Abstract
Neuronal circuitries in the mammalian visual system change as a function of experience. Sensory experience modifies neuronal networks connectivity via the activation of different physiological processes such as excitatory/inhibitory synaptic transmission, neurotrophins, and signaling of extracellular matrix molecules. Long-lasting phenomena of plasticity occur when intracellular signal transduction pathways promote epigenetic alterations of chromatin structure that regulate the induction of transcription factors that in turn drive the expression of downstream targets, the products of which then work via the activation of structural and functional mechanisms that modify synaptic connectivity. Here, we review recent findings in the field of visual cortical plasticity while focusing on how physiological mechanisms associated with experience promote structural changes that determine functional modifications of neural circuitries in V1. We revise the role of microRNAs as molecular transducers of environmental stimuli and the role of immediate early genes that control gene expression programs underlying plasticity in the developing visual cortex.
Keywords: visual cortex, plasticity, extracellular matrix, OTX2, GABAergic interneurons, epigenetics, microRNAs, chromatin remodeling, Npas4
Introduction
The neuronal representation of environmental stimuli in sensory areas as well as the selection and association of sensory input for further neuronal processing seem to be associated with the spatiotemporal dynamic synchrony of neuronal firing within and between interconnected neuronal networks in the brain.1,2 The structure and organization of sensory systems ensures the existence of both (i) feedforward connections that lie behind neurons with feature-selective receptive fields and (ii) reciprocal connections between these neurons that serve to dynamically associate them into complexes.3 This feature enables cortical regions to detect consistent associations among incoming electrical signals associated with experience and to represent such relations by grouping neuronal responses in a temporal-dependent4,5 and context-dependent6,7 manner.
The functional nature of computational operations in sensory areas of the brain relies, at least partially, on experience-dependent processes of neuronal plasticity. A general feature in the nervous system is that the remodeling of neural networks by early experience8 is actively preserved by the late appearance of structural and functional factors that restrict plasticity over the time-course.9–12 This characteristic seems to be critical in terms of adaptive functions (e.g., sensory perception, memory and learning, language acquisition, motor skills, reasoning), but determines a major decrease of plasticity in the adult brain.
Sensory experience during early life drives the consolidation of synaptic circuitries in the primary visual cortex (V1).13 The rules that control plasticity of visual representations in V1 are reasonably understood;14,15 however, structural and functional mechanisms underlying these plastic phenomena still need further research. A large number of physiological processes have been identified in parallel to the decline of plasticity that occurs with age. Modifications have been described for long-distance projection systems,16–18 for BDNF expression,19,20 for a shift in the composition and gating dynamics of glutamate NMDA receptors,21,22 for myelin associated proteins23–25 and surface recognition molecules,26 for the activity of the CRE-CREB system,27 and for the regulation of histones post-translational changes.28 More recently, attention has been focused on physiological mechanisms associated with experience that promote structural changes and determine functional modifications in V1.29–31
Previous studies have comprehensively reviewed development and plasticity of neuronal circuitries in the visual cortex.32,33 In this review, we discuss recent experimental findings in the field of plasticity and provide a concise and updated view that aims to encompass different physiological processes at the basis of plastic phenomena in V1. We focus on how intracellular signal transduction pathways associated with experience drive changes of chromatin structure that regulate gene programs underlying visual cortical plasticity.
Intracortical Inhibitory/Excitatory Balance and Visual Cortex Plasticity in Early Life
Inhibitory GABAergic networks in the brain consist of a wide variety of different interneuron cell types that show diverse physiological properties and display different innervation targets in subcellular compartments of excitatory cells.34 GABA-mediated inhibitory transmission is critical in shaping patterns of electrical activity associated with experience.35 Inhibitory circuitries that include large-basket cells expressing the calcium binding protein parvalbumin (Pv) are one kind of interneuron anatomically suited to compute this task as they send axons across large areas in the cortex while enwrapping cell bodies of pyramidal excitatory neurons and establishing inhibitory synaptic contacts enriched on GABAA-receptors containing the α1 subunit.36 Notably, studies performed in rodents revealed that development of GABAergic inhibition and the presence of GABAA-α1 receptors on perisomatic inhibitory synapses in pyramidal cells are critical for defining the critical period (CP) during early stages of development in which neuronal networks in V1 are highly sensitive to experience.14,37–39
Plasticity in the visual system is normally assessed in terms of the induction of sensory deprivation effects. The ocular dominance (OD) distribution in V1, for instance, markedly changes in favor of the open (not deprived) eye after unilateral eyelid suture (monocular deprivation, MD) during the CP but not later.40–44 The study of plasticity using this experimental design revealed that the developmental maturation of GABAergic inhibitory circuitries controls the time-course of the CP for OD plasticity. Sensory experience sets in motion a two-threshold mechanism for the time-course of the CP during development. An initial threshold of inhibition37,39 drives the CP in which neuronal circuitries in in the visual cortex are highly sensitive to experience, whereas a second inhibitory threshold19 signals the end of this phase of enhanced plasticity.14 An important issue that has been subject of recent attention is the role of parvalbumin-positive (Pv+) GABAergic neurons in the regulation of CP plasticity.
CP Plasticity and Experience-dependent Transfer of OTX2 in the Visual Pathway
The time-course of the CP for V1 plasticity critically depends on sensory experience. This notion derives from classical experiments combining sensory deprivation and electrophysiological analysis. While rearing animals in total darkness from birth delays the functional maturation of striate cortex and prolongs neuronal plasticity beyond its normal limits,43,45,46 raising animals in an environment enriched in terms of sensory-motor activity and social stimulation accelerates visual system development and promotes a precocious closure of the CP.47
How do Pv+ GABAergic neurons come into play in the regulation of the CP? There is evidence that visual experience drives the transfer of the retina-derived homeoprotein OTX2 along the visual pathway, which in turn promotes the maturation of the Pv+ subclass of cortical GABAergic interneurons critically involved in the regulation of the CP for OD plasticity.29,48,49 In rodents, OTX2 mRNA appears to not be synthesized in the visual cortex but in subcortical structures of the visual pathway: retina, lateral geniculate nucleus and superior colliculus. Yet, OTX2 protein accumulates in Pv+ GABAergic cells in the cortex, indicating that a possible source of cortical OTX2 protein may be the retina. OTX2 possesses both secretion and internalization sequences in the homeodomain, allowing the potential transfer of the protein from cell to cell,50 which appears to be relayed by retinogeniculocortical projections that form onto Pv+ interneurons over development.29
In summary, the geniculocortical transfer of OTX2 is regulated by experience, and OTX2 seems to direct Pv+ cells maturation in V1. While dark rearing decreases OTX2 as well as Pv protein levels in the cortex and delays the maturation of GABAergic inhibition, OTX2 delivery rescues Pv+ cells maturation in complete darkness.29 Notably, infusion of recombinant OTX2 in the visual cortex of young animals, before the start of the CP, causes a premature occurrence of plasticity in response to MD. Moreover, intraocular injection of biotinylated OTX2 leads to an increase of OTX2 labeled protein into Pv+ cells in V1, whereas interfering with OTX2 synthesis in the retina, by means of RNA interference, effectively prevents CP plasticity.29 These findings indicate that OTX2 regulates the time-course of the CP, most likely by controlling the maturation of GABAergic inhibition.51 In line with this, delivery of OTX2 in the visual cortex of dark- and normally-reared mice increases the number of Pv+ inhibitory interneurons.29 Moreover, OTX2 infusion in V1 of pre-CP mice reduces the excessive spike firing of single units that is normally observed in early life, indicating that OTX2 induces the maturation of inhibition.
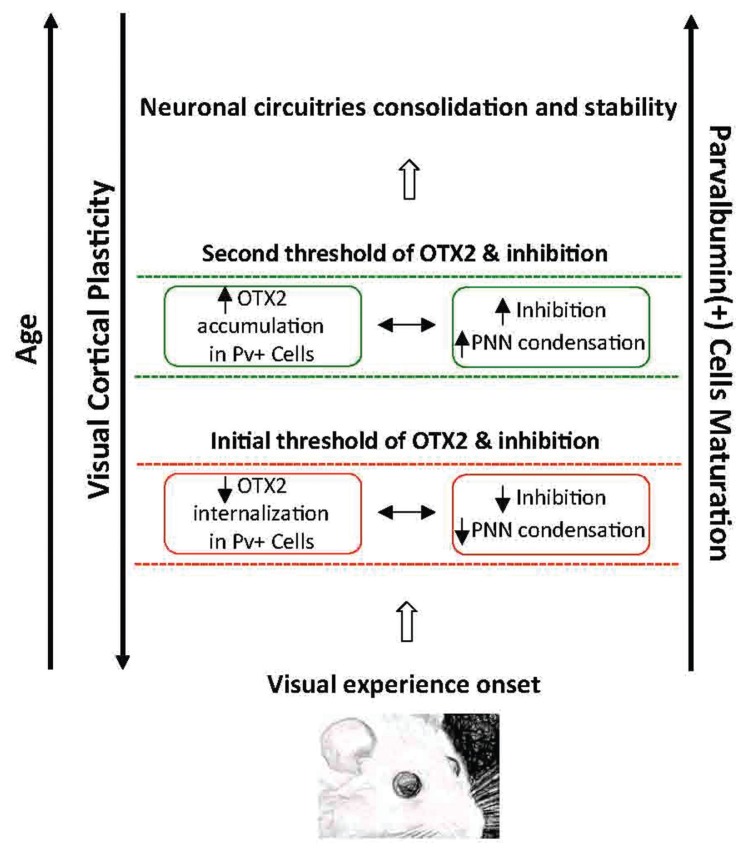
A two-threshold model for the OTX2 regulation of OD plasticity that is perfectly in line with the role of inhibition has been recently postulated.29–31 Early sensory experience is believed to drive OTX2 accumulation in cortical Pv+ GABAergic cells, thus favoring their functional maturation and CP onset (Fig. 1). This is likely to promote the condensation of extracellular matrix components around Pv+ interneurons leading to higher levels of OTX2 incorporation that, in turn, result in further maturation of intracortical inhibition and associated structural stabilization of neural circuitries that cause the end of CP plasticity in V1.
Figure 1.
A two-threshold model for OTX2 in the regulation of visual cortical plasticity during early life. Visual experience drives the initial incorporation of the OTX2 protein into Pv+ GABAergic cells. This results in the initial functional maturation of inhibition and the CP onset. As development proceeds, PNNs condense around inhibitory interneurons, leading to higher levels of OTX2 accumulation that in turn promote inhibition and eventually reduce plasticity while causing neuronal circuitries consolidation and stability in V1. OTX2 is synthesized, secreted and maintained in Pv+ GABAergic cells in the mature brain.30
Role of PNNs Organization in OTX2 Internalization by GABAergic Cells During the CP
Extracellular matrix components such as perineuronal nets (PNNs) in V1, which consist of hyaluronic acids, chondroitin sulfate proteoglycans (CSPGs) and cell adhesion molecules, aggregate and enwrap mainly around the soma and dendritic processes of Pv+ interneurons.52,53 PNNs appear late in development in concomitance with the closure of the CP for plasticity, this phenomenon being associated to the stabilization of neuronal connectivity patterns and inhibition of structural and functional plasticity of dendritic spines.54 Targeting PNNs with Chondroitinase-ABC enhances OD plasticity55 and promotes the rescue of visual functions in adult amblyopic rats.56
There is evidence that OTX2 modifies PNNs structures in V1. On the one hand, OTX2 accumulation in Pv+ GABAergic interneurons serves as a positive feedback loop in promoting PNNs maturation, which eventually favors OTX2 uptake and therefore the strengthening of somatic inhibition during the CP in the visual system. The reduction of OTX2 in the visual cortex of young OTX2flx/+ mice by CRE recombination actually delays PNNs formation while reducing the number of Pv+ interneurons.29 On the other hand, OTX2 delivery into the visual cortex of CP animals not only increases the incorporation of OTX2 in Pv+ GABA cells, but also enhances the number of PNNs enwrapping Pv+ GABAergic neurons while promoting the expression of different GABAergic markers.29 As PNNs seem to facilitate OTX2 internalization by Pv+ cells, OTX2 promotes the maturation of intracortical inhibition by its gradual appearance on Pv+ interneurons in response to experience after the onset of vision. This is a compelling example of how physiological mechanisms associated to experience promote structural changes that determine functional modifications of neural circuitries.
The role of OTX2 in mediating the recovery from the effects caused by long-term sensory deprivation in rodents has also been recently explored. A common finding in all species tested so far is that depriving animals of visual experience during the CP irreversibly alters the quality of sight in adulthood. Long-term MD leads to marked impairments of normal visual functions; binocularity, spatial acuity and contrast sensitivity are severely impaired in amblyopic animals.40–44 It has been reported that exogenous administration of a peptide that disrupts OTX2 availability in GABAergic interneurons and hampers inhibition, promotes the rescue of spatial acuity in adult amblyopic mice.31 Amblyopia recovery has also been claimed in adult OTX2flx/flx animals after deletion of the OTX2 protein in the choroid plexus by CRE recombination.30 Surprisingly, though, these electrophysiological studies found only a partial rescue of acuity in adult life (nearly 0.4 cycles per degree).30,31 Because visual acuity in normally reared, mature mice is around 0.6 cycles per degree of visual angle, this notion being consistently confirmed both electrophysiologically and behaviorally,19,57–59 the behavioral analysis of acuity in adult amblyopic animals after OTX2 deletion is likely to shed light on this important subject.
Sulfactation Patterns of CSPGs in PNNs and the Regulation of CP Plasticity
A novel role for specific sulfation patterns of chondroitin sulfates as permissive factors for visual cortical plasticity has been subject of attention in recent years.60 Chondroitin sulfate chains are long linear polysaccharides that consist of a repeating disaccharide subunit composed of glucoronic acid as well as N-acetylgalactosamine.61 While there is evidence that the functional information of CSPGs may be encoded by the specific sulfation sequence (sugar code) of chondroitin sulfate chains,62–64 OTX2 appears to recognize sugar sequences in PNNs presumably enriched in chondroitin-6-sulfate moieties.31 Notably, OTX2 possess an “arginine-lysine” binding motif in its primary sequence that recognizes PNNs sugars, preferentially glycosaminoglycans (GAGs) around Pv+ interneurons in layer IV.31 This observation sheds light on how OTX2 could be specifically internalized in PV+ GABAergic interneurons.
Recent findings suggest that a developmental increase in the 4-sulfaction/6-sulfaction ratio of CSPGs is necessary for the accumulation of OTX2 in the cortex as well as for the maturation of Pv+ interneurons and that it does lead to the end of CP plasticity in the mouse visual cortex.60 Indeed, a low 4-sulfaction/6-sulfaction ratio of CSPGs in transgenic mice that overexpress the C6ST-1 sulfotransferase enzyme prevents both the condensation of CSPGs in PNNs that enwrap Pv+ interneurons and the associated maturation of inhibition in V1. Accordingly, these transgenic animals retain juvenile-like plasticity in adult life: short- and long-term sensory deprivation by monocular occlusion results in a shift of OD in favor of the open eye.60
Epigenetic Regulation of CP Plasticity
Plasticity requires the activity-dependent activation of molecular pathways controlling gene expression. Several studies have shown that signaling pathways involving ERK, PKA, and CaMKII signaling mediate experience-dependent activation of gene expression and are required for OD plasticity in juvenile rodents.65–69 Inhibition of ERK results in a dramatic decrease of the activation of CREB-regulated transcription in the visual cortex and effectively prevents plasticity.65,70 CREB- regulated transcription appears to set in motion mechanisms that modify chromatin and chromatin-bound proteins, thus promoting the transition of heterochromatin to a permissive state for transcription. This mechanism is not specific for CREB and it is thought to occur on many activity-dependent genes. However, additional studies are needed to determine how specificity for selected genes is obtained. The experiments on the visual cortex demonstrate that global levels of epigenetic modifications of chromatin usually associated with active gene transcription (e.g., acetylation of lysine 9 and 14, dimethylation of lysine 4, and phosphorylation of Ser 3 on histone H3) are quickly upregulated by visual experience after dark rearing.28 Moreover, ERK inhibitors prevent experience-dependent induction of these marks. Intriguingly, visual stimulation is much less effective in activating CREB-mediated and epigenetic modifications in adult (>P100) mice.28 Accordingly, when histone acetylation is enhanced in adult animals using inhibitors of histone deacetylases (HDACs), reactivation of CP-like plasticity is observed.28,71 Rats monocularly deprived for three months did recover normal visual acuity after treatment with HDAC inhibitors combined with deprived eye reopening.72 Thus, the upregulation of the mechanisms mediating the action of experience on histone posttranslational modifications restore CP plasticity late in life.
These findings raise considerable interest for epigenetic mechanisms as therapeutic targets to promote functional recovery in the visual cortex and possibly also in other sensory systems. There are, however, many questions that remain to be answered in future experiments. First, there are many enzymes that add and remove histone epigenetic marks; a thorough analysis of the most relevant modifications, and the corresponding enzymes, for plasticity enhancement in the adult visual cortex would lead to elaborate specific treatments to increase plasticity and may shed light into the mechanisms by which epigenetic marks become less sensitive to visual input in the adult visual cortex. Second, a key issue in the field of epigenetics and plasticity is that most of the studies have not been able to identify the cell types in which the epigenetic regulation occurs. Considering the complexity of the visual cortical microcircuit and the specific roles that different cell types could play in plasticity, it would be important to develop tools to study epigenetic modifications in specific cells. Finally, an obvious key question is the identification of the genes regulated by epigenetic marks in OD plasticity. Answering this question will require extensive sequencing and bioinformatic analysis.
MicroRNAs as Molecular Transducers of Environmental Stimuli
In addition to epigenetic factors, another type of regulation of gene expression is mediated by microRNAs at post-translational level. MicroRNAs are short noncoding RNAs that interact with specific mRNA targets to control their stability and translation. MircoRNAs are involved in several models of plasticity and can be synthesized in an activity-dependent manner.73 Interestingly, mircroRNA stability also is regulated by mechanisms of activity that are still unknown but that are diverse for different microRNAs.74 At neuronal level, one well-studied action of microRNAs is the regulation of dendritic spine maturation. Indeed, several microRNAs are enriched in synaptodendritic compartments.73 One of these, miR134, has been shown to control size and density of dendritic spines by targeting Limk1 mRNA.75 Another microRNA that seems to be involved in synaptic plasticity is miR132.76 There is evidence that synaptic activity rapidly induces miR132 expression, which in turn promotes activity-dependent dendritic growth in vitro. These effects on dendrites morphology are mediated by miR132 translational inhibition of its target protein p250GAP, a Rho family GTPase activating protein. It has been proposed that by down-regulating p250GAP, miR132 controls dendritic growth increasing Rac signaling cascade activity.77 This protein is also involved in mediating miR132 induction of activity-dependent spine formation.78 Structural and electrophysiological analyses in hippocampal neurons in culture have shown that overexpression of miR132 promotes the maturation of dendritic spines assessed by increased presence of mushroom and stubby spines and increased miniature EPSC amplitude and frequency.79 In vivo studies of structural plasticity in newborn hippocampal neurons in adult animals have also demonstrated that conditional knock down of miR212/132 locus causes a significant decrease in total dendritic length, arborization and spine density.80
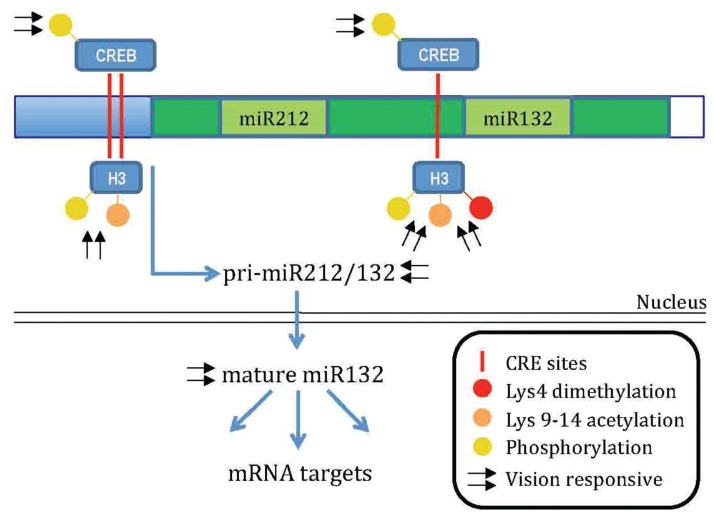
Interestingly, miR132 undergoes visual stimulation induced upregulation together with histone phoshoacetylation and dimethylation (Lys 4) on the CREB-bound sequences present in its promoter (Fig. 2). Visual induction of miR132 is reduced in adult life but it does increase after treatment with the HDAC inhibitor trichostatin.81 These results suggest that dynamic regulation of miR132 levels is mediated by epigenetic factors. Additionally, MD during the CP induces a decrease of miR132 levels in the cortex that is necessary for the plasticity process completion.81 If an exogenous miR132 mimic is administered to the visual cortex during the period of MD to counteract the reduction normally observed in monocularly deprived mice, OD plasticity is fully blocked. Interestingly, a certain level of miR132 must be preserved to have plasticity. Indeed, the OD shift induced by MD is prevented also in visual cortical neurons that were infected with a lentivirus expressing a miR132-sequestering sponge.82 Notably, microchip miRNA analysis followed by quantitative PCR confirmation revealed that 19 miRNAs are regulated by dark rearing or MD, suggesting that microRNAs could represent another layer of activity-dependent regulation of plasticity.82 As for the research on epigenetic marks, identification of the targets of miR132 relevant for OD plasticity is a challenge for future studies.
Figure 2.
Model for the vision responsive epigenetic regulation of miR132. Mir132 is produced by a bicistronic transcript containing also miR212 (primary-miR212/132). Visual experience enhances both the primary miR212/132 and mature miR132 transcript. Basal mature miR212 levels are much lower than miR132 levels due to a different processing and stability.76 Its regulation by visual experience has not been investigated. The miR212/132 gene is reported in dark green. Mature miR212 and miR132 sequence are reported in light green.
Immediate Early Genes in CP Plasticity: Activity-dependent Npas4 Expression
Recent studies suggest that the IEG Arc (activity-regulated cytoskeletal associated protein) is a candidate gene for the occurrence of experience-dependent plasticity in V1.83 The activity-dependent Arc expression has been implicated in different forms of synaptic plasticity (e.g., LTP, LTD).84–88 While transcription and translation of Arc depends on NMDA receptors,89 Arc expression modulates AMPA receptor-mediated synaptic transmission.90 These signal transduction pathways have been implicated in experience-dependent forms of neuronal plasticity.91–93 Accordingly, intrinsic signal optical imaging and electrophysiological analysis revealed that knockout Arc−/− mice show no OD plasticity in response to MD during the CP, indicating that in the absence of this IEG, synapses in V1 become insensitive to the effects of sensory experience.83 Additionally, the IEG Narp (neuronal activity regulated pentraxin), which encodes a secreted synaptic protein that can bind to and induce clustering of glutamate AMPA receptors, appears to be involved in phenomena of V1 plasticity during early life. There is evidence that Narp recruits AMPA receptors at excitatory synapses onto Pv+ interneurons to rebalance excitation/inhibition dynamics of neuronal networks after episodes of increased excitability94 and Narp expression seems to play a key role in enabling V1 plasticity during the CP.95
The experience-dependent transcription factor Npas4 appears to be another gene implicated in the occurrence of plastic phenomena in V1. This neuronal-specific IEG seems to lie behind homeostatic mechanisms of plasticity that keep neuronal firing in response to sensory experience within normal levels.96 In rodents, Npas4 expression appears to mediate that of IEGs such as c-Fos, Zif268 and Arc97 and there is evidence that Npas4 binds to the BDNF promoters I and IV, indicating that Npas4 directly mediates the activity-dependent BDNF expression.98 Interestingly, BDNF regulates the maturation of inhibition and the time-course of the CP for V1 plasticity.19 Most importantly, Npas4 drives a transcriptional program that enhances inhibition by promoting the expression of genes that direct the formation of inhibitory synaptic contacts on pyramidal neurons in V1.96 Because the maturation of intracortical inhibition triggers both the start and the end of the CP for V1 plasticity,14 it seems reasonable to speculate that Npas4 expression is likely involved in the regulation of CP plasticity. In agreement with this notion, PRMT8 (protein arginine-methyl transferase) null mice, in which Npas4 is significantly downregulated, show behavioral deficits of visual acuity.99 Because the functional development of acuity in the visual system depends on the maturation of inhibitory circuitries,43 this points toward an Npas4-mediated impairment of inhibition that could hamper plasticity in V1. In line with key role for Npas4 in mediating V1 plastic phenomena, there is evidence that the experience-dependent expression of this transcription factor regulates plasticity in the adult visual cortex.100,101
How can one reconcile Npas4 findings with previous data on the role of OTX2 in driving the maturation of intracortical inhibition? A dual action of molecular players in different cell types driving inhibitory transmission in concert is likely to be in place. On the one hand, there is evidence that homeoproteins regulate both transcription and translation processes.102–105 Hence, the observation that OTX2 is selectively internalized by Pv+ interneurons suggests that OTX2-mediated transcriptional and translational mechanisms are likely to modify axonal projections of GABAergic cells that make inhibitory synaptic contacts. On the other hand, the experience-dependent transcription factor Npas4 may act, in parallel or in series, at the level of pyramidal excitatory neurons regulating gene programs required for the formation of inhibitory synapses that match GABAergic axonal projections and lead to the functional maturation of visual cortical circuitries.
Conclusion and Future Perspectives
The nervous system translates information from the external world by analyzing electrical signals associated with sensory inputs and drives appropriate adaptive responses to changing environmental conditions. In the visual system, plasticity of neuronal circuitries is maximal during early stages of development but decreases with age. Some of the factors that restrict plasticity are structural, such as experience-dependent modifications in the extracellular matrix (e.g., condensation of PNNs, myelin associated proteins). Some others are functional (e.g., maturation of intracortical inhibition) and lead to the physiological establishment of the inhibitory/excitatory balance within neuronal circuitries. An emerging and exciting view in the field of plasticity is the novel role of epigenetic mechanisms and microRNAs as molecular transducers of environmental stimuli.
The visual system as a model of experience-dependent modifications of neuronal circuitries has been remarkably illuminating in the field of brain plasticity and continues to point the way ahead. Seminal studies in cats and monkeys laid down the physiological basis for our current view of neuronal representations of environmental input in sensory areas. The more recent introduction of the rodent visual cortex in the field provided the opportunity to use invaluable tools, from genetics to biochemistry and behavior, for the study of plasticity in a relatively less expensive model with a shorter life cycle as compared to higher species. This has confirmed the existence of feedforward connections that underlie feature-selective receptive fields, which are now known to share high similarity from mouse to man. A major challenge we have left is how to translate biological manipulations in animal experimentation into feasible and safe clinical interventions in humans. Interestingly, recent studies in animal models71,106,107 have been successfully applied to facilitate the recovery from stroke in humans108 and lead the way to develop potential therapeutic strategies that promote the recovery of sensory functions after long-term sensory deprivation.
Footnotes
COMPETING INTERESTS: The authors declare no potential conflict of interests.
Author Contributions
JFMV and TP equally contributed to this work.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
FUNDING: JFMV is supported by a research fellowship at the Italian Institute of Technology.
REFERENCES
- 1.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24(1):49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 2.Treisman A. Solutions to the binding problem: progress through controversy and convergence. Neuron. 1999;24(1):105–110. 111–125. doi: 10.1016/s0896-6273(00)80826-0. [DOI] [PubMed] [Google Scholar]
- 3.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270(5237):758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 4.Engel AK, Konig P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252(5009):1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- 5.Engel AK, Kreiter AK, Konig P, Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(14):6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel AK, Konig P, Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(20):9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Current Opinion in Neurobiology. 2000;10(1):138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 9.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nature Neuroscience. 2011;14(9):1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. The European Journal of Neuroscience. 2012;35(10):1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensch TK. Critical period regulation. Annual Review of Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 13.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 14.Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews Neuroscience. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 15.Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annual Review of Neuroscience. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 16.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasamatsu T, Pettigrew JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976;194(4261):206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- 18.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. The European Journal of Neuroscience. 1995;7(6):1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 20.Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(12):5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 23.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atwal JK, Pinkston-Gosse J, Syken J, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 25.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 26.Di Cristo G, Chattopadhyaya B, Kuhlman SJ, et al. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nature Neuroscience. 2007;10(12):1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- 27.Pham TA, Impey S, Storm DR, Stryker MP. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron. 1999;22(1):63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 28.Putignano E, Lonetti G, Cancedda L, et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53(5):747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama S, Di Nardo AA, Aizawa S, et al. Experience-dependent transfer of OTX2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134(3):508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 30.Spatazza J, Lee HH, Di Nardo AA, et al. Choroid-plexus-derived OTX2 homeoprotein constrains adult cortical plasticity. Cell Reports. 2013;3(6):1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beurdeley M, Spatazza J, Lee HH, et al. OTX2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(27):9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maya-Vetencourt JF, Origlia N. Visual cortex plasticity: a complex interplay of genetic and environmental influences. Neural Plasticity. 2012;2012:631965. doi: 10.1155/2012/631965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelsom C, Lu W. Development and specification of GABAergic cortical interneurons. Cell & Bioscience. 2013;3(1):19. doi: 10.1186/2045-3701-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(1):331–343. doi: 10.1523/JNEUROSCI.3189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nature Reviews Neuroscience. 2007;8(9):673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- 37.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282(5393):1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303(5664):1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 39.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 40.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. Journal of Neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 41.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. The Journal of Comparative Neurology. 1980;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 42.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260(5115):1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 43.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Research. 1994;34(6):709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 44.Morishita H, Hensch TK. Critical period revisited: impact on vision. Current Opinion in Neurobiology. 2008;18(1):101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. Journal of Neurophysiology. 1980;43(4):1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- 46.Blakemore C, Price DJ. Effects of dark-rearing on the development of area 18 of the cat’s visual cortex. The Journal of Physiology. 1987;384:293–309. doi: 10.1113/jphysiol.1987.sp016455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(20):4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang ZJ, Di Cristo G. Time to change: retina sends a messenger to promote plasticity in visual cortex. Neuron. 2008;59(3):355–358. doi: 10.1016/j.neuron.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Rebsam A, Mason CA. OTX2’s incredible journey. Cell. 2008;134(3):386–387. doi: 10.1016/j.cell.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 50.Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nature Cell Biology. 2004;6(3):189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- 51.Heimel JA, van Versendaal D, Levelt CN. The role of GABAergic inhibition in ocular dominance plasticity. Neural Plasticity. 2011;2011:391763. doi: 10.1155/2011/391763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends in Neurosciences. 1998;21(12):510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 53.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Developmental Neurobiology. 2011;71(11):1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 54.de Vivo L, Landi S, Panniello M, et al. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nature Communications. 2013;4:1484. doi: 10.1038/ncomms2491. [DOI] [PubMed] [Google Scholar]
- 55.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 56.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pizzorusso T, Maffei L. Plasticity in the developing visual system. Current Opinion in Neurology. 1996;9(2):122–125. doi: 10.1097/00019052-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Research. 1999;39(18):3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 59.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Research. 2000;40(16):2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 60.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nature Neuroscience. 2012;15(3):414–422. S411–412. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 61.Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Current Opinion in Structural Biology. 2007;17(5):536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 62.Gama CI, Tully SE, Sotogaku N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nature Chemical Bbiology. 2006;2(9):467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 63.Mikami T, Yasunaga D, Kitagawa H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. The Journal of biological Chemistry. 2009;284(7):4494–4499. doi: 10.1074/jbc.M809227200. [DOI] [PubMed] [Google Scholar]
- 64.Nadanaka S, Ishida M, Ikegami M, Kitagawa H. Chondroitin 4-O- sulfotransferase-1 modulates Wnt-3a signaling through control of E disaccharide expression of chondroitin sulfate. The Journal of Biological Chemistry. 2008;283(40):27333–27343. doi: 10.1074/jbc.M802997200. [DOI] [PubMed] [Google Scholar]
- 65.Di Cristo G, Berardi N, Cancedda L, et al. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292(5525):2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- 66.Ranson A, Cheetham CE, Fox K, Sengpiel F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):1311–1316. doi: 10.1073/pnas.1112204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36(3):483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 68.Rao Y, Fischer QS, Yang Y, McKnight GS, LaRue A, Daw NW. Reduced ocular dominance plasticity and long-term potentiation in the developing visual cortex of protein kinase A RII alpha mutant mice. The European journal of Neuroscience. 2004;20(3):837–842. doi: 10.1111/j.1460-9568.2004.03499.x. [DOI] [PubMed] [Google Scholar]
- 69.Beaver CJ, Ji Q, Fischer QS, Daw NW. Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nature Neuroscience. 2001;4(2):159–163. doi: 10.1038/83985. [DOI] [PubMed] [Google Scholar]
- 70.Cancedda L, Putignano E, Impey S, Maffei L, Ratto GM, Pizzorusso T. Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(18):7012–7020. doi: 10.1523/JNEUROSCI.23-18-07012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maya-Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. The European journal of Neuroscience. 2011;33(1):49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- 72.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. The European Journal of Neuroscience. 2010;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 73.Schratt G. microRNAs at the synapse. Nature Reviews Neuroscience. 2009;10(12):842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 74.Krol J, Busskamp V, Markiewicz I, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141(4):618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 75.Schratt GM, Tuebing F, Nigh EA, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 76.Tognini P, Pizzorusso T. MicroRNA212/132 family: molecular transducer of neuronal function and plasticity. The International Journal of Biochemistry & Cell Biology. 2012;44(1):6–10. doi: 10.1016/j.biocel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 77.Wayman GA, Davare M, Ando H, et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105(26):9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Impey S, Davare M, Lasiek A, et al. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43(1):146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edbauer D, Neilson JR, Foster KA, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magill ST, Cambronne XA, Luikart BW, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tognini P, Putignano E, Coatti A, Pizzorusso T. Experience-dependent expression of miR-132 regulates ocular dominance plasticity. Nature Neuroscience. 2011;14(10):1237–1239. doi: 10.1038/nn.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mellios N, Sugihara H, Castro J, et al. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nature Neuroscience. 2011;14(10):1240–1242. doi: 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF, Sur M. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nature Neuroscience. 2010;13(4):450–457. doi: 10.1038/nn.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plath N, Ohana O, Dammermann B, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 85.Messaoudi E, Kanhema T, Soule J, et al. Sustained Arc/Arg31 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(39):10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guzowski JF, Lyford GL, Stevenson GD, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shepherd JD, Rumbaugh G, Wu J, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52(3):475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30(1):227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 90.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52(3):461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1990;10(3):909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38(6):977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 93.Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience. 2005;8(3):380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- 94.Chang MC, Park JM, Pelkey KA, et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nature Neuroscience. 2010;13(9):1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM. Obligatory Role for the Immediate Early Gene NARP in Critical Period Plasticity. Neuron. 2013;79(2):335–346. doi: 10.1016/j.neuron.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Y, Bloodgood BL, Hauser JL, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455(7217):1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramamoorthi K, Fropf R, Belfort GM, et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334(6063):1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(9):3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee PKM. Poster A264. IBRO Meeting; Florence, Italy. 2011. [Google Scholar]
- 100.Maya-Vetencourt JF, Tiraboschi E, Greco D, et al. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. The Journal of Physiology. 2012;590(Pt 19):4777–4787. doi: 10.1113/jphysiol.2012.234237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maya-Vetencourt JF. Activity-Dependent NPAS4 expression and the regulation of gene programs underlying plasticity in the central nervous system. Neural Plasticity. 2013;683909 doi: 10.1155/2013/683909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dubnau J, Struhl G. RNA recognition and translational regulation by a homeodomain protein. Nature. 1996;379(6567):694–699. doi: 10.1038/379694a0. [DOI] [PubMed] [Google Scholar]
- 103.Nedelec S, Foucher I, Brunet I, Bouillot C, Prochiantz A, Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brunet I, Weinl C, Piper M, et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438(7064):94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Topisirovic I, Borden KL. Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): an unexpected relationship. Histology and Histopathology. 2005;20(4):1275–1284. doi: 10.14670/HH-20.1275. [DOI] [PubMed] [Google Scholar]
- 106.Maya-Vetencourt JF, Sale A, Viegi A, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 107.Karpova NN, Pickenhagen A, Lindholm J, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334(6063):1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurology. 2011;10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]