Abstract
Background
Initial low levels of hs cTnT and NT-proBNP identify older adults at lower risk for CV events.
Objective
To determine whether the combined trajectories of cardiac biomarkers identify those older adults with initial low levels who have an increased risk for structural heart disease, incident HF, and CV death.
Methods
Observational study among older adults without prevalent HF in the Cardiovascular Health Study. NT-proBNP and hs cTnT were measured at baseline and after 2-3 years. In those with low baseline levels, significant increase was defined as: cTnT >50%; NT-proBNP >25% increase to >190 pg/mL. LVEF and LV mass were measured by echocardiography at baseline and 5 years. Cox regression was used to estimate the association of change in biomarkers with HF and CV mortality.
Results
Among 2,008 participants with initially low biomarker concentrations, significant increases occurred in 14.8% for cTnT only, 13.2% for NT-proBNP only and 6.1% for both. After 10 years, cumulative HF incidence was 50.4% vs. 12.2% among those with both vs. neither biomarkers increased. The adjusted relative risk comparing those with increases in both vs. neither biomarkers was 3.56 for incident HF (95% confidence interval [CI]: 2.56-4.97) and 2.98 for CV mortality (95% CI: 2.98-4.26). Among 1340 participants with serial echocardiography, the frequency of new abnormal LVEF was 11.8% vs. 4.0% for those with increases in both vs. neither biomarkers (p=0.007).
Conclusions
Among older adults without HF with initially low cTnT and NT-proBNP, the long-term trajectory of both biomarkers predicts systolic dysfunction, incident HF, and CV death.
Keywords: troponin, NT-proBNP, epidemiology, elderly
Introduction
The disease burden of HF and other high-risk cardiovascular conditions falls mostly upon older adults, with those ≥65 years comprising nearly half of individuals with known cardiovascular disease. The annual incidence of HF for older adults is as high as 1 in 100 (1). With a high mortality in older adults associated with symptomatic HF, identifying those at greatest risk in the community may provide an opportunity to intervene to delay symptom onset. However, traditional risk scores based on clinical risk factors typically are less accurate in estimating cardiovascular risk in older adults compared to younger cohorts (2,3).
Blood-based biomarkers have been shown to add to traditional risk-factor based models to improve risk stratification for HF and cardiovascular death (4-10). While the use of single biomarkers is helpful, the examination of multiple biomarkers representing different pathopysiologies may have even greater predictive and prognostic value (4,9,10). However, a large proportion of individuals remain at-risk even when multiple biomarker concentrations are below previously defined thresholds. For example, we have previously shown that in older adults with initially low concentrations of either the cardiac specific biomarkers cTnT (measured by a hs assay) or NT-proBNP, an upward trajectory in one biomarker over 2-3 years is associated with a 70 to 200% increased risk of new-onset HF, respectively, compared to individuals without such an increase(5,6). Correlation between concentrations of these two biomarkers is only moderate, suggesting that the mechanisms underlying their association with adverse outcomes may differ. As such, it may be that consideration of changes in both biomarkers would provide a more complete evaluation of cardiovascular risk, characterizing an early HF phenotype preceding structural changes and symptomatic disease.
We hypothesized that in community-dwelling older adults free of HF with initially lower-risk concentrations of the biomarkers NT-proBNP and hs cTnT, the trajectories of both biomarkers measured over 2-3 years would be additive in identifying those participants at greater long-term risk for new-onset HF and CV death. Furthermore, we hypothesized that the trajectories of both biomarkers would identify those more likely to develop left ventricular structural pathology by serial echocardiography prior to symptomatic HF.
Methods
Study Population
CHS is a multicenter prospective observational cohort study of cardiovascular disease in older adults. A detailed account of the study methods, as well as a description of the study-defined co-morbidities, has been previously published (11). Participants (N=5201) initially enrolled in 1989-90, and an African-American supplemental cohort (N=687) enrolled in 1992-93. In the present analysis, we excluded those participants with a diagnosis of HF prior to the time of second biomarker measure (see below).
The CHS was approved by the institutional review boards of the University of Washington, Seattle, and the participating centers. All participants gave written informed consent. The present study was approved by the institutional review board of the University of Maryland, Baltimore.
Biomarker Measurement
NT-proBNP and cTnT were measured in serum collected in 1989-90 and 1992-93 (main cohort) or 1992-93 and 1995-96 (supplemental cohort) and stored at -70C to -80C. Samples were thawed just prior to testing (maximum of 3 freeze-thaw cycles) and measured according to previously described assay methods (6,12). The assay range for NT-proBNP is 5 to 35,000 pg/mL and for cTnT - utilizing the highly sensitive 5th generation test - is 3 to 10,000 pg/mL.
Primary Outcome Measures
Outcomes were incident HF and CV mortality. Incident HF events were ascertained by participant interview at semiannual study visits and examination of Medicare claims data. Potential HF events and determination of cause of death were determined by an expert adjudication panel (13). Details of adjudication criteria have been previously described (14).
CV mortality was defined as mortality related to atherosclerotic heart disease (fatal myocardial infarction and definite and possible fatal CHD), death following cerebrovascular disease (fatal stroke), or mortality from other atherosclerotic and CV diseases including HF, as previously described (13).
Other Covariates
Covariates including prevalent CV disease risk factors were defined at the time of the 2nd biomarker measurement as previously described (5). Race was determined by self-identification and classified as black or other. CHD was defined as a history of angina, coronary angioplasty, coronary artery bypass surgery, or myocardial infarction. Echocardiograms were also obtained; in the main cohort, this was performed in 1989- 1990 and among both cohorts was performed in 1995-1996. The measures of interest for this analysis – left ventricular mass, left atrial diameter, diastolic measures including the mitral inflow Doppler E/A ratio and semi-quantitative left ventricular ejection fraction – were defined as previously specified (15). LVH was defined as LV mass indexed to height of >46.7 g/m2.7 for women and >49.2g/m2.7 for men (16) .
Statistical Methods
Selection of study sample
In the present analysis, we excluded those participants with prevalent HF at enrollment into CHS and/or incident HF between enrollment and the time of the second biomarker measurement. Since the purpose of the present analysis was to examine the effect of change in biomarkers among those whose initial biomarkers was not already elevated, we further excluded participants with initial NT-proBNP>190 pg/mL or cTnT>13 pg/mL. The cut-point for elevated NT-proBNP represents the 70th percentile in the CHS study sample and best corresponds with increased risk of heart failure in this population. The cut-point for cTnT represents the 99th percentile in a healthy reference population (17), and identified a high-risk subgroup in three general population studies (6,7,18).
Definition of significant increase in biomarkers
A “clinically significant” increase in each biomarker was defined according to previously defined criteria: for NT-proBNP, a >25% increase from baseline to a concentration at follow-up of >190 pg/mL; for cTnT, a >50% increase from baseline. Increases of this magnitude represent a change greater than the short-term within-individual variability in healthy adults (19,20), and individually have been shown to predict a greater risk of incident HF in older adults (5,6). In the primary analysis, participants (N=950, 47%) with concentrations below the level of detection (<3 pg/mL) were imputed a value of 2.99 pg/mL.
Study participants were divided into 4 subgroups based upon their increase from baseline to follow-up in each of the two specified biomarkers (NT-proBNP and cTnT) as follows: no change, change in cTnT only, change in NT-proBNP only, or change in both cTnT and NT-proBNP. Baseline characteristics by category were compared using chi-square tests or 1-way analysis of variance, as appropriate.
Comparison of echocardiographic abnormalities
Among participants in the main cohort with complete echocardiogram data at both baseline and follow-up (1995-96) who remained free of diagnosed HF at the time of the 2nd echocardiogram, we compared the frequency of a LVEF classified as depressed (borderline [estimated as 45-54%] or abnormal [estimated as < 45%]) at follow-up across the 4 subgroups defined by change in biomarkers, after excluding those with a depressed LVEF at baseline. Likewise, after excluding those with baseline LVH, we plotted the frequency of new LVH at follow-up echocardiogram across the subgroups defined by biomarker change. The chi-squared test was used to test the hypothesis that the incidence of new LVH and a new depressed LVEF differed between biomarker change subgroups.
Comparison of incident HF and CV mortality
Cumulative incidence of HF and CV mortality in each subgroup from the time of the second biomarker measurement was estimated using the Kaplan-Meier method and compared with the log-rank test. Multivariate analyses were performed using Cox proportional hazard regression models. All models were adjusted for baseline concentrations of each biomarker. Two sets of adjustment covariates were selected which included: 1) demographics (age, sex, race [black vs. other]) and 2) cardiovascular risk factors defined a-priori from validated risk models, which are distinct for both HF and CV mortality (21,22). In addition to demographics, the models for HF were adjusted for: systolic blood pressure, smoking, heart rate, creatinine, albumin, glucose, CHD, LVH as determined by electrocardiograph (components of the Health ABC Risk model (21)). CV death models, in addition to demographics, were adjusted in accordance with the traditional CV risk factors as defined in the Framingham risk score: systolic blood pressure, diastolic blood pressure, use of anti-hypertensive medication, diabetes, CHD, smoking, total and high-density lipoprotein cholesterol levels (22).
We estimated the incremental improvement in risk classification and discrimination from the addition of change in biomarker concentrations to models containing baseline concentrations and traditional risk factors using the C-statistic (as modified for time-to-event analyses) (23), the IDI, and the NRI statistics (24). For the NRI computation, we defined a-priori 10-year risk categories of <10%, 10%-20%, and >20% as previously described, using model-based estimates of cumulative hazard. In sensitivity analyses, we also estimated net reclassification using the newer “continuous NRI” as defined by Pencina et. al. (25), with bootstrapping used to estimate the 95% confidence intervals.
Statistical analysis was performed using SPSS Statistics version 19 (IBM) and Stata v11.2 (Statacorp, College Station, TX).
Results
Characteristics of study samples
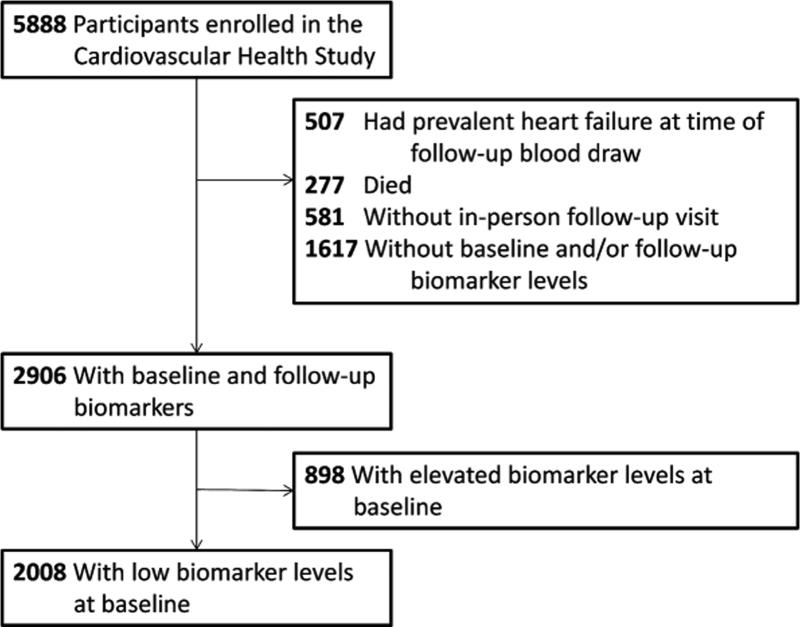
The number of participants who met inclusion criteria for this analysis is described in figure 1. Among all CHS participants, 507 (8.6%) had HF prior to the 2nd biomarker measurement, and an additional 581 (15.9%) were either deceased or did not attend an in-person follow-up study visit. Of the remaining participants, 2906 (64.2%) had sufficient sera from both the baseline and follow-up visits for cardiac biomarker measurement. A total of 898 (30.9%) had elevated concentrations of at least one cardiac biomarker at baseline and were excluded from analysis, resulting in inclusion of 2008 participants for analysis.
Figure 1. Flow Diagram.
Number of participants in the Cardiovascular Health Study and inclusion in the current analysis based on the measurement of NT-proBNP and hs cTnT.
Overall, approximately one third of the study population had an increase in at least one biomarker from baseline to second blood draw. The proportion of participants with increase in only one biomarker was relatively similar (cTnT, 14.8%; NT-proBNP, 13.2%) and 6.1% had an increase in both biomarkers (Table 1). A progressive trend in participants with a rise from none to both biomarkers could be seen with more advanced age, known CHD, hypertension, and higher creatinine values. However, several demographic characteristics and traditional risk factors, though significantly different between groups, didn't follow this linear trend including: male gender, diabetes, and cholesterol levels. On baseline echocardiography, significant differences were observed in those who had increased biomarker levels over time versus those who did not. Specifically, in those in whom biomarkers increased there was a greater prevalence of depressed LVEF, impaired diastolic relaxation, and greater left atrial diameter. Those with increases in biomarkers also had higher baseline hs cTnT and NT-proBNP concentrations.
Table 1.
Characteristics of study sample, by change in biomarker concentrations.
| Characteristics | No change (−/−) N = 1322 (65.8%) | Increased cTnT only (+/−) N = 297 (14.8%) | Increased NT-proBNP only (−/+) N = 266 (13.2%) | Increased both (+/+) N = 123 (6.1%) | p value |
|---|---|---|---|---|---|
| Age(yrs) | 73.2 (4.0) | 74.2 (4.4) | 74.8 (4.6) | 76.4 (4.8) | < 0.001 |
| Male | 436 (33.0%) | 158 (53.2%) | 74 (27.8%) | 52 (42.3%) | < 0.001 |
| African-American | 255 (19.3%) | 46 (15.5%) | 23 (8.6%) | 10 (8.1%) | < 0.001 |
| Coronary Heart Disease | < 0.001 | ||||
| None | 1171 (88.6%) | 246 (82.8%) | 199 (74.8%) | 88 (71.5%) | |
| Present at baseline | 124 (9.4%) | 44 (14.8%) | 45 (16.9%) | 27 (22.0%) | |
| Interim | 27 (2.0%) | 7 (2.4%) | 22 (8.3%) | 8 (6.5%) | |
| LV hypertrophy by EKG | 22 (1.7%) | 5 (1.7%) | 13 (5.1%) | 5 (4.1%) | 0.005 |
| HTN Medication | 538 (40.7%) | 138 (46.6%) | 141 (53.0%) | 70 (56.9%) | < 0.001 |
| Heart rate (/min) | 68.2 (10.1) | 68.4 (11.4) | 66.1 (10.3) | 67.4 (11.4) | 0.019 |
| Blood Pressure (mm Hg) | |||||
| Systolic | 132.6 (19.0) | 133.3 (18.7) | 138.9 (21.4) | 140.4 (25.1) | < 0.001 |
| Diastolic | 71.1 (10.6) | 70.9 (10.8) | 71.2 (10.8) | 71.4 (12.2) | 0.968 |
| Difference in SBP* | 0.8 (16.9) | −0.1 (15.5) | 2.5 (20.5) | 3.7 (22.8) | 0.1 |
| LDL (mg/dL) | |||||
| Total Cholesterol (mg/dL) | 214.1 (36.7) | 211.5 (38.5) | 211.0 (38.5) | 208.7 (39.9) | 0.256 |
| HDL Cholesterol (mg/dL) | 54.7 (14.1) | 51.8 (14.2) | 54.3 (14.5) | 53.1 (16.1) | 0.017 |
| Serum creatinine (mg/dL) | 0.92 (0.20) | 1.02 (0.24) | 0.96 (0.25) | 1.04 (0.33) | < 0.001 |
| Smoking Status | |||||
| Never | 610 (47.1%) | 126 (43.4%) | 114 (44.0%) | 57 (46.7%) | 0.365 |
| Former | 564 (43.6%) | 145 (50.0%) | 119 (45.9%) | 57 (46.7%) | |
| Current | 121 (9.3%) | 19 (6.6%) | 26 (10.0%) | 8 (6.6%) | |
| Diabetes | |||||
| None | 979 (74.1%) | 196 (66.0%) | 216 (81.2%) | 93 (75.6%) | 0.003 |
| Impaired Fasting Glucose | 144 (10.9%) | 40 (13.5%) | 25 (9.4%) | 16 (13.0%) | |
| Overt | 199 (15.1%) | 61 (20.5%) | 25 (9.4%) | 14 (11.4%) | |
| Serum Glucose (mg/dL) | 109.1 (34.2) | 114.4 (45.1) | 104.2 (29.4) | 105.3 (25.0) | 0.004 |
| Serum Albumin (g/dL) | 3.95 (0.27) | 3.97 (0.26) | 3.90 (0.27) | 3.85 (0.27) | < 0.001 |
| NT-proBNP (pg/mL) | |||||
| Baseline | 68.1 [38.6-102.6] | 59.5 [32.7-93.3] | 120.5 [79.2-151.8] | 102.6 [56.9-143.6] | < 0.001 |
| Follow-up | 79.9 [48.6-123.9] | 83.6 [55.5-117.2] | 268.8 [229.0-358.1] | 357.6 [251.3-492.7] | < 0.001 |
| cTnT (pg/ml) | |||||
| Baseline | 3.03 [<3.00, .40] | 3.02 [<3.00, 5.97] | 4.78 [<3.00,7.36] | 3.65 [<3.00, 7.37] | < 0.001 |
| Follow-up | 2.99 [<3.00, 5.04] | 8.46 [5.69, 12.84] | 3.83 [<3.00 −6.46] | 11.11 [7.48-18.07] | < 0.001 |
| LV EF baseline | 0.001 | ||||
| Normal | 1273 (97.0%) | 278 (93.6%) | 260 (97.7%) | 111 (91.0%) | |
| Borderline | 35 (2.7%) | 18 (6.1%) | 4 (1.5%) | 10 (8.2%) | |
| Abnormal | 4 (0.3%) | 1 (0.3%) | 2 (0.8%) | 1 (0.8%) | |
| E/A ratio | 0.016 | ||||
| < 0.7 | 193 (15.0%) | 49 (16.7%) | 54 (20.5%) | 32 (26.9%) | |
| 0.7-1.5 | 1060 (82.4%) | 237 (80.9%) | 200 (70.6%) | 83 (69.7%) | |
| > 1.5 | 34 (2.6%) | 7 (2.4%) | 9 (3.4%) | 4 (3.4%) | |
| Left Atrial Dimension (cm) | 3.75 (0.57) | 3.82 (0.62) | 3.83 (0.63) | 3.86 (0.66) | 0.039 |
| LVH at baseline | 99 (12.6%) | 21 (13.6%) | 28 (16.0%) | 14 (18.2%) | 0.4 |
Cell values represent N(%), mean(SD) or median [interquartile range].
Difference between follow-up and baseline systolic BP.
Abbreviations/definitions: Abnormal LVEF (<45%); Borderline LVEF (45-54%); EKG – electrocardiogram; HTN – hypertension; HR: heart rate; BP - blood pressure; LVEF – Left Ventricle Ejection Fraction; LVH – Left Ventricular Hypertrophy
Cardiac structural changes based on changes in biomarkers
Sequential echocardiograms from baseline and after 5 years were available in 1393 study participants from the main CHS cohort who remained free of HF, of whom 1340 (96.2%) had a normal LVEF at baseline and were included for analysis. The incidence of new depressed LVEF at follow-up differed significantly between those with vs. without changing biomarker concentrations, at 4.0% for those with a rise in neither biomarker to 11.8% for those with a rise in both cTnT and NT-proBNP (p=0.007 for test of incidence across biomarker change subgroups; Figure 2a). Sequential measures of LV mass on echocardiography were available for 815 participants in the main cohort without heart failure at the time of the 2nd echocardiogram, of whom 97 (11.9%) had baseline LVH as defined by sex-specific cut-points for LV mass indexed to height and were excluded from analysis. The incidence of LVH on follow-up did not differ significantly (p=0.1) between subgroups defined by increases in cardiac biomarkers (Figure 2b).
Figure 2a. Biomarker changes and New Depressed EF.
Incidence of new depressed LVEF at follow-up echocardiogram in CHS participants free of HF with serial echocardiograms, by change in cardiac-aspecific biomarkers (N=1340)
Figure 2b. Biomarker changes and New LVH.
Incidence of new LVH at follow-up echocardiogram in CHS participants free of HF with serial echocardiograms, by change in cardiac specific biomarkers (N=815)
Change in biomarkers and incident heart failure and cardiovascular related death
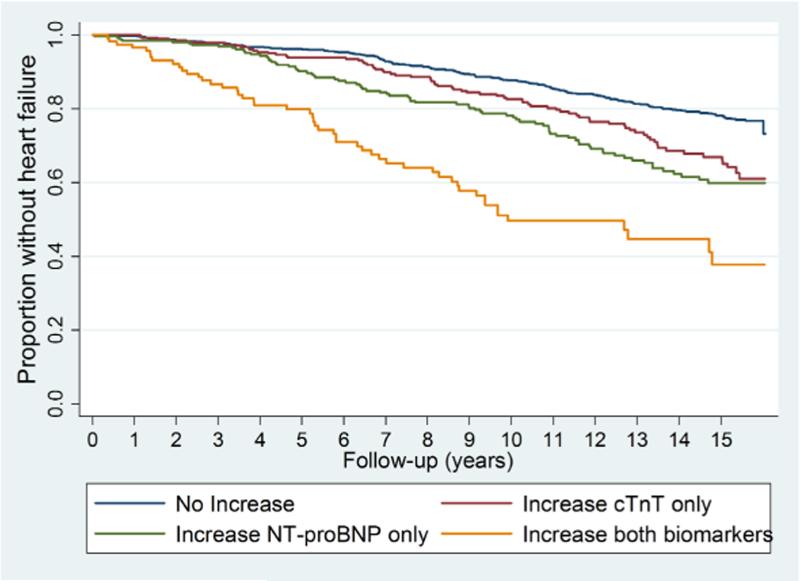
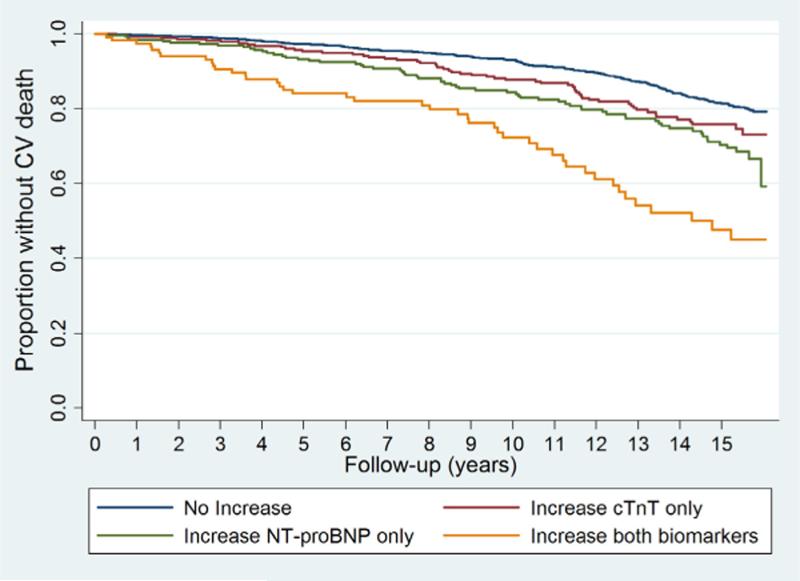
The cumulative proportion without incident HF and CV related mortality in each subgroup defined by changes in biomarkers is shown in Figures 3a and 3b, respectively. There was a graded association with respect to risk of either endpoint based on the presence of 0, 1 or 2 biomarkers that became elevated by the second blood draw. Individuals with increases in both biomarkers were at a markedly greater risk of developing HF and CV mortality than were those that had no significant increase in either biomarker (p<0.001 for both outcomes). After 10 years, roughly one-half (50.4%, 95% confidence interval [CI]: 40.4%, 61.4%) of the participants with increases in both biomarkers had developed new-onset HF. More modest but significant increments in risk were observed among those with increases in NT-proBNP or cTnT only.
Figure 3a. Biomarker changes and HF.
Risk of incident HF Cumulative proportion without HF, by change in cardiac biomarker
Figure 3b. Biomarker changes and CV death.
Cumulative CV mortality, by change in cardiac biomarker
In Cox proportional hazards models, a strong association of change in both biomarkers and the risk of incident HF was observed, with a 329% greater risk among those with both biomarkers increased compared to those with no change in either biomarker, after adjusting for baseline concentrations (Table 2). Adjustment for demographics and HF risk factors attenuated this association only modestly (HR=3.56, 95% CI: 2.56, 4.97). Individuals with increases of cTnT or NT-proBNP had an intermediate but significantly greater risk of incident HF compared with those without increases in either biomarker. The risk of HF among those with increases in both biomarkers was significantly greater than among those with increases in NT-proBNP or cTnT only (p=0.001 for both tests of differences). Adjustment for cystatin C or estimated GFR (using the Modification of Diet in Renal Disease estimating equation) in place of serum creatinine resulted in similar hazard ratios (data not shown). For CV mortality, individuals with increases in both biomarkers, after adjustment for demographics and clinical risk factors, were at a nearly 3-fold increased risk (HR, 2.96; 95% CI, 2.07-4.23). In contrast, participants with a rise in only NT-proBNP or cTnT in a fully adjusted model were not at significantly increased risk of CV mortality compared to participants with an elevation in neither (Table 3).
Table 2.
Association of change in cardiac-specific biomarkers with the risk of incident HF
| No Increase | Increased only cTnT | Increased only NT-proBNP | Increased Both | |
|---|---|---|---|---|
| Event Rate (per 100 person-years) | 1.5 (1.3-1.7) | 2.2 (1.7-2.8) | 2.8 (2.2-3.6) | 5.1 (3.8-7.0) |
| Hazard Ratio (95% CI) | ||||
| Unadjusted | 1 [Reference] | 1.66 (1.28, 2.15) | 1.71 (1.31, 2.21) | 4.29 (3.15, 5.84) |
| Demographics | 1 [Reference] | 1.46 (1.12, 1.90) | 1.66 (1.27, 2.17) | 3.77 (2.75, 5.16) |
| Demographics + Risk Factors | 1 [Reference] | 1.37 (1.04, 1.80) | 1.56 (1.18, 2.08) | 3.56 (2.56, 4.97) |
All models additionally adjusted for baseline concentrations of hs cTnt and NT-proBNP.
Demographics: Age, gender, race (African-American vs. other)
Risk Factors:: SBP, smoking, heart rate, coronary heart disease (absent, prevalent at baseline, interim between baseline and follow-up), glucose, serum creatinine, serum albumin, and LVH by EKG (risk factors comprising the HealthABC model) (21).
Table 3.
Association change in cardiac-specific biomarkers with the risk of CV mortality
| No Increase | Increased only cTnT | Increased only NT-proBNP | Increased Both | |
|---|---|---|---|---|
| Event rate (per 100 person-years) | 1.6 (1.4 - 1.7) | 2.1 (1.7 – 2.6) | 3.2 (2.7 – 3.7) | 5.4 (4.5 – 6.5) |
| Hazard Ratio (95% CI) | ||||
| Unadjusted | 1 [Reference] | 1.49 (1.11, 2.00) | 1.55 (1.16, 2.08) | 3.80 (2.72, 5.32) |
| Demographics | 1 [Reference] | 1.29 (0.95, 1.75) | 1.49 (1.11, 2.00) | 3.22 (2.29, 4.53) |
| Demographics + Risk Factors | 1 [Reference] | 1.16 (0.85, 1.59) | 1.37 (1.00, 1.87) | 2.98 (2.09, 4.26) |
All models additionally adjusted for baseline concentrations of hs cTnt and NT-proBNP.
Demographics: Age, gender, race (African-American vs. other)
Risk Factors: SBP, DBP, diabetes, anti-hypertensive medication use, smoking, coronary heart disease (absent, prevalent at baseline, interim between baseline and follow-up), total and HDL cholesterol (risk factors comprising long-term Framingham risk prediction model) (22)
Reclassification and Discrimination
Model discrimination as quantified by the C-statistic for the 10-year prediction of incident HF and CV mortality by standard risk factors and baseline biomarkers was 0.727 and 0.746, respectively. Addition of change in cardiac biomarker concentrations to these models did not significant improve the C-statistic but did significantly improve discrimination as quantified by the IDI stastistic (table 4). Addition of change in cardiac biomarkers significantly improved risk classification (as quantified by the net NRI statistic) for both HF and CV mortality; this significant improvement was observed whether risk of HF and CV death was categorized using a-priori cut-points or considered on a continuous scale (the “category-less” NRI, table 4). The latter statistic indicates that a net 21% and 30% of patients were correctly reclassified with regards to risk of incident HF and CV mortality, respectively, by the measurement of change biomarkers.
Table 4.
Net improvement in reclassification and discrimination for the prediction of CV death and incident HF at 10 years of change in biomarkers to standard risk factors and baseline biomarker concentrations
| Incident HF | CV Mortality | |
|---|---|---|
| Statistic (95% CI) | Statistic (95% CI) | |
| NRI (3 categories) | .073 (.019, 0.13) | .075 (.004, 0.146) |
| NRI (category-less) | .210 (0.062, 0.418) | .303 (.082, 0.540) |
| IDI | .020 (.009, .031) | .018 (.005, .031) |
| ΔC-statistic | .02 (−0.01, 0.02) | 0.02 (−.01, .02) |
Discussion
Results from this study show that in community-dwelling older adults free of HF and with initial lower-risk levels of NT-proBNP and hs cTnT, a rise in one or both biomarkers over the next 2-3 years is common and associated with more baseline structural cardiac abnormalities, a greater frequency of developing a depressed LVEF in the interim between biomarker measures, and increased long-term risk for both new-onset HF and CV mortality. In prior analyses, we showed that the trajectory of change of a single cardiac-specific biomarker in older adults is associated with a concordant change in CV risk (5,6). Other authors have also reported that multiple biomarkers measured at a single time point improve CV prognostication in community based cohorts (4,9,10). A recent study has also shown that the combination of BNP and hs cTnT concentrations were additive in predicting subclinical cardiac disease among primary prevention patients without overt CV disease (26).
The current results extend these previous findings in demonstrating the prognostic significance of longitudinal changes in multiple cardiac-specific markers in predicting CV morbidity and mortality independent of established risk factors. Also unique to this study, we provide evidence of a positive association between biomarker increase, and not only baseline structural cardiac pathology, but the development of LV pathology prior to onset of symptoms. While such structural changes could be identified in only a minority of participants with elevations of their biomarker levels over time, it does support the contention that both of these biomarkers represent pathophysiology of a preclinical HF phenotype and that the presence of both increasing neurohormonal activation and myocyte cell death represent an acceleration of a process that carries a very high risk of cumulating as clinical symptoms or death.
We and others have shown that the correlation between baseline levels of cardiac markers of neurohormonal activation (as measured by NT-proBNP) and myocyte cell death (as measured by cardiac specific troponin assays) are only moderately correlated (4,6). While a rise in cardiac biomarkers over time is common in those with initially lower levels of both tests, the majority have a rise in only one of the two biomarkers, associated with an intermediate increased risk of new-onset HF compared to the majority without any rise, but a significantly lower risk than those with a rise in both biomarkers. This finding, also supported by an intermediate risk of developing a depressed LVEF, suggests that in older adults there are likely multiple pathophysiologies that ultimately express themselves as a common clinical phenotype of HF, some of which represent mechanisms that predominate in cell death and others in cardiac neurohormonal activation. The heterogeneity of trajectory of biomarkers that ultimately lead to symptomatic HF in older adults should not be surprising given the heterogeneity of echocardiographic findings in those with HF with the near equal prevalence of HF with reduced or preserved LVEF in this population (27,28). One hypothesis is that an upward trajectory of levels of hs cTnT may represent a process of increased myocyte apotosis with subsequent replacement with fibrosis and increased cardiac stiffiness. Recent evidence from magnetic resonance imaging shows in a cohort of older adults a relatively high (17%; 95% CI, 14% to 19%) prevalence of unrecognized myocardial infarctions (29). Therefore, occult myocardial infarction could be one potential mechanism to account for increased hs cTnT levels. Another hypothesis is that an upward trajectory of NT-proBNP levels may represent subtle increases in fluid and sodium retention from both cardiac and non cardiac mechanisms increasing a vulnerability to symptomatic HF. Inclusion of both a rising hs cTnT level and NT-proBNP level may identify those most likely to have a cardiac specific mechanism for increasing fluid retention and potentially identify those still asymptomatic individuals to be targeted with a specific therapy such as aldosterone antagonists that could potentially reduce both cardiac fibrosis and fliud retention.
Several limitations of this study should be considered. First, stored sera at both baseline and follow-up visits were unavailable for roughly 1/3rd of participants, as they had been consumed during prior ancillary studies. We have previously shown that those participants without available sera differed modestly from those with available sera in that they were more likely be female, African-American and to have diabetes and hypertension(5,6). Therefore, differential missingness of biomarker measurements may have introduced bias. Second, although the long duration of follow-up is a strength of this study, changes in cardiovascular prevention and disease management (e.g. greater use of statins and angiotensin antangonists) have occurred from the time when participants were initially enrolled into CHS, potentially effecting the generalizability of these results. Third, serial echocardiography (and especially LV mass measures) was not performed on all CHS participants. This smaller sample size, along with the limited reproducibility of quantitative echocardiography to measure LV mass (30), may have resulted in limited statistical power to detect serial changes in LV mass in association with changes in biomarker concentrations. Fourth, we found that the combined change in both biomarkers improved discrimination when quantified by the IDI statistic but not the C-statistic. However, the C-statistic is relatively insensitive in detecting important risk predictors,(31,32) and the magnitude of the improvement in model discrimination as reflected in the IDI is greater than that previously reported for HDL cholesterol in predicting coronary heart disease events in the Framingham Offspring Study(24).
In conclusion, over one third of older adults free of HF who may be initially classified as lower risk based on lower baseline levels of the cardiac specific markers NT-proBNP and hs cTnT will have a rise in one or both of these markers over the next 2-3 years. This rise is indicative of an increased risk of both developing symptomatic HF and CV mortality, independent of traditional risk factors. Change in concentrations of either or both of these biomarkers likely represents the presence of active and ongoing cardiac pathophysiology representing a pre clinical HF phenotype that could provide a mechanism to differentiate which ACC/AHA stage A patients are most likely to move toward stage B and C and be targets for trials of disease modifying therapies.
Condensed Abstract.
We examined whether the combined trajectories of hs cTnT and NT-proBNP identified those older adults with initially low biomarker concentrations at increased risk for developing structural heart disease, incident HF, and CV death. Among 2,008 participants, significant increases occurred in 14.8% for cTnT only, 13.2% for NT-proBNP only and 6.1% for both. The adjusted relative risk comparing those with increases in both vs. neither biomarker was 3.56 for HF and 2.98 for CV mortality (p<.001). Among 1340 participants with serial echocardiography, frequency of new abnormal LVEF was 11.8% vs. 4.0% for those with increases in both vs. neither biomarkers (p=0.007).
Acknowledgements
The research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Support for measurement of cardiac biomarkers was provided by Roche Diagnostics, Inc.
Relationships with Industry: Seliger: Roche Diagnostics (research funding, consulting). deFilippi: Roche Diagnostics (research funding, consulting, advisory board). Christenson: Roche Diagnostics (research funding).
Abbreviations
- HF
heart failure
- CV
cardiovascular
- CHD
Coronary heart disease
- NT-proBNP
amino-terminal pro-B-type natriuretic peptide
- hs cTnT
highly-sensitive cardiac troponin T
- CHS
Cardiovascular Health Study
- LVEF
left ventricular ejection fraction
- LVH
left ventricular hypertrophy
- NRI
net reclassification improvement
- IDI
integrated discrimination improvement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update / 1. About 1. About These Statistics / 2. American Heart Association's 2020 Impact Goals / 3. Cardiovascular Diseases / 4. Subclinical Atherosclerosis / 5. Coronary Heart Disease, Acute Coronary Syndrome, and Angina Pectoris / 6. Stroke (Cerebrovascular Disease) / 7. High Blood Pressure / 8. Congenital Cardiovascular Defects / 9. Cardiomyopathy and Heart Failure / 10. Other Cardiovascular Diseases / 11. Family History and Genetics / 12. Risk Factor: Smoking/Tobacco Use / 13. Risk Factor: High Blood Cholesterol and Other Lipids / 14. Risk Factor: Physical Inactivity / 15. Risk Factor: Overweight and Obesity / 16. Risk Factor: Diabetes Mellitus / 17. End-Stage Renal Disease and Chronic Kidney Disease / 18. Metabolic Syndrome / 19. Nutrition / 20. Quality of Care / 21. Medical Procedures / 22. Economic Cost of Cardiovascular Disease / 23. At-a-Glance Summary Tables / 24. Glossary. Circulation. 2011;123:e18–e209. [Google Scholar]
- 2.D'Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 3.Koller MT, Leening MJG, Wolbers M, et al. Development and Validation of a Coronary Risk Prediction Model for Older U.S. and European Persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012;157:389–397. doi: 10.7326/0003-4819-157-6-201209180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenberg S, Zeller T, Saarela O, et al. Contribution of 30 Biomarkers to 10-Year Cardiovascular Risk Estimation in 2 Population Cohorts: The MONICA, Risk, Genetics, Archiving, and Monograph (MORGAM) Biomarker Project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 5.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ. Multiple biomarkers for predicting cardiovascular events: lessons learned. J Am Coll Cardiol. 2010;55:2092–5. doi: 10.1016/j.jacc.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 10.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. Journal of the American College of Cardiology. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic Cardiovascular Risk Assessment in Elderly PeopleThe Role of Repeated N-Terminal Pro–B-Type Natriuretic Peptide Testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of epidemiology. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 14.Schellenbaum GD, Heckbert SR, Smith NL, et al. Congestive heart failure incidence and prognosis: case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–22. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 17.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical Validation of a High-Sensitivity Cardiac Troponin T Assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 18.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA : the journal of the American Medical Association. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28:177–182. doi: 10.1093/eurheartj/ehl449. [DOI] [PubMed] [Google Scholar]
- 20.Wu AHB, Lu QA, Todd J, Moecks J, Wians F. Short- and Long-Term Biological Variation in Cardiac Troponin I Measured with a High-Sensitivity Assay: Implications for Clinical Practice. Clin Chem. 2009;55:52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 21.Kalogeropoulos A PB, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PW, Newman AB, Harris TB, Butler J. Validation of the Health ABC Heart Failure Model for Incident Heart Failure Risk Prediction: The Cardiovascular Health Study. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.904300. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr., Larson MG, Massaro JM, Vasan RS. Predicting the 30-Year Risk of Cardiovascular Disease: The Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadir MA, Rekhraj S, Wei L, et al. Improving the Primary Prevention of Cardiovascular Events by Using Biomarkers to Identify Individuals With Silent Heart Disease. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 27.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 28.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 29.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. Jama. 2012;308:890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. Journal of the American College of Cardiology. 2004;44:878–886. doi: 10.1016/j.jacc.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 32.deFilippi CR, Seliger SL. Biomarkers for prognostication after acute coronary syndromes: new times and statistics. J Am Coll Cardiol. 2009;54:365–7. doi: 10.1016/j.jacc.2009.04.031. [DOI] [PubMed] [Google Scholar]