Abstract
A total of 70 water samples, including tap, river, fountain and well water were collected in the Ordu province, Middle Black Sea, Turkey and investigated for the detection of Cryptosporidium oocysts. The samples were directly screened microscopically for Cryptosporidium oocysts’ detection by immunofluorescence test and subsequently DNA was extracted for the molecular detection by loop-mediated isothermal amplification (LAMP) and nested polymerase chain reaction (PCR). Eighteen out of the 70 (25.7%) water samples were found positive for Cryptosporidium spp. by immunofluorescence test and 19 (27.1%) were found positive by LAMP. Nested PCR products were not generated in any of the investigated water samples. A total of 16 randomly selected pellets were spiked with 10 Cryptosporidium oocysts to test the efficiency of the applied method. All the samples were found positive by LAMP for the presence of Cryptosporidium DNA, while the nested PCR assay was positive in only seven (43.75%) out of the 16 examined spiked samples. This is the first report on the occurrence of Cryptosporidium species in environmental and drinking water supplies in the Black Sea area.
INTRODUCTION
Giardia and Cryptosporidium are protozoan parasites that cause widespread gastrointestinal illnesses. Ninety percent of the reported outbreaks of these pathogenic protozoan occur through water, while 10% are related to food (Karanis et al., 2007a; Rose and Slifko, 1999; Baldursson and Karanis, 2011). The loop-mediated isothermal amplification (LAMP) is a recently developed technology for DNA amplification that surpasses polymerase chain reaction (PCR) in its efficiency and insensitivity to DNA contaminants. LAMP is one of the nucleic acid amplifications tests available for organism identification applications in various fields such as infection diagnosis (Notomi et al., 2000). Owing to relatively recent invention, LAMP assays are in a stage of rapid development and a recent review showed that LAMP assays have been developed and applied to detect over 100 different pathogens (Karanis and Ongerth, 2009). The successful use of LAMP has been reported for the diagnosis of waterborne protozoan including Cryptosporidium spp. (Karanis et al., 2007b), Toxoplasma gondii (Sotiriadou and Karanis, 2008) and Giardia lamblia (Plutzer and Karanis, 2009).
The aim of the present study was to investigate water supply areas in the Ordu province at Middle Black Sea area for their contamination with Cryptosporidium species. The investigations involved collection of water samples from selected sampling sites in Ordu province and examination of those samples by three detection assays to determine the occurrence and prevalence of Cryptosporidium species in environmental water resources.
MATERIALS AND METHODS
Water Sampling Sites
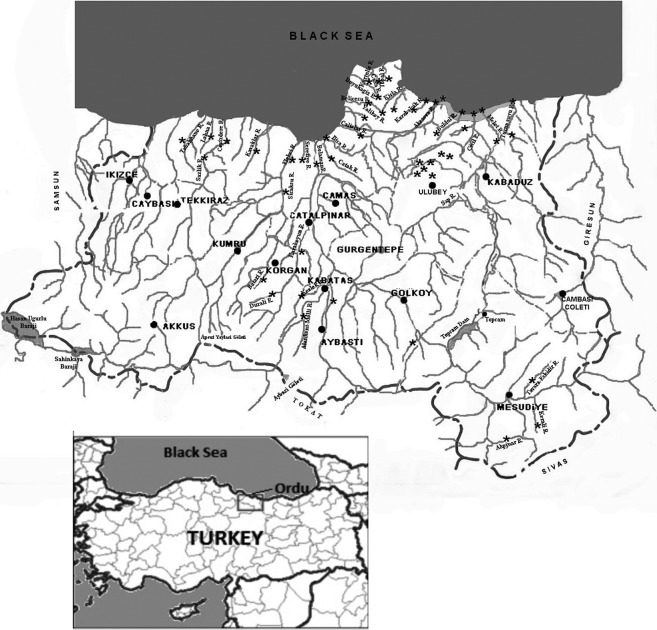
Ordu is a port city in the Middle Black Sea coast of Turkey consisting of 18 county boroughs such as Unye, Ulubey, Fatsa, Kabatas-Aybastı-Korgan, Persembe and Mesudiye-Golkoy, which have been selected as water sampling sites. Ordu generally has a temperate climate and each season is rainy with warm and humid summers and cool and damp winters. The Black Sea Basin is the largest river basin in Turkey and a significant part of rivers in Turkey flow into the Black Sea (Fig. 1).
Fig. 1.
Location of sampling sites.
Water Sample Collection and Oocysts Purification
All samples were obtained in the period between September and December 2009. The investigations involved collection of water samples from selected sampling sites in rivers Serefiye, Tabakhane, and in rivers crossing Village Akoluk and Village Durali (Fig. 1). River water samples were of particular interest due to cloudy water and the presence of cows for feeding in the investigated catchment areas. Different locations of the Ordu province and its county boroughs (Unye, Ulubey, Fatsa, Kabatas-Aybasti-Korgan, Persembe and Mesudiye-Golkoy) were also investigated in order to estimate the occurrence of oocysts in water resources from these sampling sites.
Ten liters of water from the catchment areas were collected in sterile plastic bottles without chemical additives and were immediately transferred to the lab for processing. The collected water from each sample was decanted to dark glass bottle to perform Al2(SO4)3 flocculation. All collected water samples around Ordu at the Middle Black Sea area from different sources were concentrated by Al2(SO4)3-flocculation and concentrated as described by Karanis and Kimura (2002) and as it has been applied by Kourenti et al. (2003) and Karanis et al. (2006).
Microscopic Detection and Identification of Cryptosporidium Oocysts by Immunofluorescence Test (IFT)
IFT was subsequently applied in all collected water samples and it was performed in Eppendorf tubes according to the modified protocol of Karanis et al. (2006). Briefly, defined 50 μl pellet aliquots out of 1 ml pellet, corresponding to 5% of the whole water sample, were once washed with distilled water and concentrated by centrifugation at 8000 rev/minute for 5 minutes. The resulted pellets of around 100 μl, depending of the water quality and additional debris present in the water sample, were stained with 30 μl of fluorescein isothiocyanate-conjugated monoclonal antibody (Cellabs Pty Ltd, Brookvale, Australia). After incubation at 37°C for 45 minutes, the reaction was terminated by the addition of 1 ml of phosphate saline buffer solution (pH 7.2). Consequently, all the samples were concentrated by centrifugation and the sediments were laid onto glass slides, air dried and covered in presence with mounting medium, supplied from the manufacturer. All slides were screened by the use of fluorescence microscopy (Leica, Solms, Germany) under ×20, ×40 and ×100 magnifications.
Extraction of Genomic DNA and Molecular Detection by PCR and LAMP
Total DNA, representing 50% of the sucrose purified water concentrates, was extracted by the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the modified protocol of Plutzer et al. (2008) with additional processing of 10 freeze–thaw cycles after the resuspension step in lysis buffer, supplied from the manufacturer. All samples were frozen by immersion in liquid nitrogen for 1 minute and thawed in a water bath with boiling water, until the frozen samples were completely dissolved. DNA was eluted in 50 μl TE buffer in a clean tube and kept at −20°C until PCR and LAMP reactions were performed.
The nested PCR targeting the small subunit (SSU) rRNA gene was performed as described by Xiao et al. (1999; 2001) and Plutzer et al. (2008). Both PCRs were performed in standard mixtures of 50 μl containing 200 nmoles of each primer, 200 μM dNTP of each dNTP (Finnzymes, Espoo, Finland), 1× PCR buffer containing 1.5 mM MgCl2 (Qiagen GmbH), 3 mM MgCl2 (Qiagen GmbH), 2.5 U HotstarTaq DNA polymerase (Qiagen GmbH) and 2 μl bovine serum albumin (acetylated, 10 mg/ml) (Promega, Madison, WI, USA).
The templates were subjected to an amplification program with a peqSTAR thermal cycler (PEQLAB GmbH, Erlangen, Germany) consisting of an initial step at 96°C for 15 minutes, 35 amplification cycles (94°C for 45 seconds, 55°C at primary PCR and 58°C at secondary PCR for 45 seconds and 72°C for 60 seconds) followed by one cycle of 10 minutes at 72°C according to Plutzer et al. (2008). PCR products were analyzed in 1.5% agarose gel stained with ethidium bromide solution (1 μg/ml) and visualized under UV light. LAMP assay was performed on all DNA-extracted water samples according to Karanis et al. (2007b) and Bakheit et al. (2008) targeting the SAM gene. Briefly, all LAMP reactions used in this study were performed in a final volume of 25 μl containing 12.5 μl 2× reaction buffer [40 mM Tris-HCl, 20 mM KCl, 16 mM MgSO4, 20 mM (NH4)2SO4, 0.2% Tween 20, 1.6M betaine and 2.8 mM each deoxynucleoside triphosphate], 8 U Bst DNA polymerase (New England Biolabs, Tokyo, Japan), 1.3 μl primer mixture (40 pmol each of the FIP and BIP primers, 20 pmol each of the LF and LB primers and 5 pmol each of the F3 and B3 primers), 2 μl DNA and 8.2 μl distilled water. The samples were incubated at 63°C for 60 minutes and consequently heated at 85°C for 5 minutes to terminate the reaction. Positive (C. parvum) and negative controls (distilled water) were included for each test. The LAMP products were analyzed by agarose gel electrophoresis and visualized under UV light after ethidium bromide staining as mentioned before.
Application of the LAMP and Nested PCR in Spiked Water Pellets: Efficiency of the Applied Assays
Aliquots from 14 river and two tap water sample pellets from different locations of Ordu province have been chosen and used as spiked water samples after the addition of known number of Cryptosporidium oocysts. Cryptosporidium oocysts from the stock solution were counted by hematocytometer and 10 oocysts were added in 10% aliquots of the concentrated sample pellets. After the DNA extraction, LAMP and nested PCR assays have been applied for all 16 spiked sediments as described before (Table 1).
Table 1. Results obtained by LAMP assay based on the Cryptosporidium SAM gene and by nested PCR of the SSU rRNA gene in spiked with Cryptosporidium oocysts water samples from Ordu province.
| Water type | SAM-1 LAMP assay positive/examined (spiked samples) | Nested PCR assay positive/examined (spiked samples) |
| River water | 14/14 | 6/14 |
| Tap water | 2/2 | 1/2 |
| Total | 16/16 | 7/16 |
| (%) | (100%) | (43.75%) |
Sensitivity of the Nested PCR Assay
The sensitivity of the nested PCR was assessed by spectrophotometric quantification of a reference C. parvum (Iowa) control sample. The nested PCR amplified the DNA in serial dilution steps of 10 ng to 1 pg and of 10 ng to 100 fg for the LAMP–PCR assays.
RESULTS
A total of 70 water samples from Ordu city and their boroughs were analyzed by IFT, nested PCR and LAMP assays. Altogether 18 river water samples were identified positive for the presence of Cryptosporidium oocysts, when screened by IFT under epifluorescence microscopy. Table 2 presents the results from the occurrence of Cryptosporidium oocysts in the water samples collected during October and December 2009 and analyzed by IFT, nested PCR and LAMP assays. All water samples taken from different sources in Ordu city center were negative by all examined methods. Of the nine samples collected from Ulubey borough, three river samples were found highly contaminated by detecting 30, 20 and 15 Cryptosporidium oocysts, respectively. Positive water samples for the presence of Cryptosporidium were also detected in Unye borough with 28 and 14 oocysts. The occurrences of Cryptosporidium oocysts in river water samples collected from Fatsa borough were 30, 14 and 8, respectively. From the collected river samples during November 2009, one sample from river Catak was positive with six Cryptosporidium oocysts, and six river samples from Kabatas-Aybasti-Korgan borough, collected during the same sampling period, were found positive containing from 6 to 22 Cryptosporidium oocysts per 0.5 l. In the borough of Persembe, two rivers were found positive with 9 and 10 Cryptosporidium oocysts, respectively. Two rivers from the last investigated area during December 2009 were also found positive by the detection of six and seven Cryptosporidium oocysts within the investigated volume. In contrast, no parasites were detected in 16 tap, 3 fountain and 2 well water samples.
Table 2. Cryptosporidium detection by IFT, SSU rRNA-PCR and SAM-1 LAMP assays in water samples collected from Ordu city center and Ordu province, Middle Black Sea.
| Water type (total) | Investigation month | No. of positive/examined samples by IFT* | No. of positive/examined samples by SSU rRNA-PCR assay | No. of positive/examined samples by SAM-1 LAMP assay |
| Tap water (5) | October 2009 | All water samples taken from different sources in Ordu city center were negative by all methods | ||
| Fountain water field from ground water (3) | ||||
| River water (12) | ||||
| Well water (unconfined) (2) | ||||
| River water (7) | 3/7 water from villages Akoluk (30), Kumanlar (20) and Orencik (15) | 0/7 | 3/7 water from villages Akoluk, Kumanlar and Orencik | |
| Tap water (2) | 0/2 | 0/2 | 0/2 | |
| River water (4) | 2/4 water from river Tabakhane (28) and Cevizdere (14) | 0/4 | 2/4 water from river Tabakhane and Cevizdere | |
| Tap water (1) | 0/1 | 0/1 | 0/1 | |
| River water (6) | 3/6 water from river Kavaklar (14), Sıcaksu (8) and Serefiye (30) | 0/6 | 4/6 water from river Kavaklar, Sıcaksu, Serefiye and Bolaman | |
| River water (2) | November 2009 | 1/2 water from river Catak (6) | 0/2 | 1/2 water from river Catak |
| Tap water (1) | 0/1 | 0/1 | 0/1 | |
| River water (6) | 5/6 water from river Karakoyun (7), Kabatas (11), Aybastı (19), Batari (6) and Durali (Akcekese) (22) | 0/6 | 5/6 water from river Karakoyun, Kabatas, Aybastı, Batari and village Durali (Akcekese) | |
| Tap water (3) | 0/3 | 0/3 | 0/3 | |
| River water (8) | 2/8 water from river Büyükagız (9) and Belicesu (10) | 0/8 | 2/8 water from river Büyükagız and Belicesu | |
| Tap water (2) | 0/2 | 0/2 | 0/2 | |
| River water (4) | December 2009 | 2/4 water from river Kerali (6) and Akpinar (Koykent-Mesudiye) (7) | 0/4 | 2/4 water from river Kerali and Akpinar (Koykent-Mesudiye) |
| Tap water(2) | 0/2 | 0/2 | 0/2 | |
| Total | 18/70 | 0/70 | 19/70 | |
| (%) | (25.7%) | (0%) | (27.1%) | |
*Number of Cryptosporidium oocysts detected in 0.5 l examined volume.
The SAM-1 LAMP assay amplified Cryptosporidium DNA in 18 water samples. In detail, all IFT Cryptosporidium positive river water samples were confirmed also as positive by the SAM-1 LAMP assay. Additionally, one river water sample from Bolaman River in Fatsa borough yielded positive LAMP *reaction (Fig. 2).
Fig. 2.
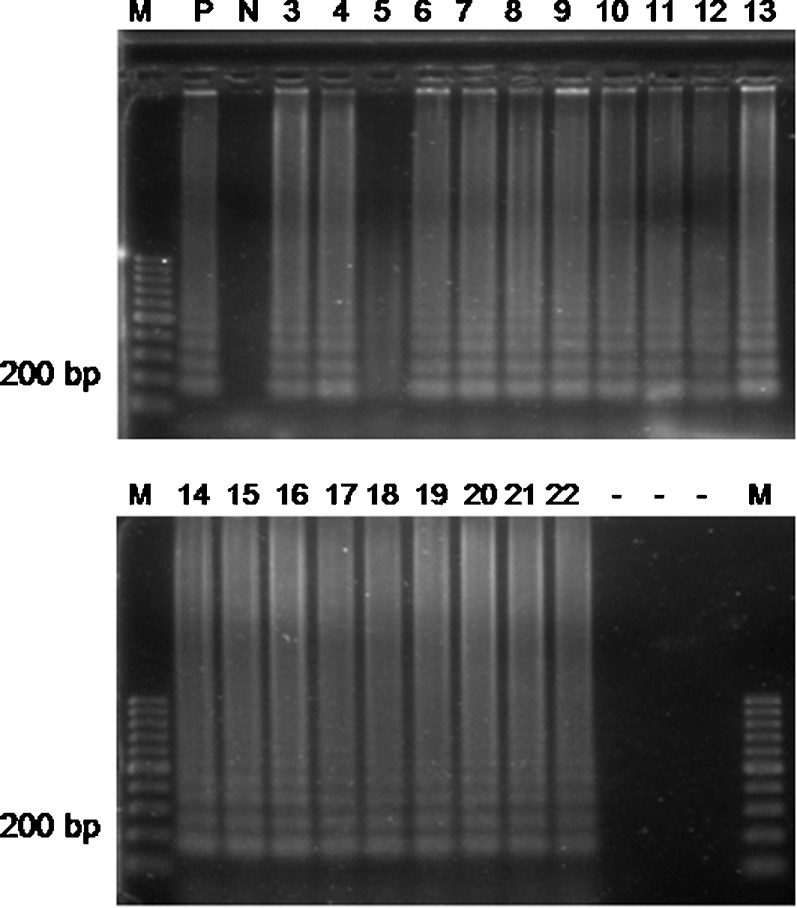
LAMP detection of Cryptosporidium spp. SAM gene. M: 100 bp ladder. P: positive control (C. parvum Iowa); N: negative control (distilled water); lanes 3–6: river water from Ulubey; lanes 7–8: river water from Unye; lanes 9–13: river water from Fatsa; lanes 14–18: river water from Kabatas-Aybastı-Korgan; lanes 19–20: river water from Persembe; lanes 21–22: river water from Mesudiye-Golkoy. -: empty slots.
By summarizing the data on the occurrence of Cryptosporidium in water resources from Ordu city and its boroughs (Tables 2 and 3), we found that 18 out of 70 samples were positive for Cryptosporidium (25.7%) when investigated by IFT and 19 out of 70 by SAM-1 LAMP (27.1%).
Table 3. Summarized table on the occurrence of Cryptosporidium in water samples collected from Ordu city and county boroughs examined by IFT and SAM-1 LAMP molecular assays.
| Water type | Location | No. of examined samples | Total no. of positive samples by IFT | Total no. of positive samples by SAM-1 LAMP |
| Tap water | All locations | All tap, well and fountain samples were negative. | ||
| Well water | ||||
| Fountain water | ||||
| Subtotal | 21 | 0 | 0 | |
| % positive | (0%) | (0%) | ||
| River water | Ordu | 12 | 0 | 0 |
| Ulubey | 7 | 3 | 3 | |
| Unye | 4 | 2 | 2 | |
| Fatsa | 8 | 4 | 5 | |
| Kabatas-Aybastı-Korgan | 6 | 5 | 5 | |
| Persembe | 8 | 2 | 2 | |
| Mesudiye-Golkoy | 4 | 2 | 2 | |
| Subtotal | 49 | 18 | 19 | |
| (%) | (36.7%) | (38.8%) | ||
| Total positive | 70 | 18 | 19 | |
| (%) | (25.7%) | (27.1%) | ||
Figure 3 demonstrates the results of testing serially diluted C. parvum DNA (Iowa). Nested PCR assays showed a detection limit of 100 fg (Fig. 3a), while the detection limit for the SAM-1 LAMP was found to be 1.8 fg (data not shown). LAMP–PCR was not used in the study, due to the small size of the amplified product and the difficult characterization of the any possible positive sample by sequencing. Nested PCR correctly amplified the 826- to 864-bp product and showed a sensitivity of 1 pg of the reference DNA. Nested PCR was used for screening all water samples, allowing the molecular characterization in case of positive result, although the method was less sensitive than LAMP–PCR. None of the samples were found positive by nested PCR (Tables 2 and 3). Interestingly, 18 river water samples (representing a ratio of 36.7%) out of 49 water samples examined, were found contaminated with Cryptosporidium oocysts by IFT and 19 (representing a ratio of 38.8%) by LAMP. However, Cryptosporidium oocysts were absent in fountain, tap and well water by IFT and SAM-1 LAMP assays.
Fig. 3.
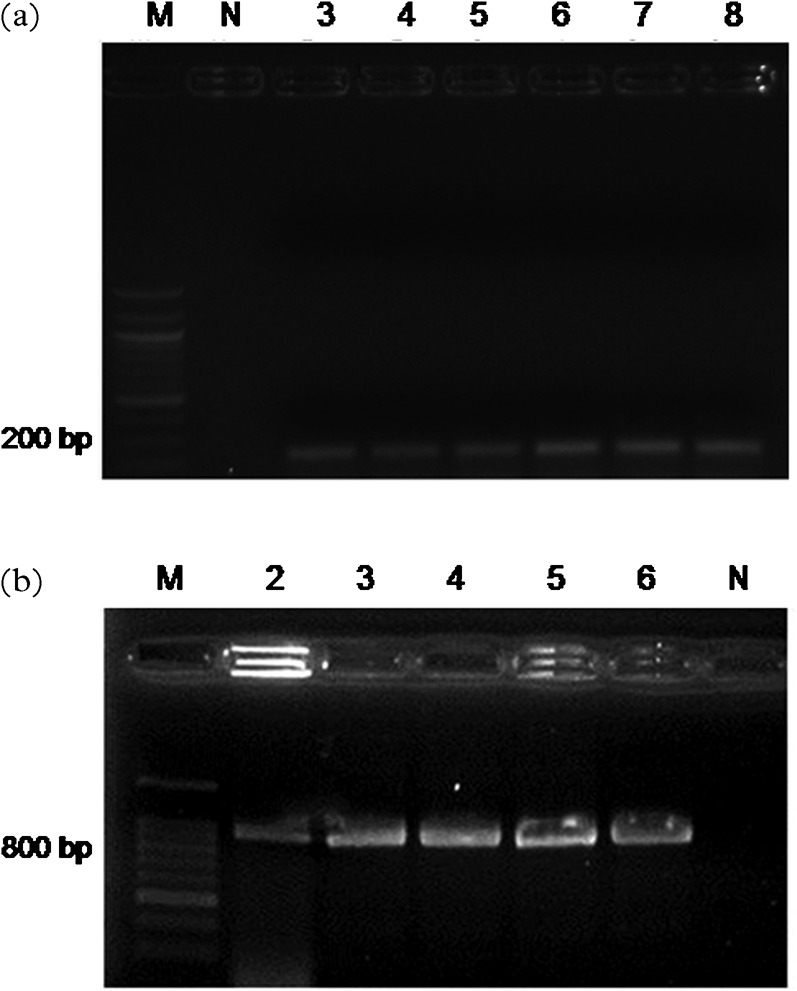
(a) Sensitivity of the LAMP–PCR for the amplification of the SAM gene on reference C. parvum DNA (Iowa). M: 100 ladder; lane 1: 100 fg; lane 2: 1 pg; lane 3: 10 pg; lane 4: 100 pg; lane 5: 1 ng; lane 6: 10 ng. (b) Sensitivity of the nested PCR for the amplification of the SSU rRNA gene on reference C. parvum DNA (Iowa). M: 100 bp ladder; lane 2: 1 pg; lane 3: 10 pg; lane 4: 100 pg; lane 5: 1 ng; lane 6: 10 ng; N: negative control (distilled water).
We evaluated the LAMP method in randomly selected pellets of 16 (14 river and two tap) spiked water samples from Ordu province, which served in the study as internal positive controls. All the examined samples by LAMP were found positive (100%) for the presence of Cryptosporidium DNA confirming the effectiveness of the method in working with difficult DNA templates. The applied nested PCR assay detected only seven (43.75%) out of 16 samples spiked water pellets based on the amplification of the SSU rRNA (Table 1).
DISCUSSION
Cryptosporidium is increasingly gaining attention as a human and an animal pathogen mainly due to its dominant involvement in worldwide waterborne outbreaks (Karanis et al., 2007a; Smith et al., 2007; Baldursson and Karanis, 2011). Most of the reported outbreaks associated with protozoan pathogens, namely Giardia and Cryptosporidium, occur through water (Craun et al., 1998; Barwick et al., 2000; Lee et al., 2002; Karanis et al., 2007a; Baldursson and Karanis, 2011). Here, we report the detection of Cryptosporidium after application of IFT as one conventional method and two molecular tools using the SSU rRNA gene for nested PCR and the SAM gene for LAMP assays. Black Sea is one of the richest areas for fishing and has complex and diverse ecosystem in the world; however, it has recently lost its value. About sixteen million people living in the coast area cities contribute to the contamination of water supplies by domestic waste and mixed unpurified sewage. The Black Sea region is abundant in streams and some of them are used as a source of drinking water. Heavy rainfalls may result in an increased faecal wash off into surface water (rivers and sea) and private wells as well. The most important running flowers are Bolaman, Melet, Turnasuyu and Elekci River originating from the northern slopes of the Black Sea Mountains. They are streams with short distance reached to marine, while river Melet is the biggest river and it is used as drinking water source supplying the main city. Water treatment in Ordu includes flocculation, sedimentation and extensive chlorination. Although there is policy to detect waterborne protozoa in Turkey and the frequency of fecal contamination in these waters is known, there is no any investigation about the presence of waterborne protozoan in this region.
There is a rising demand on developing more sophisticated and sensitive molecular methods to detect waterborne protozoan in drinking water supplies. LAMP will be one useful alternative in the future as this method is not affected by the presence of inhibitors and additional debris extracted along with the DNA. The comparison of the results obtained by the IFT and the SAM-1 LAMP assays demonstrated a clear correlation of the positive results. In 18 IFT-positive samples, LAMP products have been also generated and the positive result has been confirmed by both assays (IFT and LAMP).
Interestingly, none of the investigated samples could be found positive by PCR indicating the simultaneous presence of additional water components in the concentrates, inhibiting the PCR reaction. The LAMP assay has advantages for the detection of organisms at relatively low concentration in environmental samples. The use of three primer sets makes the procedure elegantly specific permitting amplification of target DNA extracted from crude samples containing extraneous biological material. The assays have also been shown to be insensitive to the common interferences that limit PCR application. The SAM-1 LAMP assay has been developed to amplify DNA from C. parvum, C. hominis and C. meleagridis (Bakheit et al., 2008), three of the most important human pathogenic species of Cryptosporidium. According to the abilities of the developed test, we can assume that a minimum of one of these species was present in the investigated samples.
The LAMP assay has been established in combination with ARAD filtration for improved detection sensitivity and identification of waterborne protozoan like Cryptosporidium and Giardia is as it has been reported by Plutzer et al. (2008). Extraction of high-quality DNA is a key step in PCR detection. However, inhibition of the PCR reaction by various substances commonly present in environmental samples is a well-documented disadvantage (Kourenti and Karanis, 2006). This is particularly important when applied in samples containing low parasite concentrations, typical in surface water samples and in samples from animals, suffering from chronic infections with low-level excretion of oocysts in feaces. Such inhibitors do not affect the LAMP reaction. LAMP provided detection sensitivity superior to that of PCR as it was demonstrated by Bakheit et al. (2008) in South African faecal samples of small volume (5 g) of investigated material. Since inhibition of the PCR reactions by various substances, commonly present in environmental samples and the amount of DNA extracted from environmental samples, is too low for the PCR sensitivity, we suppose that the negative findings by PCR during our investigation occurred due to the aforementioned reasons.
During our study, the spiked samples served as internal positive controls in both LAMP and nested PCR reactions in order to detect the inhibition. We found that the amplification of spiked samples has failed in 56.25% of the tests performed by nested PCR. All the inhibited nested PCR samples were found positive by LAMP, which supports the previous finding that inhibitor components have no effect on LAMP assays (Plutzer et al., 2008).
Water pollution is one of the most urgent health problems, currently facing all countries of the world, since safe drinking water is a general concern. The development of sensitive detection methods for emerging pathogens related to waterborne diseases is of increasing interest and there is a need for wider dissemination of information on waterborne and emerging diseases worldwide (Karanis et al., 2007a). There is a dramatic increase in waterborne outbreaks in the recent years (Baldursson and Karanis, 2011).
The LAMP assays have major advantages for detection of protozoan pathogens at relatively low concentration in environmental samples. Since there is no previous report about waterborne protozoan in the investigated area, the present paper will not only contribute to the initiation of protection measures for public health but also be the platform for further and more extensive studies in the Black Sea greater area.
REFERENCES
- Bakheit MA, Palomino L, Thekisoe OMM, Mate PA, Ongerth J, Karanis P.(2008)Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Veterinary Parasitology 15811–22. [DOI] [PubMed] [Google Scholar]
- Baldursson S, Karanis P.(2011)Waterborne transmission of protozoan parasites: review of worldwide outbreaks — an update 2004–2010. Water Research 456603–6614. [DOI] [PubMed] [Google Scholar]
- Barwick RS, Levy DA, Craun GF, Beach MJ, Calderon RL.(2000)Surveillance for waterborne-disease outbreaks — United States, 1997–1998. Morbidity and Mortality Weekly Report 491–21. [PubMed] [Google Scholar]
- Craun GF, Hubbs SA, Frost F, Calderon RL, Via SH.(1998)Waterborne outbreaks of cryptosporidiosis. Journal of the American Water Works Association 9081–91. [Google Scholar]
- Karanis P, Kimura A.(2002)Evaluation of three flocculation methods for the purification of Cryptosporidium parvum oocysts from water samples. Letters in Applied Microbiology 34444–449. [DOI] [PubMed] [Google Scholar]
- Karanis P, Kourenti C, Smith H.(2007a)Water-borne transmission of protozoan parasites: a review of worldwide outbreaks and lessons we learnt. Journal of Water and Health 51–38. [DOI] [PubMed] [Google Scholar]
- Karanis P, Ongerth J.(2009)LAMP — a powerful and flexible tool for monitoring microbial pathogens. Trends in Parasitology 25498–499. [DOI] [PubMed] [Google Scholar]
- Karanis P, Sotiriadou I, Kartashev V, Kourenti C, Tsvetkova N, Stojanova K.(2006)Occurrence of Giardia and Cryptosporidium in water supplies of Russia and Bulgaria. Environmental Research 102260–271. [DOI] [PubMed] [Google Scholar]
- Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N.(2007b)Development and preliminary evaluation of loop-mediated isothermal amplification (LAMP) for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Applied and Environmental Microbiology 735660–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourenti C, Heckeroth A, Tenter A, Karanis P.(2003)Development and application of different methods for the detection of Toxoplasma gondii in water. Applied and Environmental Microbiology 69102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourenti C, Karanis P.(2006)Evaluation and applicability of a purification method coupled with nested PCR for the detection of Toxoplasma oocysts in water Letters in Applied Microbiology 43475–481. [DOI] [PubMed] [Google Scholar]
- Lee SH, Levy DA, Craun GF, Beach MJ, Calderon RL.(2002)Surveillance for waterborne-disease outbreaks — United States, 1999–2000. Morbidity and Mortality Weekly Report 511–47. [PubMed] [Google Scholar]
- Notomi T, Hiroto O, Harumi M, Toshihiro Y, Watanabe K, Nobuyuki A, Hase T.(2000)Loop-mediated isothermal amplification of DNA. Nucleic Acids Research 28E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzer J, Karanis P.(2009)Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitology Research 1041527–1533. [DOI] [PubMed] [Google Scholar]
- Plutzer J, Karanis P, Domokos K, Torokne A, Marialigeti K.(2008)Detection and characterisation of Giardia and Cryptosporidium in Hungarian raw, surface and sewage water samples by IFT, PCR and sequence analysis of the SSUrRNA and GDH genes. International Journal of Hygiene and Environmental Health 211524–533. [DOI] [PubMed] [Google Scholar]
- Rose JB, Slifko TR.(1999)Giardia, Cryptosporidium, and Cyclospora and their impact on foods: a review. Journal of Food Protection 621059–1070. [DOI] [PubMed] [Google Scholar]
- Smith HV, Caccio SM, Cook N, Nichols RA, Tait A.(2007)Cryptosporidium and Giardia as food-borne zoonoses. Veterinary Parasitology 14929–40. [DOI] [PubMed] [Google Scholar]
- Sotiriadou I, Karanis P.(2008)Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test. Diagnostic Microbiology and Infectious Disease 62357–365. [DOI] [PubMed] [Google Scholar]
- Xiao L, Escalante L, Yang CF, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA.(1999)Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Applied and Environmental Microbiology 651578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Singh A, Limor J, Graczyk TK, Gradus S, Lal A.(2001)Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Applied and Environmental Microbiology 671097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]