Abstract
Objective
Thoracoabdominal aortic aneurysms (TAAAs) occur most commonly in elderly individuals, who are often suboptimal candidates for open repair because of significant comorbidities. The availability of a hybrid option, including open visceral debranching with endovascular aneurysm exclusion, may have advantages in these patients who are at high-risk for conventional repair. This report details the evolution of our technique and results with complete visceral debranching and endovascular aneurysm exclusion for TAAA repair in high-risk patients.
Methods
Between March 2005 and June 2011, 47 patients (51% women) underwent extra-anatomic debranching of all visceral vessels, followed by aneurysm exclusion by endovascular means at a single institution. A median of four visceral vessels were bypassed. The debranching procedure was initially performed through a partial right medial visceral rotation approach, leaving the left kidney posterior in the first 22 patients, and in the last 25 by a direct anterior approach to the visceral vessels. The debranching and endovascular portions of the procedure were performed in a single operation in the initial 33 patients and as a staged procedure during a single hospital stay in the most recent 14.
Results
Median patient age was 71.0 ± 9.8 years. All had significant comorbidity and were considered suboptimal candidates for conventional repair: 55% had undergone previous aortic surgery, 40% were American Society of Anesthesiologists (ASA) class 4, and baseline serum creatinine was 1.5 ± 1.3 mg/dL. The 30-day/in-hospital rates of death, stroke, and permanent paraparesis/plegia were 8.5%, 0%, and 4.3%, respectively, but 0% in the most recent 14 patients undergoing staged repair. These patients had significantly shorter combined operative times (314 vs 373 minutes), decreased intraoperative red blood cell transfusions (350 vs 1400 mL), and were more likely to be extubated in the operating room (50% vs 12%) compared with patients undergoing simultaneous repair. Over a median follow-up of 19.3 ± 18.5 months, visceral graft patency was 97%; all occluded limbs were to renal vessels and clinically silent. There have been no type I or III endoleaks or reinterventions. Kaplan-Meier overall survival is 70.7% at 2 years and 57.9% at 5 years.
Conclusions
Hybrid TAAA repair through complete visceral debranching and endovascular aneurysm exclusion is a good option for elderly high-risk patients less suited to conventional repair in centers with the requisite surgical expertise with visceral revascularization. A staged approach to debranching and endovascular aneurysm exclusion during a single hospitalization appears to yield optimal results.
The treatment of thoracoabdominal aortic aneurysm (TAAA) is one of the most formidable challenges in cardiovascular surgery. Several treatment strategies have evolved since DeBakey1 in 1956 reported a small series of repairs using a temporary passive thoracic-to-femoral shunt and aortic replacement with homografts, with 50% mortality. A larger series of 42 patients was reported in 1965, still using the shunt concept to minimize organ ischemia time, with a 26% mortality.2 Crawford introduced the concept of endoaneurysmorrhaphy with anastomosis of one or more button patches containing the orifices of the visceral vessels into the side of a Dacron graft during a period of aortic cross-clamping without a shunt.3 Although ischemic times averaged >40 minutes in that report, the mortality was a commendable 7%.
More recently, partial left heart bypass and complete cardiopulmonary bypass (CPB) with or without deep hypothermic circulatory arrest have been used in these difficult cases in an effort to minimize the risk of ischemic organ injury as well as paraplegia from spinal cord ischemia. Kouchoukos et al4 reported 211 patients who underwent repair using hypothermic CPB and circulatory arrest, with an overall 7% mortality. The mortality in extent II (Crawford classification) patients, however, was 15%. Some centers have also used partial left heart bypass without an oxygenator or heat exchanger.5 Although select high-volume centers5 report <10% mortality for open TAAA repair, larger more representative databases show that the operative mortality in the United States averages 20% to 25% and is even higher in subsets such as older patients.6
Aortic endografting has decreased the operative mortality for infrarenal aneurysms, and a similar approach to TAAA has been taken with the development of branched or fenestrated endografts.7 At the present time, however, these devices are extremely complex and require very sophisticated endovascular equipment and skills. Furthermore, the U.S. Food and Drug Administration (FDA) has not approved the devices, with only limited availability in the United States, and more widespread availability is unlikely in the near future. Finally, the durability of these devices has yet to be demonstrated, particularly in the setting of conformational changes that may occur after the successful exclusion of large aneurysms.
A third approach, a hybrid of the open and endovascular methods involving extra-anatomic bypass of the visceral vessels (debranching), was described as early as 1999 by Quiñones-Baldrich.8 Since this first report, several authors have described small series using the hybrid approach,9–12 and a larger series has presented aggregate data from three European institutions.13 This strategy avoids the need for CPB and aortic cross-clamp. It does require a major laparotomy, but because the visceral revascularization occurs serially, the individual organ ischemic times are short. The procedure uses currently available devices and familiar surgical techniques and has become our procedure of choice for patients at high risk for open TAAA repair. This report describes the largest single-institution series to date of total visceral debranching and endovascular repair for TAAAs and the techniques we have developed to perform this operation.
METHODS
This study was reviewed and approved by the Duke University Institutional Review Board, and the need for individual patient consent was waived.
Patients and data source
A prospective cohort review was performed of the 47 patients who underwent hybrid repair involving complete visceral debranching and endovascular aneurysm exclusion for Crawford extent I to III TAAA between March 2005 (date of FDA approval of the first available thoracic device in the United States) and June 2011 at Duke University Medical Center (DUMC) in Durham, North Carolina, a referral institution. During the same period, 37 patients judged to have lower risk underwent conventional open TAAA repair.
Preoperative, intraoperative, and postoperative variables were abstracted from the Duke Thoracic Aortic Surgery Database, which is a prospectively maintained clinical registry of all patients undergoing thoracic aortic surgery at DUMC since 2005. General criteria regarding patient selection for hybrid rather than the conventional TAAA repair have been described previously14 and are listed in Table I. We consider the presence of a connective tissue disorder as a contraindication to this approach, unless proximal and distal landing zones are completely within existing Dacron grafts (eg, visceral patch aneurysm after prior conventional TAAA repair).15 Crawford extent IV aneurysms are treated with conventional open repair at DUMC and are not included in this report.
Table I.
Patient selection criteria for the hybrid thoracoabdominal aortic aneurysm (TAAA) repair14
| Selection criteriaa | Comments |
|---|---|
| Age ≥65 years | Increased mortality in prior studies5 of open repair. |
| Cardiac disease | Avoids CPB ± cross-clamp, and single-lung ventilation lessens hemodynamic stress. Allows repair in patients with concomitant aortic insufficiency. |
| Pulmonary disease | No thoracotomy, single-lung ventilation, or CPB lessens pulmonary morbidity. |
| Renal insufficiency | Increased mortality in prior studies5 of open repair. Sequential revascularization strategy minimizes renal ischemia; generally modest contrast loads. |
| Prior open abdominal or descending thoracic or TAAA repair | Avoids redo-chest exposure; existing aortic grafts generally excellent landing zones. Infrarenal grafts provide good inflow source for visceral debranching graft. |
CPB, Cardiopulmonary bypass.
These criteria are relative factors in the decision-making process but are not absolute indications or contraindications. Ideally, the decision for conventional vs hybrid repair should be made by a surgical team with expertise in both techniques, and consideration of institutional results with each technique should factor heavily into the decision-making process.
All follow-up was done at the Duke University Center for Aortic Surgery. Details regarding the follow-up protocol as performed at DUMC have been described and included clinical examination and computed tomography angiography at 1, 6, and 12 months postoperatively, and annually thereafter. All procedural outcomes and complications were prospectively recorded. This report includes all data collected through the patients’ most recent follow-up visit. The Social Security Death Index was queried (http://ssdi.rootsweb.com/) to confirm patient deaths, including patients who did not return for follow-up visits. Cause of death for patients who died during follow-up was confirmed by review of medical records or family interview in all cases.
Survival analyses were performed using the Kaplan-Meier method. All data are presented in accordance with the “Reporting Standards for Endovascular Aortic Aneurysm Repair” of the Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery.16
Operative technique
The current technique has evolved14,15,17 due to experience gained during the past 6 years. We now perform the procedure (A video of the procedure is available online only) in a staged fashion: the open abdominal portion is performed first and the endovascular portion takes place 3 to 7 days later during the same hospitalization. The debranching procedure was initially performed through a partial right medial visceral rotation approach, leaving the left kidney posterior in the first 22 patients and, subsequently, through a direct anterior approach to the visceral vessels in the last 25 patients. The partial medial visceral rotation approach was abandoned because of concerns over disruption of the collateral supply to the left colon as well as the not infrequent need for incidental splenectomy. A transperitoneal approach was used in all cases because the right renal artery is not accessible from the left side using a retroperitoneal left flank approach if the visceral segment of the aorta is aneurysmal.
The debranching and endovascular portions of the procedure were performed during a single operation in the initial 33 patients, with a staged approach during the same hospital stay in the most recent 14 patients. The change to a staged approach was prompted by concerns over renal injury with visceral debranching, followed by contrast administration, as well as the report by Patel et al18 demonstrating excellent outcomes using a staged approach to perform hybrid TAAA repair. The current anterior staged approach is described below.
Patients are admitted the day before surgery for judicious hydration and gentle mechanical bowel preparation. The debranching is done through a generous midline incision. A self-retaining ringed retractor (Bookwalter) is indispensable for exposure. To minimize resetting of the retractor blades, the right renal artery is dissected first. The peritoneum lateral to the third portion of the duodenum is incised, exposing the inferior vena cava. The right renal vein is identified, and the artery is usually found cephalad and deep to it.
The preoperative imaging is studied carefully to ensure the main trunk of the artery is controlled if there is early branching. The retractor blades are then reset to expose the infrarenal abdominal aorta. A standard dissection of the anterior surface of the aorta in the region of the left renal vein is performed. The superior mesenteric artery (SMA) origin and the celiac axis are exposed by elevating the pancreas. These vessels are dissected over an adequate distance to permit clamping, division, and secure closure of the aortic stump. The aorta at the origins of these vessels is aneurysmal and often friable, so extreme care is taken to ensure an adequate and secure closure. This often includes oversewing with pledgeted vascular sutures.
Finally, the left renal artery is dissected free, which may require the division of some branches of the left renal vein. The proximal anastomosis for the multibranched visceral bypass graft used can be to the distal aorta, either iliac system if large enough, or a previously placed aortic graft or iliac limb. The left common iliac is the most common inflow source.
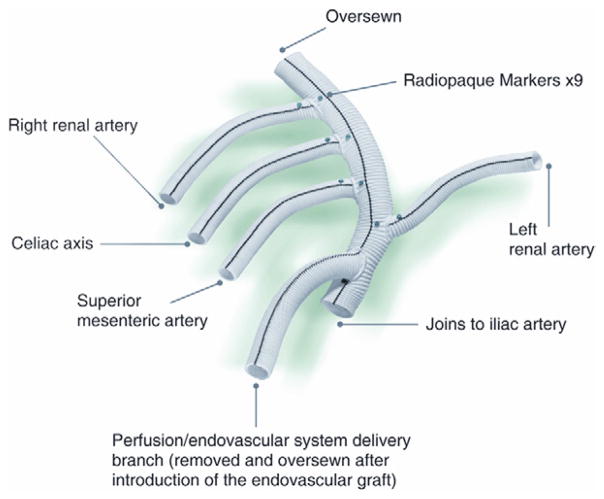
We designed a Dacron graft, which is now commercially available (Vascutek, Ann Arbor, Mich), specifically for this operation. The graft (Fig 1) has a 14-mm trunk, two 6-mm side limbs for the renal arteries, and two 8-mm side limbs for the visceral (celiac, SMA) arteries. In addition, there is a 10-mm side limb at the proximal end adjacent to the inflow anastomosis that is used as a conduit for the large sheaths during the delayed second endovascular stage. This generally avoids the need for additional vascular exposure at this second operation. At the end of the first operation, just before closing, the conduit limb designed for endograft introduction is exited in a retroperitoneal tunnel to a pocket on the abdominal wall that is positioned to afford as straight a line access to the aorta as possible. Each branch of the debranching graft has radiographic markers for identification should angiography of the bypass graft be required in the future.
Fig 1.
A custom-designed multibranched Dacron graft (Vascutek USA, Ann Arbor, Mich) was used for visceral debranching during hybrid thoracoabdominal aortic aneurysm (TAAA) repair.
After the vascular exposures have been completed, the patient is heparinized (100 U/kg) to a goal activated clotting time >200 seconds. The proximal anastomosis is done first, after trimming the graft obliquely to minimize the angle of entry of the endovascular access limb to the aorta. The origin of the bypass graft is marked with radiographic markers to allow identification of the graft origin under fluoroscopy and aid in deployment of the endovascular grafts at the second stage. The radiographic markers indicate the most distal position beyond which the endografts should not extend to avoid compromising the inflow to the visceral debranching graft.
The branches of the graft are sequentially anastomosed in an end-to-end fashion to their respective visceral branches (Fig 2). In this manner, visceral ischemia time is minimized and is usually <10 minutes per anastomosis. The right renal graft is done last because the retractor blades must be readjusted. After the three left-sided bypasses are completed, the right renal graft is passed through the root of the mesentery over the aorta and inferior vena cava to the right retroperitoneal exposure.
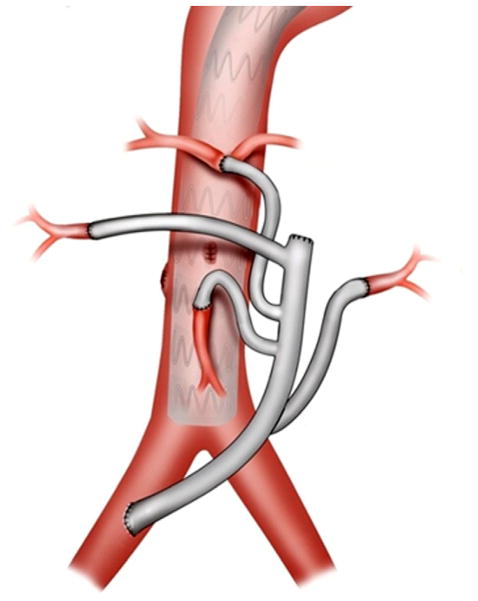
Fig 2.
Drawing demonstrates the completed hybrid thoracoabdominal aortic aneurysm (TAAA) repair. The inflow for the debranching graft in this particular case is from the right common iliac artery with end-to-end distal anastomoses to the four visceral vessels.
The heparin is reversed with protamine, and other clotting factors are given as required after the anastomoses are completed. The graft can usually be covered with peritoneum to avoid bowel adhesion to the graft. The access limb is stored in a small subcutaneous abdominal wall pocket, and the abdomen is closed in standard fashion.
Postoperatively, the patient is maintained well hydrated, and bowel and renal function are monitored, as well as blood pressure. The second stage is performed 3 to 7 days later to permit recovery of renal function and avoid simultaneous renal stress with contrast injection. Patients remain in the hospital between procedures to reduce the risk of interval rupture through a monitored care environment and by reducing the interval between procedures. The first and second stages are done in a hybrid operating room with fixed imaging equipment and full operative capability.
The second stage of the endovascular procedure is done with somatosensory and motor evoked potential electro-physiologic monitoring of spinal cord function, as previously described,19 routine lumbar cerebrospinal fluid drainage, and invasive cardiovascular monitoring in the event that evoked potential evidence of spinal cord ischemia requires manipulating the blood pressure or draining of spinal fluid.
To start the endovascular second stage, the endovascular access limb is retrieved by opening the abdominal wall pocket, and the graft limb is pulled out of the pocket and thrombectomized. The only other vascular access that is required for this stage, unless a bifurcated distal device is used (Fig 3), in which case unilateral groin exposure will also be required, is a percutaneously placed 5F sheath in either femoral artery for passage of the diagnostic angiographic catheter.
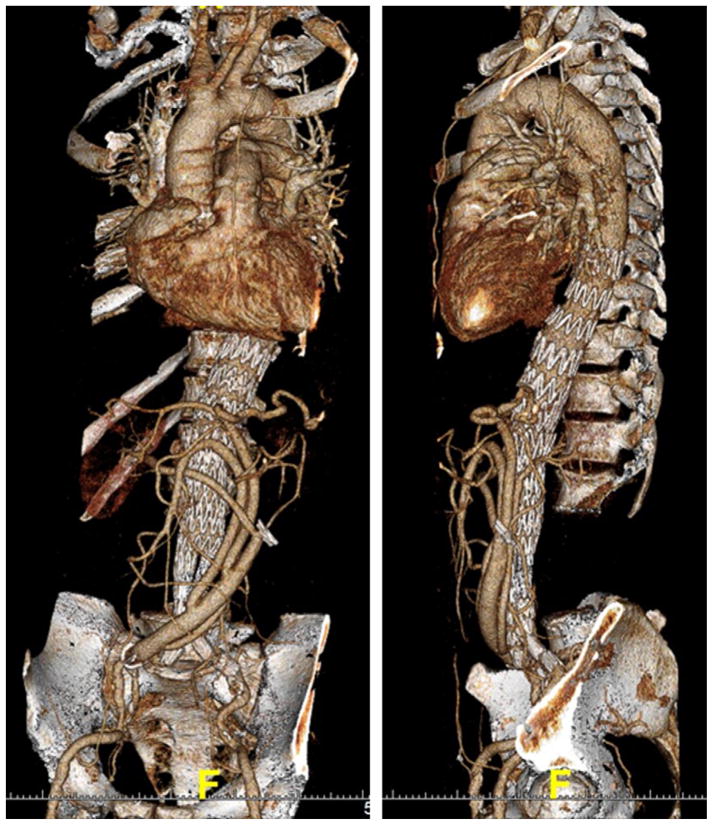
Fig 3.
Three-dimensional computed tomography angiography reconstructions show a patient with a Crawford extent III aneurysm after hybrid thoracoabdominal aortic aneurysm (TAAA) repair with deployment of a bifurcated abdominal system distally for seal within the iliac arteries.
The aneurysm is relined by deploying any of the available thoracic or abdominal endograft systems. The large-bore access sheath is inserted only a small distance into the access limb of the visceral debranching graft to avoid occlusion of the main channel feeding the viscera during endograft delivery. After endograft deployment and a completion arteriogram, the debranching graft is selectively imaged to confirm graft limb patency. At the conclusion of the endovascular second-stage procedure, the access limb is amputated and buried beneath the abdominal wall.
Data analyses
Univariate comparisons of preoperative, operative, and postoperative variables were performed between patients undergoing simultaneous or staged aneurysm repair. Continuous variables were compared using the nonparametric Wilcoxon sign test, and categoric variables were assessed by the χ2 test or the Fisher exact test. A value of P < .05 was considered statistically significant.
Postoperative outcomes were controlled for baseline covariates with univariate P < .25 using multivariable models when sufficient events were present. Continuous outcome variables were all skewed and were dichotomized at their median and used for multivariable analysis. Stepwise logistic regression was used to examine the adjusted effect of procedure type on the outcomes of interest. Variables were selected for final inclusion in the logistic regression models via a stepwise procedure with α = 0.1. Statistical analyses were performed using SAS 9.1 software (SAS Institute Inc, Cary, NC). Unadjusted survival estimates were calculated to produce a Kaplan-Meier curve for overall and aorta-specific survival, as well as simultaneous vs staged survival, using the log-rank test. Survival analyses were conducted using GraphPad Prism 4.00 software (GraphPad Software, San Diego, Calif).
RESULTS
Patient demographics
Baseline patient characteristics are presented in Table II and operative and procedural characteristics in Table III. Data are presented for the overall group and for simultaneous vs staged repair. The initial 33 patients underwent simultaneous repair, and the most recent 14 patients underwent a staged approach during a single hospital stay. The mean duration between procedures in the staged group was 5.5 days (range, 3–7 days). Median patient age was 71 years, 40% of patients were American Society of Anesthesiologists (ASA) class IV, and baseline serum creatinine for the entire cohort was 1.5 ± 1.3 mg/dL.
Table II.
Baseline patient characteristics
| Variablea | Overall (n = 47) | Type of repair
|
P | |
|---|---|---|---|---|
| Simultaneous (n = 33) | Staged (n = 14) | |||
| Age, median (SD) years | 71 (9.8) | 71 (10.3) | 71 (8.8) | .86 |
| Female | 24 (51) | 12 (36.4) | 12 (85.7) | .002 |
| Nonwhite race | 17 (36) | 13 (39.4) | 4 (28.6) | .48 |
| BMI, mean (SD) kg/m2 | 27.2 (6.1) | 28.1 (6.3) | 25.1 (5.1) | .13 |
| Pre-op steroid use | 4 (9) | 1 (3.0) | 3 (21.4) | .07 |
| Active or recent smoker | 30 (65) | 21 (65.6) | 9 (64.3) | .93 |
| Hypertension | 37 (79) | 27 (81.8) | 10 (71.4) | .43 |
| Diabetes mellitus | 6 (13) | 6 (18.2) | 0 (0) | .16 |
| History of | ||||
| GERD | 16 (34) | 11 (33.3) | 5 (35.7) | .87 |
| Ethanol abuse | 3 (6) | 1 (3.0) | 2 (14.3) | .21 |
| Stroke | 9 (19) | 7 (21.2) | 2 (14.3) | .58 |
| Myocardial infarction | 9 (19) | 4 (12.1) | 5 (35.7) | .06 |
| Congestive heart failure | 4 (9) | 4 (12.1) | 0 (0) | .30 |
| Ejection fraction, mean (SD) | 54.3 (2.1) | 54.0 (2.5) | 55.0 (0) | .18 |
| Peripheral vascular disease | 12 (26) | 8 (24.2) | 4 (28.6) | .76 |
| Chronic lung disease | 19 (40) | 11 (33.3) | 8 (57.1) | .13 |
| Connective tissue disorderb | 2 (4) | 2 (6.1) | 0 (0) | >.99 |
| Pre-op diuretic use | 19 (40) | 14 (42.4) | 5 (35.7) | .67 |
| Previous aortic surgery | 26 (55) | 19 (57.6) | 7 (50.0) | .63 |
| Previous aortic dissection | 5 (11) | 4 (12.1) | 1 (7.1) | >.99 |
| Symptomatic | 16 (34) | 11 (33.3) | 5 (35.7) | .88 |
| Pre-op laboratory values | ||||
| Hemoglobin, mean (SD) g/dL | 11.5 (2.0) | 11.3 (2.0) | 11.8 (2.0) | .50 |
| Creatinine, mean (SD) mg/dL | 1.5 (1.3) | 1.5 (1.3) | 1.7 (1.4) | .60 |
BMI, Body mass index; GERD, gastrointestinal reflux disease; SD, standard deviation.
Continuous data are shown as indicated, and categoric data as number (%).
Both were visceral patch aneurysms in patients with Marfan syndrome after prior open TAAA repair.
Table III.
Operative characteristics by procedure
| Variablea | Overall (n = 47) | Type of repair
|
P | |
|---|---|---|---|---|
| Simultaneous (n = 33) | Staged (n = 14) | |||
| Max aortic diameter, mean (SD) cm | 6.7 (1.3) | 6.8 (1.5) | 6.5 (0.8) | .47 |
| ASA classification | ||||
| 3 | 28 (59.6) | 20 (60.6) | 8 (57.1) | |
| 4 | 19 (40.4) | 13 (39.4) | 6 (42.9) | |
| Status | ||||
| Elective | 39 (83.0) | 27 (81.8) | 12 (85.7) | .75 |
| Urgent or emergency | 8 (17.0) | 6 (18.2) | 2 (14.3) | |
| Crawford classification | ||||
| Extent I | 2 (4.3) | 1 (3.0) | 1 (7.1) | |
| Extent II | 15 (31.9) | 9 (27.3) | 6 (42.9) | |
| Extent III | 30 (63.8) | 23 (69.7) | 7 (50.0) | |
| Total bypass grafts, No. | 171 | 118 | 53 | |
| Bypass grafts/patient, median (range) | 4 (2–4) | 4 (2–4) | 4 (2–4) | .31 |
ASA, American Society of Anesthesiologists.
Continuous data are shown as indicated, and categoric data as number (%).
Procedural (30-day) outcomes
All patent visceral vessels were debranched in the 47 patients, resulting in 171 visceral bypasses for a median of 4 vessels bypassed per patient (Table III). The median number of main body endograft components deployed per case was three (range 1–6). Seven patients (15%) required a bifurcated abdominal endografting system distally to obtain seal within the iliac arteries (Fig 3) due to lack of an adequate distal landing zone within the infrarenal aorta.
The 30-day/in-hospital outcomes are presented in Table IV. Operative mortality, defined as death ≤30 days of the procedure or during the same hospital admission, was 8.5% (4 of 47) for the entire cohort. No strokes occurred. The rate of permanent paraparesis/paraplegia was 4.3% (2 of 47). Although not statistically different from the simultaneous approach, there were no deaths, strokes, or permanent spinal cord ischemic events in the most recent 14 patients treated by a staged operation. Patients undergoing staged repair were significantly more likely to be extubated in the operating room (50% vs 12%) than those undergoing simultaneous repair. Further, intraoperative red blood cell transfusion (350 vs 1400 mL) and operative time (314 vs 373 minutes) were lower with the staged approach. Length of stay was longer with a staged approach by 3.5 days (13.5 vs 10 days). No aneurysm ruptures occurred during the interval before the second stage in the staged group.
Table IV.
Thirty-day/in-hospital operative outcome measures
| Variablea | Overall | Type of repair
|
Adjusted OR (95% CI)b | P (unadjusted) | |
|---|---|---|---|---|---|
| Simultaneous (n = 33) | Staged (n = 14) | ||||
| Operative time, min | 350 (302, 417) | 373 (325, 435) | 314 (293, 339)c | 0.18 (0.04–0.77) | .01 |
| Estimated blood loss, mL | 1300 (700, 3000) | 1975 (850, 3000) | 1150 (300, 1350)c | 0.28 (0.06–1.28) | .07 |
| Intra-op PRBC transfusion, mL | 1050 (350, 1925) | 1400 (700, 2100) | 350 (88, 1313)c | 0.14 (0.03–0.62) | .06 |
| Intubated to ICU | 36 (77) | 29 (88) | 7 (50)d | 0.14 (0.03–0.61) | .01 |
| Stroke >72 hours | 0 (0) | 0 (0) | 0 (0) | ||
| Permanent paraparesis/plegiae | 2 (4.3) | 2 (6.1) | 0 (0) | >.99 | |
| Ventilation >24 hours | 6 (12.8) | 6 (18.2) | 0 (0) | .16 | |
| Tracheostomy | 2 (4.3) | 2 (6.1) | 0 (0) | .35 | |
| Re-op for bleeding | 2 (4.3) | 2 (6.1) | 0 (0) | >.99 | |
| Post-op wound infection | 4 (8.5) | 2 (6.1) | 2 (14.3) | .57 | |
| Post-op MI | 1 (2.1) | 1 (3.0) | 0 (0) | >.99 | |
| New dialysis requirement | 6 (12.8) | 5 (15.2) | 1 (7.7) | .66 | |
| Length of stay, days | 12 (8, 15) | 10 (7, 14) | 13.5 (14, 15) | 5.5 (1.2–26.31) | .04 |
| In-hospital/30-day deaths | 4 (8.5) | 4 (12.1) | 0 (0) | .30 | |
CI, Confidence interval; ICU, intensive care unit; MI, myocardial infarction; OR, odds ratio, PRBC, packed red blood cells.
Data are presented as median (interquartile range) for continuous variables and as number (%) for categoric variables.
Odds ratios were adjusted for covariates selected by stepwise logistic regression (intraoperative PRBC transfusion was adjusted for sex; length of stay was adjusted for chronic obstructive pulmonary disease).
Denotes totals for stage 1 + stage 2 procedures.
Denotes whether patients remained intubated at the conclusion of either the stage 1 or stage 2 procedure.
Denotes condition present at discharge.
Follow-up outcomes
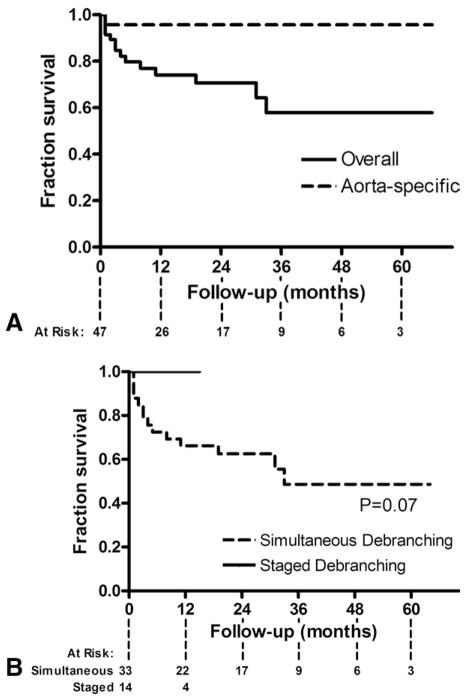
Follow-up imaging to assess graft patency was available for 37 patients (79%). Over a median follow-up of 19.3 ± 18.5 months (range, 1–66 months), with follow-up of 24 ± 3.4 months for simultaneous repair and 4.3 ± 1.4 months for staged repair, visceral graft patency was 97.0% (131 of 135). All occluded limbs were to renal vessels and clinically silent. All were detected on the 1-month follow-up scan, with no new graft occlusions developing thereafter. There have been no type I or III endoleaks, no reinterventions, and no late aortic-related deaths. Kaplan-Meier overall survival is 70.7% at 2 years and 57.9% at 5 years (Fig 4).
Fig 4.
Kaplan-Meier curves demonstrate (A) overall and aorta-specific survival and (B) simultaneous vs staged repair.
DISCUSSION
The current report represents the largest single-center series to date of hybrid TAAA repair by open visceral debranching with endovascular aneurysm exclusion. The results demonstrate that the hybrid approach we used is a safe alternative to conventional repair in older patients with significant comorbidity:
The improved operative parameters and lack of stroke, paraplegia, or death in any of the patients treated by a staged operation suggest a staged approach to repair may be superior. The latter finding may be especially true for renal function because the mandatory period of renal ischemia inherent in the debranching operation is not immediately followed by a second nephrotoxic insult with contrast administration for arteriography.
The staged strategy lessens the period of heparinization, accounting for the lower blood loss and transfusion requirements vs single-stage repair.
Having the patient fully resuscitated and medically optimized at the time of the endovascular procedure likely accounts for the lower mortality and paraplegia rates with the staged approach.
The shorter operative times of the individual stages certainly contribute to the lower incidence of mechanical ventilation >24 hours and the greater number of patients being extubated before leaving the operating room with the staged approach.
Keeping patients hospitalized and completing the second stage within 3 to 7 days after the visceral debranching allows for closer monitoring of physiologic parameters such as blood pressure. No interval ruptures occurred in patients undergoing the staged approach, unlike a previous report, in which there was a median of 20 days between stages and three ruptures occurred in the interval.13
The results we have presented are generally similar, if not superior, to those previously published with conventional open TAAA repair in younger patients in high-volume centers. For example, Wong et al20 recently published results in 509 patients treated with open surgery for TAAA between May 2006 and October 2010; this report included patients with Crawford extent I to IV aneurysms, and the authors stated the results were intended to represent contemporary outcomes for comparison with newer approaches to the treatment of TAAA. The 30-day inhospital rates of mortality, stroke, and permanent paraparesis/paraplegia in the 384 patients with extent I to III aneurysms were 7.8%, 5.5%, and 7.6%, respectively, vs 8.5%, 0%, and 4.3% in the current report.
Rates of new dialysis were similar between the two series, with major pulmonary morbidity significantly less with a hybrid approach. Risk factors for adverse outcomes with open repair in the Wong article included advanced age and pulmonary disease. Of further note is that the patients in the Wong series were nearly a decade younger than those reported here. The average age of the patients with operative mortality in that series was 72.7 years vs 62.9 years in those with operative survival. The average age of the patients who died is very similar to the median age of all patients in the current report, and as a whole, the data presented in the Wong article would advocate for an alternative treatment option, such as hybrid repair, for the older and sicker among the TAAA population.
Advantages of the hybrid approach are likely due to the avoidance of thoracotomy, CPB, and aortic cross-clamp, thus making the procedure better tolerated by patients with significant comorbidity. Despite the apparent advantages over conventional repair in high-risk patients, hybrid TAAA repair is still a significant operation, and given the complexity of the intervention, an integrated team experienced with the open and endovascular surgical techniques required, as well as the necessary infrastructure to care for these typically elderly patients with multiple comorbidities, is mandatory. The hybrid procedure does not replace conventional repair, but allows a complimentary alternative for high-risk patients who might otherwise be denied therapy for their lethal aortic disease.
Among the limitations of our study are that it is retrospective, and there is no randomized open control group for comparison. The effects of the learning curve and surgeon experience have not been accounted for and may partially contribute to the improved results observed with staged repair. The study is also underpowered, with too few adverse events to detect a statistically significant difference between the simultaneous and staged groups. In addition, the staged approach has only been in use since 2010 in this series. Although we have 100% follow-up on the staged group, many patients have not yet reached the 1-year follow-up assessment. Long-term results will need to be reviewed to determine if the advantages over the simultaneous repair are maintained.
CONCLUSIONS
Hybrid TAAA repair via complete visceral debranching and endovascular aneurysm exclusion is a good option for elderly high-risk patients less suited to conventional repair in centers with the requisite surgical expertise with visceral revascularization. A staged approach to debranching and endovascular aneurysm exclusion during a single hospitalization appears to yield optimal results.
Footnotes
Author conflict of interest: Dr Hughes is a consultant and speaker for Medtronic Vascular and W. L. Gore and Associates and has received an unrestricted research grant from W. L. Gore. Drs Hughes and McCann are consultants and speakers for Vascutek Terumo.
Presented at the 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago Ill, June 16–18, 2011.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: GH, RM
Analysis and interpretation: GH, MB, AS, NA, RM, MK, JH
Data collection: MB, AS, SB, JW, NA
Writing the article: GH, RM
Critical revision of the article: GH, MB, NA, RM
Final approval of the article: GH, RM
Statistical analysis: MB, AS, MK, JH
Obtained funding: Not applicable
Overall responsibility: RM
References
- 1.Creech O, Jr, Debakey ME, Morris GC., Jr Aneurysm of thoracoabdominal aorta involving the celiac, superior mesenteric, and renal arteries; report of four cases treated by resection and homograft replacement. Ann Surg. 1956;144:549–73. doi: 10.1097/00000658-195610000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBakey ME, Crawford ES, Garrett HE, Beall AC, Jr, Howell JF. Surgical considerations in the treatment of aneurysms of the thoracoabdominal aorta. Ann Surg. 1965;162:650–62. doi: 10.1097/00000658-196510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg. 1974;179:763–72. doi: 10.1097/00000658-197405000-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouchoukos NT, Masetti P, Murphy SF. Hypothermic cardiopulmonary bypass and circulatory arrest in the management of extensive thoracic and thoracoabdominal aortic aneurysms. Semin Thorac Cardiovasc Surg. 2003;15:333–9. doi: 10.1053/s1043-0679(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 5.Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:S862–4. doi: 10.1016/j.athoracsur.2006.10.088. discussion: S90–2. [DOI] [PubMed] [Google Scholar]
- 6.Cowan JA, Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR., Jr Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37:1169–74. doi: 10.1016/s0741-5214(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg RK, West K, Pfaff K, Foster J, Skender D, Haulon S, et al. Beyond the aortic bifurcation: branched endovascular grafts for thoracoabdominal and aortoiliac aneurysms. J Vasc Surg. 2006;43:879–86. doi: 10.1016/j.jvs.2005.11.063. discussion: 886–7. [DOI] [PubMed] [Google Scholar]
- 8.Quiñones-Baldrich WJ, Panetta TF, Vescera CL, Kashyap VS. Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg. 1999;30:555–60. doi: 10.1016/s0741-5214(99)70084-4. [DOI] [PubMed] [Google Scholar]
- 9.Fulton JJ, Farber MA, Marston WA, Mendes R, Mauro MA, Keagy BA. Endovascular stent-graft repair of pararenal and type IV thoracoabdominal aortic aneurysms with adjunctive visceral reconstruction. J Vasc Surg. 2005;41:191–8. doi: 10.1016/j.jvs.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Flye MW, Choi ET, Sanchez LA, Curci JA, Thompson RW, Rubin BG, et al. Retrograde visceral vessel revascularization followed by endovascular aneurysm exclusion as an alternative to open surgical repair of thoracoabdominal aortic aneurysm. J Vasc Surg. 2004;39:454–8. doi: 10.1016/j.jvs.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Patel R, Conrad MF, Paruchuri V, Kwolek CJ, Chung TK, Cambria RP. Thoracoabdominal aneurysm repair: hybrid versus open repair. J Vasc Surg. 2009;50:15–22. doi: 10.1016/j.jvs.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Reardon M, Peden EK, Lin PH, Lumsden AB. Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J Vasc Surg. 2006;44:688–93. doi: 10.1016/j.jvs.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Drinkwater SL, Böckler D, Eckstein H, Cheshire NJ, Kotelis D, Wolf O, et al. The visceral hybrid repair of thoracoabdominal aortic aneurysms—a collaborative approach. Eur J Vasc Endovasc Surg. 2009;38:578–85. doi: 10.1016/j.ejvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Hughes GC, McCann RL. Hybrid thoracoabdominal aortic aneurysm repair: concomitant visceral revascularization and endovascular aneurysm exclusion. Semin Thorac Cardiovasc Surg. 2009;21:355–62. doi: 10.1053/j.semtcvs.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008;136:21–8. 8e1–6. doi: 10.1016/j.jtcvs.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, McCann RL. Visceral debranching techniques for hybrid thoracoabdominal aortic aneurysm repair. In: Davies AH, editor. Vascular and Endovascular Surgery Highlights 2009–2010. 1. Oxford, UK: Health Press Limited; 2010. pp. 84–93. [Google Scholar]
- 18.Patel HJ, Upchurch GR, Jr, Eliason JL, Criado E, Rectenwald J, Williams DM, et al. Hybrid debranching with endovascular repair for thoracoabdominal aneurysms: a comparison with open repair. Ann Thorac Surg. 2010;89:1475–81. doi: 10.1016/j.athoracsur.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 19.Husain AM, Swaminathan M, McCann RL, Hughes GC. Neurophysiologic intraoperative monitoring during endovascular stent graft repair of the descending thoracic aorta. J Clin Neurophysiol. 2007;24:328–35. doi: 10.1097/WNP.0b013e31811ebf6e. [DOI] [PubMed] [Google Scholar]
- 20.Wong DR, Parenti JL, Green SY, Chowdhary V, Liao JM, Zarda S, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg. 2011;212:569–79. doi: 10.1016/j.jamcollsurg.2010.12.041. discussion: 79–81. [DOI] [PubMed] [Google Scholar]