Abstract
Objective
Using a national database, we asked whether video-assisted thoracoscopic surgery (VATS) lobectomy is beneficial in high-risk pulmonary patients.
Background
Single-institution series demonstrated benefit of VATS lobectomy over lobectomy via thoracotomy in poor pulmonary function patients [FEV1 (forced expiratory volume in 1 second) or DLCO (diffusion capacity of the lung to carbon monoxide) <60% predicted].
Methods
The STS General Thoracic Database was queried for patients having undergone lobectomy by either thoracotomy or VATS between 2000 and 2010. Postoperative pulmonary complications included those defined by the STS database.
Results
In the STS database, 12,970 patients underwent lobectomy (thoracotomy, n = 8439; VATS, n = 4531) and met inclusion criteria. The overall rate of pulmonary complications was 21.7% (1832/8439) and 17.8% (806/4531) in patients undergoing lobectomy with thoracotomy and VATS, respectively (P < 0.0001). In a multivariable model of pulmonary complications, thoracotomy approach (OR = 1.25, P < 0.001), decreasing FEV1% predicted (OR = 1.01 per unit, P < 0.001) and DLCO% predicted (OR = 1.01 per unit, P < 0.001), and increasing age (1.02 per year, P < 0.001) independently predicted pulmonary complications. When examining pulmonary complications in patients with FEV1 less than 60% predicted, thoracotomy patients have markedly increased pulmonary complications when compared with VATS patients (P = 0.023). No significant difference is noted with FEV1 more than 60% predicted.
Conclusions
Poor pulmonary function predicts respiratory complications regardless of approach. Respiratory complications increase at a significantly greater rate in lobectomy patients with poor pulmonary function after thoracotomy compared with VATS. Planned surgical approach should be considered while determining whether a high-risk patient is an appropriate resection candidate.
Keywords: thoracoscopic lobectomy, pulmonary function, non–small cell lung cancer, major lung resection, outcomes
Lung cancer remains the leading cause of cancer-related deaths in the United States of America. Estimated to account for 226,160 new diagnoses of cancer in the year of 2012,1 only 20% to 25% of patients will have resectable disease, with approximately 30,000 pulmonary resections performed per year.2
Several studies have demonstrated the benefit of thoracoscopic lobectomy over lobectomy by thoracotomy. Both single-institution series3,4 and multi-institutional meta-analyses5,6 have demonstrated that thoracoscopic lobectomy is associated with fewer complications and decreased length of stay when compared with thoracotomy. It has also been suggested that thoracoscopy patients may enjoy improved overall survival.5
During the introduction of thoracoscopic lobectomy, patients were selected with early-stage disease and fewer operative risk factors. However, more recent series have documented safety in patients with larger tumors,3,7 patients after induction therapy,8 and benefit in patients who are elderly9–12 and those with poor pulmonary function.13–15
Pulmonary complications are the most common postoperative complication in patients undergoing noncardiac thoracic surgery.16,17 In their study on the outcomes of patients with postoperative respiratory failure after general and vascular surgery, Johnson et al found that respiratory failure predicted further complications such as myocardial infarction, pneumonia, acute renal failure, and deep venous thrombosis.18 In addition, among patients with postoperative respiratory failure, 26% died within 30 days of surgery.
Using a national, multi-institutional database, we sought to determine whether a respiratory benefit existed for high-risk patients undergoing thoracoscopic lobectomy over lobectomy by thoracotomy.
Methods
After approval was granted by a local institutional review board and by the Society of Thoracic Surgeons (STS) Database Access and Publications committee, the STS General Thoracic Database was queried for patients having undergone an anatomic pulmonary resection (either lobectomy or segmentectomy) by either thoracotomy or video-assisted thoracoscopic surgery (VATS) between 2000 and 2010. Patients undergoing additional procedures (sleeve resection, en bloc chest wall resection, bronchoplasty, pulmonary arterioplasty, diaphragm resection) and those with incomplete staging (T, N) and preoperative pulmonary function testing data were excluded. In addition, because of concern over data accuracy, patients with recorded forced expiratory volume in 1 second (FEV1) predicted less than 20% and diffusion capacity of the lung to carbon monoxide (DLCO) predicted less than 20% were excluded from the study.
Demographics, significant comorbidities, smoking status, use of induction therapy, and stage of disease were harvested and analyzed. The definitions of postoperative events were based on those defined by the STS database (http://www.sts.org/sections/stsnationaldatabase).
Univariate and multivariate analyses were performed relating operative morbidity to the following patient characteristics: age, sex, coronary artery disease, congestive heart failure, FEV1 predicted, DLCO predicted, use of induction therapy, diabetes, renal insufficiency, smoking status, pathologic stage, and operative approach. Two-sample t test was used to compare continuous data and χ2 test for categorical variables. Logistic regression was used for multivariate models. The SAS statistical package (SAS Institute, Cary, NC) was used for statistical analyses.
Results
In the STS database, the number of anatomic pulmonary resections increased each year (23 patients in 2001 vs 3599 patients in 2009). Similarly, the percentage of resections performed thoracoscopically increased over the study period (8% in 2003 vs 44.7% in 2010; Fig. 1). From these patients, we identified 18,585 patients who underwent anatomic pulmonary resection between January 1, 2000, and December 31, 2010. After excluding patients with incomplete staging and pulmonary function testing data, 12,970 patients were included in this study. All patients included in the study had a pulmonary resection for a primary lung cancer. A total of 12,255 (94.5%) patients underwent lobectomy (n = 7877 thoracotomy; n = 4378 VATS), and 715 (5.5%) patients underwent a segmentectomy (n = 562 thoracotomy; n = 153 VATS).
Figure 1.
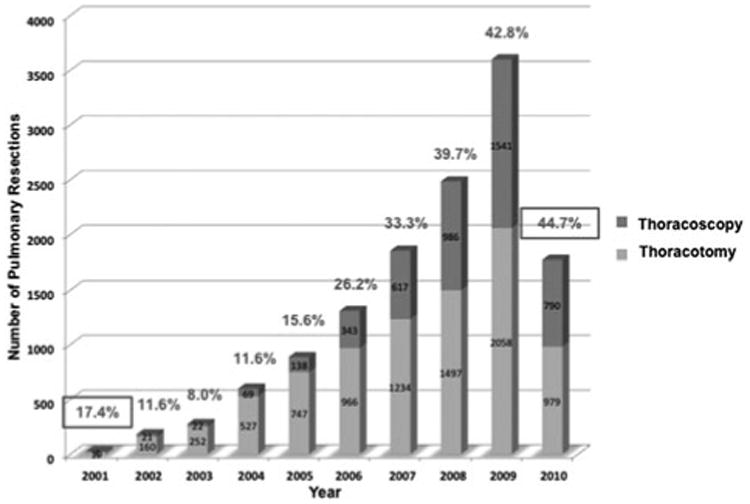
Volume of anatomic pulmonary resections in STS General Thoracic Database. The annual number of recorded anatomic pulmonary resections (either lobectomy or segmentectomy) in the STS database increased over the study period. In addition, the percentage of procedures performed thoracoscopically increased each year during the study period. Complete data for the year of 2010 were not available at the time of this study.
Demographic, baseline characteristics, pathologic staging, and comorbid conditions are shown in Table 1. Patients undergoing pulmonary resection were similar in the thoracotomy and thoracoscopy groups with regard to diabetes and preoperative renal function. Despite a slightly higher age, patients undergoing thoracoscopy were slightly healthier from a respiratory standpoint than patients undergoing thoracotomy: either never or former smokers (3399/4531 (75%) vs 6118/8439 (72.5%), P = 0.002), and had a higher predicted FEV1 (mean 83.7% vs 79.3%, P < 0.001) or DLCO (mean 74.9% vs 72.4%, P < 0.001). Patients with higher clinical stage and who received induction chemotherapy or radiation therapy were more likely to undergo resection via thoracotomy.
Table 1. Demographic and Preoperative Baseline Characteristics of Patients Undergoing Pulmonary Resection.
| Characteristic | Open n = 8439 n (%) | VATS n = 4531 n (%) | Total n = 12970 n (%) | P |
|---|---|---|---|---|
| Age at time of surgery | n = 8436 | n = 4525 | n = 12961 | 0.001 |
| Mean ± SD | 66.9 ± 10.4 | 67.5 ± 10.3 | 67.1 ± 10.4 | |
| Sex | n = 8432 | n = 4530 | n = 12962 | <0.001 |
| Female | 4193 (49.7) | 2529 (55.8) | 6722 (51.9) | |
| Male | 4239 (50.3) | 2001 (44.2) | 6240 (48.1) | |
| Diabetes | n = 8333 | n = 4483 | n = 12816 | 0.014 |
| 1384 (16.6) | 670 (14.9) | 2054 (16.0) | ||
| Creatinine ≥ 2.0 | 195 (2.3) | 93 (2.1) | 288 (2.2) | 0.34 |
| Coronary artery disease | n = 7497 | n = 4108 | n = 11605 | <0.001 |
| 1924 (25.7) | 887 (21.6) | 2811 (24.2) | ||
| Congestive heart failure | n = 7465 | n = 4095 | n = 11560 | 0.003 |
| 302 (4.0) | 121 (3.0) | 423 (3.7) | ||
| Current smoker | 0.002 | |||
| Never or past smoker | 6118 (72.5) | 3399 (75.0) | 9517 (73.4) | |
| Current smoker | 2321 (27.5) | 1132 (25.0) | 3453 (26.6) | |
| COPD | n = 2937 | n = 2258 | n = 5195 | <0.001 |
| 1083 (36.9) | 730 (32.3) | 1813 (34.9) | ||
| FEV predicted | <0.001 | |||
| Mean ± SD | 79.3 ± 20.4 | 83.7 ± 20.9 | 80.8 ± 20.7 | |
| DLCO predicted | <0.001 | |||
| Mean ± SD | 72.4 ± 21.4 | 74.9 ± 21.8 | 73.2 ± 21.6 | |
| Pathologic stage | n = 8097 | n = 4425 | n = 12522 | <0.001 |
| I | 5482 (67.7) | 3472 (78.5) | 8954 (71.5) | |
| II | 1337 (16.5) | 474 (10.7) | 1811 (14.5) | |
| III | 1080 (13.3) | 414 (9.4) | 1494 (11.9) | |
| IV | 198 (2.4) | 65 (1.5) | 263 (2.1) | |
| Preoperative chemotherapy | n = 7470 | n = 4082 | n = 11552 | <0.001 |
| 1155 (15.5) | 327 (8.0) | 1482 (12.8) | ||
| Preoperative X-ray therapy | n = 7475 | n = 4103 | n = 11578 | <0.001 |
| 890 (11.9) | 253 (6.2) | 1143 (9.9) |
Patients undergoing thoracotomy were more likely to have at least one pulmonary complication than those undergoing thoracoscopy [1832/8439 (21.7%) vs 806/4531 (17.8%), P < 0.001]. Atelectasis requiring bronchoscopy, postoperative pneumonia, adult respiratory distress syndrome, initial ventilatory support of more than 48 hours, and reintubation were more common following thoracotomy than thoracoscopy (Table 2).
Table 2. Postoperative Pulmonary Events.
| Open | VATS | Total | ||
|---|---|---|---|---|
| n = 8439 | n = 4531 | n = 12970 | ||
| Characteristic | n (%) | n (%) | n (%) | P |
| Pulmonary complication (at least one) | 1832 (21.7) | 806 (17.8) | 2638 (20.3) | <0.001 |
| Atelectasis requiring bronchoscopy | 369 (4.4) | 121 (2.7) | 490 (3.8) | <0.001 |
| Postoperative pneumonia | 382 (4.5) | 148 (3.3) | 530 (4.1) | <0.001 |
| Adult respiratory distress syndrome | 101 (1.2) | 28 (0.6) | 129 (1.0) | 0.002 |
| Bronchopleural fistula | 45 (0.5) | 14 (0.3) | 59 (0.5) | 0.070 |
| Initial vent support >48 h | 69 (0.8) | 16 (0.4) | 85 (0.7) | 0.002 |
| Reintubation | 372 (4.4) | 119 (2.6) | 491 (3.8) | <0.001 |
| Tracheostomy | 110 (1.3) | 42 (0.9) | 152 (1.2) | 0.057 |
| Other pulmonary event | 327 (3.9) | 171 (3.8) | 498 (3.8) | 0.78 |
On univariate analysis using the variables listed in Table 3, increasing age, male gender, thoracotomy approach, decreasing FEV1 predicted and decreasing DLCO predicted, preoperative renal insufficiency, smoking status, coronary artery disease, congestive heart failure and chronic obstructive pulmonary disease independently predicted pulmonary complications. On multivariable logistic regression with presence of at least 1 pulmonary complication as the outcome, increasing age (odds ratio 1.02 per year, P < 0.001), decreasing FEV1 predicted (odd ratio 1.01 per unit, P < 0.001), decreasing DLCO predicted (odds ratio 1.01 per unit, P < 0.001), preoperative creatinine ≥ 2.0 (odds ratio 1.76, P < 0.001), and current smoking (odds ratio 1.56, P < 0.001) remained significant predictors of pulmonary morbidity (Table 3). When all of these factors were controlled, surgical approach remained a significant predictor of pulmonary complications.
Table 3. Univariate and Multivariate Analysis Relating Pulmonary Complications to age, Gender, Pulmonary Function, Diabetes, Preoperative Renal Function, Coronary Artery Disease, Congestive Heart Failure, COPD, Pathologic Stage and Induction Chemotherapy, or Radiotherapy.
| Univariate | Multivariate (n = 11123) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P |
| Approach | <0.001 | <0.001 | ||||
| Open | 1.00 | 1.00 | ||||
| VATS | 0.699 | (0.622, 0.785) | 0.797 | (0.702, 0.906) | ||
| Age at time of surgery | 1.018 | (1.013, 1.024) | <0.001 | 1.026 | (1.019, 1.032) | <0.001 |
| Sex | <0.001 | 0.002 | ||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.267 | (1.140, 1.408) | 1.210 | (1.072, 1.366) | ||
| FEV predicted | 0.982 | (0.979, 0.984) | <0.001 | 0.989 | (0.985, 0.992) | <0.001 |
| DLCO predicted | 0.980 | (0.977, 0.983) | <0.001 | 0.986 | (0.983, 0.990) | <0.001 |
| Creatinine ≥ 2.0 | <0.001 | <0.001 | ||||
| Creatinine <2.0 | 1.00 | 1.00 | ||||
| Creatinine ≥ 2.0 | 2.111 | (1.591, 2.801) | 1.760 | (1.299, 2.385) | ||
| Diabetes | 0.035 | 0.56 | ||||
| No diabetes | 1.00 | 1.00 | ||||
| Diabetes | 1.163 | (1.012, 1.337) | 1.046 | (0.900, 1.216) | ||
| Current smoker | <0.001 | <0.001 | ||||
| Never or past smoker | 1.00 | 1.00 | ||||
| Current smoker | 1.558 | (1.393, 1.742) | 1.557 | (1.368, 1.772) | ||
| Coronary artery disease | <0.001 | 0.16 | ||||
| 0 = No | 1.00 | 1.00 | ||||
| 1 = Yes | 1.385 | (1.227, 1.564) | 1.103 | (0.964, 1.262) | ||
| Congestive heart failure | 0.003 | 0.29 | ||||
| 0 = No | 1.00 | 1.00 | ||||
| 1 = Yes | 1.505 | (1.164, 1.947) | 1.161 | (0.884, 1.526) | ||
| COPD | <0.001 | - | - | - | ||
| 0 = No | 1.00 | - | - | - | ||
| 1 = Yes | 2.031 | (1.712, 2.409) | - | - | - | |
| Pathologic stage | 0.019 | 0.032 | ||||
| I | 1.00 | 1.00 | ||||
| II | 1.166 | (1.002, 1.356) | 1.095 | (0.930, 1.289) | ||
| III | 1.244 | (1.059, 1.461) | 1.258 | (1.057, 1.498) | ||
| IV | 1.234 | (0.863, 1.764) | 1.383 | (0.951, 2.011) | ||
| Preoperative chemotherapy | 0.24 | 0.64 | ||||
| 0 = No | 1.00 | 1.00 | ||||
| 1 = Yes | 1.103 | (0.939, 1.295) | 0.947 | (0.756, 1.187) | ||
| Preoperative X-ray therapy | 0.21 | 0.015 | ||||
| 0 = No | 1.00 | 1.00 | ||||
| 1 = Yes | 1.231 | (1.035, 1.465) | 1.356 | (1.064, 1.728) | ||
In examining patients with limited pulmonary function (defined as FEV1 < 60% predicted), thoracotomy patients had increasing pulmonary complications with decreasing FEV1 predicted when compared with patients approached with thoracoscopy (Fig. 1, P = 0.023). A significant difference in rate of pulmonary complications between thoracotomy and thoracoscopy patients was not noted with FEV1 greater than 60% predicted.
Discussion
In this study, we demonstrate that the thoracotomy approach independently predicted pulmonary complications in patients undergoing pulmonary resection as compared to thoracoscopy. Moreover, in patients with limited pulmonary function (defined as FEV1 predicted <60%), the rate of increase in pulmonary complications with decreasing FEV1 predicted in patients undergoing resection by thoracotomy was significantly higher than in patients undergoing resection by thoracoscopy. Therefore, while patients with limited pulmonary function are at an elevated risk of pulmonary complications regardless of approach, they were less likely to have a pulmonary complication if they were approached by thoracoscopy.
McKenna et al19 published the first series on the feasibility and safety of thoracoscopic lobectomy. Since then, numerous large-scale single institution3,4,20 and multi-institution5,6,21,22 studies have repeatedly demonstrated the benefit of thoracoscopic lobectomy over lobectomy by thoracotomy in terms of decreased postoperative complications (atrial fibrillation, pneumonia, respiratory failure, prolonged air leak), chest tube duration, overall length of stay, and decreased hospital cost with the same overall and disease-free survival. The present study confirms that patients undergoing thoracoscopic lobectomy had significantly fewer pulmonary complications than those undergoing lobectomy by thoracotomy (17.8% vs 21.7%, P < 0.001). Although the rate of pulmonary complications and the benefit of thoracoscopy on pulmonary complications in this study are lower than in prior single-institution series,13,14,15 our results are similar to other multi-institution studies comparing thoracoscopic lobectomy with open lobectomy. Paul et al6 reported a 4.6% benefit (7.6% vs 12.2%, P = 0.001) on all pulmonary complications and Swanson et al22 noted a 1% benefit on pneumonia in patients undergoing lobectomy by thoracoscopy. In addition, VATS lobectomy techniques (number of incisions, length of incision, use of rib spreaders) and postoperative management (pain management regimen, pulmonary care-maps) are not standardized in multi-institution databases, which may also account for the differences in rate of pulmonary complications and benefit of thoracoscopy on pulmonary complications reported in single-institution series and multi-institution studies.
Preoperative pulmonary function tests (PFTs) are used to assess risk of pulmonary complications after lobectomy, with FEV1 and DLCO being the most useful predictors of postoperative morbidity.23 Unfortunately, patients with limited pulmonary function have the highest incidence of lung cancer.24 This study reported a lower pulmonary complication rate for patients undergoing thoracoscopic resection with an FEV1 predicted of 20% to 30% than those with an FEV1 predicted of 30% to 40% (Fig. 2). However, this result is similar to a prior study by Berry et al13 that demonstrated that while PFTs predict pulmonary complications in patients undergoing lobectomy by thoracotomy, this correlation is not as strong in patients with poor pulmonary function undergoing thoracoscopic lobectomy. This finding is likely in part due to decreased pain and preservation of chest wall mechanics in patients undergoing thoracoscopy. Further studies have suggested that postoperative pulmonary function is preserved after thoracoscopic lobectomy.25 Because of these data and the findings of the present study, we strongly believe that patients with marginal pulmonary function should be referred for minimally invasive resection and that thoracotomy should be avoided when technically feasible. We recognize that there remain situations where a thoracoscopic approach may not be feasible (ie, large tumors, proximal tumors requiring intrapericardial control of vascular structures).
Figure 2.
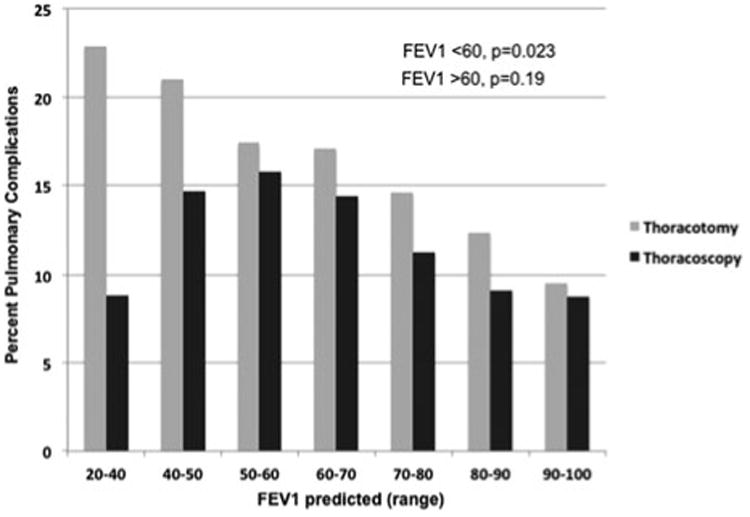
Respiratory complications by FEV1 predicted. In patients with FEV1 predicted less than 60%, the rate of increase in pulmonary complications with decreasing FEV1 predicted is significantly greater in patients undergoing pulmonary resection by thoracotomy than in patients undergoing thoracoscopy (P = 0.023).
In 2012, patients with limited pulmonary function who are deemed high operative risk are often referred for sublobar resection, radiofrequency ablation (RFA), or stereotactic body radiation therapy. Sublobar resection (segmentectomy or wedge) has been discouraged in patients who can tolerate a lobectomy due to higher local recurrence rates.26 More recent studies, however, have demonstrated that an anatomic segmentectomy or extended wedge resection with particular attention paid to surgical margin and lymph node assessment can have equivalent results to lobectomy.27,28,29 Although there is no dispute that a sublobar resection should only be undertaken in patients who are unable to tolerate a lobectomy, an anatomic sublobar resection is a reasonable oncologic approach in high-risk patients. Our study would point to the benefits of a minimally invasive approach.
Nonsurgical approaches are gaining favor because of the perceived operative risk of lobectomy. Although RFA is generally associated with a low morbidity and mortality rate, oncologic efficacy is not optimal. A study of RFA of 18 pulmonary lesions that were subsequently resected for pathologic analysis demonstrated that only 39% of lesions were completely ablated and only 50% of patients had more than 90% tumor ablation.30 Local recurrence rates as high as 38% to 42%31,32 have been reported, and higher rates are reported in the treatment of lesions larger than 3 cm.32 A recent study on stereotactic body radiation therapy reported 0% mortality in comparison with 10% mortality in historical patients treated surgically.33 This study also reported 89% local control at 3-years and overall survival that was equivalent to patients treated surgically. These impressive early results require longer-term follow-up because of inability to treat interlobar nodal metastases or to provide access for hilar or mediastinal lymph node staging. Prior studies have demonstrated that even peripheral lesions smaller than 1 cm have a 7% incidence of occult nodal spread.34 Given these considerations, we believe that our data justify thoracoscopic anatomic resection for many of these high-risk patients.
As Figure 2 demonstrates, patients with FEV1 less than 60% predicted disproportionately benefit from a minimally invasive approach to resection. In fact, the rate of pulmonary complications decreases as respiratory function worsens in the thoracoscopy group. The reasons for this are unclear, but possible explanations are many. These include possible performance of thoracoscopic lobectomy on poor-PFT patients in high-volume centers with low complication rates and possible extra attention to these high-risk patients postoperatively. An analysis focused on high-volume centers may be entertained in the future to evaluate these issues.
We recognize that there are important limitations to this analysis. There are inherent weaknesses in any large voluntary national database regarding data accuracy and missing data. However, of the patients included in the study, only 1.3% had missing data in variables used during univariate and multivariate analyses and were excluded from these analyses. The STS database does not mandate technique, and there may be institutional differences in thoracoscopic techniques (number of port sites, use of a rib spreader). However, no one institution disproportionately contributed to the data set. When examining data in a very particular subset of patients, such as patients with poor pulmonary function, inherent biases in patient selection may exist. A randomized trial of thoracoscopic and open lobectomy will never be performed. Propensity matching reduces selection bias but does not eliminate it. We believe that our multivariable model controls for most factors involved in patient survival. Next, complications are self-reported in the database and, therefore, may be underreported. Once again, because no one institution disproportionately contributed to the data set, this potential underreporting bias should be equal between the thoracotomy and thoracoscopy groups. Surgeon volume and experience may play a role in outcomes following thoracoscopic lobectomy, and higher hospital volume may result in improved outcomes for high-risk pulmonary patients. Although no one institution or surgeon disproportionately contributed to the data set, our analysis does not take these factors into account. Future studies may investigate the effect of surgeon experience and hospital volume on thoracoscopic versus thoracotomy lobectomy by treating these variables as instrumental variables. Last, our study does not include long-term outcomes, as these data are not included in the STS database.
Despite the growing evidence of the benefits of thoracoscopic lobectomy over the open approach, this approach accounts for only 20% to 30% of pulmonary resections.22,35 This has increased to above 40% in the last year of the present study. Difficulty in teaching and learning this operative approach36 and fear of poor hemorrhage control have been cited as barriers to the adoption of this surgical technique. As more trainees from programs with high-volume thoracoscopic institutions graduate from training, however, there will be a growing number of surgeons who will be increasingly comfortable with the thoracoscopic lobectomy.37 In addition, the robotic platform may serve as an alternative minimally invasive approach for open surgeons who were initially resistant to learning the technique of thoracoscopic lobectomy.
Our study provides further support of increasing the application of thoracoscopic lobectomy. In particular, to our knowledge, our study is the first multi-institutional study to demonstrate a benefit from thoracoscopic lobectomy over the open approach in higher-risk patients with limited pulmonary function. In patients with limited pulmonary function who may not be offered surgical resection, approach should be considered before referring patients for alternative therapy. Moreover, in patients with poor pulmonary function being considered for surgical resection, thoracotomy should be avoided in favor of thoracoscopy when technically feasible.
Discussant
Dr. Douglas Wood (Seattle, WA)
There are a couple of points that I would like to emphasize. First is the importance of a physician-run clinical database. In 1989, the Society of Thoracic Surgeons (STS) had the foresight to develop an adult cardiac surgical database; investing substantial STS funds before outcomes data were fashionable and at a time when clinical outcomes were not yet scrutinized.
Today, nearly every cardiac surgeon in the United States is a member and nearly all cardiac surgery operations are captured, now totaling over 4.7 million cardiac surgery cases. The STS database is a model of voluntary clinical data collaboration, has resulted in marked continuous improvements in patient outcomes, and is now used by the government, insurers, industry, and physician groups to evaluate and improve the delivery of cardiothoracic surgery.
The STS has now extended clinical databases to include general thoracic surgery and congenital cardiac surgery. Although the general thoracic database utilized in this research has not yet reached the penetration or maturity of the cardiac database, there are now 222 sites, representing 781 surgeons and 312,000 surgical cases.
The STS also recognized that some general surgeons practicing thoracic surgery would benefit from participation, and extended the STS general thoracic database to include general surgeons, several of whom have chosen to join. At a time when cardiothoracic surgery has been challenged on many fronts, the presence of high-quality clinical databases that allow this type of research and quality assessment has been a powerful tool in health services research and the development of partnerships that have ultimately benefited the patients we serve.
I wish also to recognize the benefits of surgical advances, in this case, minimally invasive thoracic surgery, on the patients that need it most; those at high risk for surgical intervention. As Dr. Ceppa and colleagues have pointed out, those patients with poor pulmonary reserve, frequently a majority of our lung resection patients are the ones that benefit the most from VATS lobectomy, and, in fact, patients with better pulmonary function do not show significant differences in morbidity and mortality when comparing VATS to open techniques.
Dr. Ceppa, I have two questions for you. You have done a good job in your multivariate analysis to show that the benefit of VATS technique is independent of other clinical variables. Thoracotomy patients were more likely to have other significant comorbidities, lower pulmonary function, higher cancer stage, and more frequent preoperative chemotherapy and radiation. However, once you corrected for this, although the benefit of VATS remained independently significant, the difference on minimizing pulmonary complications was fairly small, 22% versus 18%.
Were you surprised by the relatively modest benefits conferred by VATS, given the more impressive reports from several single- institution studies comparing the two techniques?
Although you did a good job of correcting for other clinical variables, you have not analyzed the impact of individual surgeon experience or hospital volume on these outcomes. It is clear that VATS lobectomies are generally performed by higher volume and more experienced surgeons, and so I would venture a hypothesis that your results may be skewed by the surgeons and institutions performing VATS resections. In fact, the benefit may be related to surgeon experience and volume rather than the surgical technique. Have you considered this unmeasured, but potentially confounding variable? Is there a way you can distinguish between the surgical approach and the surgeons who utilize that approach? Is it VATS, or is it the surgeons who use VATS that really count?
Dr. Duykhanh Ceppa
I could not agree with you more about the importance of physician-run clinical databases, especially in this environment of pay for performance that we are progressively entering.
To answer your question, I am not surprised; obviously, that we have demonstrated there is a benefit to thoracoscopic lobectomy in patients over thoracotomy, and I am certainly not surprised that there is more of a benefit in patients with limited pulmonary function.
To be quite honest, I do not know how to specifically address the question of whether or not I am surprised at the benefit that we saw is numerically smaller than that found by other institutions. Whenever we investigate single-institution findings on a multi-institutional level, there is typically a “watering-down” on account of the variability of differing institutions. The data, unfortunately, are what they are, and the message still remains the same, that thoracoscopy is better than thoracotomy.
Now, to address your other question regarding the effect of surgeon experience and higher-volume institutions in looking at our data set, no one institution, no single surgeon disproportionately contributed to the data set, and to the best of my knowledge, this bias does not exist. However, this could be further investigated by evaluating the surgeon or institution as an instrument variable in future studies.
Discussant
Dr. Stephen Swisher (Houston, Texas)
I was wondering if you knew the conversion rate from lobectomy, or from thoracoscopic lobectomy to open lobectomy, and if so, how did you group those patients? Were they grouped under the open lobectomy or the thoracoscopic group?
Dr. Duykhanh Ceppa
The STS database basically just categorizes patients as having undergone thoracoscopy or thoracotomy. Depending on the version of the database, some cases were categorized as both. Unfortunately, one cannot easily decipher whether a case that was categorized as both was a wedge for diagnosis with a planned thoracotomy for resection or a conversion from VATS to open. Therefore, in short, there is not an easy way to delineate the number of cases that started off as VATS and were converted to thoracotomies. Finally, these patients were likely grouped in the thoracotomy group.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Buie VC, Owings MF, DeFrances CJ, et al. National Hospital Discharge Survey: 2006 Summary. Hy-attsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2010. (Vital and Health Statistics, Series 13, No. 168). [PubMed] [Google Scholar]
- 3.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg. 2006;81:421–426. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 4.Villamizar N, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–425. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2018. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol. 2011;18:3732–3736. doi: 10.1245/s10434-011-1834-9. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg. 2006;82:214–219. doi: 10.1016/j.athoracsur.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 9.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88:1093–1099. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothor Surg. 2011;39:989–994. doi: 10.1016/j.ejcts.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramification of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg. 2011;92:1951–1957. doi: 10.1016/j.athoracsur.2011.06.082. [DOI] [PubMed] [Google Scholar]
- 12.Amer K, Khan AZ, Vohra H, et al. Is it safe to include octogenarians at the start of a video-assisted thoracic surgery lobectomy programme? Eur J Cardiothorac Surg. 2012;41:346–352. doi: 10.1016/j.ejcts.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg. 2010;89:1044–1052. doi: 10.1016/j.athoracsur.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient: the benefits of avoiding the gold standard. Eur J Cardiothorac Surg. 2010;38:6–13. doi: 10.1016/j.ejcts.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Kachare S, Dexter EU, Nwogu C, et al. Perioperative outcomes of thoracoscopic anatomic resections in patients with limited pulmonary reserve. J Thorac Cardiovasc Surg. 2011;141:459–462. doi: 10.1016/j.jtcvs.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson MK, Gaisset HA, Grab JD, et al. Pulmonary Complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg. 2009;138:1297–1301. doi: 10.1016/j.jtcvs.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Agostini P, Cleslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65:815–818. doi: 10.1136/thx.2009.123083. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–1198. doi: 10.1016/j.jamcollsurg.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 19.McKenna RJ. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac and Cardiovasc Surg. 1994;107:879–881. [PubMed] [Google Scholar]
- 20.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–425. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson SJ, Herndon JE, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: a report of the CALGB 39802: a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–4997. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 22.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoraco-scopic lobectomy is less costly and morbid than open lobectomy: a retrospective multi-institutional database analysis. Ann Thorac Surg. 2012;93:1027–1032. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson MK. Preoperative assessment of pulmonary risk. Chest. 1999;115:58S–63S. doi: 10.1378/chest.115.suppl_2.58s. [DOI] [PubMed] [Google Scholar]
- 24.Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25-year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev. 1994;3:289–298. [PubMed] [Google Scholar]
- 25.Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg. 2000;70:1644–1646. doi: 10.1016/s0003-4975(00)01909-3. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 27.Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of non-small cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2004;77:415–420. doi: 10.1016/S0003-4975(03)01511-X. [DOI] [PubMed] [Google Scholar]
- 28.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 29.Shuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–933. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Schneider T, Warth A, Herpel E, et al. Intraoperative radiofrequency ablation of lung metastases and histologic evaluation. Annals of Thorac Surg. 2009;87:379–384. doi: 10.1016/j.athoracsur.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 31.Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2007;134:857–864. doi: 10.1016/j.jtcvs.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 32.Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg. 2012;92:921–988. doi: 10.1016/j.athoracsur.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Palma D, Lagerwald F, Rodrigues G, et al. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Miller DL, Rowland CM, Deschamps C, et al. Surgical treatment of non-small cell lung cancer 1cm or less in diameter. Ann Thorac Surg. 2002;73:1541–1545. doi: 10.1016/s0003-4975(02)03525-7. [DOI] [PubMed] [Google Scholar]
- 35.Boffa DJ, Allen MS, Grab JD, et al. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg. 2010;34:2368–2372. doi: 10.1007/s00268-010-0661-7. [DOI] [PubMed] [Google Scholar]
- 37.Boffa DJ, Gangadharan S, Kent M, et al. Self-perceived video-assisted thoracic surgery lobectomy proficiency by recent graduates of North American thoracic residencies. Interact Cardiovasc Thorac Surg. 2012;14:797–800. doi: 10.1093/icvts/ivr098. [DOI] [PMC free article] [PubMed] [Google Scholar]


