Abstract
Flap creation is a critical step in laser in situ keratomileusis (LASIK). Efforts to improve the safety and predictability of the lamellar incision have fostered the development of femtosecond lasers. Several advantages of the femtosecond laser over mechanical microkeratomes have been reported in LASIK surgery. In this article, we review common considerations in management and complications of this step in femtosecond laser–LASIK and concentrate primarily on the IntraLase laser because most published studies relate to this instrument.
In the early 1960s, Barraquer introduced the concept of lamellar refractive procedures in Bogotá, Colombia.1 In the 1990s, Pallikaris et al.2,3 and Buratto et al.4 conceived of techniques combining lamellar procedures with excimer laser ablation. These advances led to the development of modern laser in situ keratomileusis (LASIK) procedures.
Laser in situ keratomileusis has several advantages over photorefractive keratectomy (PRK) when performed properly in appropriate eyes. These include faster visual recovery, less discomfort after surgery, and milder and more predictable wound healing with less risk for corneal stromal opacity (haze). Many advantages are related to preservation of the central corneal epithelium and epithelial basement membrane in LASIK.5
Lamellar corneal flap formation is the critical step in successful LASIK surgery. Improper flap formation, including improper flap geometry, decentration, irregularity of the cut, and epithelial damage, can lead to myriad LASIK complications. Considerable progress has been made over the years in producing safer instruments for LASIK flap formation since the Automated Corneal Shaper was adapted to LASIK. Thus, more reliable and safer mechanical microkeratomes contributed to the explosive growth of refractive surgery over the past 15 years. Despite these advances, complications such as incomplete or partial flaps, free flaps, buttonholes, and small irregular flaps continue to plague refractive surgeons who perform LASIK with a microkeratome.6–8 There are also significant limitations to the eyes that can safely have lamellar flap formation performed with a mechanical microkeratome, including corneas that are too steep (likely to have buttonhole flaps), too flat (likely to have small diameter flaps), or relatively thin (more likely to have low residual stromal bed).
The femtosecond laser became available for LASIK flap formation approximately 10 years ago. Since the early femtosecond laser models were introduced, considerable progress has been made in improving flap geometry and limiting complications of LASIK performed with the laser. This has led to increasing popularity of LASIK performed with the femtosecond laser, to the point that different sources estimate that 30% to 50% of LASIK procedures in the United States in 2008 are performed using a femtosecond laser.A
Previous reviews9–13 delineated the advantages and disadvantages of both the mechanical microkeratome and the femtosecond laser. This article focuses on considerations that are unique to LASIK surgery performed with the femtosecond laser, including wound-healing differences compared with the microkeratome and conditions that are specific to LASIK performed with a femtosecond laser.
Recently, new femtosecond laser models were introduced. These include the Femtec (20/10 Perfect Vision AG), the Femto LDV (Zeimer Group), and the VisuMax (Carl Zeiss Meditec). Little has been published, however, on the designs or outcomes of these newer femtosecond lasers. Therefore, much of the information in this review focuses on the commonly used IntraLase 60 kHz femtosecond laser (Abbott Medical Optics, Inc.).
LASER ENERGY LEVEL AND PULSE RATES
All 4 commercially available femtosecond laser systems use ultrashort pulses of laser and produce corneal tissue cutting using a photodisruption process.14 However, energy parameters and pulse rates differ between the laser systems. In a recent overview of commercially available femtosecond lasers, Lubatschowski14 classified the systems into 2 groups. One group was characterized by high pulse energy and low pulse frequency (including the IntraLase and the Femtec), and the other group was characterized by low pulse energy and high pulse frequency (including the Femto LDV).
To create the lamellar flap, the IntraLase laser generates pulses of femtosecond (10−15 seconds) laser at a near-infrared (1053 nm) wavelength and delivers closely spaced 3 μm spots, which are focused at variable depths to photodisrupt stromal tissue. When a high peak power is reached, hot plasma is generated, initiating a process of tissue ionization that is commonly called laser-induced optical breakdown. The hot plasma expands in shock waves and creates an intrastromal cavitation bubble composed primarily of water and carbon dioxide. Multiple cavitation bubbles coalesce, and an intrastromal cleavage plane is created. The laser delivers a series of pulses in a specified pattern to create the lamellar intrastromal cut and then extends the cleavage to the surface with a side cut to complete the flap.
The IntraLase femtosecond laser with a speed of 6 kHz first became commercially available in the U.S. in 2000. Subsequent evolution of the technology led to the sequential introduction of 10 kHz, 15 kHz, 30 kHz, and 60 KHz lasers. In some cases, more than 1 model with a particular speed was marketed. The 60 kHz was introduced in 2006 and is the model that is most widely used throughout the world. IntraLase Corp. recently introduced the 150 kHz iFS Advanced femtosecond laser; however, there is little published information on this new model.
Pulse rates with the IntraLase femtosecond laser are key determinates of the efficacy and safety of flap formation in LASIK. The slower the pulse rate, the more energy required to generate the flap. The use of high energy, for example with the 6 kHz, 10 kHz, and 15 kHz models, produces larger shock waves that result in more tissue damage and in larger cavitation bubbles that can block following pulses and interfere with the cutting process.15 With the introduction of the 60 kHz model, the total energy delivered to the cornea was markedly reduced; therefore, the level of inflammation and associated complications were minimized. In addition, with the higher pulse repetition rate, there can be closer spot and line separation, which enhances the quality of the cleavage plane, making it easier to lift the flap. Studies show that improvements in laser optics and overall design of the femtosecond cuts improve results with the 60 kHz model over the 15 kHz and 30 kHz models.16 For example, the width of the epithelial cut during the side cut was decreased. This results in less release of epithelial proinflammatory cytokines and therefore less inflammation.16 No studies have reported these parameters with sue of the 150 kHz model.
FLAP THICKNESS AND SAFETY
Femtosecond lasers are designed to make thinner LASIK flaps, with a tighter range of thickness around the mean. Femtosecond laser flaps also tend to be more uniform in thickness from the center to the periphery than microkeratome flaps.16
The flap shape is typically thicker in the periphery and thinner in the center with mechanical microkeratomes. This meniscus-shaped flap increases the incidence of buttonhole perforation. In flaps created with the IntraLase femtosecond laser, there is more even thickness across the flap, producing a planar-shaped flap.16 A more planar flap morphology increases the safety of flap formation and, as will be discussed, improves custom ablation outcomes. Thus, it is exceedingly rare to encounter a buttonhole flap with this femtosecond laser.
Previous studies17–19 report the SD of flap thickness with mechanical microkeratomes in the range of ±20 to ±40 μm. Recent studies9,20,21 found that the femtosecond laser produced flaps with less variability in flap thickness. Most of these studies found that most eyes had a flap thickness within ±20 μm of the intended result. Stahl et al.22 used anterior segment optical coherence tomography to evaluate flaps created with a femtosecond laser and found them to be highly predictable and reproducible. In a recent study,23 our group found the SD of flaps to be ±25 μm with the Hansatome microkeratome (Bausch & Lomb and ±14.5 μm with the IntraLase femtosecond laser. Thinner mean flap thickness and a lower SD for flap thickness with the femtosecond laser LASIK to be performed in more eyes with relatively thinner corneas.
Poor predictability of flap diameter is also an issue with the mechanical microkeratome. With these devices, the diameter of the flap is a function of corneal power, with steeper corneas having wider diameter flaps that may impinge on the limbus and flatter corneas having smaller diameter flaps that may not allow the full ablation to be delivered (eg, an ablation with a blend zone out to 9.0 mm) and that may be predisposed to greater halos, glare, and other visual disturbances in eyes with larger pupils.13 According to Binder,9,13 femtosecond flaps are more accurate in achieved flap diameter and flap thickness than microkeratome flaps. Our experience with the IntraLase femtosecond laser flap diameter is similar. We observed little variability in flap diameter in corneas with a high mean curvature compared with those with a low mean curvature; this is not true of microkeratomes, with which there tends to be a positive correlation between mean corneal curvature and LASIK flap diameter.9,13
Another advantage of femtosecond laser is that the surgeon can select the position and diameter of the hinge. Thus, some surgeons prefer a superior-hinged flap, while others prefer a horizontal-hinged or oblique-hinged flap.
Among the problems that can occur during flap creation, loss of suction has the potential to lead to serious complications. Possible causes of loss of vacuum with the IntraLase femtosecond laser include eyelid squeezing, tight orbits, inadequate positioning of the suction ring on the eye, and penetrating edematous conjunctiva under the ring.24 If suction is lost with this laser, the laser focal plane jumps to the surface without further extension of the lamellar cut. In many cases, the suction ring can be reapplied and the treatment repeated at the same corneal depth. This recommendation is based on the nature of the process of flap creation with this laser, in which the generated high-pressure bubbles expand and disperse, creating their own pathway.25 Thus, it is reasonable to presuppose that the original lamellar cut represents an effortless pathway for these bubbles to expand, leading to separation of the preexisting resection plane.25 However, there is no absolute agreement on how and when to retreat these patients; some authors recommend immediate retreatment them, while others suggest waiting for up to 1 month.24
If the suction is lost during the side cut, the laser can be programmed to repeat only the side cut, again after the suction ring is placed in the same position it occupied during the initial cut, making a smaller flap diameter.24,26 Conversely, it is risky to attempt to extend a partial flap with a mechanical microkeratome.13
FLAP CENTRATION
Appropriate flap centration is crucial for the success of small-diameter flaps, such as microkeratome-generated flaps. Thus, the latest tendency has been to try to create flaps with the largest possible diameter, attempting to compensate for any decentration.27 As a consequence, flap centration tends to be less of an issue with femtosecond lasers than with microkeratomes, especially when large-diameter (9.3 mm) flaps are used routinely. These large diameter flaps are centered on the limbus and provide ample bed for application of excimer laser treatments centered on the entrance pupil. Flap centration on the actual pupil clearly becomes more important for surgeons who wish to use smaller diameter LASIK flaps with the femtosecond laser.
Ertan and Karacal28 suggest that flap centration is influenced by patient and surgeon factors as well. According to the authors, improper patient head position may lead to inadequate ring placement and applanation. They also state that the applanation point should be very well centered and that the glass lens should not be moved once the cornea is touched because the friction between the surfaces may cause decentration. On the other hand, with the IntraLase femtosecond laser, small adjustments in flap centration can be made with the computer mouse after suction is achieved.24
STROMAL WOUND HEALING AND INFLAMMATION
Corneal wound healing after refractive procedures is a complex process and a major determinate of the efficacy and safety of surgery.29,30 Significant improvements to commercially available lasers have been made since differences in the wound-healing response between the femtosecond laser and microkeratomes were first noted in 1997.31
Netto et al.16 compared early postoperative wound healing in LASIK flaps created with 3 femtosecond laser models (IntraLase 15 kHz, 30 kHz, and 60 kHz) with flaps created with a Hansatome microkeratome (Bausch & Lomb). They found that flap cuts with the 15 kHz femtosecond laser triggered more cell death, stromal cell proliferation, and inflammatory cell infiltration than flaps cut with the 30 kHz or 60 kHz laser or the microkeratome. They also found that cell death and central corneal inflammation were not significantly different between the 60 kHz femtosecond laser and the microkeratome.16 An important finding in this study was confirmation that the mode of stromal cell death triggered directly by the femtosecond laser 24 hours after surgery was necrosis versus apoptosis with the microkeratome. Cellular necrosis promotes inflammation. Therefore, procedures that trigger more keratocyte necrosis are likely to cause greater inflammation. A subsequent study by de Medeiros et al.32 found a direct correlation between the level of energy used in flap formation and stromal cell death as well as corneal inflammatory cell infiltration.
Kim et al.11 report stronger healing at the flap edge and in the flap interface with femtosecond laser flaps than with microkeratome flaps. Laboratory results show a similar difference between the femtosecond laser and microkeratome.16 This difference becomes very small when comparing the newer 60 kHz femtosecond laser and the Hansatome microkeratome.16 Although there are no studies of the new 150 kHz IntraLase laser or other new brands, we believe the correlations between energy level delivered to the cornea and stromal cell death and inflammation will remain fundamental to the application of femtosecond laser technology to LASIK.
The corneal wound-healing response after refractive procedures has been well documented, and it is known to be stronger after PRK than after LASIK, especially in high corrections.33 Accordingly, the risk for stromal haze development and a decrease in corrected distance visual acuity (CDVA) is higher after surface ablation.5 However, in a recent study, Rocha et al.34 found that the generation of ultrathin (90 μm) femtosecond laser flaps was associated with greater risk for haze development after LASIK. The authors propose that stromal haze after thin-flap LASIK is attributable to epithelium and basement membrane damage caused by the proximity of the laser-induced photodisruption of tissue (Figure 1). Based on animal studies of haze generation,16,35,36 increased myofibroblast generation in these eyes likely results from a combination of basement membrane damage and proximity of the myofibroblast progenitor cells to the epithelial cell source of transforming growth factor-β and other cytokines that drive modulate myofibroblast development.
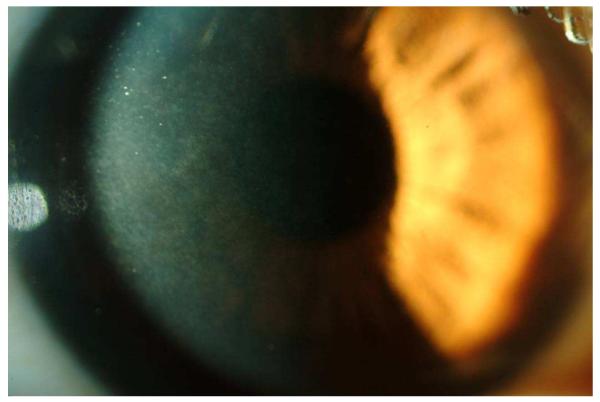
Figure 1.
Slitlamp photograph showing trace interface haze after ultrathin-flap (90 μm) femtosecond laser LASIK. Although the patient had no decrease in CDVA, a light-scattering test (C-Quant) was abnormal in the eye (courtesy of Karolinne M. Rocha, MD).
Published data on femtosecond laser tissue interactions show that earlier models of femtosecond lasers, such as the 6 kHz and 15 kHz IntraLase models, led to an increased incidence of diffuse lamellar keratitis (DLK)37 and slower visual recovery. This is likely a result of the more intense inflammatory response generated with the early models.38 More recent models, such as the 60 kHz IntraLase, induce a small inflammatory response that is similar to that with microkeratomes.16
Epithelial preservation is another concern in LASIK because epithelial defects are also associated with increased inflammation and DLK.39 They also cause greater postoperative pain, a higher rate of epithelial ingrowth, and slower visual recovery.10 It is uncommon to generate epithelial defects after LASIK performed with a femtosecond laser, even in eyes with anterior basement membrane dystrophy. However, in some cases, there will be epithelial damage overlying the stromal pocket produced by the laser. When damage to the epithelium is noted, it is important to increase the frequency of corticosteroidal agents during the first 24 to 48 hours after surgery because there will be increased risk that DLK will develop.
STROMAL BED QUALITY AND WAVEFRONT ANALYSIS
Higher-order aberrations (HOA) commonly increase after LASIK procedures.40–42 Customized wavefront-driven ablation is effective in limiting these increases, and reductions in aberrations have been noted in some cases. Laser ablation itself induces increases in HOAs after LASIK, although some increases are attributable to flap formation alone.43–46
Regardless of the device used to fashion a LASIK flap, it is important that the ablation surface be smooth to limit the generation of HOAs.46 Several studies have correlated better visual and refractive outcomes with more regular bed surfaces.47,48 Several recent studies49–53 report less induction of HOAs with the femtosecond laser than microkeratomes during LASIK. For example, Medeiros et al.50 compared the Moria-M2 and Hansatome microkeratomes with the 15 kHz and 30 kHz IntraLase femtosecond lasers and found that, on average, the femtosecond laser group had significantly less induced aberrations than the microkeratome group.
Surgically induced astigmatism also affects the outcomes of LASIK.54 At least 1 study comparing microkeratomes and femtosecond laser microkeratomes10 found less induced astigmatism with the femtosecond laser.
CORNEAL ECTASIA AND BIOMECHANICS
Iatrogenic corneal ectasia is a relatively rare complication of LASIK. It does occur, however, especially in eyes with predisposing factors such as forme fruste keratoconus.55 One strategy to prevent corneal ectasia is to preserve as much posterior stromal bed as possible, although cases of ectasia have been reported in eyes with a posterior residual bed of 300 μm or greater.55 Conversely, a flap that is too thin increases the risk for a flap tear, striae, or even a buttonhole or intraepithelial flap. Thus, predictability of flap thickness is a key factor in LASIK safety. A major advantage of the femtosecond laser is the capacity to reproducibly generate thinner flaps than with microkeratomes, with lower variability.19,20,22 For example, with the Hansatome microkeratome and a 180 μm head, we have obtained flaps that were 131 ± 25 μm thick.23 In contrast, when using the femtosecond laser and aiming for a flap 100 to 110 μm thick, we obtained flaps that were 112 ± 15 μm thick.23 Thus, not only are the flaps, on average, thinner with the femtosecond laser, there is also much less variation in flap thickness than with microkeratomes. Hence, when using a femtosecond laser rather than a microkeratome, the surgeon is less likely to encounter very thin flaps and very thick flaps. It follows that there is less likelihood of producing flaps that threaten the biomechanical stability of the cornea or disrupt the epithelium when the femtosecond laser is used for LASIK.27,56,57 Knorz and Vossmerbaeumer58 evaluated and compared the adhesion strength of flaps created with a mechanical microkeratome and a femtosecond laser. They found that flap adhesion was stronger with a femtosecond laser flap. This likely reduces the risk for flap dislocation associated with trauma and, possibly, the risk for corneal ectasia.
OPAQUE BUBBLE LAYER
As discussed previously, there are differences in technical features of commercially available femtosecond laser systems.14 These include, gas-bubble formation during the cutting process, which is reported to be less or nonexistent in systems that use low-energy pulses.14
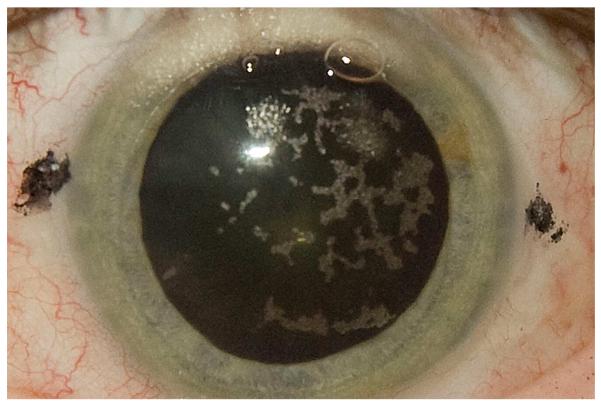
The photodisruptive process generated by the IntraLase laser produces cavitation bubbles that expand to create a cleavage plane in the corneal tissue. These high-pressure bubbles tend to expand into a path of least resistance and produce what has been termed the opaque bubble layer (OBL)59 (Figure 2). Typically, expansion of the bubbles produced during the central cut takes place in a naturally occurring stromal interlamellar space, creating a cleavage plane connected to the surface via the side cut. Thus, when the flap is lifted, little tissue disruption occurs in the central corneal stroma. A peripheral pocket is produced peripheral to the hinge with the laser to facilitate clearing of the cavitation bubbles from the flap. The components of these bubbles, primarily water and carbon dioxide gas, are cleared from the cornea over several minutes to hours by a combination of diffusion and endothelial pump action. An excessive OBL can interfere with the tracking mechanism during excimer laser ablation, especially if it is located in the pupil.59
Figure 2.
Diffuse OBL 5 minutes after flap creation using the IntraLase 60 kHz femtosecond laser. Over 35 minutes, all the bubbles were cleared from the cornea and refractive ablation was performed with no interference with the tracking system of the excimer laser.
Such bubbles were more common with the 15 kHz IntraLase laser, especially when thicker and smaller diameter flaps were generated,59 but has been noted with the 60 kHz model on occasion.
Gas bubbles infrequently appear in the anterior chamber after application of the femtosecond laser. These bubbles are probably associated with formation of the pocket and expansion through posterior corneal or stromal layers into the anterior chamber. Lifshitz et al.60 suggest that bubbles are occasionally pushed posteriorly and into the anterior chamber by shock waves if the stromal lamellae are weak and the endothelial cell tight junctions leaky. Whatever the mechanism, these bubbles are typically benign. They may interfere with the eye tracker of the excimer laser if they are multiple or large. In this situation, the surgeon can delay excimer laser application for a few hours, or even to the next day, until the bubbles are reabsorbed.
Vertical subepithelial gas breakthrough is also an uncommon complication during flap creation with the femtosecond laser. It occurs when gas bubbles escape into the epithelial space, usually when the surgeon is attempting to cut a thin flap.61 The real cause is uncertain, although some authors propose that local defects in the Bowman membrane61 or altered epithelium60 contribute to it because they can act as a low-resistance pathway for the bubbles to expand.62 Accordingly, fibrotic corneal tissue may increase the resistance to an effective plane of separation and may be associated with this complication.62 When this occurs, it is best to allow the OBL to clear completely and then recut the flap at thickness that is somewhat deeper than the initial attempt. If the surgeon aims more superficial for the recut, there is a tendency for the laser pulses to find the initial lamellar cut and for the complication to recur.
TRANSIENT LIGHT-SENSITIVITY SYNDROME
Transient light-sensitivity syndrome (TLSS) is a unique side effect of femtosecond laser that is primarily associated with earlier 6 kHz and 15 kHz models of the IntraLase laser but has been noted rarely with the 30 kHz and 60 kHz models.57 It syndrome represents a group of symptoms that develop days to weeks after LASIK in which the flap is created with a femtosecond laser. It usually presents with the onset of intense light sensitivity 2 to 6 weeks after uneventful LASIK, despite good uncorrected distance visual acuity and an unremarkable slitlamp examination.63 Although the real etiology is unknown, most possible mechanisms proposed to explain this syndrome correlate it to an inflammatory origin. Migration of cytokines from the interface to the iris and sclera,64 deposit of necrotic debris or subproducts of gas bubbles,64 keratocyte activation in the interface,63 and irritation of the ciliary body63 have been suggested as possible causes of inflammation. Symptoms usually regress with intensive topical corticosteroid treatment, at times supplemented with oral corticosteroidal agents, which corroborates the idea of an inflammatory origin.64
RAINBOW GLARE
Rainbow glare is another side effect unique to the femtosecond laser. This phenomenon has been described as an optical effect that is believed to be a consequence of diffractive light scattering.65 It is reported by patients as a spectrum of colored bands radiating from a white-light source when in a dark environment, although it does not appear to interfere with the patient’s visual acuity.66
A recent study by Bamba et al.66 found that rather than faster pulse frequencies or lower raster energies, it is the quality of the beam and the dimension of the numerical aperture of the focusing optics that seem to be the most important factors in reducing this symptom. Thus, it is extremely important to have regular laser maintenance to ensure beam quality and alignment and avoid this side effect.
DRY EYE
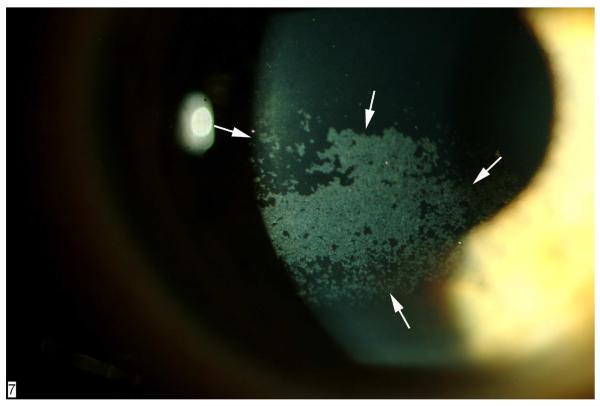
Dry eye is the most common complication after LASIK and a major cause of patient and surgeon dissatisfaction.67,68 Ambrósio et al.69 describe several possible mechanisms of LASIK-associated dry eye, including damage to afferent sensory nerves, reduction of blinking reflex and tear production, increased tear evaporation, and injury to goblet cells at the limbus. Transsection of afferent sensory nerves in the anterior third of the stroma during the lamellar cut is considered the most important factor in the pathophysiology of LASIK-induced dry eye70–72 (Figure 3). Although the neurotrophic effect of LASIK is probably the dominant factor in the symptoms and signs after surgery, it is likely that many patients who develop the disorder have underlying subclinical inflammatory dry before surgery, accounting for the favorable response to topical cyclosporine A in most cases.69 The stromal nerves regenerate into the flap 5 to 8 months after LASIK in most eyes, leading to a reduction in symptoms and signs and a decreased need for pharmacologic treatment.69,73,74
Figure 3.
Slitlamp documentation of severe punctate epithelial erosions (arrows) in the cornea of an eye that developed LASIK-induced neurotrophic epitheliopathy 1 week after surgery. The epitheliopathy involved the pupillary area, and there was some decrease in CDVA, which resolved 6 months after surgery.
A decrease in the incidence of LASIK-induced dry eye when the flap is created with a femtosecond laser has been reported. For example, we found a 9% incidence of LASIK-induced dry eye with a femtosecond laser compared with 46% with a microkeratome.23 We found a corresponding decrease in the need for treatment with cyclosporine A and other modalities. Possible explanations for the decrease include less damage to corneal nerves with the femtosecond laser, attributable to thinner flaps, or less damage to limbal cells, including goblet cells, produced by the femtosecond fixation ring.
As mentioned, using the femtosecond laser allows the surgeon to select the hinge position, likely with fewer complications than when a mechanical microkeratome is used.10 Some authors suggest that more nerves penetrate the stroma in horizontal meridians75,76 and, therefore, nasal hinged flaps better preserve the corneal innervation and induce less neurotrophic dry eye.77 However, recent studies found no significant difference in dry eye development between superior-hinged flaps and nasal-hinged flaps.78 Also, anatomic studies of human eyes76 found that nerve trunks providing sensation to the cornea are equally distributed for 360 degrees around the cornea. Further studies are necessary to confirm whether hinge position or other factors are responsible for the lower incidence of neurotrophic dry eye with the use of the femtosecond laser.
STRIAE AND FOLDS
Striae and folds are among the postoperative flap complications that can arise after LASIK. They can be responsible for symptoms such as halos, star bursts, and diplopia as well as decreased visual acuity.79 Striae are more frequently associated with flap irregularities and extremely thin or thick flaps.80 Proposed causes include trauma, flap desiccation and contraction, misalignment, and modification in the corneal contour, especially with higher myopic ablations, which may create a disparity in the arc length between the excimer laser–ablated bed and the posterior surface of the flap.79,80
Flap adhesion might play a role in reducing the occurrence of striae because it is important to minimize the risk for trauma. As discussed, femtosecond flaps have stronger adhesion than microkeratome flaps,58 which may provide additional protection from the lower eyelid and inadvertent manipulations by the patient during the early postoperative period. When flap striae occur after LASIK performed with the femtosecond laser, management is similar to that with LASIK performed with the microkeratome. Kuo et al.79 report 2 cases of flap striae that were resistant to multiple treatments; PRK was performed as a helpful alternative.
DISCUSSION
The application of femtosecond laser technology to LASIK surgery has progressed rapidly over the past few years. It seems likely that femtosecond LASIK will become more common throughout the world with the introduction of several new femtosecond lasers. Improvements in flap thickness reproducibility,20,22 wavefront measurements,50 stromal bed quality,49 and biomechanical outcomes56,57 are some of the advantages that may carry this tendency forward.
It is important to mention the limitations of this study. The most important is that we only compared 1 type of femtosecond laser and microkeratome. Therefore, our conclusions may not be applicable to all femtosecond lasers and mechanical microkeratomes. Another important consideration is cost. Femtosecond lasers are a more expensive alternative; thus, not all centers can offer this technology.
Although some complications, such as TLSS, are specific to femtosecond lasers, they are rare with newer models of the IntraLase femtosecond laser. Several new femtosecond laser models were recently introduced, and they have functions other flap creation alone. The VisuMax, for example, has been used in a new procedure for lenticule extraction in which the laser is used for flap creation as well as for the actual correction; thus, an excimer laser is not required. In a recent study, Blum et al.81 report 6-month results in 108 myopic eyes treated with this procedure and found it to be highly satisfactory. Further clinical experience is needed to evaluate the efficacy and safety of these new instruments.
Acknowledgments
Supported in part by United States Public Health Service grants EY010056 and EY015638 from the National Eye Institute, Bethesda, Maryland, and Research to Prevent Blindness, New York, New York, USA.
Biography

Footnotes
Dr. Wilson is a consultant to Allergan, Inc., Irvine, California, USA.
Other Cited Material A. 2008 Comprehensive Report on the Global Ophthalmic Laser Market. St. Louis, MO, Market Scope, October 2008
Financial Disclosure: Neither author has a financial or proprietary interest in any material or method mentioned. Additional disclosure is found in the footnotes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barraquer JI. Basis of refractive keratoplasty—1967. Refract Corneal Surg. 1989;5:179–193. [PubMed] [Google Scholar]
- 2.Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A. Laser in situ keratomileusis. Lasers Surg Med. 1990;10:463–468. doi: 10.1002/lsm.1900100511. [DOI] [PubMed] [Google Scholar]
- 3.Pallikaris IG, Papatzanaki ME, Siganos DS, Tsilimbaris MK. A corneal flap technique for laser in situ keratomileusis; human studies. Arch Ophthalmol. 1991;109:1699–1702. doi: 10.1001/archopht.1991.01080120083031. [DOI] [PubMed] [Google Scholar]
- 4.Buratto L, Ferrari M, Rama P. Excimer laser intrastromal keratomileusis. Am J Ophthalmol. 1992;113:291–295. doi: 10.1016/s0002-9394(14)71581-8. [DOI] [PubMed] [Google Scholar]
- 5.Ambrósio R, Jr, Wilson SE. LASIK vs LASEK vs PRK: advantages and indications. Semin Ophthalmol. 2003;18:2–10. doi: 10.1076/soph.18.1.2.14074. [DOI] [PubMed] [Google Scholar]
- 6.Knorz MC. Flap and interface complications in LASIK. Curr Opin Ophthalmol. 2002;13:242–245. doi: 10.1097/00055735-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Lichter H, Stulting RD, Waring GO, III, Russell GE, Carr J. Buttonholes during LASIK: etiology and outcome. J Refract Surg. 2007;23:472–476. doi: 10.3928/1081-597X-20070501-09. [DOI] [PubMed] [Google Scholar]
- 8.Ambrósio R, Jr, Wilson SE. Complications of laser in situ keratomileusis: etiology, prevention, and treatment. J Refract Surg. 2001;17:350–379. doi: 10.3928/1081-597X-20010501-09. [DOI] [PubMed] [Google Scholar]
- 9.Binder PS. One thousand consecutive IntraLase laser in situ keratomileusis flaps. J Cataract Refract Surg. 2006;32:962–969. doi: 10.1016/j.jcrs.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804–811. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Kim MJ, Kim T-I, Choi H-J, Pak JH, Tchah H. [Accessed March 15, 2010];A femtosecond laser creates a stronger flap than a mechanical microkeratome. Invest Ophthalmol Vis Sci. 2006 47:599–604. doi: 10.1167/iovs.05-0458. Available at: http://www.iovs.org/cgi/reprint/47/2/599. [DOI] [PubMed] [Google Scholar]
- 12.Montés-Micó R, Rodríguez-Galietero A, Alió JL. Femtosecond laser versus mechanical keratome LASIK for myopia. Ophthalmology. 2007;114:62–68. doi: 10.1016/j.ophtha.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- 14.Lubatschowski H. Overview of commercially available femtosecond lasers in refractive surgery. J Refract Surg. 2008;24:S102–S107. doi: 10.3928/1081597X-20080101-18. [DOI] [PubMed] [Google Scholar]
- 15.Ratkay-Traub I, Juhasz T, Horvath C, Suarez C, Kiss K, Ferincz I, Kurtz R. Ultra-short pulse (femtosecond) laser surgery; initial use in LASIK flap creation. Ophthalmol Clin North Am. 2001;14(2):347–355. [PubMed] [Google Scholar]
- 16.Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Jr, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbelaez MC. Nidek MK 2000 microkeratome clinical evaluation. J Refract Surg. 2002;18:S357–S360. doi: 10.3928/1081-597X-20020502-15. [DOI] [PubMed] [Google Scholar]
- 18.Uçakhan Ö Ö . Corneal flap thickness in laser in situ keratomileusis using the Summit Krumeich-Barraquer microkeratome. J Cataract Refract Surg. 2002;28:798–804. doi: 10.1016/s0886-3350(01)01304-9. [DOI] [PubMed] [Google Scholar]
- 19.Shemesh G, Dotan G, Lipshitz I. Predictability of corneal flap thickness in laser in situ keratomileusis using three different microkeratomes. J Refract Surg. 2002;18:S347–S351. doi: 10.3928/1081-597X-20020502-13. [DOI] [PubMed] [Google Scholar]
- 20.Kim J-H, Lee D, Rhee K-I. Flap thickness reproducibility in laser in situ keratomileusis with a femtosecond laser: optical coherence tomography measurement. J Cataract Refract Surg. 2008;34:132–136. doi: 10.1016/j.jcrs.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Sutton G, Hodge C. Accuracy and precision of LASIK flap thickness using the IntraLase femtosecond laser in 1000 consecutive cases. J Refract Surg. 2008;24:802–806. doi: 10.3928/1081597X-20081001-06. [DOI] [PubMed] [Google Scholar]
- 22.Stahl JE, Durrie DS, Schwendeman FJ, Boghossian AJ. Anterior segment OCT analysis of thin IntraLase femtosecond flaps. J Refract Surg. 2007;23:555–558. doi: 10.3928/1081-597X-20070601-03. [DOI] [PubMed] [Google Scholar]
- 23.Salomão MQ, Ambrósio R, Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35:1756–1760. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedlaender MH. LASIK surgery using the IntraLase femtosecond laser. Int Ophthalmol Clin. 2006;46(3):145–153. doi: 10.1097/00004397-200604630-00013. [DOI] [PubMed] [Google Scholar]
- 25.Tran DB, Binder PS, Brame CL. Lasik flap revision using the IntraLase femtosecond laser. Int Ophthalmol Clin. 2008;48(1):51–63. doi: 10.1097/IIO.0b013e31815eae43. [DOI] [PubMed] [Google Scholar]
- 26.Soong HK, Malta JB. Femtosecond lasers in ophthalmology. Am J Ophthalmol. 2009;147:189–197. doi: 10.1016/j.ajo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Slade SG. The use of the femtosecond laser in the customization of corneal flaps in laser in situ keratomileusis. Curr Opin Ophthalmol. 2007;18:314–317. doi: 10.1097/ICU.0b013e3281bd88a0. [DOI] [PubMed] [Google Scholar]
- 28.Ertan A, Karacal H. Factors influencing flap and INTACS decentration after femtosecond laser application in normal and keratoconic eyes. J Refract Surg. 2008;24:797–801. doi: 10.3928/1081597X-20081001-05. [DOI] [PubMed] [Google Scholar]
- 29.Netto MV, Ambrósio R, Jr, Chalita MR, Krueger RR, Wilson SE. [Accessed March 16, 2010];Resposta cicatricial corneana em diferentes modalidades de cirurgia refrativa. Corneal wound healing response following different modalities of refractive surgical procedures]. Arq Bras Ophthalmol. 2005 68:140–149. doi: 10.1590/s0004-27492005000100027. Available at: http://www.scielo.br/pdf/abo/v68n1/23276.pdf. [DOI] [PubMed] [Google Scholar]
- 30.Wilson SE, Netto M, Ambrósio R., Jr Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol. 2003;136:530–536. doi: 10.1016/s0002-9394(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SE. Molecular cell biology for the refractive corneal surgeon: programmed cell death and wound healing. J Refract Surg. 1997;13:171–175. doi: 10.3928/1081-597X-19970301-15. [DOI] [PubMed] [Google Scholar]
- 32.de Medeiros FW, Kaur H, Agrawal V, Chaurasia SS, Hammel J, Dupps WJ, Jr, Wilson SE. Effect of femtosecond laser energy level on corneal stromal cell death and inflammation. J Refract Surg. 2009;25:869–874. doi: 10.3928/1081597X-20090917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan RR, Hutcheon AEK, Choi R, Hong JW, Lee JS, Mohan RR, Ambrósio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 34.Rocha KM, Kagan R, Smith SD, Krueger RR. Thresholds for interface haze formation after thin-flap femtosecond laser in situ keratomileusis for myopia. Am J Ophthalmol. 2009;147:966–972. doi: 10.1016/j.ajo.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur H, Chaurasia SS, Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. 2009;88:960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaloy J, Vidal MT, Abdelrahman AM, Artola A, Alió JL. Confocal microscopy comparison of IntraLase femtosecond laser and Moria M2 microkeratome in LASIK. J Refract Surg. 2007;23:178–187. doi: 10.3928/1081-597X-20070201-10. [DOI] [PubMed] [Google Scholar]
- 38.Chang JSM. Complications of sub-Bowman’s keratomileusis with a femtosecond laser in 3009 eyes. J Refract Surg. 2008;24:S97–S101. doi: 10.3928/1081597X-20080101-17. [DOI] [PubMed] [Google Scholar]
- 39.Shah MN, Misra M, Wilhelmus KR, Koch DD. Diffuse lamellar keratitis associated with epithelial defects after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:1312–1318. doi: 10.1016/s0886-3350(00)00570-8. [DOI] [PubMed] [Google Scholar]
- 40.Oshika T, Klyce SD, Applegate RA, Howland HC, El Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 1999;127:1–7. doi: 10.1016/s0002-9394(98)00288-8. [DOI] [PubMed] [Google Scholar]
- 41.Bühren J, Kohnen T. Factors affecting the change in lower-order and higher-order aberrations after wavefront-guided laser in situ keratomileusis for myopia with the Zyoptix 3.1 system. J Cataract Refract Surg. 2006;32:1166–1174. doi: 10.1016/j.jcrs.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 42.Subbaram MV, MacRae SM, Slade SG, Durrie DS. Customized LASIK treatment for myopia: relationship between preoperative higher order aberrations and refractive outcome. J Refract Surg. 2006;22:746–753. doi: 10.3928/1081-597X-20061001-04. [DOI] [PubMed] [Google Scholar]
- 43.Pallikaris IG, Kymionis GD, Panagopoulou SI, Siganos CS, Theodorakis MA, Pallikaris AI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refract Surg. 2002;28:1737–1741. doi: 10.1016/s0886-3350(02)01507-9. [DOI] [PubMed] [Google Scholar]
- 44.Chalita MR, Chavala S, Xu M, Krueger RR. Wavefront analysis in post-LASIK eyes and its correlation with visual symptoms, refraction, and topography. Ophthalmology. 2004;111:447–453. doi: 10.1016/j.ophtha.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Waheed S, Chalita MR, Xu M, Krueger RR. Flap-induced and laser-induced ocular aberrations in a two-step LASIK procedure. J Refract Surg. 2005;21:346–352. doi: 10.3928/1081-597X-20050701-08. [DOI] [PubMed] [Google Scholar]
- 46.Porter J, MacRae S, Yoon G, Roberts C, Cox IG, Williams DR. Separate effects of the microkeratome incision and laser ablation on the eye’s wave aberration. Am J Ophthalmol. 2003;136:327–337. doi: 10.1016/s0002-9394(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 47.Vinciguerra P, Azzolini M, Airaghi P, Radice P, De Molfetta V. Effect of decreasing surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis on optical and functional outcomes. J Refract Surg. 1998;14:S199–S203. doi: 10.3928/1081-597X-19980401-12. [DOI] [PubMed] [Google Scholar]
- 48.Vinciguerra P, Azzolini M, Radice P, Sborgia M, De Molfetta V. A method for examining surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis: predictor of optical and functional outcomes. J Refract Surg. 1998;14:S204–S206. doi: 10.3928/1081-597X-19980401-13. [DOI] [PubMed] [Google Scholar]
- 49.Sarayba MA, Ignacio TS, Binder PS, Tran DB. Comparative study of stromal bed quality by using mechanical, IntraLase femtosecond laser 15- and 30- kHz microkeratomes. Cornea. 2007;26:446–451. doi: 10.1097/ICO.0b013e318033e7cc. [DOI] [PubMed] [Google Scholar]
- 50.Medeiros FW, Stapleton WM, Hammel J, Krueger RR, Netto MV, Wilson SE. Wavefront analysis comparison of LASIK outcomes with the femtosecond laser and mechanical microkeratomes. J Refract Surg. 2007;23:880–887. doi: 10.3928/1081-597X-20071101-03. [DOI] [PubMed] [Google Scholar]
- 51.Buzzonetti L, Petrocelli G, Valente P, Tamburrelli C, Mosca L, Laborante A, Balestrazzi E. Comparison of corneal aberration changes after laser in situ keratomileusis performed with mechanical microkeratome and IntraLase femtosecond laser: 1- year follow-up. Cornea. 2008;27:174–179. doi: 10.1097/ICO.0b013e31815a50bf. [DOI] [PubMed] [Google Scholar]
- 52.Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis; prospective contralateral eye study. J Cataract Refract Surg. 2005;31:120–126. doi: 10.1016/j.jcrs.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Tran DB, Sarayba MA, Bor Z, Garufis C, Duh Y-J, Soltes CR, Juhasz T, Kurtz RM. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: potential impact on wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:97–105. doi: 10.1016/j.jcrs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 54.Sharma N, Pangtey MS, Vajpayee RB, Dada T, Aggarwal T, Dada VK, Pandey RM. Surgically induced astigmatism after laser in situ keratomileusis for spherical myopia. J Refract Surg. 2002;18:239–244. doi: 10.3928/1081-597X-20020501-05. [DOI] [PubMed] [Google Scholar]
- 55.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 56.Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stonecipher K, Ignacio TS, Stonecipher M. Advances in refractive surgery: microkeratome and femtosecond laser flap creation in relation to safety, efficacy, predictability, and biomechanical stability. Curr Opin Ophthalmol. 2006;17:368–372. doi: 10.1097/01.icu.0000233957.88509.2d. [DOI] [PubMed] [Google Scholar]
- 58.Knorz MC, Vossmerbaeumer U. Comparison of flap adhesion strength using the Amadeus microkeratome and the IntraLase iFS femtosecond laser in rabbits. J Refract Surg. 2008;24:875–878. doi: 10.3928/1081597X-20081101-04. [DOI] [PubMed] [Google Scholar]
- 59.Kaiserman I, Maresky HS, Bahar I, Rootman DS. Incidence, possible risk factors, and potential effects of an opaque bubble layer created by a femtosecond laser. J Cataract Refract Surg. 2008;34:417–423. doi: 10.1016/j.jcrs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 60.Lifshitz T, Levy J, Klemperer I, Levinger S. Anterior chamber gas bubbles after corneal flap creation with a femtosecond laser. J Cataract Refract Surg. 2005;31:2227–2229. doi: 10.1016/j.jcrs.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan S, Herzig S. Sub-epithelial gas breakthrough during femtosecond laser flap creation for LASIK [video report] Br J Ophthalmol. 2007;91:1373. doi: 10.1136/bjo.2007.129213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seider MI, Ide T, Kymionis GD, Culbertson WW, O’Brien TP, Yoo SH. Epithelial breakthrough during IntraLase flap creation for laser in situ keratomileusis. J Cataract Refract Surg. 2008;34:859–863. doi: 10.1016/j.jcrs.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 63.Stonecipher KG, Dishler JG, Ignacio TS, Binder PS. Transient light sensitivity after femtosecond laser flap creation: clinical findings and management. J Cataract Refract Surg. 2006;32:91–94. doi: 10.1016/j.jcrs.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Muñoz G, Albarrán-Diego C, Sakla HF, Javaloy J, Alió JL. Transient light-sensitivity syndrome after laser in situ keratomileusis with the femtosecond laser; incidence and prevention. J Cataract Refract Surg. 2006;32:2075–2079. doi: 10.1016/j.jcrs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Krueger RR, Thornton IL, Xu M, Bor Z, van den Berg TJTP. Rainbow glare as an optical side effect of IntraLASIK. Ophthalmology. 2008;115:1187–1195. doi: 10.1016/j.ophtha.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Bamba S, Rocha KM, Ramos-Esteban JC, Krueger RR. Incidence of rainbow glare after laser in situ keratomileusis flap creation with a 60 kHz femtosecond laser. J Cataract Refract Surg. 2009;35:1082–1086. doi: 10.1016/j.jcrs.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent corneal erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27:577–584. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 68.Jabbur NS, Sakatani K, O’Brien TP. Survey of complications and recommendations for management in dissatisfied patients seeking a consultation after refractive surgery. J Cataract Refract Surg. 2004;30:1867–1874. doi: 10.1016/j.jcrs.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 70.Wilson SE, Ambrósio R., Jr Laser in situ keratomileusis-induced neurotrophic epitheliopathy. Am J Ophthalmol. 2001;132:405–406. doi: 10.1016/s0002-9394(01)00995-3. [DOI] [PubMed] [Google Scholar]
- 71.Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108:1230–1235. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 72.Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001;108:1082–1087. doi: 10.1016/s0161-6420(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 73.Latvala T, Linna T, Tervo T. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int Ophthalmol Clin. 1996;36(4):21–27. doi: 10.1097/00004397-199603640-00005. [DOI] [PubMed] [Google Scholar]
- 74.Linna TU, Pérez-Santonja JJ, Tervo KM, Sakla HF, Alió y Sanz JL, Tervo TMT. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66:755–763. doi: 10.1006/exer.1998.0469. [DOI] [PubMed] [Google Scholar]
- 75.Müller LJ, Vrensen GFJM, Pels L, Cardozo BN, Willekens B. [Accessed March 15, 2010];Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997 38:985–994. Available at: http://www.iovs.org/cgi/reprint/38/5/985. [PubMed] [Google Scholar]
- 76.Muller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]; 2003;77:253. errata. [Google Scholar]
- 77.Donnenfeld ED, Solomon K, Perry HD, Doshi SJ, Ehrenhaus M, Solomon R, Biser S. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. 2003;110:1023–1029. doi: 10.1016/S0161-6420(03)00100-3. discussion by CJ Rapuano, 1029–1030. [DOI] [PubMed] [Google Scholar]
- 78.Vroman DT, Sandoval HP, Fernández de Castro LE, Kasper TJ, Holzer MP, Solomon KD. Effect of hinge location on corneal sensation and dry eye after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2005;31:1881–1887. doi: 10.1016/j.jcrs.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 79.Kuo IC, Jabbur NS, O’Brien TP. Photorefractive keratectomy for refractory laser in situ keratomileusis flap striae. J Cataract Refract Surg. 2008;34:330–333. doi: 10.1016/j.jcrs.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Biser SA, Bloom AH, Donnenfeld ED, Perry HD, Solomon R, Doshi S. Flap folds after femtosecond LASIK. Eye Contact Lens. 2003;29:252–254. doi: 10.1097/01.icl.0000081600.43438.4B. [DOI] [PubMed] [Google Scholar]
- 81.Blum M, Kunert K, Schröder M, Sekundo W. Femtosecond lenticule extraction for the correction of myopia: preliminary 6-month results. Graefes Arch Clin Exp Ophthalmol. 2010 Feb 4; doi: 10.1007/s00417-009-1293-1. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]