Abstract
Ongoing transmission of lymphatic filariasis (LF) was assessed in five Samoan villages by measuring microfilaraemia (Mf), circulating filarial antigen (CFA) and antibody prevalence. Compared to the other villages, Fasitoo-Tai had a significantly higher Mf prevalence (3.2%), CFA prevalence (14.6%) and antibody prevalence in children (62.0%) (P<0.05). Puapua had a significantly lower CFA prevalence (2.5%), no detectable Mf-positive individuals and significantly low antibody prevalence in children (7.9%) (P<0.05). Siufaga, previously believed to be LF-free, recorded >1% CFA prevalence and a high antibody prevalence in children (46.6%). Overall, antibody prevalence in children appeared to reflect the transmission dynamics in the villages and, in Siufaga, identified an area of ongoing transmission. The Filariasis Cellabs Enzyme-Linked Immunosorbent Assay (CELISA), based on recombinant antigen Bm14, to detect antibodies, could potentially be a promising diagnostic tool for inclusion in future surveillance in the South Pacific.
INTRODUCTION
Lymphatic filariasis (LF) is a mosquito-transmitted parasitic disease caused by the filarial nematodes Wuchereria bancrofti, Brugia malayi and Brugia timori (Ottesen, 2006). In 1997 the 50th World Health Assembly approved a resolution calling for the elimination of LF as a public health problem (WHA50.29) (WHO, 2005). The resolution acknowledged the morbidity and socioeconomic costs of LF including the general lack of awareness of disease and the potential for its eradication (CDC, 1993). The Global Program to Eliminate Lymphatic Filariasis (GPELF) was developed in 1999 based on a comprehensive strategy to rid countries of LF as a public health problem by the year 2020 (WHO, 2005). The Pacific counterpart of GPELF, formed in 1999, was named the Pacific Program for the Elimination of Lymphatic Filariasis (PacELF) (Ichimori and Crump, 2005).
Prevalence of bancroftian LF, caused by the parasite W. bancrofti, throughout the Pacific was historically high (PacELF, 2006). Sixteen of the 22 countries falling under the jurisdiction of PacELF were classified as endemic following baseline prevalence surveys. They were: American Samoa, the Cook Islands, the Federated States of Micronesia, Fiji, French Polynesia, Kiribati, the Marshall Islands, New Caledonia, Palau, Papua New Guinea, Samoa, Solomon Islands, Tonga, Tuvalu, Vanuatu, and Wallis and Futuna (WHO, 2007). Originally, PacELF set the target of LF elimination as a public health problem by 2010, demonstrated by <1% circulating filarial antigen (CFA) prevalence of the population or <0.1% CFA prevalence in children (PacELF, 2006; Ichimori et al., 2007a). The strategic plan focused on the scheduling of a minimum of five rounds of mass drug administration (MDA), depending on baseline prevalence in each country and the results of post-MDA surveys (PacELF, 2006). Since then, post-campaign prevalence surveys have revealed persistent ongoing transmission in certain countries, such as Samoa, suggesting the need to intensify efforts (Chanteau and Roux, 2008; Huppatz et al., 2009). Subsequently, at the 2007 PacELF meeting, a 5-year plan was drafted (WHO, 2007). Countries on the brink of elimination entered monitoring and surveillance mode until 2012, whereas other countries with >1% CFA prevalence planned further control efforts (WHO, 2007). The target date for the elimination of LF from PNG was lengthened until 2020 (WHO, 2007).
One country to persistently detect >1% CFA prevalence in the population is Samoa (Huppatz et al., 2009; Joseph et al., 2011a). Samoa has a long history of filariasis control; initial filariasis surveys began as early as the 1920s, with attempts at control program in the 1940s (Ichimori and Crump, 2005). In 1966, MDAs began, and Samoa completed 10 rounds of MDA before the establishment of PacELF (Burkot et al., 2002; Ichimori et al., 2007b). In 1999, Samoa was the first country to implement the MDA regime under the direction of the World Health Organization (Ichimori and Crump, 2005) and a further seven rounds of MDA were completed from 1999 to 2008 (PacELF, 2006; Huppatz et al., 2009). The reported MDA coverage for the five rounds conducted from 1999 to 2003 was 90%, 57%, 68%, 60% and 80%, respectively (Huppatz et al., 2009). MDA coverage was defined as the number of people provided with medication divided by the total population that was reported by an official government census (Huppatz et al., 2009). This was not a directly observed treatment administration (Huppatz et al., 2009). A stratified cluster nationwide survey of Samoa in 2003, carried out following five rounds of MDA, demonstrated an overall microfilariae (Mf) prevalence of 0.4% with a CFA prevalence of 1.1% (Huppatz et al., 2009). This corresponded to a 75.6% reduction in CFA-positive individuals since the implementation of the PacELF (Ichimori and Crump, 2005; Ichimori et al., 2007a). The promising decline in CFA prevalence led to a sixth round of MDA in 2006, with a high coverage rate of 93%, with the goal of further lowering the prevalence below the recommended threshold of <1% CFA (Huppatz et al., 2009). Unfortunately, follow-up post-MDA surveys in 2007 detected persistent antigenaemia (Joseph et al., 2011a) leading to the seventh round of MDA administered following the research described in this paper.
To assess the effectiveness of MDAs in Samoa, as well as implement successful surveillance strategies in previously LF endemic countries, it is crucial to apply sensitive diagnostic assays which are capable of identifying these areas of residual endemnicity or resurgence early. This phase of low prevalence poses particular challenges: ‘hot spots’ may be scattered and ill-defined and the diagnostic tools measuring Mf and CFA that were successful in the earlier phase of the program may no longer be adequate because of issues with sensitivity, the requirement for larger sampling sizes, and lag phases before Mf or CFA are detectable in newly infected persons (Burkot et al., 2002; Ramzy, 2002; Durrheim et al., 2003; Lammie et al., 2004; Melrose et al., 2004; Rawlins et al., 2004; Grady et al., 2007; Weil and Ramzy, 2007; Ramaiah et al., 2009). The addition of antibody serology as a complementary diagnostic tool when prevalence is low may provide an earlier warning system, since children born after the interruption of transmission would be antibody negative (Ramzy et al., 1995; Lammie et al., 1998, 2004; Supali et al., 2004; Weil and Ramzy, 2007; Weil et al., 2008; Mladonicky et al., 2009). In order to incorporate serology into the LF program, a standardized commercial assay must be implemented, such as the Filariasis Cellabs Enzyme-Linked Immunosorbent Assay (CELISA) (Weil et al., 2010). The Filariasis CELISA measures anti-filarial IgG4 in a recombinant antigen system based on the Bm14 research-based assay prototype (Chandrashekar et al., 1994). The assay is adaptable for filter paper sampling (Joseph and Melrose, 2010; Weil et al., 2010) and has successfully been implemented in both spatial epidemiological studies and seroprevalence studies in the Pacific (Joseph et al., 2011a, b). Therefore, the aim of this epidemiological study was to assess the potential for antibody prevalence measured in children born after commencement of MDA to identify persistent areas of residual endemnicity in Samoa and its relationship to Mf and CFA.
MATERIALS AND METHODS
Study Area
This research was conducted in May 2008, before the seventh MDA round in June 2008, on both islands of Samoa (Fig. 1). Study areas chosen on the island of Savai’i were Tafua and Puapua. Study areas chosen on the island of Upolu included Fasitoo-Tai, Siufaga and Falefa. These five villages have been used as sentinel sites not more than twice since 1999 and were selected to give a range of infection prevalences based on the previous C survey completed in 2007 (Table 1). The C survey was a countrywide prevalence survey, testing randomly selected clusters, to assess if the prevalence of CFA had fallen below the required 1% after the fifth round of MDA. Unfortunately, CFA prevalence was still >1% (Joseph et al., 2011a), which prompted the requirement for this study. Siufaga was chosen as being representative of a LF-free village, as it was previously thought that LF transmission had been interrupted since CFA prevalence was recorded as <1% in previous years.
Fig. 1.
Locations of the five study villages in Samoa. On Savai’i, the two villages were Tafua and Puapua. On Upolu, the three villages chosen were Fasitoo-Tai, Siufaga and Falefa. The capital city, Apia, is included on the map as a reference.
Table 1. Data collected from the 2007 survey in Samoa, kindly provided by the Pacific Program to Eliminate Lymphatic Filariasis (PacELF).
| Island | Village | Mf (%) | CFA (%) | Ab in children (%) |
| Savai’i | Tafua | 0.5 | 14.8 | 71 |
| n = 92 | ||||
| Puapua | 0.2 | 16.7 | 40 | |
| n = 29 | ||||
| Upolu | Falefa | 0.5 | 10.7 | 44 |
| n = 122 | ||||
| Fasitoo-Tai | 0.6 | 21.5 | 25 | |
| n = 65 | ||||
| Siufaga† | 0 | 0 | ND |
Mf, microfilariae; CFA, circulating filarial antigen; Ab, antibody; n, number of participants; ND = not done.
†Chosen as a negative control village (ceased transmission).
Study Population
It was the aim of the research to screen every individual residing in the villages of Tafua, Puapua and Siufaga ⩾2 years, and coverage rates achieved ranged from 79% to 84% of the population (Table 2). This was based on the most recent population census, at the time of the study, conducted in 2006. The villages of Fasitoo-Tai and Falefa had populations exceeding 1000 and it was the aim of the study to screen a minimum of 500 residents. The selection criteria for the latter villages related to the previous 2007 survey (Table 1). An individual from each village, who tested CFA positive in the previous 2007 survey, was randomly selected. Their household of residence was deemed the central point and, radiating out, every household was included in the survey until approximately 500 individuals were registered and screened. Since surveying occurred during the daytime, school children registered in the study by their guardians, after visiting their household of residence, were tested at their respective primary schools.
Table 2. Demographics of the five Samoan villages chosen for the study.
| Upolu | Savai’i | ||||
| Characteristic | Fasitoo-Tai | Falefa | Siufaga | Puapua | Tafua |
| Male | 316 | 286 | 270 | 229 | 178 |
| Female | 301 | 284 | 225 | 219 | 166 |
| Children ⩽10 years | 158 | 167 | 131 | 126 | 86 |
| Total tested | 617 | 570 | 495 | 448 | 344 |
| % coverage of village* | 44% | 41% | 79% | 81.1% | 84% |
| Median age (years) | 19 | 18 | 23 | 18 | 20 |
| Age range (years) | 2–90 | 2–86 | 2–92 | 2–85 | 2–84 |
*Based on population census 2006. Note that Fasitoo-Tai and Falefa had populations of 1393 and 1388, respectively; it was the aim of the study to test at least 500 individuals radiating from a central house.
In this research, any statement regarding ‘children’ will refer to participants ⩽10years. The reasoning for choosing a target population of ⩽10 years was due to the timing of the initial MDA. MDAs, under the guidance of PacELF, began in Samoa in 1999 (Ichimori and Crump, 2005) and targeting children born after the initial MDA placed their age at approximately 9 years at the time of the study. Unfortunately, in most situations, it was apparent that dates of birth were not recorded for children; thus, the selection was based on grade level for children who attended school. Children aged 9 or 10 years corresponded to grade 5: thus, any child equivalent to grade 5 was included in the study.
Informed consent was given verbally and individuals were registered for the study with a unique identification number linked to their household of residence. Demographic information was recorded including age and gender. The study was conducted under human ethics approval number H1423, as approved by the James Cook University Research Human Ethics Committee. The study protocol was also approved by the Samoan Health Research Committee before commencement.
Blood Collection
Following registration, 160 μl of blood was collected by fingerprick. One hundred microlitres was used for antigen testing in the field and the remaining blood was collected onto a Tropbio filter paper disc (Tropbio Pty Ltd, Townsville, Qld, Australia). All six protrusions were saturated with blood, to give a volume of 10 μl of blood on each protrusion, thus a total of 60 μl. These were dried in the field, placed in ziplock bags and transported back to Australia for storage at −20°C for antibody testing. In the village of Puapua, blood for antibody testing was drawn only from children, whereas antibody testing was done on every participating individual in the other four villages. If individuals tested antigen positive in the field test, a further 120 μl of blood was collected by fingerprick. Sixty microlitres was used to make a three-line thick blood smear for Mf examination and the remaining was soaked onto the six protrusions of a filter paper disc, dried, placed in ziplock bags and transported back to Australia for storage at −20°C until confirmatory antigen testing.
Antigen Testing
The field test used to detect CFA was the NOW® filariasis immunochromatographic test and performed according to the manufacturer’s instructions (Binax, Portland, ME, USA). Briefly, the collected 100 μl of blood was transferred onto the absorbent pad and the result was read at exactly 10 minutes and recorded as positive, negative or invalid. Positive tests were confirmed in the laboratory using the Og4C3 antigen capture ELISA (Tropbio Pty Ltd) as previously described (Hoti et al., 2002). Any positives were followed up for treatment by Ministry of Health staff during the MDA scheduled following this study.
Mf Testing
Blood taken from a fingerprick was drawn into three lines, approximately 20 μl thick, onto a microscope glass slide using a capillary as previously described (Sasa, 1976). The slides were left to dry for 48 hours then wrapped for transport. In the laboratory, each slide was stained in 10% Giemsa stain (20 minutes), washed in water, dried, then coverslipped. The slide was examined under the microscope (×200) and Mf were counted. The number of Mf per millilitre of blood was calculated based on the initial 60 μl volume. Mf testing was performed during daylight hours, between 0800 and 2000 hours according to peak levels of Mf and biting tendencies of Aedes polynesiensis (Ramalingam, 1968).
Antibody Testing
Anti-filarial IgG4 antibodies were detected using the commercially available Filariasis CELISA kit (Cellabs Pty Ltd, Manly, NSW, Australia). One protrusion of filter paper was eluted overnight at 4°C in 500 μl of sample diluent. The following morning the elution was thoroughly vortexed and assayed in duplicate, according to the manufacturer’s instructions. The washing steps were performed with an automated plate washer (MultiDrop® Combi nL; Pathtec, Preston, Vic., Australia) using 200 μl per well. Plates were read at a dual wavelength of 450 and 650 nm with a Multiskan EX Type 355 Primary V.2.1-0 (Pathtec) using the software Labsystems Genesis Version 3.00 (Pathtec). Negative samples were defined as optical density (OD) absorbance value <0.26 and positive samples were defined as OD absorbance value ⩾0.400 (Joseph and Melrose, 2010). Samples with values between these OD absorbance values were repeated, in accordance with the manufacturer’s instructions, and if <0.400, they were considered negative.
Statistical Analysis
All data were entered into SPSS Statistical Software Package Version 17.0. Prevalence rates were calculated using the descriptive options in SPSS. The three analyses used were the Chi-square, scatter plots with Pearson’s correlation coefficient and the Mann–Whitney U non-parametric analysis. Confidence intervals (95% CI) were determined using the Binomial Stats program ‘JavaStat’ (Clopper and Pearson, 2005).
RESULTS
Prevalence
The overall prevalence of Mf, CFA and antibody for the five villages are tabulated (Table 3). To account for the possibility of including antibody positive children born prior to the 1999 MDA, data were re-analysed for children ⩽9 years (Table 3). No significant difference was observed between the two antibody prevalence rates (P>0.05).
Table 3. Prevalence of microfilariae (Mf), circulating filarial antigen (CFA) and antibodies (Ab) in each of the five villages (%) including 95% CI. Antibody prevalence was re-calculated to include only children ⩽9 years to account for the potential of inclusion of antibody positive children born prior to the 1999 MDA. There was no significant difference between the two prevalence rates (P>0.05).
| Upolu | Savai’i | ||||
| Fasitoo-Tai | Falefa | Siufaga | Puapua | Tafua | |
| Mf prevalence (%) | 3.2 | 0* | 0* | 0* | 0.6 |
| (2.0–5.0) | (0–0.7) | (0–0.7) | (0–0.8) | (0.1–2.1) | |
| CFA prevalence (%) | 14.6 | 5.1 | 1.6 | 2.5 | 8.4 |
| (11.9–17.6) | (3.4–7.2) | (0.7–3.2) | (1.2–4.4) | (5.7–11.9) | |
| Total Ab prevalence (%) | 74.9 | 64.9 | 64.8 | ND | 34.3 |
| (71.3–78.3) | (60.8–68.8) | (60.5–69.1) | (29.3–39.6) | ||
| Ab prevalence children (%) | 62 | 51.5 | 46.6 | 7.9 | 12.8 |
| (54.0–69.6) | (43.6–59.3) | (37.8–55.5) | (3.9–14.1) | (6.6–21.7) | |
| Ab prevalence ⩽9 years (%) | 63.1 | 49.3 | 45.1 | 8.9 | 14.3 |
| (54.2–71.4) | (41.0–57.7) | (36.1–54.4) | (4.3–15.7) | (7.4–24.1) | |
| CFA prevalence children (%) | 9.5 | 4.2 | 0 | 0.8 | 3.5 |
| (5.4–15.2) | (1.7–8.5) | (0–2.8) | (0.2–4.3) | (0.7–9.9) | |
ND, not done.
*Mf testing was only performed on CFA positive individuals and not the entire population.
Fasitoo-Tai recorded a significantly higher Mf prevalence (P<0.01), CFA prevalence (P<0.001), total antibody prevalence (P<0.001) and antibody prevalence in children (P<0.001) (Table 3). Although Fasitoo-Tai had a significantly higher number of Mf cases than Tafua (n = 20 versus n = 2), there was not a significant difference between the two villages for the Mf load in carriers (P>0.05) (data not shown). There was no correlation between Mf load/ml and antibody titre (data not shown). OD absorbance values were assumed to correlate with titre (Dylewski et al., 1984).
The lowest CFA prevalence rates were recorded for Puapua and Siufaga, both of which were significantly lower than the other three villages (P<0.001) (Table 3). Puapua had lower antibody prevalence in children than all other villages, significantly lower than Fasitoo-Tai, Falefa and Siufaga (P<0.001). Except for Siufaga, CFA positive children were observed in four of the villages (Table 3), all of which exceeded the threshold of 0.1% antigenaemia.
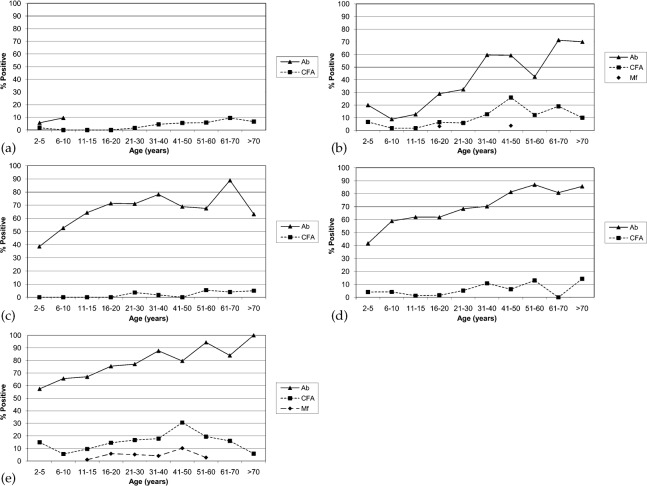
Microfilaraemic persons were identified in both Tafua and Fasitoo-Tai (Table 3) and prevalence appeared to increase with age (Fig. 2b and e), but this did not reach significance (P>0.05). CFA prevalence increased significantly with age for all villages (P<0.05) as did the total antibody prevalence (P<0.001; Fig. 2).
Fig. 2.
Age specific prevalence of microfilaraemia (Mf), antigenaemia (CFA) and total antibody (Ab) for each of the five villages: (a) Puapua, (b) Tafua, (c) Siufaga, (d) Falefa and (e) Fasitoo-Tai. CFA and antibody positivity increased significantly with age (P<0.05). Although it was observed that Mf prevalence also increased with age, this did not reach statistical significance (P>0.05). In Puapua, antibodies were only measured in children.
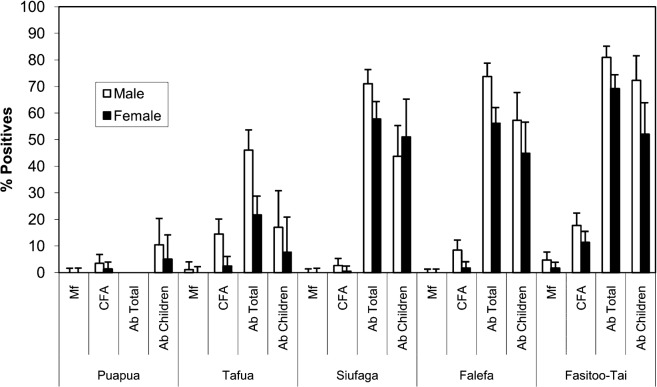
Prevalences of Mf, CFA and antibodies were higher among males for each village (Fig. 3). The Mf prevalences among males in Tafua and Fasitoo-Tai (1.1% and 4.7%, respectively) were higher than females (0% and 1.7%, respectively) albeit not significant (P>0.05). Despite a higher Mf load observed for males, there was no significant difference between males (436 Mf/ml blood) and females (100 Mf/ml) (P>0.05). CFA was significantly higher in males than females for Tafua (14.1% versus 2.4%; P<0.05), Falefa (8.4% versus 1.8%; P<0.05) and Fasitoo-Tai (17.7% versus 11.3%; P<0.05). Although higher in males for Puapua and Siufaga, the difference did not reach statistical significance (P>0.05). Total antibodies were significantly higher in the male population for the four villages studied (P<0.05). Male children had a higher antibody prevalence, but this was only significant for Fasitoo-Tai (P<0.05).
Fig. 3.
Gender-specific prevalence of microfilaraemia (Mf), antigenaemia (CFA) and antibodies (Ab) for the total population and children ⩽10 years. For each parameter for every village, the prevalence was greater in males.
DISCUSSION AND CONCLUSIONS
Elimination of LF in the South Pacific poses many challenges including geographical remoteness, funding, baseline prevalence of LF, vectors present, threat of resurgence and MDA compliance (Chanteau et al., 1995; Esterre et al., 2001; Ichimori and Crump, 2005; Kyelem et al., 2008; Joseph et al., 2010). Samoa has a long history of LF control. Mf prevalence in Samoa was reduced to 0.14% in the 1970s, but resurged to 2.1% 2 years following cessation of MDAs (Ichimori, 2001). Following the 2007 C survey, a countrywide prevalence of 2.6% was recorded with certain health districts recording a Mf prevalence >0.1% (Joseph et al., 2011a). These results suggested the need for further MDAs and to identify pockets of residual endemnicity within the health districts. Current surveying and diagnostic methods are ill-equipped to identify such areas and there is the growing need for innovative sampling, monitoring and surveillance strategies (Ramaiah et al., 2009). Recently it was demonstrated, using the Filariasis CELISA, that exposed antibody-positive individuals could be spatially related to infected individuals (Joseph et al., 2011b). The comprehensive epidemiological study described in the current research allows identification of some of these residual areas in Samoa and the potential for incorporation of the new diagnostic tool, Filariasis CELISA, into the repertoire of LF diagnostic assays during low prevalence settings.
The five villages chosen for this research (Fig. 1) differed in their infection prevalence levels (Table 3), but overall antibody rates were higher than CFA rates, which in turn were higher than Mf rates. Antibody prevalence levels in relation to the other LF markers were comparable to previous studies using the research-based Bm14 assay prototype (Weil et al., 1999, 2008; Njenga et al., 2007; Tisch et al., 2008; Mladonicky et al., 2009). The levels of antigen and antibody prevalence in children born post-MDA will be used as a proxy for determining ongoing transmission in this study. That is, prevalence levels above the CFA threshold of 1% (WHO, 2007) will be defined in this research as indicative of ongoing transmission.
The data indicated that ongoing transmission was occurring in all five villages as total CFA prevalence was greater than the defined threshold of 1% and there were detectable exposed antibody positive children (Table 3) (WHO, 2007). The presence of antibody responses in children where CFA prevalence is >1% shows that there is a strong relationship between CFA prevalence of 1% or greater and ongoing transmission. This was of a concern since Siufaga was chosen to represent a village where transmission was believed to be interrupted. This observation is not surprising for Samoa, where resurgence has historically been recorded (Kimura et al., 1985; Ichimori, 2001). Similarly, in French Polynesia where the same mosquito vector is present, resurgence has been well documented (Esterre et al., 2001). Therefore, at least in Ae. polynesiensis endemic areas, a CFA threshold of 1% would support ongoing transmission in these areas.
The linear relationship observed between infection (CFA positivity) and exposure (antibody positivity) suggests that levels of exposure could correlate with the intensity of transmission, concurring with previous studies (Tisch et al., 2008). The highest transmission was observed for Fasitoo-Tai, whereby significantly higher prevalence was observed for all three parameters measured, including CFA and antibody positivity in children (Table 3). The villages with the lowest levels of transmission, Puapua and Siufaga, recorded a significantly lower CFA prevalence and, for Puapua, antibody prevalence in children (Table 3).
The relationship between antigen and antibody prevalence did not conform to expectations in Siufaga or Tafua. In Siufaga, a relatively low CFA prevalence (1.6%) was coupled with a relatively high (46.6%) antibody response in children, whereas in Tafua, higher levels of Mf (0.6%) and CFA (8.4%) were associated with a lower (12.8%) antibody prevalence in children. Follow-up epidemiological studies are required to analyse the factors responsible for these differences.
The potential reservoir of ongoing transmission could be the older age group, in particular males, since CFA prevalence and antibody prevalence significantly increased with age (Fig. 2) and there was a higher measure of infection in males in all parameters (Fig. 3). Increasing CFA and/or antibody prevalence with age has been noted previously, both in high prevalence and low prevalence settings (Tisch et al., 2001; Beuria et al., 2003; Njenga et al., 2007; Mladonicky et al., 2009). In Samoa, male predisposition to infection has been documented in the past, whereby males had a three- to five-fold higher prevalence of Mf than females (Mahoney and Kessel, 1971; Ichimori et al., 2007b). Previous studies have speculated that the likelihood of older men working on plantations may increase their chances of exposure and, thus, infection (Mahoney and Kessel, 1971). Therefore, future MDA campaigns should especially target the older age group and/or male population as a potential reservoir of infection.
The data also impact upon future surveillance in the Pacific. Preliminary studies in the Pacific suggested that measuring CFA prevalence in children alone, in accordance with the ‘draft LF active surveillance strategy for the Pacific Islands and Communities (PICT)’ (WHO, 2007), would be inadequate to detect all areas of residual endemnicity (Joseph et al., 2011a). The data from this study concur with the preliminary study, since if the proposed LF active surveillance strategy for the PICT was implemented in the current research, Siufaga would have been declared LF-free. This was not the case since the CFA prevalence in individuals >10 years exceeded 1%, which is defined as ongoing transmission (WHO, 2007), and there was a high prevalence of antibody positive children consistent with ongoing exposure (Tisch et al., 2008). This highlights the possibility for complementing the current strategy with antibody testing now that a standardized assay is available.
Despite the commercial availability of the Filariasis CELISA and reproducibility across different laboratories using serum samples (Weil et al., 2010), there have been recent unpublished reports of a high number of false positive results and lack of reproducibility when using eluted blood from filter papers. These problems became evident partway through the aforementioned unpublished study. These unpublished reports concluded that the Filariasis CELISA kit in its current format would be unreliable as a programmatic tool for defining interruption of LF transmission. It must be emphasized that such data were collected following the manufacturer’s decision to alter the development process of the kits. Changes in the manufacturing process have appeared to adversely affect results for eluted blood from filter paper, but not serum samples. These alterations to the manufacturing process occurred after the current study in Samoa and previous successful studies (Joseph et al., 2010, 2011a, b) and, therefore, had no adverse effect on data collection. However, for future studies, these manufacturing and quality control issues that have recently been raised require immediate attention in order for this particular antibody assay to have any use as a standardized diagnostic tool in the LF elimination programme. The authors are confident that once these problems are solved, the Filariasis CELISA could be a promising diagnostic assay for defining cessation of transmission and future surveillance as evidenced by the promising results from the current study.
The epidemiological assessment identified residual foci in Samoa and highlighted the need for strengthened control efforts in these areas. Importantly, the previously declared LF-free village of Siufaga would not have been identified as endemic if using CFA testing alone in children. Future studies need to validate the Filariasis CELISA in other epidemiological settings and to include mathematical modelling to determine the best sampling strategy and thresholds if it were incorporated into the LF program (Lammie et al., 2004; Michael et al., 2006; Tisch et al., 2008).
Acknowledgments
We would like to thank the staff of the Samoan Ministry of Health for approving this research and the World Health Organization, Samoa, for their participation in the field work. We would also like to thank Dr Petra Buttner for her statistical advice. We would like to thank Phil Bright, from the Secretariat of the Pacific Community, for providing the ArcGIS map of Samoa. Lastly, we would like to thank GlaxoSmithKline for their generous ongoing financial support of the LF support centre at James Cook University.
REFERENCES
- Beuria MK, Bal MS, Mandal NN, Das MK.(2003)Age-dependent prevalence of asymptomatic amicrofilaraemic individuals in a Wuchereria bancrofti-endemic region of India. Transactions of the Royal Society of Tropical Medicine and Hygiene 97297–298. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Taleo G, Toeaso V, Ichimori K.(2002)Progress towards, and challenges for, the elimination of filariasis from Pacific-island communities. Annals of Tropical Medicine and Parasitology 96S61–S69. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention(1993)Recommendations of the International Task Force for Disease Eradication. Morbidity and Mortality Weekly Reports Recommendations and Reports 421–38. [PubMed] [Google Scholar]
- Chandrashekar R, Curtis KC, Ramzy RM, Liftis F, Li BW, Weil GJ.(1994)Molecular cloning of Brugia malayi antigens for diagnosis of lymphatic filariasis. Molecular and Biochemical Parasitology 64261–271. [DOI] [PubMed] [Google Scholar]
- Chanteau S, Glaziou P, Plichart C, Luquiaud P, Moulia-Pelat JP, N’Guyen L, Cartel JL.(1995)Wuchereria bancrofti filariasis in French Polynesia: age-specific patterns of microfilaremia, circulating antigen, and specific IgG and IgG4 responses according to transmission level. International Journal for Parasitology 2581–85. [DOI] [PubMed] [Google Scholar]
- Chanteau S, Roux JF.(2008)[Bancroftian lymphatic filariasis: toward its elimination from the Pacific?]. Bulletin de la Societe de Pathologie Exotique 101254–260. [PubMed] [Google Scholar]
- Clopper C, Pearson E.(2005)JavaStat: Exact Binomial and Poisson Confidence Intervals [document on the Internet]. Available at: http://statpages.org/confint.html [accessed 25 May 2009]
- Durrheim DN, Nelesone T, Speare R, Melrose W.(2003)Certifying lymphatic filariasis elimination in the Pacific — the need for new tools. Pacific Health Dialog 10149–154. [PubMed] [Google Scholar]
- Dylewski JS, Rasmussen L, Mills J, Merigan TC.(1984)Large-scale serological screening for cytomegalovirus antibodies in homosexual males by enzyme-linked immunosorbent assay. Journal of Clinical Microbiology 19200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterre P, Plichart C, Sechan Y, Nguyen NL.(2001)The impact of 34 years of massive DEC chemotherapy on Wuchereria bancrofti infection and transmission: the Maupiti cohort. Tropical Medicine and International Health 6190–195. [DOI] [PubMed] [Google Scholar]
- Grady CA, de Rochars MB, Direny AN, Orelus JN, Wendt J, Radday J, Mathieu E, Roberts JM, Streit TG, Addiss DG, Lammie PJ.(2007)Endpoints for lymphatic filariasis programs. Emerging Infectious Diseases 13608–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoti SL, Elango A, Radjame K, Yuvaraj J, Pani SP.(2002)Detection of day blood filarial antigens by Og4C3 ELISA test using filter paper samples. National Medical Journal of India 15263–266. [PubMed] [Google Scholar]
- Huppatz C, Capuano C, Palmer K, Kelly PM, Durrheim DN.(2009)Lessons from the Pacific Programme to Eliminate Lymphatic Filariasis: a case study of 5 countries. BMC Infectious Diseases 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimori K.(2001)Entomology of the filariasis control programme in Samoa, Aedes polynesiensis and Ae. samoanus. Medical Entomology and Zoology 5211–21. [Google Scholar]
- Ichimori K, Crump A.(2005)Pacific collaboration to eliminate lymphatic filariasis. Trends in Parasitology 21441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimori K, Graves PM, Crump A.(2007a)Lymphatic filariasis elimination in the Pacific: PacELF replicating Japanese success. Trends in Parasitology 2336–40. [DOI] [PubMed] [Google Scholar]
- Ichimori K, Tupuimalagi-Toelupe P, Toeaso Iosia V, Graves PM.(2007b)Wuchereria bancrofti filariasis control in Samoa before PacELF (Pacific Programme to Eliminate Lymphatic Filariasis). Tropical Medicine and Health 35261–269. [Google Scholar]
- Joseph H, Clough A, Peteru A, Crawley S, Pulu T, Maiava F, Melrose WD.(2010)Exploratory study investigating factors influencing mass drug administration (MDA) compliance for lymphatic filariasis in Samoa. Samoa Medical Journal 212–25. [Google Scholar]
- Joseph H, Maiava F, Naseri T, Taleo F, ‘Ake M, Capuano C, Melrose WD.(2011a)Application of the Filariasis CELISA anti-filarial IgG4 antibody assay in surveillance in lymphatic filariasis elimination programmes in the South Pacific. Journal of Tropical Medicine 2011492023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph H, Moloney J, Maiava F, McClintock S, Lammie P, Melrose W.(2011b)First evidence of spatial clustering of lymphatic filariasis in an Aedes polynesiensis endemic area. Acta Tropica 120S39–S47. [DOI] [PubMed] [Google Scholar]
- Joseph HM, Melrose WD.(2010)Applicability of the filter paper technique for detection of antifilarial IgG4 antibodies using the Bm14 Filariasis CELISA. Journal of Parasitology Research 2010594687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Penaia L, Spears GF.(1985)Epidemiology of subperiodic Bancroftian filariasis in Samoa 8 years after control by mass treatment with diethylcarbamazine. Bulletin of the World Health Organization 63869–880. [PMC free article] [PubMed] [Google Scholar]
- Kyelem D, Biswas G, Bockarie MJ, Bradley MH, El-Setouhy M, Fischer PU, Henderson RH, Kazura JW, Lammie PJ, Njenga SM, Ottesen EA, Ramaiah KD, Richards FO, Weil GJ, Williams SA.(2008)Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. American Journal of Tropical Medicine and Hygiene 79480–484. [PMC free article] [PubMed] [Google Scholar]
- Lammie PJ, Reiss MD, Dimock KA, Streit TG, Roberts JM, Eberhard ML.(1998)Longitudinal analysis of the development of filarial infection and antifilarial immunity in a cohort of Haitian children. American Journal of Tropical Medicine and Hygiene 59217–221. [DOI] [PubMed] [Google Scholar]
- Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E.(2004)Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis — a multicenter trial. Filaria Journal 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney LE, Kessel JF.(1971)Treatment failure in filariasis mass treatment programmes. Bulletin of the World Health Organization 4535–42. [PMC free article] [PubMed] [Google Scholar]
- Melrose WD, Durrheim DD, Burgess GW.(2004)Update on immunological tests for lymphatic filariasis. Trends in Parasitology 20255–257. [DOI] [PubMed] [Google Scholar]
- Michael E, Malecela-Lazaro MN, Maegga BT, Fischer P, Kazura JW.(2006)Mathematical models and lymphatic filariasis control: monitoring and evaluating interventions. Trends in Parasitology 22529–535. [DOI] [PubMed] [Google Scholar]
- Mladonicky JM, King JD, Liang JL, Chambers E, Pa’au M, Schmaedick MA, Burkot TR, Bradley M, Lammie PJ.(2009)Assessing transmission of lymphatic filariasis using parasitologic, serologic, and entomologic tools after mass drug administration in American Samoa. American Journal of Tropical Medicine and Hygiene 80769–773. [PubMed] [Google Scholar]
- Njenga SM, Wamae CN, Mwandawiro CS, Molyneux DH.(2007)Immuno-parasitological assessment of Bancroftian filariasis in a highly endemic area along the River Sabaki, in Malindi district, Kenya. Annals of Tropical Medicine and Parasitology 101161–172. [DOI] [PubMed] [Google Scholar]
- Ottesen EA.(2006)Lymphatic filariasis: treatment, control and elimination. Advances in Parasitology 61395–441. [DOI] [PubMed] [Google Scholar]
- PacELF(2006)The PacELF Way: Towards Elimination of Lymphatic Filariasis 1999–2005 Geneva: World Health Organization [Google Scholar]
- Ramaiah KD, Thiruvengadam B, Vanamail P, Subramanian S, Gunasekaran S, Nilamani N, Das PK.(2009)Prolonged persistence of residual Wuchereria bancrofti infection after cessation of diethylcarbamazine-fortified salt programme. Tropical Medicine and International Health 14870–876. [DOI] [PubMed] [Google Scholar]
- Ramalingam S.(1968)The epidemiology of filarial transmission in Samoa and Tonga. Annals of Tropical Medicine and Parasitology 62305–324. [DOI] [PubMed] [Google Scholar]
- Ramzy RM.(2002)Recent advances in molecular diagnostic techniques for human lymphatic filariasis and their use in epidemiological research. Transactions of the Royal Society of Tropical Medicine and Hygiene 96S225–S259. [DOI] [PubMed] [Google Scholar]
- Ramzy RM, Helmy H, Faris R, Gad AM, Chandrashekar R, Weil GJ.(1995)Evaluation of a recombinant antigen-based antibody assay for diagnosis of Bancroftian filariasis in Egypt. Annals of Tropical Medicine and Parasitology 89443–446. [DOI] [PubMed] [Google Scholar]
- Rawlins SC, Siung-Chang A, Baboolal S, Chadee DD.(2004)Evidence for the interruption of transmission of lymphatic filariasis among schoolchildren in Trinidad and Tobago. Transactions of the Royal Society of Tropical Medicine and Hygiene 98473–477. [DOI] [PubMed] [Google Scholar]
- Sasa M.(1976)Human Filariasis: A Global Survey of Epidemiology and Control Baltimore, MD: University Park Press [Google Scholar]
- Supali T, Rahmah N, Djuardi Y, Sartono E, Ruckert P, Fischer P.(2004)Detection of filaria-specific IgG4 antibodies using Brugia Rapid test in individuals from an area highly endemic for Brugia timori. Acta Tropica 90255–261. [DOI] [PubMed] [Google Scholar]
- Tisch DJ, Bockarie MJ, Dimber Z, Kiniboro B, Tarongka N, Hazlett FE, Kastens W, Alpers MP, Kazura JW.(2008)Mass drug administration trial to eliminate lymphatic filariasis in Papua New Guinea: changes in microfilaremia, filarial antigen, and Bm14 antibody after cessation. American Journal of Tropical Medicine and Hygiene 78289–293. [PMC free article] [PubMed] [Google Scholar]
- Tisch DJ, Hazlett FE, Kastens W, Alpers MP, Bockarie MJ, Kazura JW.(2001)Ecologic and biologic determinants of filarial antigenemia in Bancroftian filariasis in Papua New Guinea. Journal of Infectious Diseases 184898–904. [DOI] [PubMed] [Google Scholar]
- Weil GJ, Curtis KC, Fischer PU, Won KY, Lammie PJ, Joseph H, Melrose WD, Brattig NW.(2010)A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia malayi antigen Bm-14. Acta Tropica 120S19–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil GJ, Kastens W, Susapu M, Laney SJ, Williams SA, King CL, Kazura JW, Bockarie MJ.(2008)The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on Bancroftian filariasis in Papua New Guinea. PLoS Neglected Tropical Diseases 2e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil GJ, Ramzy RM.(2007)Diagnostic tools for filariasis elimination programs. Trends in Parasitology 2378–82. [DOI] [PubMed] [Google Scholar]
- Weil GJ, Ramzy RM, El Setouhy M, Kandil AM, Ahmed ES, Faris R.(1999)A longitudinal study of Bancroftian filariasis in the Nile Delta of Egypt: baseline data and one-year follow-up. American Journal of Tropical Medicine and Hygiene 6153–58. [DOI] [PubMed] [Google Scholar]
- WHO(2005)Monitoring and epidemiological assessment of the programme to eliminate lymphatic filariasis at implementation unit level. Report no. WHO/CDS/CPE/CEE 2005.50. Available at: http://www.searo.who.int/LinkFiles/New_Lymphatic_Filariasis_OMS_LF_ME_Assessment.pdf (accessed October 2010)
- WHO(2007)Report of the ninth workshop for Pacific lymphatic filariasis programme managers. RS/2007/GE/20(FIJ). June 2007. Fiji. Available at: http://www.wpro.who.int/NR/rdonlyres/68DCB443-20CD-4E7E-96F5-0FB73092123C/0/MR_NinthWorkshopPacificLympFilariasisProgramme.pdf (accessed October 2010)