Abstract
Renal citrate metabolism and urinary citrate excretion in the infant rat.
Background
Although hypercalciuria has the same prevalence in children as adults, children rarely develop renal stones. This may be explained by a greater urinary citrate excretion in infants compared with adults. The present study examines the renal excretion of citrate and renal cortical citrate metabolism in infant and adult rats.
Methods
Adult male and newly weaned infant rats were acclimated to metabolic cages and fed synthetic diets. Urine was collected after two days, and renal cortical citrate metabolism was assayed.
Results
Infant rats had a lower plasma [HCO3−] and higher plasma [K+] and had a fourfold higher urinary citrate:creatinine ratio and a twofold higher concentration of citrate in their urine compared with adult rats. This higher urinary citrate excretion was not due to a difference in renal proximal tubular Na/citrate cotransporter activity, nor renal cortical citrate synthase or ATP citrate lyase activities in infants as compared with adults. However, infant rat kidneys had significantly lower mitochondrial aconitase (m-aconitase) activity. Renal cortical citrate concentrations were comparable in infant and adult rats. Manipulation of plasma [K+] to adult levels did not affect the higher urinary citrate excretion in infant rats.
Conclusions
Urinary citrate excretion in infant rats is greater than in adults but does not parallel tissue [citrate]. Thus, this higher urinary citrate is likely due to maturational differences in the proximal tubule, other than Na/citrate cotransport, that directly affect citrate transport.
Keywords: urolithiasis, potassium, renal development, ATP citrate lyase, aconitase, hypercalciuria
The incidence of renal stones in children with hypercalciuria is approximately 5 to 15%1,2, and is much lower than the 60 to 90% that is observed in adults with hypercalciuria3. This lower incidence of urolithiasis in children with hypercalciuria may be due to higher urinary concentrations of inhibitors that prevent stone formation4. In support of this hypothesis, Hoppe et al reported that from infancy to adolescence, urinary citrate excretion decreases with a concomitant increase in urinary Ca-oxalate saturation5. However, the mechanism for this effect is unexplained.
Urinary citrate plays an important role in inhibiting the formation of calcium-containing kidney stones by chelating calcium, directly preventing the crystallization and precipitation of calcium6 and interacting with Tamm-Horsfall proteins to inhibit Ca-oxalate crystallization7. Low urinary citrate occurs in approximately half of adult patients with renal stones8. Because the filtered load of citrate remains relatively constant, proximal tubular citrate reabsorption regulates urinary citrate excretion9. Chronic regulation of proximal tubular citrate reabsorption has been shown to be associated with changes in the apical membrane Na/citrate cotransporter (abstract; Aruga et al, J Am Soc Nephrol 8:2A, 1997)10,11, cytosolic ATP citrate lyase12, and mitochondrial aconitase (m-aconitase)13.
The purpose of this study was to characterize the metabolism of citrate in the renal proximal tubule of infant rats. The results demonstrate that infant rats excrete urine with a higher total concentration of citrate and a higher citrate:creatinine ratio than adult rats. This higher urinary citrate excretion is not a result of the higher plasma potassium seen in infant rats and is associated with a decrease in renal m-aconitase activity.
METHODS
Experimental design
Male Sprague-Dawley rats (Harlan, 180 to 200 g) were used as adults. Infant Sprague-Dawley rats were received as 18-day-old litters and were weaned two days later. Adult rats and 22-day-old rats were placed in metabolic cages for two days on ad libitum water and a synthetic diet [(in g) 180 casein, 200 cornstarch, 500 sucrose, 35 corn oil, 35 peanut oil, 10 CaHPO4, 6 MgSO4, 6 NaCl, 8.3 K2HPO4, and 10 vitamin fortification mixture; ICN Pharmaceutical, Cleveland, OH, USA]. During the third day, 24-hour urine collections were obtained in the presence of thymol14, and the animals were sacrificed after intraperitoneal anesthesia (100 mg/kg body wt of Inactin; Promonta, Germany). Blood was obtained first by aortic puncture through an abdominal incision in adults and by decapitation in infants. Subsequently, kidneys were removed through an abdominal incision, decapsulated and freeze clamped, or placed in ice-cold phosphate buffered saline (PBS; pH 7.4).
For studies comparing infant rats receiving normal and low-potassium diets, 22-day-old rats were allowed to acclimate in metabolic cages for two days on a synthetic diet that contained a similar amount of K+[(in g) 180 casein, 200 cornstarch, 500 sucrose, 35 corn oil, 35 peanut oil, 10 CaHPO4, 6 MgSO4, 6 NaCl, 6.8 Na2HPO4, 7.1 KCl, and 10 vitamin-fortification mixture]. Subsequently, rats were randomly divided either to continue receiving the control potassium diet or to receive a low-potassium diet wherein one half of the KCl was replaced with NaCl. All animals were pair-fed and 24-hour urines were collected during day 3; subsequently, the rats were sacrificed, and blood was obtained.
Blood gas determinations were performed using a CMS System 1304 blood gas analyzer (Instrumentation Laboratory, Lexington, MA, USA); electrolytes and creatinine were measured on an Astra-7 electrolyte analyzer (Beckman, Palo Alto, CA, USA). Citrate measurements were performed using a citric acid enzymatic kit (Boehringer Mannheim, Indianapolis, IN, USA) on the Cobas Fara II chemistry system (Roche Diagnostic Systems, Somerville, NJ, USA). Urinary calcium was measured using the Beckman Synchron CX3 autoanalyzer.
Measurement of renal cortical apical membrane Na/citrate cotransporter
The renal cortex was dissected and minced on an iced Petri dish and was homogenized in 15 mL of buffer [300 mmol/L mannitol, 16 mmol/L HEPES/20 mmol/L Tris, pH 7.4, 5 mmol/L ethyleneglycoltetraacetic acid (EGTA), and 100 μg/mL phenylmethylsulfonyl fluoride (PMSF)] with a Polytron (Brinkman Industries, Westbury, NY, USA). Brush border membrane vesicles were isolated by Mg2+ precipitation and differential centrifugation as previously described by our laboratory12. Membranes were pelleted and resuspended to a protein concentration of approximately 5 mg/mL in intravesicular buffer that contained valinomycin (50 to 60 μg/mg protein).
Na-dependent citrate transport was determined in brush border membrane vesicles as previously described using the difference between 1,5-14C-citrate (New England Nuclear, Boston, MA, USA) uptake in Na-free [(in mmol/L) 100 cholineCl, 50 KCl, 0.1 K3citrate, 16 HEPES/12 Tris, pH 7.4] and Na-containing [(in mmol/L) 100 NaCl, 50 KCl, 0.1 K3citrate, 16 HEPES/12 Tris, pH 7.4] extravesicular solutions15. We measured Na-dependent citrate uptake during the linear period over 10 seconds using the Millipore filtration technique with 0.65 μm pore size cellulose nitrate filters (DAWP 2500; Millipore, Bedford, MA, USA) at room temperature. Enrichment of the vesicle preparation was determined using γ-glutamyltransferase activity15,16. Vesicle volumes were calculated from 14C-citrate uptake at two hours using the known citrate concentration in the extravesicular solution.
Mitochondrial preparation and enzyme assays
Mitochondria were harvested using differential centrifugation as described previously by our laboratory13. One gram of renal cortical tissue was homogenized in 10 mL ice-cold homogenization solution [300 mmol/L sucrose, 5 mmol/L KH2PO4, 1 mmol/L EGTA, 0.1% bovine serum albumin, 10 mmol/L Na3citrate, 5 mmol/L 3-[N-morpholino] propanesulfonic acid (MOPS), pH 7.4; 50 μg/mL PMSF, and 4 μg/mL aprotinin] with four strokes of a Potter-Elvehjem. Large pieces of cellular debris were pelleted at 700 ×g for 10 minutes, and the remaining supernatant was centrifuged at 7500 ×g for 10 minutes. The resultant supernatant was decanted and used for the cytosolic preparation to measure ATP citrate lyase activity, as described in the following section. The remaining mitochondrial pellet was resuspended to a protein concentration of approximately 10 mg/mL in 150 mmol/L KCl and 5 mmol/L MOPS, pH 7.4 with KOH, and frozen at −70°C.
Mitochondrial suspensions were freeze thawed twice at −70°C to disrupt the mitochondrial membranes17. For the citrate synthase assay, 5 μL of the freeze-thawed mitochondrial suspension were added to 470 μL of 5 mmol/L Tris-HCl, pH 8.1, containing 0.17 mmol/L 5,5′-dithio-bis-(2-nitrobenzoic) acid and 0.40 mmol/L acetyl CoA. Twenty-five microliters of 10 mmol/L Na2oxaloacetate (in 1 mol/L Tris-HCl, pH 8.1) started the reaction. The generation of CoA was determined spectrophotometrically at 412 nm with an extinction coefficient of 13.6/μmol17.
Mitochondrial aconitase activity was determined spectrophotometrically on freeze-thawed mitochondria by measuring aconitate generation at 240 nm18. Fifty microliters of the mitochondrial suspension were added to 950 μL of 100 mmol/L Tris-HCl, pH 8.0, that contained 50 μmol of D,L Na3isocitrate. An extinction coefficient of 3.6/mmol was used to calculate the aconitate produced.
ATP citrate lyase assay
The supernatant obtained from differential centrifugation described in the preceding section was centrifuged at 48,000 ×g× 20 minutes at 4°C. The resultant supernatant was then placed at −20°C overnight in the presence of 6.5 mmol/L dithiothreitol to reduce the sulfhydryl groups of the enzyme19. ATP citrate lyase activity was measured using the hydroxamate assay as described previously12.
Measurement of renal citrate
Tissue citrate concentration was determined using the freeze-clamp method20. In anesthetized adult rats, the kidneys were exposed through an abdominal incision. The cortex of the kidney was pinched, in situ, and was immediately frozen using brass clamps cooled in liquid nitrogen. Because of the size of the infant kidneys, the entire organ was removed and frozen in the clamps. Approximately 100 mg of frozen tissue was placed in 2 mL of 1 mol/L perchloric acid to inactivate tissue enzymes. After homogenization with a Polytron, the supernatant was neutralized with NaOH to pH 7.0. The citrate concentration was determined as for urine samples. Lactate concentration, as an index of tissue hypoxia, was measured using a commercially available kit from Sigma (St. Louis, MO, USA).
Materials
Sigma supplied all chemicals unless otherwise noted. All protein concentrations were determined using the bicinchoninic acid assay method (Pierce, Rockford, IL, USA).
Statistical analysis
Data are expressed as mean ±SE and are compared using the unpaired t-test.
RESULTS
Plasma and urine composition
Table 1 shows that infant rats had a significantly lower plasma [HCO3−], and pH, and a higher plasma [K+] compared with adults. Table 1 also shows that infant rats excreted less total urinary citrate compared with adults. This is not an unexpected observation because of the lower glomerular filtration rate in infants. However, Table 1 also shows that infant rats excreted urine with a higher citrate concentration. This would be of more physiologic significance because the concentration of inhibiting and promoting compounds determines the saturation characteristics of the urine. Furthermore, infant rats had a higher citrate:creatinine ratio and, notwithstanding the higher plasma citrate, a greater percentage fractional excretion of citrate. The citrate:creatinine ratio and the fractional excretion citrate correct for changes in the glomerular filtration rate and give a more accurate measure of tubular citrate reabsorption. Thus, these studies demonstrate that infant rats have higher urinary citrate excretion and a lower tubular citrate reabsorption.
Table 1. Plasma and urine values for adult and infant rats.
| Adults | Infants | |
|---|---|---|
| Plasma | ||
| pH | 7.42±0.01 | 7.39±0.01a |
| pCO2 mm Hg | 40.0±1.3 | 39.5±1.6 |
| HCO3 mEq/L | 26.2±0.5 | 23.7±0.9a |
| Na mEq/L | 142±1 | 140±1 |
| K mEq/L | 3.7±0.1 | 4.9±0.2a |
| Cl mEq/L | 107±1 | 107±1 |
| Citrate mg/L | 40.6±2.5 | 47.6±2.1 |
| Urine | ||
| Citrate mg/day | 2.76±0.47 | 1.60±0.21a |
| Citrate mg/L | 158±24 | 318±36a |
| Creatinine mg/day | 11.19±0.62 | 1.45±0.08b |
| Citrate:creatinine | 0.25±0.05 | 1.12±0.13b |
| %FE citrate | 4.1±0.5 | 19.1±2.1b |
N = 7 for both groups.
P < 0.05
P < 0.001
Na/citrate cotransporter activity in infants and adults
To determine if a difference in proximal tubular apical membrane Na/citrate cotransporter activity accounted for the higher urinary citrate excretion in infants, we measured 14C-citrate uptake in brush border membrane vesicles. As can be seen in Figure 1, there was no difference in Na-dependent citrate uptake (Na/citrate cotransporter activity; in pmol citrate/mg protein/10 s) between adults (28.2 ± 1.0) and infants (31.2 ± 1.7). There was also no difference in brush border membrane vesicle enrichments (adults 11.5 ± 2.1, infants 8.1 ± 1.2) or volumes (in μL/mg protein; adults 1.18 ± 0.20, infants 0.99 ± 0.11).
Figure 1. Sodium (Na)/citrate cotransporter activity was determined in adult (□) and infant ( ) rats.
) rats.
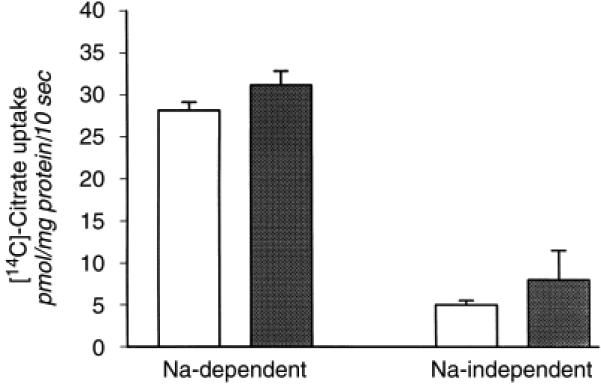
Na-dependent (left bars) and Na-independent (right bars) 14C-citrate uptake was determined in renal cortical brush border membrane vesicles (N = 6).
Citrate metabolism in infant and adult rat kidneys
Because renal citrate metabolism has been shown to play an important role in regulating urinary citrate excretion12,13, we examined whether a difference in renal cortical enzyme activities was associated with the increased urinary citrate excretion in infants. As shown in Figure 2, there was no difference in renal cortical citrate synthase activity (in nmol citrate/mg protein/minute; adults 332.8 ± 28.8, infants 301.0 ± 15.0).
Figure 2. Citrate synthase activity in adult and infant rats.
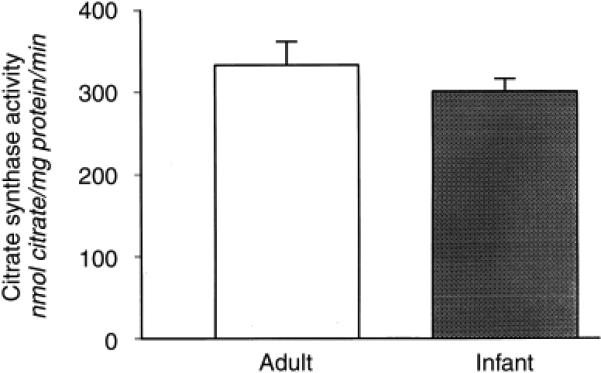
Citrate synthase activity was determined spectrophotometrically as the appearance of CoA from acetyl CoA and oxaloacetate in mitochondrial suspensions (N = 6).
Adenosine 5′-triphosphate (ATP) citrate lyase activity serves an important role in the hypocitraturia of metabolic acidosis12. This cytosolic enzyme cleaves citrate to oxaloacetate and acetyl CoA and is prevalent in kidney tissue21. We used the hydroxamate assay because the kidney contains high native NADH dehydrogenase activity that would interfere with the malate dehydrogenase-coupled assay12. Figure 3 shows that infant rat kidneys had a significantly higher ATP citrate lyase activity as compared with adult kidneys (in nmol acetylhydroxamate/mg mito protein/30 min; 41.2 ± 9.0 vs. 81.6 ± 9.6, P < 0.05).
Figure 3. Adenosine 5′-triphosphate (ATP) citrate lyase activity in adult and infant rats.
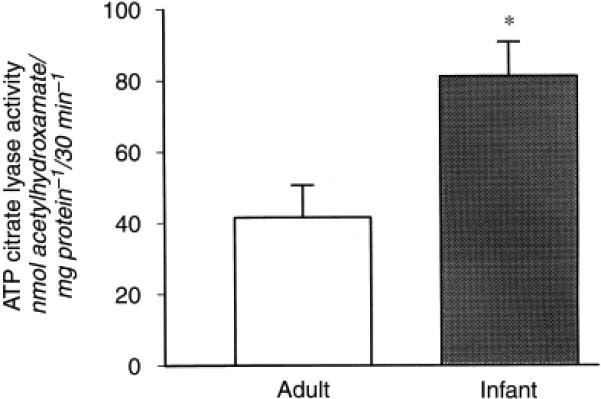
ATP citrate lyase activity was measured spectrophotometrically as the generation of acetylhydroxamate (N = 6). *P < 0.05.
Because an increase in ATP citrate lyase activity would not be expected to increase urinary citrate excretion, we measured m-aconitase activity, a regulated step in renal cortical mitochondria citrate metabolism. We have shown previously that regulation of m-aconitase partially mediates acidosis-induced hypocitraturia and alkalosis-induced hypercitraturia13. As shown in Figure 4, infant rat kidneys had a significantly lower renal cortical m-aconitase activity compared with adults (in nmol aconitate/mg mito protein/min; 163.7 ± 12.6, 51.5 ± 4.7, P < 0.001). This lower renal m-aconitase activity in infants may be responsible for hypercitraturia in these animals.
Figure 4. Mitochondrial-aconitase (m-aconitase) activity in adult and infant rats.
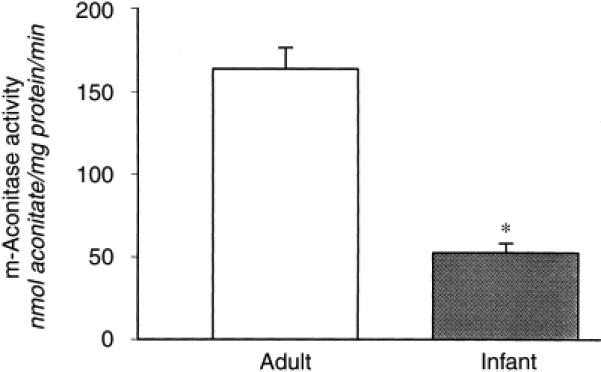
m-Aconitase activity was determined spectrophotometrically as aconitate generation from isocitrate in mitochondrial suspensions (N = 6). *P < 0.05.
Renal citrate concentration in infant and adult rats
As a result of the difference in renal citrate metabolism, in animals with hypocitraturia or hypercitraturia, there is a decrease or increase, respectively, in renal citrate concentration22. We reasoned that infant rats would have a higher renal citrate compared with adults. However, Figure 5 shows that there is no difference in renal citrate concentration (in μmol/g kidney wet weight; adults 0.54 ± 0.04 vs. infants 0.58 ± 0.06). This finding was not a result of a difference in specimen handling because renal lactate concentrations were similar (in μmol/g kidney wet weight; adults 0.71 ± 0.05 vs. infants 0.62 ± 0.09).
Figure 5. Renal cortical citrate concentration in adult and infant rats.
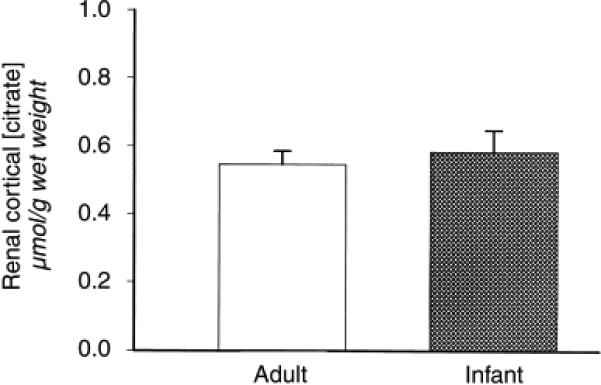
Kidneys were freeze clamped in vivo, and pulverized under liquid N2. Citrate levels were measured spectrophotometrically (N = 9).
Urinary citrate excretion in infant rats on a low-potassium diet
Because hypokalemia can cause hypocitraturia, we reasoned that the higher plasma potassium in infant rats might cause the higher urinary citrate excretion. To reduce the plasma [K+], we lowered the potassium content of the diet. Between the time of weaning and completion of the study, animals that received the low-potassium diet gained 17 ± 3 g, which was not different from control animals that gained 15 ± 2 g, or from animals that remained on the commercial diet, which were not placed in metabolic cages and gained 16 ± 2 g. As can be seen in Table 2, infant rats fed the low-K diet had a significantly lower plasma [K+], which was not different from the adults, and had a higher plasma [HCO3−]. Additionally, decreasing plasma [K+] was associated with a higher urine output and total urinary citrate excretion without affecting urinary citrate concentration. Furthermore, the low-K infant rats had a higher urinary citrate: creatinine ratio, but was not statistically significant. However, these rats had a significant decrease in both urinary calcium excretion and urinary calcium:creatinine ratio compared with controls. Thus, the higher urinary citrate excretion in infant rats, compared with adult rats, was not due to hyperkalemia.
Table 2. Plasma and urine values for control and infant rats fed a low-K diet.
| Control | Low-K | |
|---|---|---|
| Plasma | ||
| pH | 7.35±0.02 | 7.35±0.02 |
| pCO2 mm Hg | 44.4±2.6 | 45.5±1.7 |
| HCO3 mEq/L | 24.2±0.9 | 25.3±1.1 |
| Na mEq/L | 137±1 | 140±1a |
| K mEq/L | 4.6±0.2 | 3.9±0.1b |
| Cl mEq/L | 107±1 | 107±1 |
| Urine | ||
| Volume mL/day | 4.5±0.3 | 8.9±0.7b |
| Citrate mg/day | 1.40±0.14 | 3.00±0.82 |
| Creatinine mg/day | 0.94±0.04 | 1.11±0.18 |
| Citrate:creatinine | 1.50±0.14 | 2.58±0.56 |
| Calcium mg/day | 0.75±0.13 | 0.18±0.09b |
N = 6 for both groups.
P < 0.05
P < 0.01
DISCUSSION
This study demonstrates that infant rats have a significantly higher urinary citrate:creatinine ratio, as well as urinary citrate concentration, compared with adult rats. Hoppe et al found similar results in children in whom they also showed progressively higher urinary calcium oxalate saturation that peaks in prepubescence5.
The proximal tubule serves as the principal segment for renal tubular citrate reabsorption and provides the major site for the regulation of renal citrate absorption and excretion9. The first step in proximal tubular citrate absorption is citrate transport across the apical membrane on the Na/citrate cotransporter. Chronic metabolic acidosis causes hypocitraturia and an adaptive increase in Na/citrate cotransporter activity11, protein, and mRNA abundance (abstract; ibid). In our study, Na/citrate cotransporter activity in infant rats was no different than in adults. A similar observation occurs associated with the increased urinary citrate excretion of chronic alkali feeding11.
Although proximal tubule Na/citrate cotransporter activity increases in chronic metabolic acidosis, the tissue citrate concentration decreases22. This would suggest that citrate metabolism plays an important role in the regulation of urinary citrate excretion. Cytosolic citrate metabolism through the enzyme ATP citrate lyase plays an important role in decreasing urinary citrate excretion in metabolic acidosis and hypokalemia12. However, in this study, infant rats have higher renal cortical ATP citrate lyase activity when compared with adults, a change in the wrong direction to explain the hypercitraturia. Because rat kidneys continue to grow and to develop from birth to four weeks of age23, the higher activity of ATP citrate lyase may be explained by the fact that synthesis of lipids for growing tissue requires this enzyme24. Additionally, to supply the high energy demanded by proximal tubular transport processes, infant rat kidney transitions from anaerobic glycolysis to fatty acid oxidation during the first postnatal month25,26. Because ATP citrate lyase is a crucial step in the formation of fatty acids, its activity may also reflect these changes in energy substrate demand.
The mitochondrial tricarboxylic acid cycle serves as an important pathway for renal citrate metabolism. Chronic metabolic acidosis and chronic alkali feeding regulate the activity and protein abundance of m-aconitase, the first step for mitochondrial citrate metabolism13. In addition, the prostate synthesizes and secretes citrate, an effect mediated by the inhibition of m-aconitase27. We show here that similar to observations in chronic alkali feeding and prostate, infant rat kidneys excrete more citrate and have a lower m-aconitase activity than adults.
There was no difference in renal cortical citrate concentration between infant and adult rats. A decrease in citrate catabolism, as seen in animals with metabolic alkalosis, results in an increase in renal citrate concentration22. This may decrease the driving force for citrate transport across the apical membrane and result in hypercitraturia. However, proximal tubule citrate uptake depends on a sodium gradient as well, and may be affected by other factors such as basolateral Na+,K+-ATPase activity. Although we demonstrate that the Na/citrate cotransport activity was not different in infant and adult brush border membrane vesicles, the Na+,K+-ATPase is lower in infant kidneys28,29. Thus, the increase in urinary citrate excretion in infant rats likely reflects a relative immaturity of proximal tubular transport possibly through a change in the sodium gradient30.
The lower plasma [HCO3−] observed in infant rats is similarly observed in children31 and would be expected to result in lower urinary citrate excretion12. However, human infants, compared with adolescents, excrete twice as much urinary citrate when corrected for weight5. Similarly, in our study, despite the lower plasma [HCO3−], infant rats excrete fourfold more citrate when corrected for creatinine in their urine compared with adult rats. However, this study examined only a single time point in renal development. The mechanism for the increased urinary citrate in humans may differ from that presented in infant rats.
Because hypokalemia decreases urinary citrate excretion15, the relative hyperkalemia in infants may increase urinary citrate excretion even in the presence of low plasma [HCO3]. However, decreasing infant rat plasma [K+] to that seen in adults resulted in a slight increase in plasma [HCO3−] and a further increase in urinary citrate:creatinine. Thus, the higher urinary citrate excretion in infant rats does not result from their higher plasma [K+]. The infant rats fed a low-K diet also had a lower rate of urinary calcium excretion than the rats on the control diet. This is in contrast to results in adult humans32. The cause for the lower rate of calcium excretion in rats fed the low-K diet is unclear.
In summary, this study has shown that infant rats, despite mild metabolic acidosis, excrete more citrate in their urine than adult rats. Furthermore, we have shown that this difference in urinary citrate excretion is associated with lower renal cortical m-aconitase activity but not renal cortical citrate concentration. Our results suggest that there is a maturational difference in renal citrate metabolism and transport in infants that results in a higher urinary citrate excretion, which likely accounts for the paucity of urolithiasis in the pediatric population.
Acknowledgments
These studies were supported by National Institutes of Health grants R01-DK41612 (M.B.) and P01-DK20543 (R.J.A.). J.Z.M. was supported by a National Institutes of Health institutional training grant T32-DK07659 and a grant from the Northwestern University. The authors gratefully acknowledge the advice and assistance from Drs. Paul Srere and Moshe Levi, and the technical help from Ms. Paulette Padalino and Ms. Virginia Ho.
REFERENCES
- 1.Bennett AH, Colodny AH. Urinary tract calculi in children. J Urol. 1973;109:318–320. doi: 10.1016/s0022-5347(17)60416-6. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton FB, Southwest Pediatric Nephrology Study Group Idiopathic hypercalciuria: Association with isolated hematuria and risk for urolithiasis in children. Kidney Int. 1990;37:807–811. doi: 10.1038/ki.1990.49. | Article. [DOI] [PubMed] [Google Scholar]
- 3.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Arch Intern Med. 1977;87:404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 4.Elliot JS. Calcium oxalate urinary calculi: Clinical and chemical aspects. Medicine. 1983;62:36–43. doi: 10.1097/00005792-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe B, Jahnen A, Bach D, Hesse A. Urinary calcium oxalate saturation in healthy infants and children. J Urol. 1997;158:557–559. | Article | PubMed | ChemPort |. [PubMed] [Google Scholar]
- 6.Pak CY. Citrate and renal calculi. Miner Electrolyte Metab. 1987;13:257–266. [PubMed] [Google Scholar]
- 7.Hess B, Zipperle L, Jaeger P. Citrate and calcium effects on Tamm-Horsfall glycoprotein as a modifier of calcium oxalate crystal aggregation. Am J Physiol. 1994;265:F784–F791. doi: 10.1152/ajprenal.1993.265.6.F784. [DOI] [PubMed] [Google Scholar]
- 8.Nicar MJ, Skurla C, Sakhaee K, Pak CY. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8–14. doi: 10.1016/0090-4295(83)90113-9. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 9.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735. doi: 10.1038/ki.1990.265. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 10.Brennan TS, Hering-Smith KS, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol. 1988;255:F301–F306. doi: 10.1152/ajprenal.1988.255.2.F301. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: Effects of dietary acid and alkali loading. Am J Physiol. 1985;249:F590–F595. doi: 10.1152/ajprenal.1985.249.4.F590. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 12.Melnick JZ, Srere PA, Elshourbagy NA, Moe OW, Preisig PA, Alpern RJ. Adenosine triphosphate citrate lyase mediates hypocitraturia in rats. J Clin Invest. 1996;98:2381–2387. doi: 10.1172/JCI119051. | PubMed | ISI | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melnick JZ, Preisig PA, Moe OW, Srere PA, Alpern RJ. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitraturia. Kidney Int. 1998;54:160–165. doi: 10.1046/j.1523-1755.1998.00974.x. | Article | PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Nicar MJ, Hsu MC, Johnson T, Pak CY. The preservation of urine samples for determination of renal stone risk factors. Clin Chem. 1987;18:382–384. doi: 10.1093/labmed/18.6.382. [DOI] [PubMed] [Google Scholar]
- 15.Levi M, McDonald LA, Preisig PA, Alpern RJ. Chronic K depletion stimulates rat renal brush-border membrane Na-citrate cotransporter. Am J Physiol. 1991;261:F767–F773. doi: 10.1152/ajprenal.1991.261.5.F767. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 16.Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1996;15:124–136. [PubMed] [Google Scholar]
- 17.Robinson JB, Jr, Brent LG, Sumegi B, Srere PA. An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In: Darley-Usmar VM, Rickwood D, Wilson MT, editors. Mitochondria: A Practical Approach. IRL Press; Washington D.C.: 1987. p. 153. [Google Scholar]
- 18.Plank DW, Howard JB. Identification of the reactive sulfhydryl and sequences of cysteinyl-tryptic peptides from beef heart aconitase. J Biol Chem. 1988;263:8184–8189. [PubMed] [Google Scholar]
- 19.Cottam GL, Srere PA. The sulfhydryl groups of citrate cleavage enzyme. Arch Biochem Biophys. 1969;130:304–311. doi: 10.1016/0003-9861(69)90037-x. | Article. [DOI] [PubMed] [Google Scholar]
- 20.Wollenberger A, Ristau O, Schoffa G. Eine einfache technik der extrem schnellen abkuhlung groberer gewebestucke. Pflügers Arch. 1960;270:399–412. | Article | ChemPort |. [PubMed] [Google Scholar]
- 21.Srere PA. The citrate cleavage enzyme. J Biol Chem. 1959;234:2544–2547. | PubMed | ISI | ChemPort |. [PubMed] [Google Scholar]
- 22.Simpson DP. Citrate excretion: A window on renal metabolism. Am J Physiol. 1983;244:F223–F234. doi: 10.1152/ajprenal.1983.244.3.F223. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 23.Arataki M. On the postnatal growth of the kidney with special reference to the number and size of the glomeruli (albino rat) Am J Anat. 1926;36:399–436. | Article. [Google Scholar]
- 24.Abraham S, Madvig P. Enzyme activities during development of some organs of the rat. J Nutr. 1980;110:100–104. doi: 10.1093/jn/110.1.100. [DOI] [PubMed] [Google Scholar]
- 25.Djouadi F, Bastin J, Kelly DP, Merlet-Benichou C. Transcriptional regulation by glucocorticoids of mitochondrial oxidative enzyme genes in the developing rat kidney. Biochem J. 1996;315:555–562. doi: 10.1042/bj3150555. | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastin J, Delaval E, Freund N, Razanoelina M, Djouadi F, Bismuth J, Geloso JP. Effects of birth on energy metabolism in the rat kidney. Biochem J. 1988;252:337–341. doi: 10.1042/bj2520337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello LC, Franklin RB. Concepts of citrate production and secretion by prostate 1: Metabolic relationships. Prostate. 1991;18:25–46. doi: 10.1002/pros.2990180104. | PubMed |. [DOI] [PubMed] [Google Scholar]
- 28.Aperia A, Larsson L. Induced development of proximal tubular NaKATPase, basolateral cell membranes and fluid reabsorption. Acta Physiol Scand. 1984;121:133–141. doi: 10.1111/j.1748-1716.1984.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Evan AP. Development of solute transport in rabbit proximal tubule. III. Na-K-ATPase activity. Am J Physiol. 1984;246:F845–F852. doi: 10.1152/ajprenal.1984.246.6.F845. | PubMed |. [DOI] [PubMed] [Google Scholar]
- 30.Baum M, Quigley R. Ontogeny of proximal tubule acidification. Kidney Int. 1995;48:1697–1704. doi: 10.1038/ki.1995.467. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 31.Edelmann CM, Jr, Soriano JR, Boichis H, Gruskin AB, Acosta MI. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967;46:1309–1317. doi: 10.1172/JCI105623. | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemann J, Jr, Pleuss JA, Gray RW, Hoffman RG. Potassium administration increases and potassium deprivation reduces urinary calcium excretion in healthy adults. Kidney Int. 1991;39:973–983. doi: 10.1038/ki.1991.123. | Article | PubMed | ISI. [DOI] [PubMed] [Google Scholar]


