Abstract
Background:
Thrombocytopenia has been reported in the majority of malaria studies. Some but not all studies suggest the possible role of platelets in the pathology of severe malaria. We assess the association of admission platelet count with malaria complications and mortality in vivax and falciparum malaria.
Methods:
This is a prospective, observational study of patients aged 18 years and above admitted in a tertiary care teaching hospital from August 2004 to July 2006 in Manipal, India. Malaria was diagnosed based on clinical features along with positive Quantitative Buffy Coat method (QBC MP) or thin blood smear examination (Giemsa stain). Platelet counts were measured using Coulter LH 756 Analyser. Thrombocytopenia was defined as a platelet count <150×109/l.
Results:
A total of 131 consecutive patients were included. Sixty patients (46%) were infected with Plasmodium vivax and the rest with Plasmodium falciparum. Forty-six (35%) patients had non-severe and 24 (18%) had severe falciparum infection. The prevalence of thrombocytopenia was similar in vivax and falciparum malaria. Patients with severe falciparum malaria had a statistically significant lower platelet count (P = 0.01) compared to non-severe falciparum malaria. Severe malaria patients with renal failure (P = 0.02) or hyperparasitaemia (P = 0.03) had a statistically significant lower mean platelet count compared to non-severe falciparum malaria. Patients with involvement of more than one organ system had a lower mean platelet count compared to those with single organ involvement.
Conclusions:
The incidence of thrombocytopenia was similar in vivax and falciparum malaria. The admission platelet count is significantly lower in patients who have hyperparasitaemia and acute renal failure compared to patients without complications.
INTRODUCTION
India reports annually nearly 636 million cases of febrile illness in a population of about 1152 million. Among these cases, 1.78 million (0.28%) are confirmed malaria of which 838 533 (47%) are caused by Plasmodium falciparum, 946 554 (53%) are caused by Plasmodium vivax and 3475 (0.2%) are mixed infections. A total of 15 008 malarial deaths were documented in 2006, suggesting a case fatality rate of about 0.8% (WHO, 2008). Thrombocytopenia and anaemia may be linked to haemolysis, decreased cell deformity of parasitized cells, increased splenic uptake, decreased survival of platelets and decreased production of platelets (Butthep and Bunyaratvej, 1992; Bradley et al., 1996; WHO, 2000). Although Luxemburger et al. observed that severe malaria is 4.2 times less common in patients with mixed falciparum and vivax infections than among those with falciparum infection alone, suggesting that co-infection with P. vivax decreases the severity of P. falciparum malaria (Luxemburger et al., 1997), there have been reports of non-sequestration-related complications from vivax malaria (Verma and Magotra, 1976; Mishra and Singh, 1989; Valecha et al., 1992; Beg et al., 2002). There is a need to study these complications associated with vivax malaria (Sina, 2002). Thrombocytopenia has been reported in the majority of malaria studies (Beale et al., 1972; Pongponratn et al., 1985; Pukrittayakamee et al., 1989; Emuchay and Usanga, 1997; Lee et al., 1997; Erhabor et al., 2006). Gerardin et al. have studied the utility of thrombocytopenia as a prognostic marker in falciparum malaria alone (Gerardin et al., 2002). There have been few studies in the Indian subcontinent on thrombocytopenia in vivax malaria, but none of them have attempted to correlate thrombocytopenia with the complication rate (Jadhav et al., 2004; Kochar et al., 2005). The objective of this study is to investigate the proportion and severity of thrombocytopenia in falciparum malaria and vivax malaria and to assess the association of thrombocytopenia with malaria complications and mortality.
METHODS
This is a prospective, observational study in patients admitted in a tertiary care teaching hospital from August 2004 to July 2006. We included all in-patients with plasmodium-positive blood smear/Quantitative Buffy Coat (QBC) identified in Kasturba Hospital, Manipal, Karnataka, above the age of 18 years. Karnataka state is a low malaria transmission zone in India. The diagnosis was based on clinical features and positive QBC system for malarial parasites (QBC MP/Microtube Agglutination stained by acridine orange) or thin blood smear examination using Giemsa stain. Parasitaemia was reported as percentage of infected erythrocytes. We excluded patients with malaria treated outside the hospital, no platelet count available before starting treatment, known HIV-positive status, known thrombocytopenia/platelet disorders, mean corpuscular volume (MCV) <75 (suggesting iron deficiency anaemia), known chronic renal failure or clinical diagnosis of malaria without slide positivity. Blood smear for speciation of plasmodium, plasma glucose, bilirubin, liver enzymes, blood urea, creatinine, lactate levels, chest X ray, G6PD levels and HIV testing were done in all patients. Prothrombin time and activated partial thromboplastin time were done in all patients with thrombocytopenia. Arterial blood gas analysis was done when clinically indicated.
Baseline platelet counts were measured using Coulter LH 756 Analyser and were done on the day of admission along with routine investigations. A platelet count <50×109/l was considered severe thrombocytopenia and 100×109–150×109/l was considered as mild thrombocytopenia (Hoffbrand et al., 2005). The patients were categorized into non-severe and severe falciparum malaria based on the World Health Organization Definition of Severe Malaria of 2000 (WHO, 2000). The presence of one or more of the following clinical or laboratory features classifies the patient as suffering from severe malaria. The same severity criteria were applied to P. vivax cases as well.
Major:
unrousable coma;
generalized convulsions >2 episodes within 24 hours or evidence of continued seizure activity;
haematocrit <15% or haemoglobin <5 g/dl, normochromic, normocytic;
urine output <400 ml/24 hours not improving with rehydration or serum creatinine >3 mg/dl;
non-cardiogenic pulmonary oedema or acute respiratory distress syndrome;
blood glucose <40 mg/dl or 2.2 mmol/l;
systolic pressure <80 mmHg with circulatory failure;
spontaneous bleeding and laboratory evidence of disseminated intravascular coagulation;
pH <7.25 or serum bicarbonate <15mmol/l; venous lactate level >5 mmol/l
macroscopic haemoglobinuria.
Other:
impaired consciousness but arousable;
extreme weakness;
serum bilirubin >3 mg/dl;
parasitemia level >5%.
The study patients were treated according to the clinical judgement of the attending physician and national treatment guidelines. The data were analysed using the SPSS 11.0 Statistical Software Package for Windows. The comparative analysis was done using the Chi-square test for categorical variables. The t-test was applied to compare the mean of continuous variables. The correlation coefficient due to Karl Pearson was used to find the correlation.
RESULTS
A total of 131 patients were included in the study with a diagnosis of malaria. We excluded 11 of the screened patients by the exclusion criteria. The majority of study participants 112 (86%) were male. None of the study patients were pregnant. The mean age of the patients was 39 years. Sixty-two per cent of the patients were in the age group of 20–40 years. Sixty patients (46%) were infected with P. vivax, while 46 (35%) had non-severe and 24 (18%) had severe falciparum infection who met the WHO 2000 severity criteria at any time during hospitalization. There was one (1%) case of mixed infection with both P. vivax and P. falciparum; none of the P. vivax cases had complicated malaria. The mean platelet count in patients with vivax malaria was 94×109/l±48, in patients with uncomplicated falciparum malaria, 100×109/l±64, and in patients with complicated malaria, 43×109/l±24 (Fig. 1 and Table 1). The platelet count in the patient with mixed infection was 18 000×109/l. There was no significant statistical difference in mean platelet count between the patients with vivax malaria and non-severe falciparum malaria. Compared to non-severe falciparum malaria, severe falciparum malaria was associated with a significantly lower mean platelet count (43×109/l versus 101×109/l; P = 0.01). There was an inverse relationship between the percentage of infected erythrocytes in falciparum malaria and platelet count (P = 0.03) (Fig. 2).
Fig. 1.
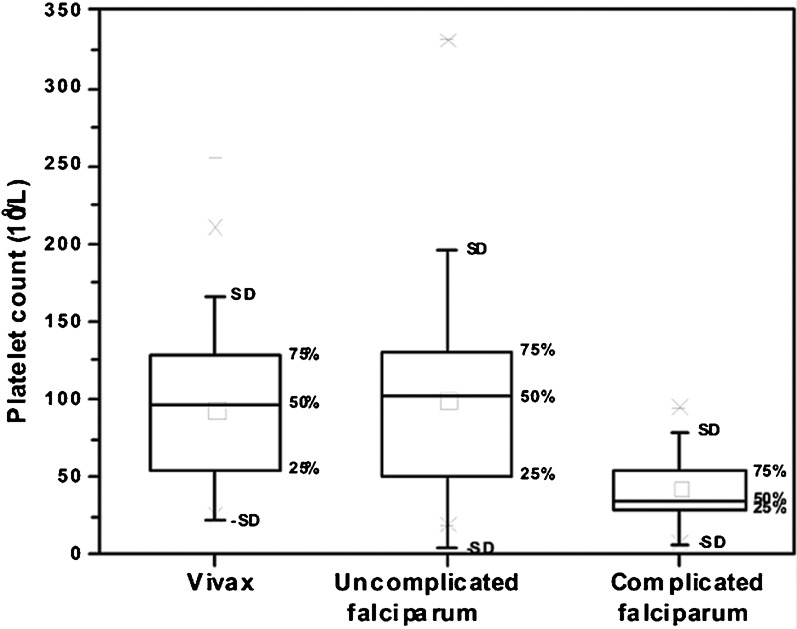
Platelet count in vivax and falciparum malaria.
Table 1. Comparison between platelet counts in vivax and falciparum malaria.
| Vivax malaria (n = 60) | Uncomplicated falciparum malaria (n = 46) | Complicated falciparum malaria (n = 24) | |
| Mean platelet count | 94×109/l±48 | 100×109/l±64 | 43×109/l±24 |
| Lowest | 22×109/l | 19×109/l | 7×109/l |
| Highest | 255×109/l | 331×109/l | 95×109/l |
Patients with severe falciparum malaria had a statistically significant lower platelet count (P = 0.01) compared to non-severe falciparum malaria.
Fig. 2.
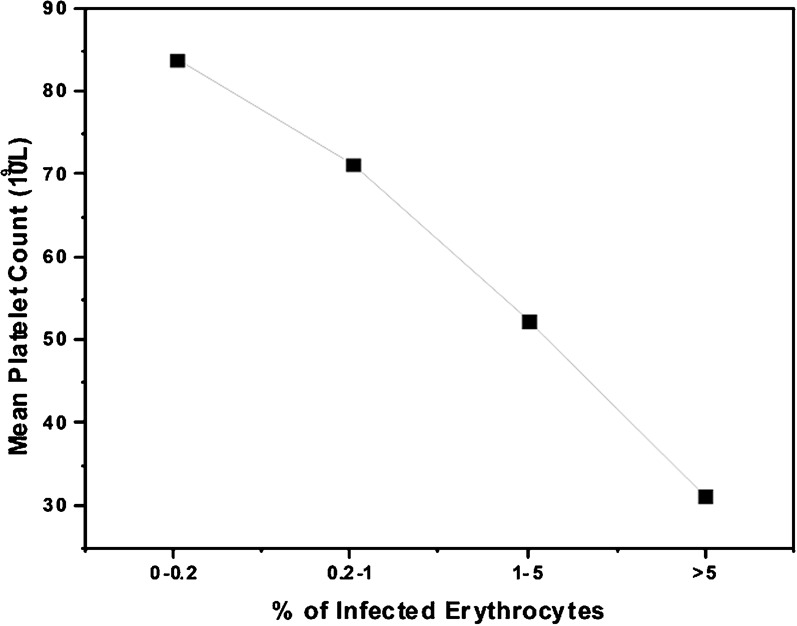
Correlation between parasitaemia and platelet count in falciparum malaria.
Two patients had cerebral malaria and two patients died of suspected overwhelming bacterial sepsis. The distribution of the complications among the 24 patients suffering from severe falciparum malaria is shown in Table 2. The mean platelet count was found to be significantly lower in patients with acute renal failure (P = 0.02) or hyperparasitaemia (P = 0.03). Among 36 patients with platelet count <50×109/l, 4 (11%) patients had spontaneous bleeding. Two of them had epistaxis, one had haemoptysis and one had haematuria. The platelet counts of these patients were 17×109, 19×109, 43×109 and 45×109/l, respectively. No patients with platelet count >50×109/l had spontaneous bleeding. Patients with involvement of more than one organ system had a lower mean platelet count (35.75×109/l) compared to those with single organ involvement (51×109/l). There was no meaningful correlation between haemoglobin and platelet count.
Table 2. Comparison of mean platelet counts in severe falciparum malaria with respect to each complication.
| Characteristics | No. of patients (n = 24) | Mean platelet count patients with this complication (×109/l) | Mean platelet count the remaining patients without this complication (×109/l) | P value |
| Cerebral malaria | 2 (8%) | 31.5 | 43.1 | 0.61 |
| Severe acidosis | 4 (17%) | 40.7 | 44.1 | 0.71 |
| Severe anaemia | 3 (13%) | 31.5 | 44.9 | 0.61 |
| Renal failure | 4 (17%) | 18.3 | 48.1 | 0.02 |
| Acute pulmonary oedema/ARDS | 6 (25%) | 24.8 | 46.1 | 0.22 |
| Shock | 4 (17%) | 26.0 | 46.3 | 0.17 |
| Spontaneous bleeding/DIC | 4 (17%) | 24.0 | 47.8 | 0.07 |
| Jaundice | 19 (79%) | 41.78 | 50.4 | 0.49 |
| Hyperparasitaemia | 5 (21%) | 20.5 | 48.5 | 0.03 |
| Death | 4 (17%) | 35.0 | 44.5 | 0.6 |
DISCUSSION
We found that the majority of vivax patients in our study (88%) had some level of thrombocytopenia. Several international studies have found the prevalence of thrombocytopenia in vivax malaria between 72% and 86% (Srichaikul et al., 1975; Kelton et al., 1979; Makkar et al., 2002). Jadhav et al. found that 65% of vivax malaria patients had platelet count between 50×109 and 150×109/l (Jadhav et al., 2004). In an African study, the mean platelet count in falciparum malaria was 115×109/l (Erhabor et al., 2006). The mean platelet count in vivax malaria in the study by Jadhav et al. was also 115×109/l (Jadhav et al., 2004). There were no patients with vivax malaria whose platelet count was <20×109/l in our study group. Although there are reports of platelet counts <10×109/l seen in vivax malaria, in our study all patients with platelet count <20×109/l had either falciparum or mixed malaria. The mean platelet count in patients with severe malaria was found to be 43×109/l and all had a platelet count of less than 100×109/l. Some but not all studies have shown that there is strong association between thrombocytopenia and severity of malaria (Ladhani et al., 2002; Reyburn et al., 2005). The discrepancies between the two studies were thought to be due to the presence of confounding factors, e.g. ventilation or iron deficiency anaemia. In our study, ventilator care was accessible to all patients and all patients with suspected iron deficiency anaemia were excluded so as to avoid confounding factors as much as possible.
It is possible that the discrepancies in mean platelet count could be due to variable involvement of different organ systems due to varying affinity of the parasitized erythrocytes or affected platelets in different vascular beds. We compared the mean platelet count in patients with involvement of one organ system to those without the involvement of the same system. The study compared platelet counts on presentation in patients with and without complications. Patients with renal failure had a significantly lower mean platelet count (P = 0.02) compared to those without renal failure (18×109/l versus 48×109/l). There was an inverse relation between parasitemia and platelet count and patients with high percentage of falciparum-infected erythrocytes having lower platelet counts which has been previously observed (Kelton et al., 1979; Horstmann and Dietrich, 1985; Pukrittayakamee et al., 1989; Touze et al., 1990; Ohtaka et al., 1993).
Overall thrombocytopenia was seen in 88% of vivax malaria and 89% of falciparum malaria patients which shows that thrombocytopenia is as common in vivax malaria as in falciparum malaria. The mean platelet count of vivax malaria and uncomplicated falciparum malaria were comparable. Patients with involvement of more than one organ system had a lower mean platelet count compared to those with single organ involvement although not statistically significant. The limitation of the study is that the platelet counts were not serially monitored and hence, its relation to severity or outcome could not be assessed. Larger studies will be needed to judge the extent of complications in vivax malaria and to assess the platelet count as a prognostic marker in complicated vivax malaria.
Acknowledgments
The authors would like to acknowledge Deepak Devarajan for his help in collection of data, Lorenz von Seidlein, Menzies School of Health Research, Australia, for his help in editing the article, and Dr Mahadeva Bhat N for his help in submission process.
REFERENCES
- Beale PJ, Cormack JD, Oldrey TB.(1972)Thrombocytopenia in malaria with immunoglobulin (IgM) changes. British Medical Journal 1345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA., Jr(2002)Cerebral involvement in benign tertian malaria. The American Journal of Tropical Medicine and Hygiene 67230–232. [DOI] [PubMed] [Google Scholar]
- Bradley D, Newbold CI, Warrell DA.(1996)Malaria. Oxford Textbook of Medicine Weatherall D.J., Ledingham J.G.G., Warrell D. A.Oxford: Oxford University Press; 835–863. [Google Scholar]
- Butthep P, Bunyaratvej A.(1992)An unusual adhesion between red-cells and platelets in falciparum malaria. Journal of the Medical Association of Thailand 75(Suppl. 1)195–202. [PubMed] [Google Scholar]
- Emuchay CI, Usanga EA.(1997)Increased platelet factor 3 activity in Plasmodium falciparum malaria. East African Medical Journal 74527–529. [PubMed] [Google Scholar]
- Erhabor O, Babatunde S, Uko KE.(2006)Some haematological parameters in plasmodial parasitized HIV-infected Nigerians. Nigerian Journal of Medicine 1552–55. [DOI] [PubMed] [Google Scholar]
- Gerardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P.(2002)Prognostic value of thrombocytopenia in African children with falciparum malaria. The American Journal of Tropical Medicine and Hygiene 66686–691. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV, Catovsky D, Tuddenham EGD.(eds.)(2005)Postgraduate Haematology Malden, MA: Blackwell Publishing Inc [Google Scholar]
- Horstmann RD, Dietrich M.(1985)Haemostatic alterations in malaria correlate to parasitaemia. Blut 51329–335. [DOI] [PubMed] [Google Scholar]
- Jadhav UM, Patkar VS, Kadam NN.(2004)Thrombocytopenia in malaria — correlation with type and severity of malaria. Journal of the Association of Physicians of India 52615–618. [PubMed] [Google Scholar]
- Kelton JG, Neame PB, Gauldie J, Hirsh J.(1979)Elevated platelet-associated IgG in the thrombocytopenia of septicemia. The New England Journal of Medicine 300760–764. [DOI] [PubMed] [Google Scholar]
- Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A.(2005)Plasmodium vivax malaria. Emerging Infectious Diseases 11132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR.(2002)Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. British Journal of Haematology 119839–847. [DOI] [PubMed] [Google Scholar]
- Lee SH, Looareesuwan S, Chan J, Wilairatana P, Vanijanonta S, Chong SM, Chong BH.(1997)Plasma macrophage colony-stimulating factor and P-selectin levels in malaria-associated thrombocytopenia. Thrombosis and Haemostasis 77289–293. [PubMed] [Google Scholar]
- Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ.(1997)The epidemiology of severe malaria in an area of low transmission in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene 91256–262. [DOI] [PubMed] [Google Scholar]
- Makkar RP, Mukhopadhyay S, Monga A, Gupta AK.(2002)Plasmodium vivax malaria presenting with severe thrombocytopenia. Brazilian Journal of Infectious Diseases 6263–265. [DOI] [PubMed] [Google Scholar]
- Mishra VN, Singh D.(1989)Cerebral malaria by Plasmodium vivax. Journal of the Association of Physicians of India 37411. [PubMed] [Google Scholar]
- Ohtaka M, Ohyashiki K, Iwabuchi H, Iwabuchi A, Lin KY, Toyama K.(1993)[A case of vivax malaria with thrombocytopenia suggesting immunological mechanisms]. Rinsho Ketsueki 34490–492. [PubMed] [Google Scholar]
- Pongponratn E, Riganti M, Harinasuta T, Bunnag D.(1985)Electron microscopy of the human brain in cerebral malaria. The Southeast Asian Journal of Tropical Medicine and Public Health 16219–227. [PubMed] [Google Scholar]
- Pukrittayakamee S, White NJ, Clemens R, Chittamas S, Karges HE, Desakorn V, Looareesuwan S, Bunnag D.(1989)Activation of the coagulation cascade in falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 83762–766. [DOI] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM.(2005)Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 2931461–1470. [DOI] [PubMed] [Google Scholar]
- Sina B.(2002)Focus on Plasmodium vivax. Trends in Parasitology 18287–289. [DOI] [PubMed] [Google Scholar]
- Srichaikul T, Puwasatien P, Karnjanajetanee J, Bokisch VA, Pawasatien P.(1975)Complement changes and disseminated intravascular coagulation in Plasmodium falciparum malaria. Lancet 1770–772. [DOI] [PubMed] [Google Scholar]
- Touze JE, Mercier P, Rogier C, Hovette P, Schmoor P, Dabanian C, Campiadgi S, Laroche R.(1990)[Platelet antibody activity in malaria thrombocytopenia]. Pathology Biology (Paris) 38678–681. [PubMed] [Google Scholar]
- Valecha N, Bagga A, Chandra J, Sharma D.(1992)Cerebral symptoms with P. vivax malaria. Indian Pediatrics 291176–1178. [PubMed] [Google Scholar]
- Verma KC, Magotra ML.(1976)Vivax cerebral malaria in Jammu. Indian Pediatrics 13229–231. [PubMed] [Google Scholar]
- WHO(2000)Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(Suppl. 1)1–51. [PubMed] [Google Scholar]
- WHO(2008)World Malaria Report 2008. WHO/HTM/GMP/2008.1Geneva: WHO [Google Scholar]


