Abstract
PILRα is an immune inhibitory receptor possessing an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain enabling it to deliver inhibitory signals. Binding of PILRα to its ligand CD99 is involved in immune regulation; however, whether there are other PILRα ligands in addition to CD99 is not known. Here, we report that a novel molecule, PILR-associating neural protein (PANP), acts as an additional ligand for PILRα. Transcription of PANP was mainly observed in neural tissues. PILRα-Ig fusion protein bound cells transfected with PANP and the transfectants stimulated PILRα reporter cells. Specific O-glycan structures on PANP were found to be required for PILR recognition of this ligand. These results suggest that PANP is involved in immune regulation as a ligand of the PILRα.
Keywords: O-glygan, Sialic acids, Paired receptor, Immune regulatory molecule
1. Introduction
Paired receptors are transmembrane-anchored proteins closely related to one another in their extracellular domains, characterized by one receptor transducing inhibitory signals, and the other activating signals [1-3]. Inhibitory receptors possess an immunoreceptor tyrosine-based inhibitory motif (ITIM) in their cytoplasmic domain, whereas activating receptors have short cytoplasmic domains, but associate with adapter molecules having immunoreceptor tyrosine-based activating motifs (ITAM). Paired receptors are mainly expressed on immune cells such as NK cells and macrophages. Most inhibitory receptors recognize self-antigens such as MHC class I, whereas their corresponding activating receptors typically show weak or no affinity for self antigens [4]. Interestingly, some viral proteins interact with inhibitory or activating receptors [5-7]; such interactions seem to play an important role in host resistance to viral infection.
The paired immunoglobulin-like type 2 receptors (PILR) comprise the inhibitory receptor PILRα and the activating receptor PILRβ [8]. We previously cloned PILRβ from a cDNA library of mouse NK cells as a molecule that associates with the ITAM-containing DAP12 adapter protein. PILRs are well-conserved among most mammals [9]. The inhibitory PILRα is mainly expressed on macrophages, dendritic cells, and granulocytes, whereas the activating PILRβ is mainly on activated NK cells [8]. Both mouse PILRα and PILRβ recognize mouse CD99 as a ligand. However, the affinity of binding of activating PILRβ with CD99 is much lower than with PILRα; this is similar to the situation with other paired receptors [10]. Mouse NK cells expressing PILRβ mediate cytotoxicity against CD99-positive target cells, suggesting that this receptor may be involved in NK cell recognition [8]. Sialylated O-linked sugar chains on CD99 are essential for the recognition of CD99 by PILR [11]. Point mutations of two O-glycosylation sites on CD99 significantly reduced its recognition by PILRα, which also binds herpes simplex virus (HSV-1) glycoprotein B in an O-glycan-dependent manner and is involved in HSV-1 entry into the cells [7,12,13]. Hence, interactions between PILRα and its ligands are involved in both immune regulation and viral infection. Therefore, it is important to understand ligand recognition by PILRα.
Here, we report a new ligand for PILRα, PILR-associating neural protein (PANP), the function of which has not yet been characterized. Because PANP is mainly expressed in neural tissues and is well conserved in most mammals, the PILRα–PANP interaction might be involved in immune regulation in neural tissues.
2. Materials and methods
2.1. Retrovirus cDNA library construction
A retrovirus cDNA library was constructed as reported previously [14]. Briefly, cDNA was generated from mRNA purified from the B16 melanoma cell line using the Superscript plasmid system (Invitrogen) and was cloned into Sal I and Not I sites of the pMx-SalI retrovirus vector [14]. The complexity of the cDNA library was more than 1 × 106.
2.2. Cloning of PANP and generation of PANP transfectants
Twenty million Ba/F3 cells were transfected with a retrovirus cDNA library from B16 cells with an efficiency of approximately 30%. Two days after infection, these transfected cells were stained with a PILRα-Ig fusion protein and PE-conjugated goat anti-human IgG and sorted using a FACSAria. After three rounds of sorting, single cell clones that were recognized by PILRα-Ig were isolated. Library-derived genes were amplified by PCR by using sense primer (5′-GGTGGACCATCCTCTAGACT-3′) and antisense primer (5′-TTTATTTTATCGTCGATCGACC-3′). Amplified cDNA was cloned into the pMx-SalI retrovirus vector, and nucleic acid sequences were determined. PANP cDNA (GenBank accession number: NM_175696) cloned into pMx-SalI retrovirus expression vector was subcloned into pME18S expression vector. Human PANP (GenBank accession number: NM_153685) cDNA was amplified by PCR and cloned into pME18S expression vector. They were transfected into 293T cells using PEI MAX (Polyscience).
2.2.1. IgG fusion proteins
PILRα-Ig and PILRβ-Ig fusion proteins have been described previously [8,15]. The cDNA-encoding the extracellular domain of PANP (amino acid residues 28–178) was cloned into an expression vector for Ig-fusion protein production [8]. These plasmids were transfected into COS-7 cells using PEI MAX and the culture supernatants were collected after 72 h.
2.2.2. Anti-PANP mAb
Wistar rats (Japan SLC) were immunized with mouse PANP-Ig fusion protein using TiterMax Gold (TiterMax) as an adjuvant. Two weeks after immunization, lymph node cells were fused with SP2/0, and single cell clones producing specific mAb that recognize mouse PANP-transfected Ba/F3 cells were obtained.
2.2.3. Reporter cell assays
NFAT-GFP reporter cells expressing DAP12 have been generated using Ba/F3 cells as previously described [5]. The cDNAs encode the extracellular domain of PILRα (amino acid residues 29–200) and the transmembrane and intracellular domains of PILRβ (amino acid residues 192–225). Chimeric PILRα- and wild-type PILRβ-expressing reporter cells (5 × 104 cells) were cocultured with PANP- or CD99-transfected cells (5 × 104 cells) for 16 h and GFP expression was analyzed by flow cytometry.
2.2.4. Real-time quantitative PCR
Quantitative PCR was performed with a SYBER green PCR kit (QIAGEN) and analyzed on an iCycler (Bio-Rad Laboratories). Primers used for amplification were as follows: β-actin, sense primer (5′-TCTACAATGAGCTGCGTGTG-3′) and antisense primer (5′-GGTACGACCAGAGGCATACA-3′); mouse PANP, sense primer (5′-GCTCAACTGGTGTCCCAACT-3′) and antisense primer (5′-ACAA CCTGCCGACGTGATCTT-3′); human PANP, sense primer (5′-GGCCA TTCCTGTTCGGGGGC-3′) and antisense primer (5′-CCTGCCTC-AGG GCACCTTGC-3′).
2.2.5. Neuraminidase treatment
PANP-transfected Ba/F3 and mock-transfected Ba/F3 cells were incubated in the presence of neuraminidase from Arthrobacter ureafaciens (1 U/ml, Roche) at 37 °C for 3 h. Thereafter, cells were analyzed by flow cytometry.
2.2.6. Mutagenesis
PANP mutants were generated using QuikChange Multi Site-Directed Mutagenesis Kits (Stratagene).
2.2.7. Surface biotinylation and immunoprecipitation
Cell surface biotinylation was performed as described previously [16]. Briefly, PANP-transfectants were washed with ice-cold PBS and were incubated with sulfo-NHS-LC-biotin (Pierce Biotechnology) at 100 μg/ml for 15 min at room temperature. Thereafter, biotinylated cells were washed with ice-cold PBS and were disrupted with lysis buffer. Flag-PANP was immunoprecipitated by protein G-coupled Sepharose preincubated with anti-Flag M2 mAb. The precipitates were separated on 5–20% SDS–PAGE gels under reducing condition and were transferred onto polyvinylidene difluoride membranes. Biotinylated proteins were detected by using peroxidase-conjugated streptavidin (Sigma–Aldrich).
3. Results
We have previously shown that both PILRα and PILRβ recognize CD99 expressed on the EL4 lymphoma cell line. However, when we analyzed PILR-ligand expression on several cell lines using PILRα-IgG Fc fusion protein (PILRα-Ig), we found that B16 melanoma cells were stained by PILRα-Ig despite their lack of expression of CD99 (Fig. 1A). This suggested that PILRα recognizes molecules on B16 cells other than CD99. In order to identify these molecules, we employed expression cloning using a retrovirus cDNA library from B16 cells. We introduced this library into Ba/F3 cells, which do not express PILRα ligands. We then sorted the cells stained with PILRα-Ig and obtained single cell clones that were stained with PILRα-Ig (Fig. 1B). cDNA derived from the B16 library were then amplified by PCR and were sequenced. This revealed a novel gene, which we designated PANP (official gene name C530028O21Rik). We transfected this cDNA into 293T cells and found that they were specifically stained with PILRα-Ig (Fig. 1D). This gene encodes a membrane protein that is highly conserved among mammals (Fig. 1C). As in mice, human PILRα-IgG fusion protein also bound to human PANP transfectants (Fig. 1D). PANP expression on the 293T cell surface was confirmed by staining with an anti-PANP mAb that recognize both human and mouse PANP (Fig. 1D). Similarly, PANP-Ig fusion protein bound to mouse PILRα correspondingly (Fig 1E). These data indicated that PANP is specifically recognized by PILRα.
Fig. 1.
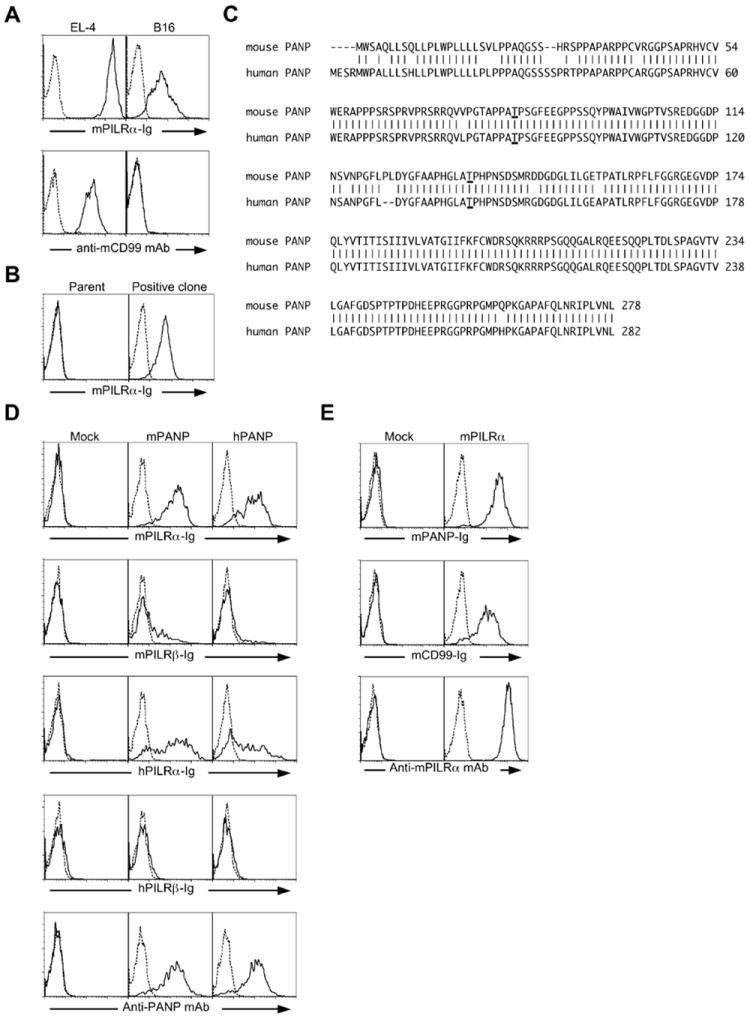
Molecular cloning of PANP as an additional ligand for PILRα. (A) EL-4 and B16 cells were stained with mouse PILRα-Ig (mPILRα, solid line) or control-Ig (dashed line). These cell lines were also stained with anti-mouse CD99 (mCD99, solid line) or control mAb (dashed line). (B) A single cell clone derived from Ba/F3 cells transfected with a B16 cDNA retroviral library was stained with mouse PILRα-Ig (mPILRα, solid line) or control-Ig (dashed line). (C) Amino acid sequences of mouse and human PANP. GenBank accession numbers for human and mouse PANP are NM_175696 and NM_153685, respectively. Possible O-glycosylation sites at threonines 83 and 136 were underlined. (D) Mouse PANP (mPANP), human PANP (hPANP) or mock-transfected Ba/F3 cells were stained with mouse or human PILR-Ig (mPILR-Ig or hPILR-Ig, solid line) or control-Ig (dashed line). The transfectants were also stained with anti-PANP mAb (solid line) or control mAb (dashed line). (E) Mouse PILRα (mPILRα) transfected 293T cells were stained with mouse PANP-Ig (mPANP-Ig, solid line), mouse CD99-Ig (mCD99-Ig, solid line), or control-Ig (dashed line). The transfectants were also stained with anti-PILRα mAb (solid line) or control mAb (dashed line).
We have previously shown that sialylated O-glycan structures on CD99 are essential for both PILRα and PILRβ binding to this molecule. In order to analyze the role of PANP glycans for PILRα binding, we first tested the effects of neuraminidase treatment of PANP. As shown in Fig 2A, the treatment completely abrogated the recognition of PANP by PILRα without affecting the cell surface expression of PANP itself. These data indicate that sialylated glycans on PANP are required for its binding to PILRα.
Fig. 2.
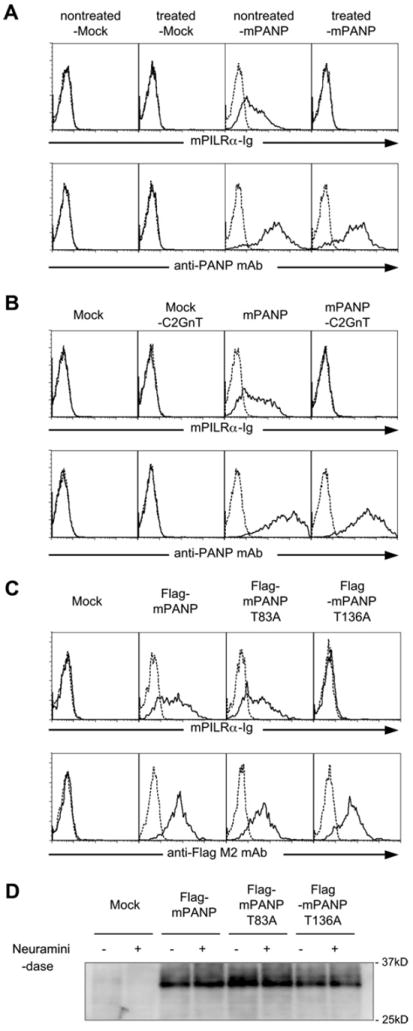
Sialic acids on PANP are essential for PANP recognition by PILRα. (A) Mouse PANP-transfected 293T cells (-mPANP) or mock-transfectants (-Mock) were treated with neuraminidase for 30 min at 37 °C. The neuraminidase-treated cells were stained with mouse PILRα-Ig (mPILRα-Ig, solid line), control-Ig (dashed line), anti- PANP mAb (solid line) or control mAb (dashed line). (B) Mock- or mouse PANPtransfected 293T cells (Mock or mPANP) were further transfected with C2GnT or control vector. The transfectants were stained with mouse PILRα-Ig (mPILRα-Ig, solid line), control-Ig, anti-PANP mAb (solid line) or control mAb (dashed line). (C) Mock-, Flag-PANP-, Flag-PANP-T83A-, or Flag-PANP-T136A-transfected 293T cells were stained with mouse PILRα-Ig (mPILRα-Ig, solid line), control-Ig (dashed line), anti-Flag-M2 mAb (solid line) or control mAb (dashed line). (D) Western blot analysis of neuraminidase-treated cells. Mock-, Flag-PANP, Flag-PANP-T83A or Flag- PANP-T136A-transfected 293T cells were incubated in the presence (±) or absence (−) with neuraminidase. Cell-surface molecules were biotinylated, and lysates of cells were precipitated with anti-Flag M2 mAb. The precipitates were separated by SDS–PAGE, and biotinylated proteins were detected by using peroxidase-conjugated streptavidin.
We previously reported that C2GnT (which generates the core 2 O-glycan branch) inhibits recognition of mouse CD99 by PILRα. This suggested that specific sialylated O-glycan structures on CD99 need to be recognized by PILR. In order to test whether core 2 O-glycans also inhibit the recognition of PANP by PILRα, we transfected C2GnT into PANP-expressing 293T cells. As shown in Fig. 2B, expression of C2GnT markedly reduced the binding of PILRα to PANP-expressing cells (Fig. 2B).
O-glycosylation is limited to threonine or serine residues [17]. We analyzed PANP using the NetOGlyc 3.1 algorithm (www.cbs.dtu.dk/services/NetOGlyc/) [18], which predicted that several serine or threonine residues were possible O-glycosylation sites. It has been proposed that areas rich in serine or threonine residues near those rich in prolines are likely to be O-glycosylated [19]. The O-glycosylation sites of CD99 and HSV gB were indeed shown to be threonine residues located in a proline-rich region. In order to test these predictions, we generated a series of Flagtagged PANP mutants–the individual serine or threonine residues of which were changed to alanines. 293T cells transfected with Flag-tagged PANP in which threonine residue at position 136 was mutated to alanine (PANP-T136A) were not stained by PILRα-Ig, whereas 293T cells transfected with wild-type PANP and PANP in which threonine residue at position 83 was mutated to alanine (PANP-T83A) were stained (Fig. 2C). These data indicate that a threonine residue at position 136 is essential for PILRα recognition of PANP.
We then examined the molecular weight of PANP expressed on 293T cells. PANP transfected cells were treated with neuraminidase and molecular weight of cell surface PANP was analyzed by SDS–PAGE. As shown in Fig. 2D, the molecular weight of wild-type PANP was about 30 kDa and we could not detect significant change in molecular weight by neuraminidase treatment. Similarly, mutant PANP showed the same molecular weight with wild-type PANP. We have previously shown that molecular weight of mouse CD99 but not HSV gB is dramatically decreased by neuraminidase treatment [11,12]. This suggested that PANP might have certain O-glycosylation pattern similar to HSV-gB.
We also tested whether PANP binding led to transduction of signals via inhibitory PILRα or activating PILRβ, using NFAT-GFP reporter cells. Because the activating PILRβ associates with ITAM-bearing DAP12 adapter proteins, it delivers NFAT activating signals upon ligand recognition [8]. On the other hand, signals from inhibitory PILRα do not activate NFAT. Therefore, in order to detect ligand recognition of inhibitory PILRα by means of NFAT activation, transmembrane and intracellular domains of PILRα were substituted with those of activating PILRβ. The chimeric PILRα and wild-type PILRβ were transfected into NFAT-GFP reporter cells carrying the DAP12 adapter molecule. When the PILRα and PILRβ NFAT-GFP reporter cells were stimulated with anti-PILRα or anti-PILRβ mAb, the transfectants expressed GFP (data not shown). We cocultured these reporter cells with PANP- or CD99- expressing cells. As shown in Fig. 3, both PANP and CD99 transfectants, but not mock transfectants, stimulated the PILRα NFAT-GFP reporter cells to express GFP. On the other hand, CD99, but not PANP, transfectants stimulated PILRβ NFAT-GFP reporter cells, consistent with the binding analysis in which PILRα-Ig but not PILRβ-Ig bound to PANP transfectants.
Fig. 3.
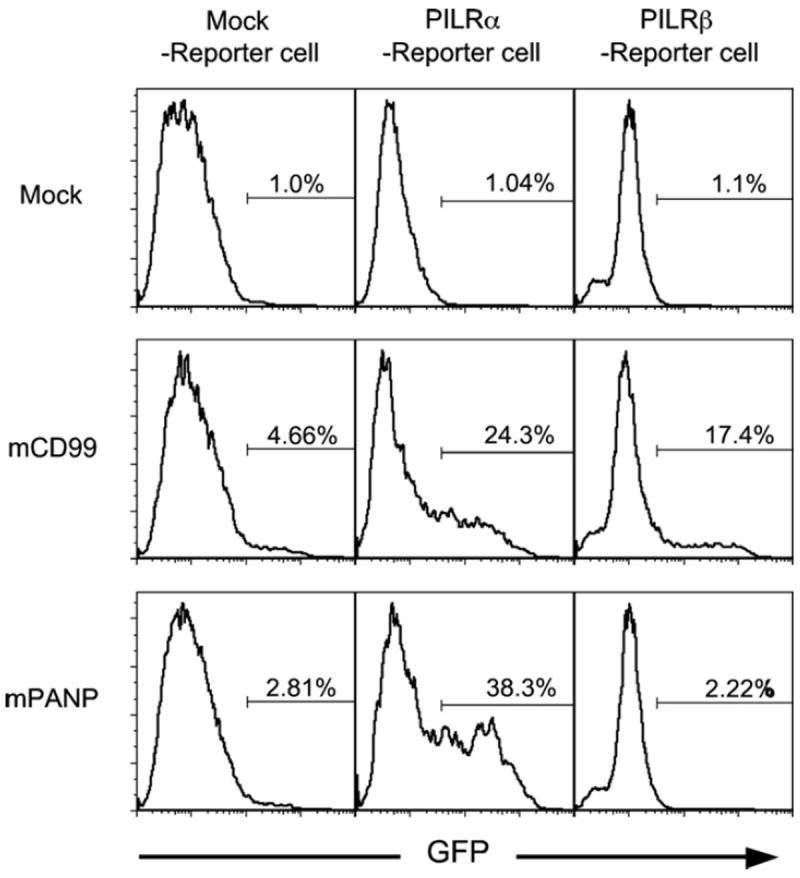
Stimulation of PILR reporter cells by PANP. PILRα or PILRβ NFAT-GFP reporter cells were co-cultured with mouse CD99-transfectants (mCD99), mouse PANP-transfectants (mPANP) or mock-transfectants (Mock) for 16 h and GFP expression in the reporter cells was analyzed by flow cytometry.
We then analyzed the expression of PANP transcripts in human and mouse tissues by real-time quantitative PCR. Interestingly, both human and mouse PANP transcripts were relatively abundant in neural tissues (Fig. 4). In the case of mouse, weak transcription of PANP was detected in spleen and lymph node. However, splenic T cells, B cells, and dendritic cells were not stained with anti-PANP mAb (data not shown).
Fig. 4.
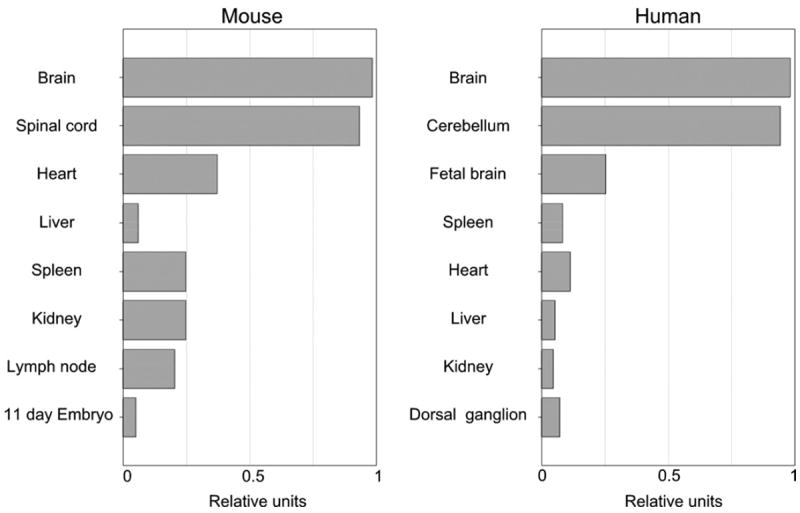
Tissue distributions of PANP transcription. Transcription of mouse and human PANP in different tissues was analyzed by real-time quantitative PCR. Transcription of β-actin was used as a standard and expression levels of PANP in each tissue relative to the brain are shown.
4. Discussion
In this study, we have demonstrated that PILRα recognizes a newly identified molecule designated PANP, which does not bind PILRβ. Because CD99 was recognized by both PILRα and PILRβ, there is presumably a difference in affinity between them for PANP and CD99 binding. One possibility accounting for this difference between PANP and CD99 may be their different glycan structures, which might affect the binding affinity of PILRβ. Alternatively, amino acid sequence differences around O-glycosylation sites might also affect the affinity of PILRβ. Because the exact glycan and protein structures recognized by PILRα and PILRβ are still unclear, further analyses of the glycan structures on PANP and CD99 are needed to clarify the molecular requirements for PILR recognition.
Generally, immune inhibitory receptors recognize endogenous ligands and downregulate immune responses to self. PILRα is predominantly expressed on dendritic cells, granulocytes, and macrophages. In addition, PILRα expression is also detected in neural tissues. Because PANP is mainly expressed in neural tissues, interactions between PILRα and PANP might play a role therein. In particular, PANP and PILRα interactions might be involved in neural immune disorders such as multiple sclerosis. Further analyses using PILRα- or PANP-deficient mice may reveal novel regulatory functions of PILRα and PANP.
Acknowledgments
We thank K. Shida, R. Hirohata, and M. Matsumoto for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (H.A.) and NIH grant AI068129. L.L.L. is an American Cancer Society Professor.
Abbreviations
- PILR
paired Ig-like type 2 receptor
- PANP
PILR-associating neural protein
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITAM
immunoreceptor tyrosine-based activating motif
- HSV-1
herpes simplex virus-1
- C2GnT
core 2 β-1,6-N-acetylglucosaminyltransferase
References
- 1.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanietsky N, Mandelboim O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett. 2010;584:4890–4900. doi: 10.1016/j.febslet.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Cheent K, Khakoo SI. Natural killer cells: integrating diversity with function. Immunology. 2009;126:449–457. doi: 10.1111/j.1365-2567.2009.03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 5.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 6.Shiratori I, Yamaguchi M, Suzukawa M, Yamamoto K, Lanier LL, Saito T, Arase H. Down-regulation of basophil function by human CD200 and human herpesvirus-8 CD200. J Immunol. 2005;175:4441–4449. doi: 10.4049/jimmunol.175.7.4441. [DOI] [PubMed] [Google Scholar]
- 7.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. PILRα is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiratori I, Ogasawara K, Saito T, Lanier LL, Arase H. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J Exp Med. 2004;199:525–533. doi: 10.1084/jem.20031885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson MD, Cheung J, Martindale DW, Scherer SW, Koop BF. Comparative analysis of the paired immunoglobulin-like receptor (PILR) locus in six mammalian genomes: duplication, conversion, and the birth of new genes. Physiol Genomics. 2006;27:201–218. doi: 10.1152/physiolgenomics.00284.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tabata S, Kuroki K, Wang J, Kajikawa M, Shiratori I, Kohda D, Arase H, Maenaka K. Biophysical characterization of O-glycosylated CD99 recognition by paired Ig-like type 2 receptors. J Biol Chem. 2008;283:8893–8901. doi: 10.1074/jbc.M709793200. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Shiratori I, Satoh T, Lanier LL, Arase H. An essential role of sialylated O-linked sugar chains in the recognition of mouse CD99 by paired Ig-like type 2 receptor (PILR) J Immunol. 2008;180:1686–1693. doi: 10.4049/jimmunol.180.3.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Fan Q, Satoh T, Arii J, Lanier LL, Spear PG, Kawaguchi Y, Arase H. Binding of herpes simplex virus glycoprotein B (gB) to paired immunoglobulin-like type 2 receptor alpha depends on specific sialylated O-linked glycans on gB. J Virol. 2009;83:13042–13045. doi: 10.1128/JVI.00792-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arii J, Wang J, Morimoto T, Suenaga T, Akashi H, Arase H, Kawaguchi Y. A single-amino-acid substitution in herpes simplex virus 1 envelope glycoprotein B at a site required for binding to the paired immunoglobulinlike type 2 receptor {α} (PILR{α}) abrogates PILR{α}-dependent viral entry and reduces pathogenesis. J Virol. 2010;84:10773–10783. doi: 10.1128/JVI.01166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arase H, Saito T, Phillips JH, Lanier LL. The mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (α2 Integrin, very late antigen-2) J Immunol. 2001;167:1141–1144. doi: 10.4049/jimmunol.167.3.1141. [DOI] [PubMed] [Google Scholar]
- 15.Arthos J, Cicala C, Steenbeke TD, Chun TW, Cruz CD, Hanback DB, Khazanie P, Nam D, Schuck P, Selig SM, Van Ryk D, Chaikin MA, Fauci AS. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein. Implications for therapeutic and vaccine strategies. J Biol Chem. 2002;277:11456–11464. doi: 10.1074/jbc.M111191200. [DOI] [PubMed] [Google Scholar]
- 16.Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRγ is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 18.Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj J. 1998;15:115–130. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- 19.Wilson IB, Gavel Y, von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]


