRI) confirmed hyperintense cysts in the left-lower-lobe pulmonary artery and its segmentar branches [Fig. 1 (c)] and the absence of cardiac cysts. Given the clinical presentation and the patient’s medical history, a cyst-related pulmonary embolism was suspected, especially after the patient was found seropositive for anti-echinococcal antibodies in an ELISA. A course of oral albendazole (400 mg twice a day for 4 months) was started. The patient was asymptomatic after 1 month of treatment and CT scans at her 2-year follow-up revealed no abnormalities.
Figure 1.
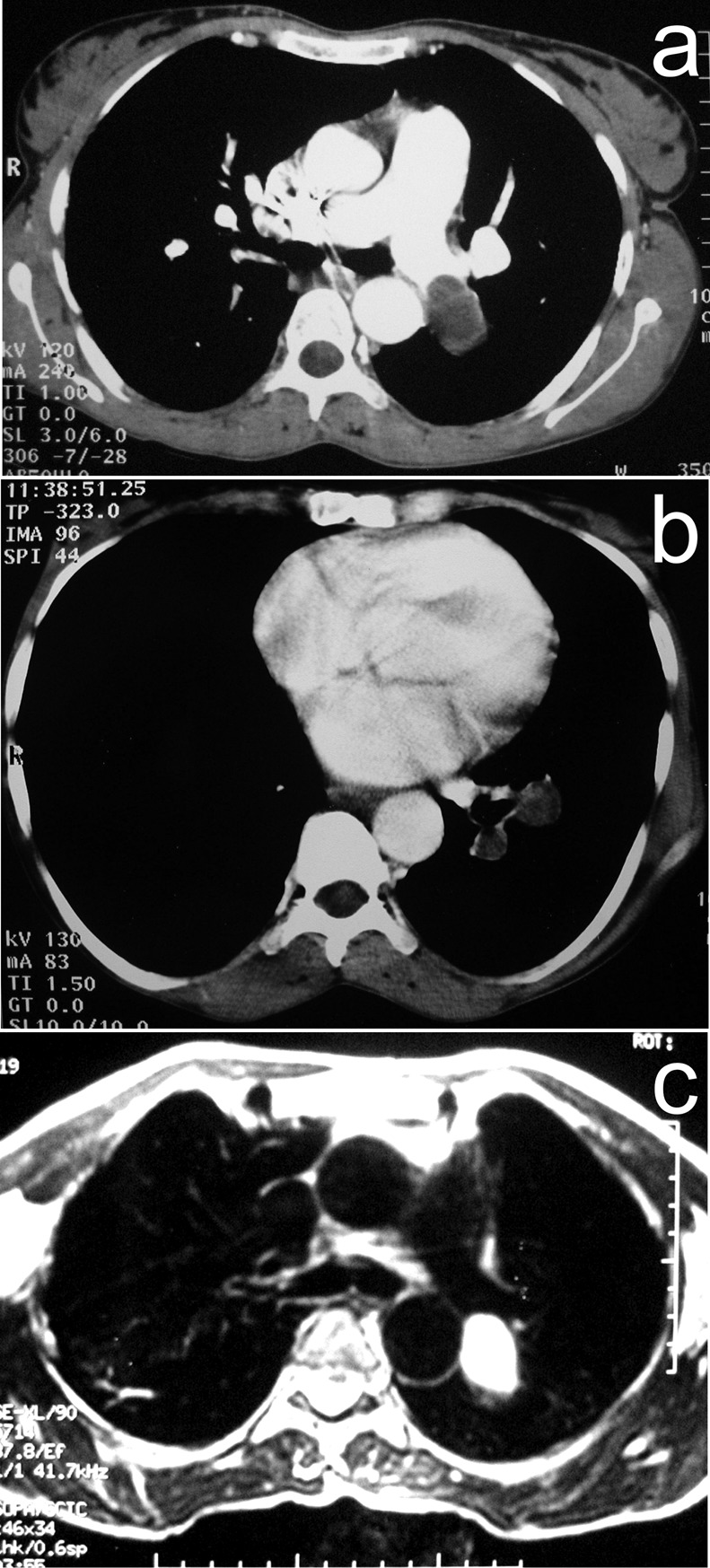
Images of the chest of Case 1, showing scans produced using either angiography based on computerized tomography [(a) and (b)] or magnetic-resonance imaging (c).
Case 2
In December 2004, a 42-year-old man was admitted because of basal right-chest pain. Six years earlier, he had had multiple hydatid cysts in both lungs and a cyst on the cardiac interventricular septum excised. Except for tachycardia (92 beats/min), a clinical examination did not reveal any abnormalities (breathing rate = 20/min; no pulmonary rales at auscultation; blood pressure = 120/70 mmHg). Arterial-blood pO2 and pCO2 were 82 and 46 mmHg, respectively. The results of routine laboratory investigations were all within normal limits. Sinus tachycardia was revealed by electrocardiography. A pulmonary embolism was suspected. A chest X-ray showed a round, well-margined opacity in each of the lower lobes of the lungs while a CT scan of the chest revealed cystic lesions in both the right and the left pulmonary parenchyma [Fig. 2 (a)] and another cystic lesion inside the pulmonary artery of the left lower lobe [Fig. 2 (b)]. The patient was found ELISA-positive for anti-echinococcal antibodies in his serum. As surgery was considered very risky, the patient was only given oral albendazole (400 mg twice a day for 4 months). His symptoms completely resolved within 2 weeks. At a 3-year follow-up, a CT scan of the patient’s chest showed stability of the abnormalities detected at presentation.
Figure 2.
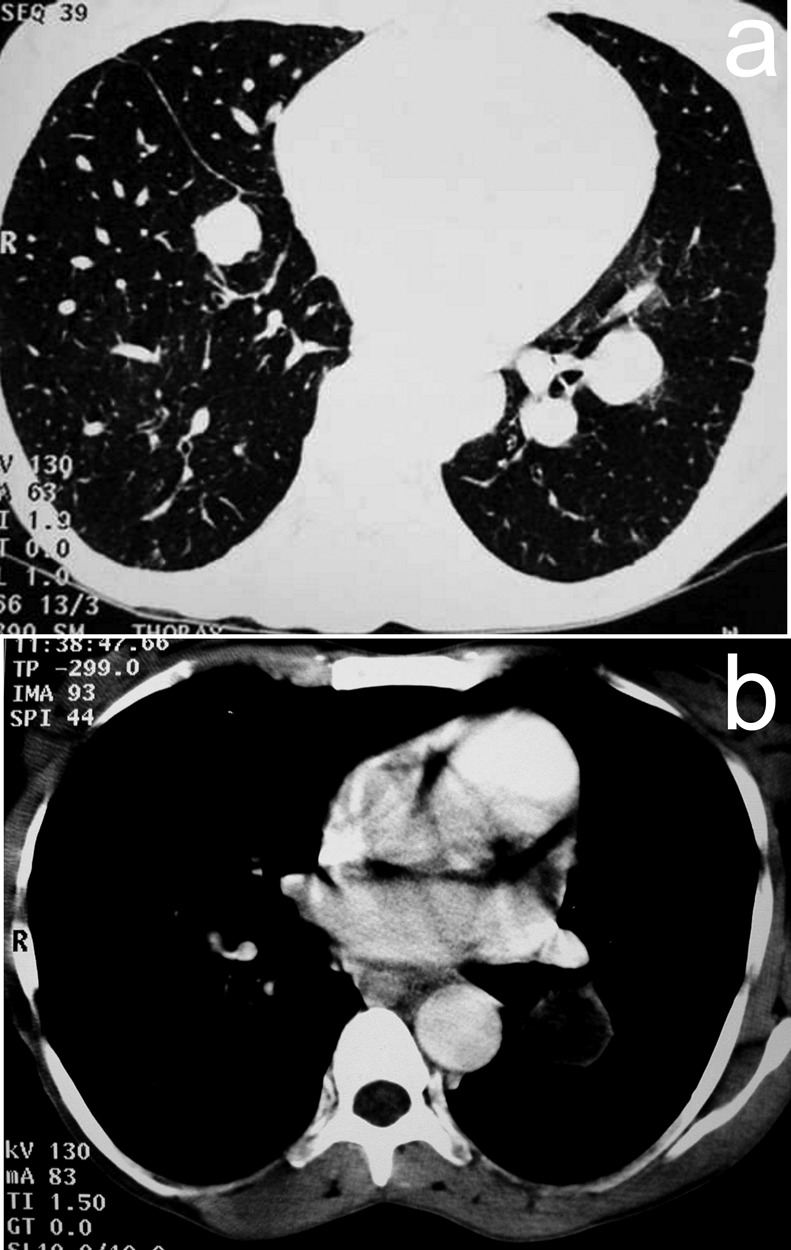
Images of the chest of Case 2, showing scans produced using computerized tomography [(a) and (b)].
DISCUSSION
In human CE, cysts can occur anywhere in the body, although the most commonly affected organs in adults are the liver and the lungs (Karantas et al., 2000). About 10%–15% of the embryos that emerge from ingested eggs of E. granulosus pass through the liver and lungs and reach the systemic circulation (Yagüe et al., 1998), allowing cysts to develop at almost any site. Although the formation of a cyst in a pulmonary artery appears to be a very rare event (Bayraktaroglu et al., 2009), the prevalence of such infection, which is nearly always detected post-mortem, is probably under-estimated (Gilsanz et al., 1977). The left pulmonary artery is more likely to suffer an Echinococcus-related embolism than the right (Lioulias et al., 2001). A pulmonary-artery cyst may result from the rupture of hepatic or abdominal cysts, into the hepatic veins or the inferior vena cava, or, more frequently, directly from the rupture of a cyst in the right cardiac chambers (Bayraktaroglu et al., 2009).
The pulmonary embolism detected in Case 1 almost certainly resulted from the accidental injury to the case’s inferior vena cava during earlier liver surgery, which may have allowed the spread of daughter cysts within the systemic circulation. Although, when admitted to the Fattouma Bourguiba University Hospital in Monastir, Case 2 did not appear to have any cardiac cysts, his pulmonary-artery cyst was presumably the result of the rupture of the cyst previously detected in his heart. It is, however, possible that parasites crossed the arterial wall through small breaks in the intima or by entering the vas nutritia (Dursun et al., 2008).
Although transthoracic echography is often used to check for cardiac cysts, it rarely allows the direct visualization of pulmonary embolisms (Buz et al., 2007). Transoesophageal echocardiography may reveal such embolisms if they are located in the main pulmonary artery (Jaafari et al., 2009). Perhaps the most useful imaging technique in the investigation of a Echinococcus-related pulmonary embolism is CT-based angiography, which may not only reveal the embolism but also indicate its parasitic cause (from the cystic appearance of the filling defect) and reveal other cysts (Buz et al., 2007). Peripheral enhancement of the cyst, corresponding to the pericyst, is often visible (Karantanas et al., 2000). Typically, a pulmonary-artery cyst appears quite similar to lung cysts. Cyst-wall calcification is also best seen by CT (Dursun et al., 2008).
For the examination of the heart and great vessels, MRI-based angiography has many advantages over a spiral CT scan (Lioulias et al., 2001). On MRI, a hydatid cyst usually appears as a characteristic oval lesion with either a low signal intensity (in T1-weighted scans) or a high signal intensity (in T2-weighted scans) (Dursun et al., 2008). A previous history of CE, previous or current residence in a CE-endemic area, and seropositivity for anti-echinococcal antibodies (Ortiz-Saracho et al., 1998) should all increase the suspicion that a pulmonary embolism may be the result of CE. Classically, CE-related pulmonary embolism has been clinically divided into three groups: acute fatal embolism, subacute pulmonary hypertension (that results in death within 1 year of diagnosis), and chronic pulmonary hypertension (Mahouachi et al., 2007). The two cases reported here differ from those previously described in lacking any signs of pulmonary hypertension, and it seems possible that their pulmonary perfusion was maintained via the bronchial arteries (Aribas et al., 2007).
For patients with hydatid cysts causing pulmonary embolisms, surgical treatment is commonly recommended. Although chemotherapy may not be curative when used alone, it remains useful for patients with recurrent or disseminated CE and whenever surgery is contra-indicated (Mahouachi et al., 2007). For both of the Tunisian patients described above, who had cysts in their lung parenchyma as well as a pulmonary-artery cyst each, surgery was considered inappropriate, given the risk of spillage of the cystic contents, further embolisms, anaphylactic shock and pseudo-aneurysm of the pulmonary artery (Koksal et al., 2006). Treatment was therefore confined to a course of oral albendazole, which left both patients asymptomatic, with cysts that appeared unchanged or reduced in size over a few years of follow-up. Although chronic obstruction of the pulmonary artery may induce pulmonary hypertension, this is quite rare (Bulman et al., 2007). As yet, the Tunisian cases described here have shown no post-treatment complications and, particularly, no clinical or radiological evidence of pulmonary hypertension.
In summary, pulmonary embolism caused by an E. granulosus cyst should be considered in patients who have undergone surgery for CE hepatic and/or cardiac cysts, especially in regions where CE is endemic. In addition to clinical history and endemic context, CT- and/or MRI-based angiography can show pathognomonic features leading to a correct diagnosis. If surgery is not possible, chemotherapy alone can at least halt the evolution of the disease and resolve symptoms.
REFERENCES
- 1.Aribas OK, Kanat F, Turk-Aribas E, Erayman I, Yuksek T.(2007)Netherlands Journal of Medicine 65109–111. [PubMed] [Google Scholar]
- 2.Bayraktaroglu S, Ceylan N, Sava? R, Nalbantgil S, Alper H.(2009)European Radiology 192083–2086. [DOI] [PubMed] [Google Scholar]
- 3.Bulman W, Coyle CM, Brentjens TE, Horn EM, Dickstein ML, Wilt JS, Emond J, Kawut SM.(2007)Chest 1321356–1358. [DOI] [PubMed] [Google Scholar]
- 4.Buz S, Knosalla C, Mulahasanovic S, Meyer R, Hetzer R.(2007)Annals of Thoracic Surgery 842108–2110. [DOI] [PubMed] [Google Scholar]
- 5.Dursun M, Terzibasioglu E, Yilmaz R, Cekrezi B, Olgar S, Nisli K, Tunaci A.(2008)American Journal of Radiology 190226–232. [DOI] [PubMed] [Google Scholar]
- 6.Gilsanz V, Campo C, Cue R, Estella J, Estrada RV, Perez-Oteiza C, Rabago G, Rebollar JL, Zarco P.(1977)British Heart Journal 39553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaafari A, Nedia A, Bukhriss B, Ehlem B, Moez T, Habib BM.(2009)Annales de Cardiologie et d’Angéiologie 58125–128. [DOI] [PubMed] [Google Scholar]
- 8.Karantanas AH, Bitsios G, Karaiskou E.(2000)Computerized Medical Imaging and Graphics 24265–267. [DOI] [PubMed] [Google Scholar]
- 9.Koksal C, Baysungur V, Okur E, Sarikaya S, Halezeroglu S.(2006)Annals of Thoracic and Cardiovascular Surgery 12349–351. [PubMed] [Google Scholar]
- 10.Lioulias A, Kotoulas C, Kokotsakis J, Konstantinou M.(2001)European Journal of Cardio-thoracic Surgery 20197–199. [DOI] [PubMed] [Google Scholar]
- 11.Mahouachi R, Berraies A, Taktak S, Chtourou A, Ben Khede A.(2007)Respiratory Medicine Extra 3192–194. [Google Scholar]
- 12.Ortiz-Saracho J, Zarza LP, Sanchez J(1998)Chest 114309–310. [DOI] [PubMed] [Google Scholar]
- 13.Yagüe D, Lozano MP, Lample C, Nuñez ME, Sánchez F.(1998)European Radiology 81170–1172. [DOI] [PubMed] [Google Scholar]


