Abstract
Background
Increased eligibility guidelines of antiretroviral therapy (ART) may lead to greater routine viral load monitoring. However, in resource-constrained settings, the additional resources required by greater routine viral load monitoring may impair ability to comply with expanded eligibility guidelines for ART.
Objective
We use a published validated computer simulation of the HIV epidemic in East African countries (expanded to include transmission as well as disease progression) to evaluate the cost–effectiveness of routine viral load monitoring.
Methods
We explored alternative scenarios regarding cost, frequency, and switching threshold of routine viral load monitoring (including every 6 or every 12 months; and switching thresholds of 1000, or 10 000 copies/ml), as well as alternative scenarios regarding ART initiation (200, 350, 500 cells/μl, and no CD4+ cell threshold). For each ART initiation strategy, we sought to identify the viral load monitoring strategy at which the incremental cost–effectiveness ratio (ICER) of more frequent routine viral load testing became more favorable than the ICER of more expansive ART eligibility. Cost inputs were based on data provided by the Academic Model Providing Access to Healthcare (AMPATH), and disease progression inputs were based on prior published work. We used a discount rate of 3%, a time horizon of 20 years, and a payer perspective.
Results
Across a wide range of scenarios, and even when considering the beneficial effect of virological monitoring at reducing HIV transmission, earlier ART initiation conferred far greater health benefits for resources spent than routine virological testing, with ICERs of approximately $1000 to $2000 for earlier ART initiation, versus ICERs of approximately $5000 to $25 000 for routine virological monitoring. ICERs of viral load testing were insensitive to the cost of the viral load test, because most of the costs originated from the downstream higher costs of later regimens. ICERs of viral load testing were very sensitive to the relative cost of second-line compared with first-line regimens, assuming favorable value when the costs of these regimens were equal.
Conclusion
If all HIV patients are not yet treated with ART starting at 500 cells/μl and costs of second regimens remain substantially more expensive than first-line regimens, resources would buy more population health if they are spent on earlier ART rather than being spent on routine virological testing.
Keywords: antiretroviral therapy, cost-effectiveness, HIV, monitoring, sub-Saharan Africa, viral load testing
Introduction
WHO guidelines for adult patient monitoring in 2010 recommended that patients be switched to second-line antiretroviral therapy (ART) [1] based on clinical criteria, immunologic criteria, or virologic criteria (viral load over 5000 copies/ml while on treatment). Viral load monitoring can be either routine or targeted; in the latter case, it is used to confirm virological failure in patients meeting either clinical or immunologic criteria, and in the former case it is performed routinely irrespective of other clinical or immunologic results. However, viral load tests are currently more expensive than CD4+ cell count tests, and the high cost of second-line therapy can drive up costs associated with viral load testing as a result of earlier and more frequent switching to second-line therapy. For this reason, the 2013 WHO guidelines overlay considerable debate about the benefits and frequency of routine viral load testing.
When allocating resources in low-resource settings, the benefits of more frequent viral load monitoring need to be balanced against its costs and disadvantages. The benefits of more frequent routine virological monitoring include timely switching to second-line therapy for those failing to suppress virus and therefore at higher risk of transmitting infection to others; avoiding unnecessary switches to second-line therapy for patients who do not need a new regimen; and limiting the development and onward transmission of virus resistant to first-line regimens. The costs and disadvantages of more frequent routine virological monitoring include those of tests, equipment, staff time, decrements in patient adherence associated with lengthy travel to sites where tests can logistically occur, and subsequent switches to more expensive second-line and third-line regimens.
It is particularly important to consider any additional costs of routine virological monitoring in light of its opportunity costs; that is, the gains that could be had by devoting resources alternatively to other simultaneously resource-constrained decisions, such as increasing the eligibility for first-line ART by raising the CD4+ cell initiation criteria, and/or by adopting more aggressive measures to pursue more complete ART coverage. These tradeoffs are complex. Mathematical modeling allows for systematic and detailed consideration of the costs and benefits of a broad potential repertoire of strategies over a range of timescales, which would be impossible to do experimentally with clinical trials.
Accordingly, our aim was to use a published validated computer simulation of the HIV epidemic in Kenya, now enhanced to incorporate HIV transmission as well as disease progression, to evaluate the cost–effectiveness of a wide spectrum of routine viral load monitoring strategies, seeking to identify the intensity of routine viral load monitoring at which its incremental cost–effectiveness ratio (ICER) became more favorable than the ICER of more expansive ART eligibility (in other words, when additional routine viral load monitoring bought more ‘health’ than using those resources, alternatively, to expand ART eligibility).
Methods
We used a computer simulation to explore alternative scenarios regarding cost, frequency, and switching threshold of alternative laboratory monitoring strategies, including routine viral load monitoring (every 6 months or every 12 months; switching thresholds of 1000 or 10 000 copies/ml), routine CD4+ cell monitoring, and routine CD4+ cell monitoring with confirmatory viral load. In addition, we explored alternative scenarios regarding ART initiation thresholds (CD4+ cell count of 200, 350, 500 cells/μl, and no CD4+ cell threshold). This model is different from our previous publications [2,3] because it considers HIV transmission as well as disease progression. For each ART initiation threshold, we sought to identify the viral load monitoring strategy at which the ICER of more frequent routine viral load testing became more favorable than the ICER of more expansive ART eligibility (in other words, when additional routine viral load monitoring bought more ‘health’ than using those resources, alternatively, to more fully comply with expanded ARTeligibility). Cost inputs were based on previously reported expenditure data from a large healthcare system in western Kenya (Academic Model Providing Access to Healthcare, or AMPATH) [2], and disease progression and transmissibility inputs were based on prior published work. We used a discount rate of 3%. We used a payer perspective in base case analyses, exploring a societal perspective in sensitivity analyses. We used a time horizon of 20 years. Outcome measures included cost per quality-adjusted life year gained. For analyses in this article, we assumed two ART regimens were available; however, the simulation has the ability to consider alternative scenarios regarding numbers of ART regimens available.
We sought to identify ‘efficient frontiers’, defined as those strategies delivering the greatest health benefit given a particular budget scenario [4]. Strategies within an efficient frontier confer the greatest benefit for a specified budget. Strategies outside this frontier are unable to deliver the greatest benefit regardless of budget, and therefore are not preferred choices regardless of the magnitude of available resources. We identified efficient frontiers by calculating the ICERs of HIV treatment initiation and monitoring strategies. ICERs measure the additive benefit of each strategy compared with its next best alternative, and interpret this benefit together with its additive cost.
Simulation overview
This simulation is composed of a stochastic progression module (e.g., hypothetical patients are followed over time in a microsimulation based on a state transition model, and depending on ARTadherence and other factors, may be more or less likely to die of AIDS versus other causes) that provides data to inform a deterministic transmission module (e.g., hypothetical compartments of persons interact with one another, and HIV-infected compartments may transmit the infection to non-HIV-infected groups).
Notable features of our simulation include that it can examine nearly infinite combinations of testing strategies and ART initiation scenarios, explicitly considers ART adherence, explicitly considers accumulation and transmission of resistance mutations, can perform evaluations over long time horizons (thereby capturing delayed harms that may not be evident in shorter term analyses), can partition effects to transmission versus progression, captures important heterogeneity in sexual mixing patterns, and has been well validated based on clinical and epidemiological data.
The simulation projects the course of the HIV epidemic over varying time horizons, and tracks the benefits of potential interventions using a variety of outcome measures, including life expectancy, measured in life years; quality-adjusted life expectancy, measured in quality-adjusted life years (QALYs); and HIV infections averted.
HIV progression module
Disease progression was modeled by evaluating mortality rates and trajectories of CD4+ cell counts and HIV-1 viral load within a previously described HIV progression simulation calibrated and validated on East African populations. This model explicitly represents the main causes of ART failure, nonadherence, and the accumulation of genotypic resistance, and has been well validated in multiple populations (see Table 1 [2,5–29] for selected model input parameters). Details of this module are described elsewhere [2].
Table 1.
Inputs into computer simulation after calibration.
| Variable | Value | Source |
|---|---|---|
| Characteristics of initial progression model cohort | ||
| Mean age, years | 39 (SD 9) | [2] |
| Mean CD4+ cell count (cells/μl) | 126 (SD 127) | [2] |
| Mean viral load (Log 10 units) | 4.5 (SD 1) | [2] |
| % Male | 38% | [2] |
| Probabilities and rates | ||
| Probability of adherence to ART regimen | 0.85 | [5] |
| Probability that mutation potentially causing resistance, results in resistance, NRTI or PI | 0.50 | [6] |
| Probability mutation potentially causing resistance, results in resistance, NNRTI | 0.90 | [6] |
| Probability of cross-resistance to other NRTI, given NRTI mutation conferring resistance (zidovudine or stavudine) | 1.0 | [6] |
| Probability of cross-resistance to other NRTI, given NRTI mutation conferring resistance (other) | 0.48 | [6] |
| Probability of cross-resistance to other PI, given PI mutation causing resistance | 0.24 | [6] |
| Probability of cross-resistance to other NNRTI, given NNRTI mutation causing resistance | 0.88 | [6] |
| Rate of accumulating resistance mutations, per year | 0.18 | [7] |
| Viral load decrement with cART consisting of nevirapine + 2 NRTIs (100% adherence) | 2.22 | [8] |
| Viral load decrement with cART consisting of boosted PI (100% adherence) | 2.68 | [8] |
| Utilities | ||
| Decrease in utility with cART | 0.053 | [8] |
| Utility with CD4+ cell count <100 cells/μl | 0.81 | [9] |
| Utility with CD4+ cell count between 100 and 200 cells/μl | 0.87 | [9] |
| Utility with CD4+ cell count 200 cells/μl and above | 0.94 | [9] |
| HIV epidemiology and transmission | ||
| Population at start of calibration | 20 479 400 | [10] |
| Age range, years | 0–50 | |
| Adult HIV prevalence (1997) | 10.60% | [11] |
| Probability of transmission per sex acta | 0.000067–0.0073 | [12,13] |
| Untreated non-HIV STI prevalence | 6% | [14] |
| Probability of HIV testing (per annum) | 16% | [15–17] |
| Probability of linkage to HIV care and treatment | 68% | [18] |
| Calibration parametersb | ||
| Multiplier on probability of transmission per sex act | 1.71 | |
| Multiplier on HIV mortality | 0.47 | |
| Sexual risk behaviors | ||
| Proportion abstinent (M/F) - Class 1 | 5%/10% | [19–23] |
| Proportion in stable, monogamous relationship (M/F) - Class 2 | 31%/69% | [23–25] |
| Proportion in multiple, concurrent relationships (if nonmonogamous) (M/F) - Class 3 | 56%/17% | Assumption |
| Proportion in multiple, concurrent relationships (if nonmonogamous) (M/F) - Class 4 | 8%/4% | [26,27] |
| Desired frequency of sex acts (per year)f | 104 | Assumption |
| Duration of relationshipc | 0.5 to 3 years | Assumption |
| Median number of concurrent partners (Class 3) | 3 | [25] |
| Median number of concurrent partners (Class 4) | 10 | [25] |
| Probability of consistent condom use | 34% | [28] |
| Relative risk of unsafe sex (condom nonuse most or all of the time) if aware of HIV status | 0.47 | [29] |
| Costs (2008 US$) | ||
| Cost of outpatient care, annually, without cART ($/month) | $288 | [2] |
| Cost of care per hospitalization | $390 | [2] |
| Cost of cART, annually, first regimend | $189 | [2] |
| Cost of cART annually, second regimene | $1361 | [2] |
| Cost of viral load test | $70 | [2] |
| Cost of CD4+ cell test | $11 | [2] |
cART, combination antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Transmission probability varies according to both sex (M/F) and HIV viral load of the infected person. These values are adjusted by a multiplier during calibration.
Varied during model calibration.
Duration dependent upon class of sexual risk behavior with stable, monogamous relationships having the longest duration and multiple, concurrent relationships having the shortest.
First-line cART regimen consists of nevirapine + either zidovudine or stavudine + other NRTI.
Second-line cART consists of a boosted PI + two NRTIs other than those in initial regimen.
See Appendix A – Technical Appendix for description of partner balancing algorithm, http://links.lww.com/QAD/A428.
HIV transmission module
A dynamic compartmental model of HIV transmission was developed, specified by sets of differential equations. The model includes heterosexual transmission, but does not include homosexual transmission or transmission from needle-sharing during injection drug use.
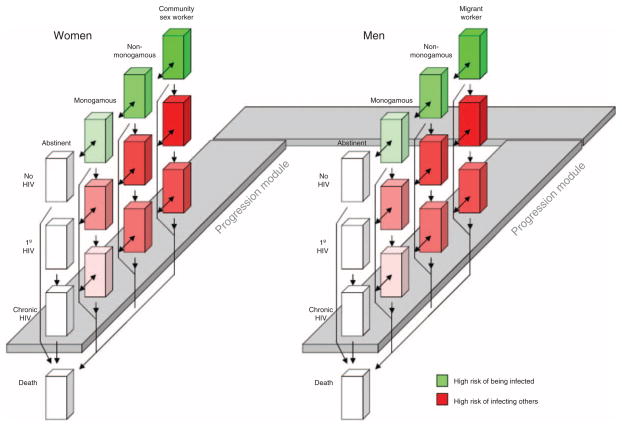
At any particular time, hypothetical people in the module occupy one among a set of mutually exclusive and collectively exhaustive compartments (Fig. 1). These compartments describe health characteristics as well as behavioral risk characteristics. As time proceeds, hypothetical people may change the compartment that they occupy, for example, proceeding from being uninfected to having a primary HIV infection to having a chronic HIV infection to death. (Alternatively they may die without ever contracting HIV). In addition, groups of people in the model may alternatively be abstinent (in which case they will not contract HIV), monogamous, nonmonogamous, or may occupy an even higher risk group [community sex workers (CSWs) if women, and migrant workers if men], with correspondingly greater chances of contracting or spreading HIV. Women in any of the nonabstinent states may ‘mix’ (have sexual contact) with men in any of the nonabstinent states. Probability of transmission is a function of multiple factors, including rate of acquiring new partners, duration of partnership, frequency of sexual contact within a partnership, and likelihood of condom use. For example, CSWs are especially likely to transmit HIV to nonmonogamous men because the likelihood of initiating contact is high, condom use is low, and duration of the relationships may be high.
Fig. 1. Schematic of computer simulation.
The progression module provides data to inform the transmission module. Additionally, there are important simulation features that are not depicted in the diagram. The probability of transmission is higher from men to women, and lower from women to men. Additionally, mixing may be asymmetric by age (greater from older men to younger women; lower from younger men to older women). Although similarly sized cubes are used to designate different states of the simulation, this is not meant to suggest that the number of individuals in each state is similar: the proportion may vary from state to state, and may also vary between corresponding states of men and women. 1° HIV, primary HIV infection.
The HIV-infected population in the transmission simulation at baseline was divided into compartments based on CD4+ cell and viral load strata. Five CD4+ cell strata were represented (<50, 50–200, 200–350, 350–500, >500 cells/μl) and five log viral load logarithmic strata were represented (<2.5, 2.5–3.5, 3.5–4.5, 4.5–5.5, >5.5 log units/ml). Data regarding transition rates and trajectories by treatment and adherence level were captured from the progression model and interfaced with the transmission simulation in the form of rate tables. The spectrum of infection and care was modeled as a stepwise progression from HIV acquisition/primary infection to chronic infection, to HIV detection through testing, followed by linkage to care, and finally initiation of treatment with ART. Details of this module are described in Appendix A – Technical Appendix, http://links.lww.com/QAD/A428.
Other design features include the following:
All sexually active persons may have untreated sexually transmitted infections (STIs), and this increases HIV transmission risk. Risk of having untreated STIs, like HIV itself, will increase with greater numbers of sexual contacts and with unhealthy alcohol use.
Men may or may not be circumcised. Men who are circumcised have a decreased chance of being infected with HIV.
Likelihood of transmission per sexual encounter will vary with viral load (greater with higher viral load). For this reason, individuals who are receiving antiretroviral treatment will have a lower risk of transmission than individuals who are not receiving antiretroviral treatment, and individuals with primary HIV infection (and a correspondingly high viral load) or AIDS will be especially likely to transmit the virus.
Mixing patterns
Whereas many transmission models assume ‘homogenous mixing’ (i.e., each hypothetical individual has an equal chance of transmitting the infection to each other hypothetical individual) or homogenous mixing stratified by age (i.e., each hypothetical individual within a particular age stratum has an equal chance of transmitting the infection to each other hypothetical individual within that age stratum), our transmission module assumes heterogeneous mixing patterns that are informed by sub-Saharan African data. Therefore, it represents the common phenomenon of assortative mixing, for example, individuals who engage in risky sex may be more likely to partner with other people who have risky sex than with people who do not engage in risky sex, even after controlling for the increase in partnering opportunities that may be available. Additionally, because older men may be more likely to have sexual contact with younger women than vice versa, the model includes a parameter that can be varied to include this age asymmetry of mixing.
Decomposition of risk per partnership
Transmission models often assume a single, aggregate risk that accumulates from all contacts that occur over the duration of a partnership. However, this approach has the disadvantage of implicitly assuming that the number of acts per partnership is static, and is independent of the number of concurrent partnerships. In other words, if a person has 10 times as many simultaneously sexual partners, conventional model approaches may implicitly assume that each person has 10 times as many sexual encounters in a particular time period. However, data suggest that people with many simultaneous partners do not have a proportionate increase in frequency of sexual encounters per unit time, and therefore this implicit assumption may exaggerate the impact of concurrency on transmission risk [30].
We chose not to represent the composite risk that accumulates from all events over the duration of a partnership, but instead represented the underlying determinates of this composite risk: frequency of sexual contacts, concurrency of sexual partnerships, and duration of sexual partnerships. Each characteristic is a separate ‘dial’ that can be increased or decreased without directly affecting the other ‘dials’; however, it is important to note that there may be important correlations between these inputs so they should only be varied independently with great caution, such as in exploratory analyses. For example, increasing the frequency of sexual encounters without changing the concurrency or duration of partnerships will increase the transmission risk per partnership without altering the partner change rate. As another example, increasing a person’s concurrency of sexual relationships without increasing their overall sexual frequency or duration of those relationships will reduce the transmission risk per partnership because of fewer sexual acts per partnership. However, the transmission risk per unit time may increase because the number of simultaneous partnerships increases. Decomposing risk per partnership into its constituent characteristics is particularly important for modeling the sub-Saharan HIV pandemic because nonmonogamous contacts (e.g., CSWs) may have long durations [31]. The model’s calibration is described in Appendix B – Model Calibration, http://links.lww.com/QAD/A429.
Comparing results with and without transmission
As a validity test of our ability to discriminate how results varied with versus without transmission, we ran the transmission model in a mode that would approximate the fixed-sized, noninteractive cohort of the progression model by turning off births and transmission. We found that, by only incorporating results from known HIV-infected patients, the transmission model results converged with corresponding results from the progression module alone.
Other sensitivity analyses
We varied input assumptions that were likely to impact which strategies lied on the efficient frontier, based on our previous published work [2]. In particular, we varied assumptions regarding the cost of second-line ART relative to first-line ART, the expense of the viral load test, and both of these together. We also varied assumptions regarding the overall transmissibility of HIV (likelihood of transmission per sexual encounter of specified risk).
Results
Our model suggested different efficient frontiers regardless of whether our simulation considered the implications of different monitoring strategies on disease progression alone, or whether it also considered the implications of different monitoring strategies on transmissibility.
Considering progression alone
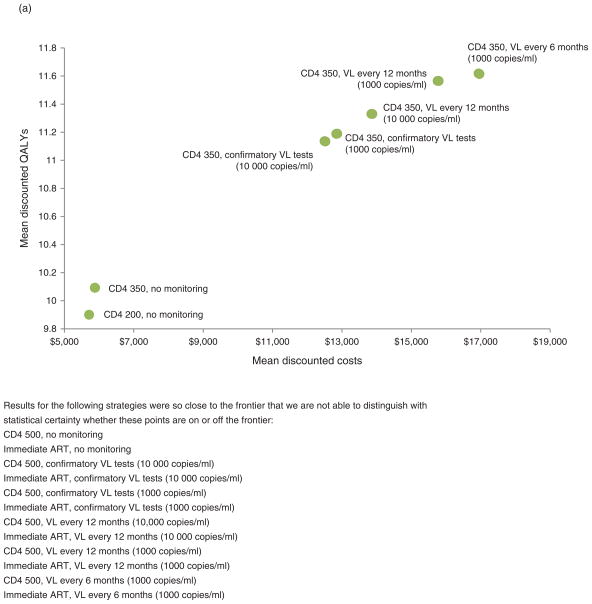
When our simulation evaluated different monitoring strategies considering only its impact on progression, efficient frontiers increased stepwise, beginning at strategies that did not involve any laboratory monitoring, increasing to strategies that involved CD4+ cell testing with follow-up virological testing upon positive results, and further increasing to strategies that involved progressively more frequent routine virological testing with progressively lower switching thresholds (Fig. 2a). The ICER of earlier ART (CD4+ of 350 cells/μl rather than 200 cells/μl, $857–2388 per QALY, considering progression only; Appendix C – Additional Results, http://links.lww.com/QAD/A430) was more favorable than any of the routine virological testing strategies, which started at $6557 per QALY, and rose to as high as $25 370 per QALY (Table 2a–d). In other words, it would only maximize health to obtain routine virological testing in situations in which coverage with first-line ART is already at goal, and in situations in which ART can be started widely at CD4+ cell count of 350 cells/μl rather than 200 cells/μl.
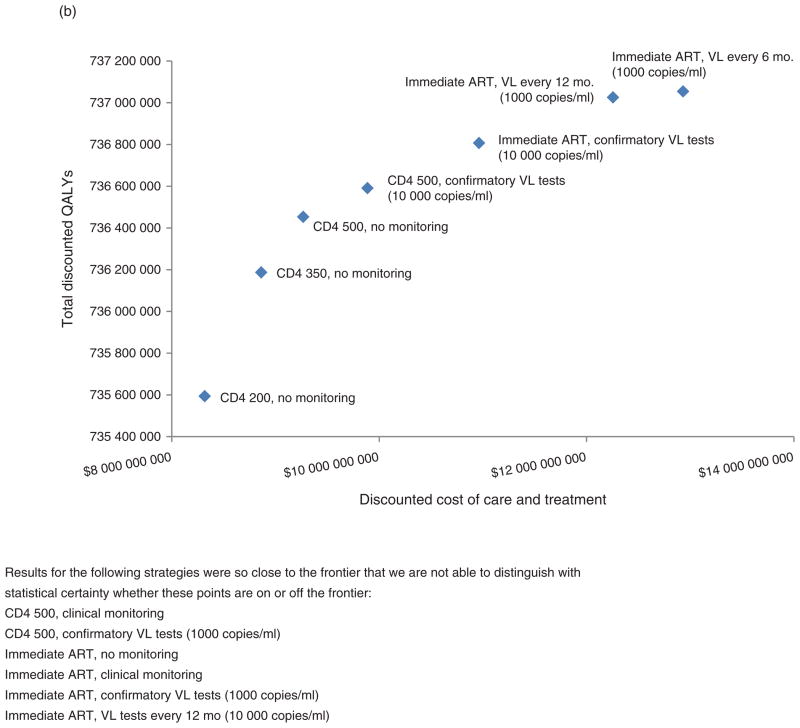
Fig. 2. Efficient frontiers comparing benefits of earlier antiretroviral therapy and more aggressive laboratory monitoring.
(a) Considering progression alone and (b) considering transmission as well as progression. Note that (a) models only HIV-infected persons and shows mean per-person costs and QALYs, whereas (b) models all persons, regardless of HIV infection, and shows total population costs and ART, antiretroviral therapy; VL, viral load.
Table 2.
Incremental cost–effectiveness ratios of different monitoring strategies.
| Monitor strategy | Viral load threshold (copies/ml) | Frequency (months) | ICER ($/QALY)
|
|
|---|---|---|---|---|
| Progression model | Transmission model | |||
| (A) Start ART at CD4+ cell count 200 cells/μl | ||||
| None | N/A | N/A | Reference | Reference |
| Clinical | N/A | 6 | ext dom | ext dom |
| CD4+ cell | N/A | 6 | ext dom | ext dom |
| Confirmatory | 10 000 | 6 | ext dom | 3641 |
| Confirmatory | 1000 | 6 | 6104 | ext dom |
| Virologic | 10 000 | 12 | 6557 | 4723 |
| Virologic | 1000 | 12 | 8055 | 5684 |
| Virologic | 10 000 | 6 | ext dom | ext dom |
| Virologic | 1000 | 6 | 25 370 | 22 188 |
| (B) Start ART at CD4+ cell count 350 cells/μl | ||||
| None | N/A | N/A | Reference | Reference |
| Clinical | N/A | 6 | ext dom | ext dom |
| CD4+ cell | N/A | 6 | ext dom | ext dom |
| Confirmatory | 10 000 | 6 | 6341 | 4083 |
| Confirmatory | 1000 | 6 | 6506 | ext dom |
| Virologic | 10 000 | 12 | 7123 | 5139 |
| Virologic | 1000 | 12 | 8135 | 5661 |
| Virologic | 10 000 | 6 | ext dom | ext dom |
| Virologic | 1000 | 6 | 22 219 | 24 731 |
| (C) Start ART at CD4+ cell count 500 cells/μl | ||||
| None | N/A | N/A | Reference | Reference |
| Clinical | N/A | 6 | ext dom | ext dom |
| CD4+ cell | N/A | 6 | ext dom | ext dom |
| Confirmatory | 10 000 | 6 | ext dom | 4489 |
| Confirmatory | 1000 | 6 | 6362 | ext dom |
| Virologic | 10 000 | 12 | 7175 | 5599 |
| Virologic | 1000 | 12 | 8165 | 5775 |
| Virologic | 10 000 | 6 | ext dom | ext dom |
| Virologic | 1000 | 6 | 23 539 | 25 521 |
| (D) Start ART upon detection (regardless of CD4+ cell count) | ||||
| None | N/A | N/A | Reference | Reference |
| Clinical | N/A | 6 | ext dom | ext dom |
| CD4+ cell | N/A | 6 | ext dom | ext dom |
| Confirmatory | 10 000 | 6 | ext dom | 4743 |
| Confirmatory | 1000 | 6 | 6363 | ext dom |
| Virologic | 10 000 | 12 | 7211 | ext dom |
| Virologic | 1000 | 12 | 8138 | 5898 |
| Virologic | 10 000 | 6 | ext dom | dom |
| Virologic | 1000 | 6 | 23 543 | 23 529 |
(A) Assumes antiretroviral therapy (ART) is initiated at CD4+ cell count of 200 cells/μl; (B) assumes ART is initiated at CD4+ cell count of 350 cells/μl, (C) assumes ART is initiated at CD4+cell count of 500 cells/μl, and (D) assumes ART is initiated upon detection, irrespective of CD4+cell count. Confirmatory, viral load test performed only if CD4+ cell meets WHO criteria for changing ART regimen based on CD4+ cell count (CD4+ cell count falls below baseline or falls >50% from peak on antiretroviral therapy); Dom, dominated strategy, meaning it is less effective and more costly than at least one alternative; Ext dom, extended dominance, less cost-effective than the next most effective strategy; ICER, incremental cost–effectiveness ratio; QALY, quality-adjusted life years.
Considering transmission as well as progression alone
When our simulation evaluated different monitoring strategies considering transmission as well as progression, the value of routine virological monitoring increased greatly, with ICERs cut by approximately one-third (decreasing from $6557/QALY to $4723/QALY; Table 2a–d). However, the value of routine virological monitoring strategies remained less favorable than that of earlier ART initiation (CD4+ cell count of 350 versus 200 cells/μl, $919 per QALY; CD4+ cell count of 500 versus 350 cells/μl, $1522 per QALY; considering transmission as well as progression; Table 3). Considering transmission in addition to progression increased the value of routine virological testing by taking into account the reduced infectiousness of patients who are effectively managed (lowering the average number of infections per infected person per year assuming immediate ART from 0.0679 to 0.0631), and decreased the number of new infections over 20 years by 9.9% from 1 268 591 to 1 142 956 (Table 3). Efficient frontiers (Fig. 2b) continued to increase stepwise, beginning at strategies that did not involve any laboratory monitoring, increasing to strategies that involved CD4+ cell testing with follow-up virological testing upon positive results, and further increasing to strategies that involved progressively more frequent routine virological testing with progressively lower switching thresholds.
Table 3.
Monitoring strategies combined with earlier antiretroviral therapy initiation thresholds that are on efficient frontier (transmission model results).
| ART initiation threshold (CD4+ cells/μl) | Monitor strategy | Viral load switch threshold (copies/ml) | Monitor freq. (mos.) | Discounted cost of care and treatment (USD) | Total discounted life years | Total discounted QALYs | New infections over 20 years | Died of AIDS over 20 years | New infections per infected per year | Cost of care and treatment per infected per year ($) | ICER ($/QALY) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | None | N/A | N/A | 8316 591 361 | 737 061 369 | 735 593 979 | 1679 374 | 807 636 | 0.0819 | 545 | Reference |
| 350 | None | N/A | N/A | 8862 218 073 | 737 664 045 | 736 187 460 | 1523 526 | 721 609 | 0.0764 | 596 | 919 |
| 500 | None | N/A | N/A | 9266 466 258 | 737 934 710 | 736 453 092 | 1423 498 | 678 783 | 0.0730 | 636 | 1522 |
| 500 | Confirmatory | 10 000 | 6 | 9887 138 505 | 738 075 583 | 736 591 360 | 1421 997 | 659 793 | 0.0726 | 677 | 4489 |
| Instant | Confirmatory | 10 000 | 6 | 10 962 792 206 | 738 334 816 | 736 807 010 | 1268 591 | 617 720 | 0.0679 | 783 | 4988 |
| Instant | Virologic | 1000 | 12 | 12 254 684 858 | 738 514 990 | 737 026 042 | 1142 956 | 579 853 | 0.0631 | 899 | 5898 |
| Instant | Virologic | 1000 | 6 | 12 930 942 330 | 738 540 370 | 737 054 783 | 1131 146 | 575 109 | 0.0626 | 952 | 23 529 |
More aggressive viral load monitoring prevents infections in Uganda, decreasing infections over 20 years from 1 268 591 to 1 142 956, a decrease of 125 635 infections or 9.9% of baseline. Earlier antiretroviral therapy (ART; from 500 cells/μl rather than 200 cells/μl) prevents an additional 255 876 infections, a decrease of 15%. Combining instantaneous ART with the most aggressive viral load monitoring could prevent 548 228 infections over 20 years, a synergistic decrease of 33%. ICER, incremental cost–effectiveness ratio; QALY, quality-adjusted life years.
As a result, even when considering transmission as well as progression, and considering the resulting decrease in infectiousness that more aggressive monitoring could yield, frequent virological routine monitoring would only be preferred in those settings in which coverage of first-line regimens is already maximized, and in settings in which coverage is already initiated at earlier CD4+ cell counts (Table 3).
Sensitivity analyses
Considering transmission along with progression, when we assumed that second-line ARTwas no more expensive than first-line ART (and, therefore, reducing the penalization of testing regimens that had frequent false positives and would consequently result in far more expensive ART), we found that routine viral load testing became far more attractive. The strategies on the frontier not involving routine viral load testing became sparse, and routine viral load testing offered favorable value (as low as $1331/QALY), now in the range of earlier ART initiation (data not shown).
When we assumed that viral load testing was extremely cheap, as might happen with advances in technology and point-of-care testing, the ICER of routine virological testing decreased but not nearly as much as when we assumed that second-line ART was no more expensive than first-line ART. Routine viral load testing every 6 months now had an ICER of $2386/QALY if the switching threshold was 10 000 copies/ml, rising to $5917/QALY if the switching threshold was 1000 copies/ml (data not shown). However, these values remained less favorable than earlier ART initiation.
When we assumed that second-line ART was no more expensive than first-line ART, and when we also assumed that viral load testing was extremely cheap, we found that routine virologic testing was clearly the preferred choice. Indeed, there was only one strategy on the efficient frontier, and this strategy specified the most frequent viral load testing (every 6 months) and the least conservative viral load switching threshold (1000 copies/ml).
Discussion
Our analyses underscore the difficult tradeoffs that are necessary to maximize health when there are simultaneous resource-constrained decisions. Notably, routine virological testing only achieved favorable expectancy after coverage with first-line ART was already at goal levels, and after initiation was achieved at a CD4+ cell count of 500 cells/μl. This remained true regardless of whether we considered progression alone, or whether we considered transmission as well as progression.
However, considering transmission in addition to progression substantially improved the value of routine viral load testing. The approximately one-third drop in ICER of routine viral load testing once transmission is considered is not entirely surprising, as more vigilant monitoring and switching would be expected to reduce community viral load and to reduce transmission. Indeed, our results underscore the important relationship between HIV treatment and progression. Our results suggest that HIV monitoring strategies that treat HIV more aggressively have the potential to prevent 9.9% of new HIV infections if ART is initiated instantly. However, this health improvement arose at a sufficiently high cost per benefit that our results suggest that it would only maximize health benefit to use routine virological testing in situations in which ART is already initiated at 500 cells/μl and goal levels of coverage are already met. The only exception to this inference occurred in sensitivity analyses when we assumed that first-line and second-line ART regimens had the same price, because most of the costs of viral load testing originate from downstream costs from more expensive ART regimens, rather than from the costs of the tests themselves.
It is likely that our results are generalizable to East African regions with similar HIV epidemics and similar cost structures, particularly as calibration of the model required varying only two parameters, lessening the danger that it was ‘overfit’ to one particular country. Additionally, most input data were from Kenya, whereas most calibration data were from Uganda, further increasing the likelihood that the model is generalizable across East African regions. However, we make this observation with considerable caution, as there is extensive variability between pandemic epidemiology and cultural factors among different regions in East African countries.
Our results are consistent with recent analyses of three mathematical models by Keebler et al. [3] examining the cost–effectiveness of different laboratory monitoring strategies, one of which is the progression module of the simulation described in the current report except applied to different sub-Saharan countries and using country-specific costs estimated by WHO rather than AMPATH. Although these three models were developed independently with different assumptions and structures, they consistently found that routine viral load testing delivered lesser incremental health benefits than using those same resources to increase ART coverage and initiating ARTat earlier thresholds. We found that our current estimates for the ICER of earlier ART were consistent with estimates by Eaton et al. [32] of the ICER of earlier ART (ranging from approximately $200/DALYaverted to $3800/DALY averted). Our progression-only estimate of the ICER of earlier ART (between $857/QALY and $2388/QALY) was more favorable than our own prior estimate of the ICER of earlier ART initiation ($2600/QALY) [2] because the prior analysis made different assumptions regarding available ART regimens and used a different initial CD4+ cell distribution for the cohort.
Our results have substantial limitations. All results reflect the assumption that two ART regimens were available, and preferred monitoring strategies clearly vary with the number of available regimens. On the one hand with only one regimen available, no virological testing is favored, as there are no decisions to be made as a result of that testing. On the other hand, when more regimens are available, more virological testing may be preferred because there is less of a danger of ‘burning through’ available regimens. Our model does not consider transmission through MSM or IDU mechanisms. While these mechanisms are likely to account for a fairly small proportion of infections in East Africa overall, same sex is very stigmatized and therefore difficult to estimate its true importance, and IDU is concentrated in the eastern Coastal region, where our results may be less generalizable.
In summary, our results suggest an important role of routine virological testing in improving life expectancy and quality-adjusted life expectancy of individuals with HIV, as well as a potential important role of routine virological testing in preventing new HIV infections in East Africa if the cost of second-line ART regimens were to decrease. However, if all HIV patients are not yet treated with ART starting at 500 cells/μl, resources would buy more health for the population if they are spent on heading toward the ART goal rather than being spent on increasing the frequency of routine virological testing.
Acknowledgments
This work is supported by National Institute of Allergy and Infectious Disease Award UO1AI069911–01 (IeDEA East Africa) and by the National Institute of Alcohol Abuse and Alcoholism award R01 AA017385, US National Institutes of Health. This work has utilized computing resources at the High Performance Computing Facility of the Center for Health Informatics and Bioinformatics at New York University Langone Medical Center.
Footnotes
Conflicts of interest
None.
References
- 1.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 revision. [PubMed] [Google Scholar]
- 2.Braithwaite RS, Nucifora KA, Yiannoutsos CT, Musick B, Kimaiyo S, Diero L, et al. Alternative antiretroviral monitoring strategies for HIV-infected patients in East Africa: opportunities to save more lives? J Int AIDS Soc. 2011;14:38. doi: 10.1186/1758-2652-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keebler D, Revill P, Braithwaite S, Phillips A, Blaser N, Borquez A, et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Health. 2014;2:e35–e43. doi: 10.1016/S2214-109X(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz H. Portfolio selection. J Finance. 1952;53:405–411. [Google Scholar]
- 5.Bajunirwe F, Arts EJ, Tisch DJ, King CH, Debanne SM, Sethi AK. Adherence and treatment response among HIV-1-infected adults receiving antiretroviral therapy in a rural government hospital in southwestern Uganda. J Int Assoc Physicians AIDS Care (Chic) 2009;8:139–147. doi: 10.1177/1545109709332470. [DOI] [PubMed] [Google Scholar]
- 6.Johnson VA, Brun-Vezinet F, Clotet B, Gunthard HF, Kuritzkes DR, Pillay D, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17:138–145. [PubMed] [Google Scholar]
- 7.Braithwaite RS, Shechter S, Chang CC, Schaefer A, Roberts MS. Estimating the rate of accumulating drug resistance mutations in the HIV genome. Value Health. 2007;10:204–213. doi: 10.1111/j.1524-4733.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination anti-retroviral therapies. AIDS. 2007;21:1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedberg KA, Scharfstein JA, Seage GR, 3rd, Losina E, Weinstein MC, Craven DE, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–136. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 10.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: the 2008 Revision. 2010 [Google Scholar]
- 11.WHO. The UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. 2008. Epidemiological Fact Sheet on HIV and AIDS, Uganda. [Google Scholar]
- 12.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 14.WHO. World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections. 2001 [PubMed] [Google Scholar]
- 15.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- 16.Irungu TK, Varkey P, Cha S, Patterson JM. HIV voluntary counselling and testing in Nakuru, Kenya: findings from a community survey. HIV Med. 2008;9:111–117. doi: 10.1111/j.1468-1293.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 17.Mossdorf E, Stoeckle M, Vincenz A, Mwaigomole EG, Chiweka E, Kibatala P, et al. Impact of a national HIV voluntary counselling and testing (VCT) campaign on VCT in a rural hospital in Tanzania. Trop Med Int Health. 2010;15:567–573. doi: 10.1111/j.1365-3156.2010.02490.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapiga SH, Sam NE, Mlay J, Aboud S, Ballard RC, Shao JF, et al. The epidemiology of HIV-1 infection in northern Tanzania: results from a community-based study. AIDS Care. 2006;18:379–387. doi: 10.1080/09540120500465012. [DOI] [PubMed] [Google Scholar]
- 20.Kapiga SH, Sam NE, Shao JF, Renjifo B, Masenga EJ, Kiwelu IE, et al. HIV-1 epidemic among female bar and hotel workers in northern Tanzania: risk factors and opportunities for prevention. J Acquir Immune Defic Syndr. 2002;29:409–417. doi: 10.1097/00126334-200204010-00013. [DOI] [PubMed] [Google Scholar]
- 21.Ao TT, Sam NE, Masenga EJ, Seage GR, 3rd, Kapiga SH. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis. 2006;33:163–169. doi: 10.1097/01.olq.0000187204.57006.b3. [DOI] [PubMed] [Google Scholar]
- 22.Mbizvo MT, Machekano R, McFarland W, Ray S, Bassett M, Latif A, et al. HIV seroincidence and correlates of seroconversion in a cohort of male factory workers in Harare, Zimbabwe. AIDS. 1996;10:895–901. doi: 10.1097/00002030-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Quigley M, Munguti K, Grosskurth H, Todd J, Mosha F, Senkoro K, et al. Sexual behaviour patterns and other risk factors for HIV infection in rural Tanzania: a case-control study. AIDS. 1997;11:237–248. doi: 10.1097/00002030-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Mishra V. Concurrent sexual partnerships and HIV infection: evidence from national population-based surveys. DHS Working Papers; 2009; Calverton, MD.. [Google Scholar]
- 25.Volle J, Letsatsi T, Tan A. A baseline survey of multiple and concurrent sexual partnerships among Basotho men in Lesotho. Washington DC: C-Change/AED; 2009. [Google Scholar]
- 26.Mmbaga EJ, Leyna GH, Mnyika KS, Klepp KI. Sexually transmitted infections knowledge and its impact in the practice of risky sexual behaviours and HIV serostatus: results from rural Kilimanjaro, Tanzania. Sex Transm Infect. 2008;84:224–226. doi: 10.1136/sti.2007.029488. [DOI] [PubMed] [Google Scholar]
- 27.Vandepitte J, Lyerla R, Dallabetta G, Crabbe F, Alary M, Buve A. Estimates of the number of female sex workers in different regions of the world. Sex Transm Infect. 2006;82(Suppl 3):iii18–iii25. doi: 10.1136/sti.2006.020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westercamp N, Mattson CL, Madonia M, Moses S, Agot K, Ndinya-Achola JO, et al. Determinants of consistent condom use vary by partner type among young men in Kisumu, Kenya: a multilevel data analysis. AIDS Behav. 2010;14:949–959. doi: 10.1007/s10461-008-9458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 30.Sawers L, Isaac AG, Stillwaggon E. HIV and concurrent sexual partnerships: modelling the role of coital dilution. J Int AIDS Soc. 2011;14:44. doi: 10.1186/1758-2652-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voeten HA, Egesah OB, Ondiege MY, Varkevisser CM, Habbema JD. Clients of female sex workers in Nyanza province, Kenya: a core group in STD/HIV transmission. Sex Transm Dis. 2002;29:444–452. doi: 10.1097/00007435-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2:e23–e34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]