Abstract
Fas-associated death domain protein is a key component of the extrinsic apoptotic pathway. In addition, in animal models, Fas-associated death domain protein phosphorylation at serine 194 has been shown to affect cell proliferation, especially in T lymphocytes. The importance of Fas-associated death domain protein phosphorylation at serine 194 for the proliferation of B lymphocytes, however, is uncertain. Here we show in reactive lymph nodes that serine 194 phosphorylated Fas-associated death domain protein is expressed predominantly in the dark (proliferative) zone of germinal centers. In B-cell non–Hodgkin lymphoma cell lines, serine 194 phosphorylated Fas-associated death domain protein levels are substantially higher in highly proliferating cells and lower in serum-starved cells. We also used immunohistochemical analysis to assess Fas-associated death domain protein phosphorylation at serine 194 expression in 122 B-cell non–Hodgkin-type lymphomas. The mean percentage of serine 194 phosphorylated Fas-associated death domain protein positive tumor cells was 81% in Burkitt lymphoma, 41% in diffuse large B-cell lymphoma, 18% in follicular lymphoma, 18% in plasma cell myeloma, 12% in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue, 11% in mantle cell lymphoma, and 2% in chronic lymphocytic leukemia/small lymphocytic lymphoma (P < .0001, Kruskal-Wallis test). Furthermore, in chronic lymphocytic leukemia/small lymphocytic lymphoma, serine 194 phosphorylated Fas-associated death domain protein was detected predominantly in proliferation centers. In the entire study group, the percentage of cells positive for serine 194 phosphorylated Fas-associated death domain protein correlated significantly with the proliferation index Ki-67 (Spearman R = 0.9, P < .0001). These data provide evidence that serine 194 phosphorylated Fas-associated death domain protein is involved in the proliferation of normal and neoplastic B cells and has features of a novel proliferation marker.
Keywords: FADD, Phosphorylation, Proliferation, B cell, Lymphomas
1. Introduction
Fas-associated death domain protein (FADD), a molecule of 22 kd, plays a central adaptor role in conveying apoptotic stimuli from death receptors containing a death domain (DD) motif in the extrinsic apoptotic pathway and particularly Fas [1,2]. FADD contains 2 evolutionary highly conserved modules: a carboxy-terminal DD, through which it associates with homologous DDs contained in death receptors, and an amino-terminal death-effector domain (DED), through which it interacts with homologous DEDs contained in the initiator caspases 8/10, and FADD is essential for the nucleation of the death-inducing signaling complex [3,4]. However, a series of experiments with mice bearing a variety of specific FADD mutations suggested that FADD is also involved in nonapoptotic processes, including innate immune signaling, hematopoiesis, and cell cycle regulation in lymphocytes [5,6].
FADD can be phosphorylated at serine 194 residue (ser194p-FADD), localized at the carboxy-terminal region adjacent to the DD. Regulation of ser194p-FADD phosphor-ylation is critical for nonapoptotic functions of FADD, including cell cycle progression, and for its subcellular localization in the nucleus [7-11]. Although many kinase signaling systems have been implicated in FADD phosphorylation, a recent study demonstrated that casein kinase Iα (CKIα) is responsible for serine 194 phosphorylation of FADD at the G2-M transition of the cell cycle [7,12,13].
FADD phosphorylation has been shown to be dysregulated in a variety of epithelial cancers including lung, breast, prostate, and gastric adenocarcinomas [14-17], and ser194p-FADD expression has been correlated with prognosis, tumor progression, or response to chemotherapy [14-17]. For example, Chen and colleagues [14] showed that increased expression of ser194p-FADD, assessed by immunohistochemistry, correlated positively with adverse prognosis in patients with lung adenocarcinoma. Also, in vitro studies using carcinoma cells have shown that increased ser194p-FADD levels augment the antitumor activity of various chemotherapeutic agents [13]. However, the patterns and potential biologic significance of ser194p-FADD expression in reactive B cells and B-cell lymphomas are unknown.
In this study, we hypothesized that serine 194 phosphorylation of FADD may play a role in cell proliferation and cell cycle progression in B cells. To address this idea, we assessed ser194p-FADD expression in reactive lymphoid tissues and in B-cell non–Hodgkin lymphoma (NHL) cell lines and patient tumor specimens. Our data show that ser194p-FADD may represent a novel proliferation marker in normal and neoplastic B cells.
2. Materials and methods
2.1. Cell lines
Eight B-cell NHL cell lines were assessed in this study including SP-53, Mino, and Jeko-1 (typical mantle cell lymphoma, or MCL), JMP-1 and Z-138 (blastoid variant of MCL), Pfeiffer (diffuse large B-cell lymphoma, or DLBCL), Raji (Burkitt lymphoma, or BL), and REH (B acute lymphoblastic leukemia/lymphoma; B-ALL). All cell lines were maintained under conditions of exponential growth as described previously [18].
For serum deprivation studies, Mino cells were incubated in 20% (control), 10%, or 0.01% fetal bovine serum (FBS) for 24 hours before harvesting. We also reincubated previously serum-starved, synchronized JMP-1 cells with 15% FBS and harvested at various time points.
In another experiment, SP-53 cells were incubated with increasing concentrations of the CKIα-specific inhibitor CKI-7 (Toronto Research Chemicals, Ontario, Canada) up to 250 μmol/L for 8 hours and were harvested for analysis of ser194p-FADD levels by Western blot analysis.
2.2. Western blot analysis
Cells in log-phase growth were collected, washed twice in cold phosphate-buffered saline, and lysed at 4°C in lysis buffer using protease and phosphatase inhibitors as previously described [19]. Western blot analysis of protein extracts was performed as described elsewhere [20]. The antibodies used included rabbit polyclonal antibody specific for ser194p-FADD and total FADD (Cell Signaling Technology, Beverly, MA). β-Actin (Sigma, St Louis, MO) was used as control for protein load and integrity.
2.3. Bromodeoxyuridine incorporation and immunofluorescence
Mino and Z-138 cells were incubated with bromodeoxyuridine (BrdU) at a final concentration of 10 μmol/L in cell culture medium for 1 hour at 37°C. After washing in cold phosphate-buffered saline, cells were fixed with 100 μL Cytofix/Cytoperm buffer for 20 minutes on ice and then treated with DNase for 1 hour at 37°C. After washings, cell suspensions were used for cytospin preparations on coated glass slides. The slides were then incubated with total and ser194p-FADD antibodies overnight at 4°C and with fluorescein isothiocyanate–labeled anti-BrdU antibody for 1 hour at room temperature. After appropriate washings, the slides were incubated with a goat antirabbit secondary antibody (Alexa Fluor 594; Invitrogen Corporation, Carlsbad, CA) diluted in 1% bovine serum for 30 minutes. 4,6-Diamidino-2-phenylindole (DAPI) (Invitrogen Corporation) was used as a counterstain.
2.4. Tissue samples and immunohistochemical methods
Two reactive lymph nodes, 2 reactive tonsils, and 122 B-cell NHLs were examined including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL; n = 25); follicular lymphoma (FL; n = 16) including grade 1 (n = 3), grade 2 (n = 11), and grade 3 (n = 2); extranodal marginal zone lymphoma of mucosa-associated lymphoid (MZL) tissue (n = 5); MCL (n = 15) including 10 typical and 5 blastoid/pleomorphic variants; DLBCL (n = 32); BL (n =12); and plasma cell myeloma (PCM; n = 17).
Immunohistochemical methods were performed as have been described previously [21]. Full tissue sections (n = 20 tumors) and tissue microarrays (n = 102 tumors) were used. A rabbit polyclonal antibody specific for ser194p-FADD (Cell Signaling Technology) was used. Proliferation index was assessed using the MIB1 (Ki-67) antibody (Immunotech, Westbrook, ME). Irrespective of intensity, any staining of tumor cells for ser194p-FADD was considered positive. The percentage of positive cells was calculated by counting at least 500 tumor cells.
2.5. Statistical analysis
As a continuous variable, the percentage of ser194p-FADD–positive tumor cells in different lymphoma types was analyzed using the nonparametric Mann-Whitney and Kruskal-Wallis tests. The Spearman R correlation coefficient was used to assess the association between the percentage of ser194p-FADD–positive tumor cells and percentage of Ki-67–positive tumor cells (proliferation index) as continuous variables. P < .05 was considered significant. All calculations were carried out using the StatView software (Abacus Concepts, Inc, Berkeley, CA).
3. Results
3.1. ser194p-FADD is expressed in the nucleus and is associated with proliferation in B-cell NHL cell lines
Western blot analysis showed ser194p-FADD expression in all 8 lymphoma cell lines that included 4 different B-cell NHL lymphoma types, with the levels of phosphorylation being higher in blastoid MCL, DLBCL, BL, and B-ALL cells as compared with typical MCL cells (Fig. 1A, upper panel). The level of FADD phosphorylation inversely correlated with doubling time in these lymphoma cell lines. For example, ser194p-FADD was expressed at high levels in Pfeiffer and Raji cells, which have a short doubling time (25-30 hours). By contrast, ser194p-FADD expression levels were relatively low in SP-53 and Jeko-1 cells that show a doubling time of 72 and 50 hours, respectively.
Fig. 1.
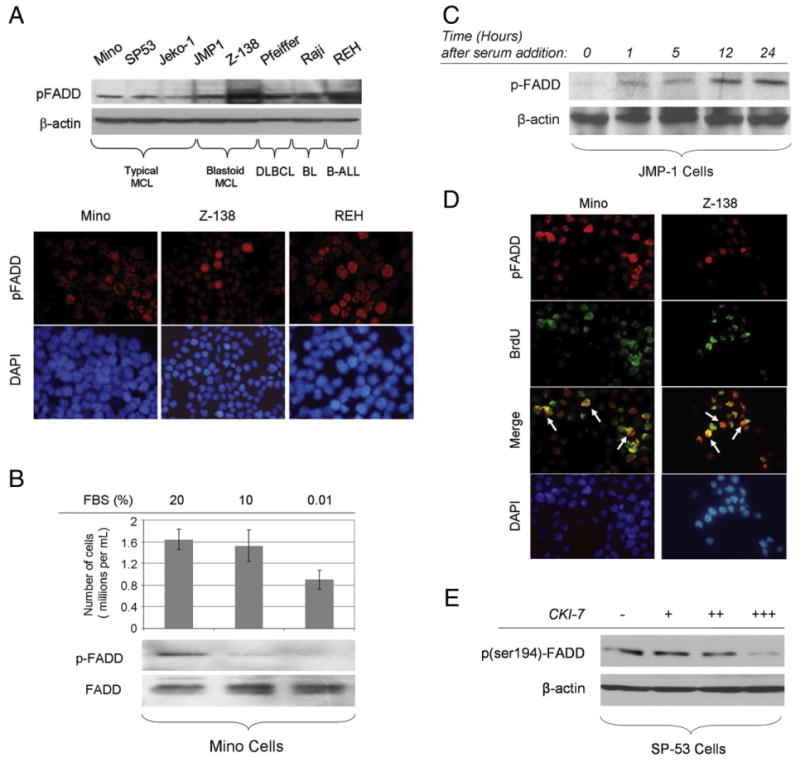
A, ser194p-FADD is expressed in B-cell NHL cell lines tested at variable levels (upper panel). ser194p-FADD protein is detected in the nucleus of Mino, Z-138, and REH cells using immunofluorescence in cytospin preparations (lower panel). B, Mino cells were incubated in 20% (control), 10%, or 0.01% FBS for 24 hours. Western blot analysis shows that the levels of ser194p-FADD expression are diminished after 24 hours of serum starvation. The total number of viable cells per volume (in milliliters) is decreased following serum deprivation. C, JMP-1 cells were initially serum starved for cell synchronization, then incubated with FBS, and collected at various time points. The expression levels of ser194p-FADD increased gradually over time in these synchronized cells. D, Mino and Z-138 cells were incubated with BrdU for 1 hour at 37°C, and double immunofluorescence was performed using cytospin preparations, antibodies specific for ser194p-FADD and BrdU, and DAPI as counterstain. ser194p-FADD and BrdU were detected using different secondary antibodies visible as red and green, respectively. A subset of cells was positive for both ser194p-FADD and BrdU as shown with yellow color in merged images, indicating that FADD phosphorylation at serine 194 is more prominent in the BrdU incorporating (proliferating) cell fraction (white arrows). E, Incubation of proliferating SP-53 cells, growing in 15% FBS, with increasing concentrations of the CKIα-specific inhibitor, CKI-7, up to 250 μmol/L for 8 hours, resulted in gradual decrease of ser194p-FADD levels, as shown by Western blot analysis.
To study the subcellular localization of ser194p-FADD, immunofluorescence was performed on cytospins prepared from 3 cell lines, Mino, Z-138, and REH. ser194p-FADD was detected primarily in the nucleus of B-cell lymphoma cells with variable staining intensity ranging from faint to strong in individual cells. Higher numbers of strongly positive cells were found in B-ALL (REH) and blastoid MCL (Z-138) than in typical MCL (Mino) (Fig. 1A, lower panel).
We examined the ser194p-FADD expression level in the Mino cell line under serum deprivation conditions resulting in growth arrest and decreased total cell numbers (Fig. 1B). Cell counting with trypan blue stain verified that any decreased number of cells was not associated with significant cell death. As shown in Fig. 1B, ser194p-FADD expression levels appear to gradually decrease in Mino cells grown in decreasing concentrations of serum (serum starvation), whereas the total FADD levels remained constant. Conversely, JMP-1 cells were first serum starved and then incubated with FBS and collected at various time points. As shown in Fig. 1C, the levels of ser194p-FADD expression increased over time in previously synchronized JMP-1. These findings suggest that ser194p-FADD expression is associated with cell proliferation.
To investigate the level of FADD phosphorylation in the proliferating fraction of cell lines, BrdU incorporation and double-immunofluorescence studies were performed using Mino and Z-138 cells. As shown in Fig. 1D, a subset of nuclei was positive for both ser194p-FADD and BrdU indicating that FADD is highly phosphorylated at serine 194 in the proliferating (BrdU incorporating) cell fraction.
As stated earlier, CKIα has been reported to be responsible for FADD phosphorylation at serine 194 [12,13]. To verify the association of FADD phosphorylation in B-lymphoma cells with CKIα activity, we treated SP-53 cells, growing in 15% FBS, with the CKIα-specific inhibitor CKI-7, and analyzed ser194p-FADD levels by Western blot analysis. As shown in Fig. 1E, incubation with increasing concentrations of CKI-7 resulted in gradually decreased ser194p-FADD protein levels. This result indicates that CKIα activity is essential for FADD phosphorylation at serine 194 in proliferating B-lymphoma cells and supports the results of others [12,13].
3.2. ser194p-FADD is highly expressed in the proliferating B-cell compartment in reactive lymphoid tissues
We assessed histologic sections of reactive lymph nodes and tonsils to determine ser194p-FADD expression in benign lymphocytes (Fig. 2). ser194p-FADD protein was expressed predominantly in the nuclei of germinal center B cells including centroblasts, immunoblasts, and some centrocytes, as well as occasional lymphocytes in the interfollicular areas. Cytoplasmic ser194p-FADD expression was identified in lymphocytes in the process of mitosis, in either metaphase or anaphase (Fig. 2).
Fig. 2.
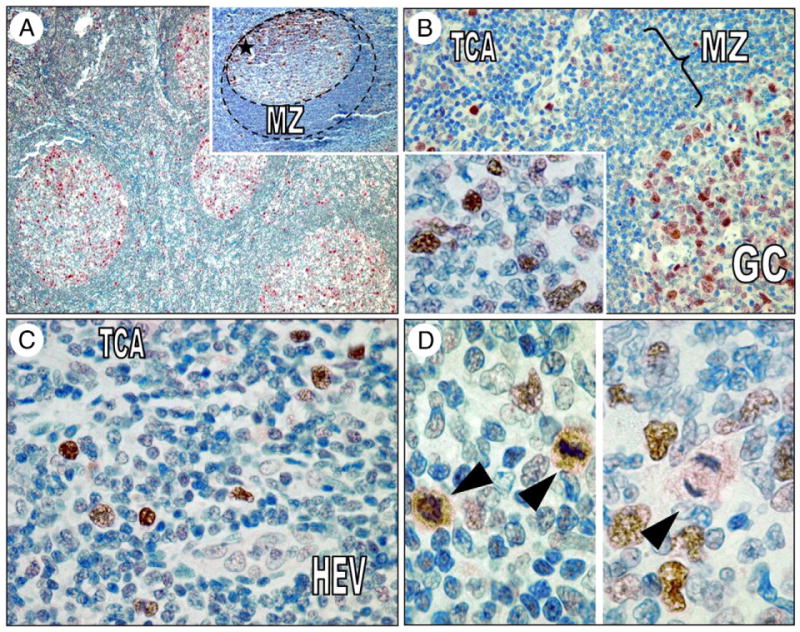
A, ser194p-FADD is detected predominantly in germinal center B cells in reactive lymph nodes and less frequently in the interfollicular areas. Note the accentuation of expression in the outer limits of germinal centers and the polarization toward the dark zone (inset, asterisk). B, ser194p-FADD protein is expressed in the nuclei of many centroblasts and some centrocytes (inset) in reactive germinal centers at variable intensity. C, In the interfollicular T-areas, ser194p-FADD expression highlights larger lymphocytes, a pattern reminiscent of Ki-67 immunostaining. D, All cells showing cytoplasmic ser194p-FADD expression were in various stages of mitosis (left and right, arrowheads). GC indicates germinal center; MZ, mantle zone; TCA, interfollicular T-cell area; HEV, high endothelial venule.
3.3. ser194p-FADD is differentially expressed in B-cell NHLs
In B-cell NHLs, ser194p-FADD was detected in tumor cell nuclei (Fig. 3A). The number of ser194p-FADD expressing cells in different lymphoma types was highly variable, ranging from 0.2% to 95%, and staining intensity was also variable, ranging from weak to strong. The mean (range) percentage of ser194p-FADD–positive tumor cells in different B-cell NHL types was 81% (55%-95%) in BL, 41% (14%-79%) in DLBCL, 18% (8%-38%) in FL, 18% (6%-38%) in PCM, 12% (5%-23%) in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue, 11% (3%-42%) in MCL, and 2% (0.2%-3.5%) in CLL/SLL. ser194p-FADD immunostaining highlighted increased positive cells within the proliferation centers of CLL/SLL (Fig. 3B).
Fig. 3.
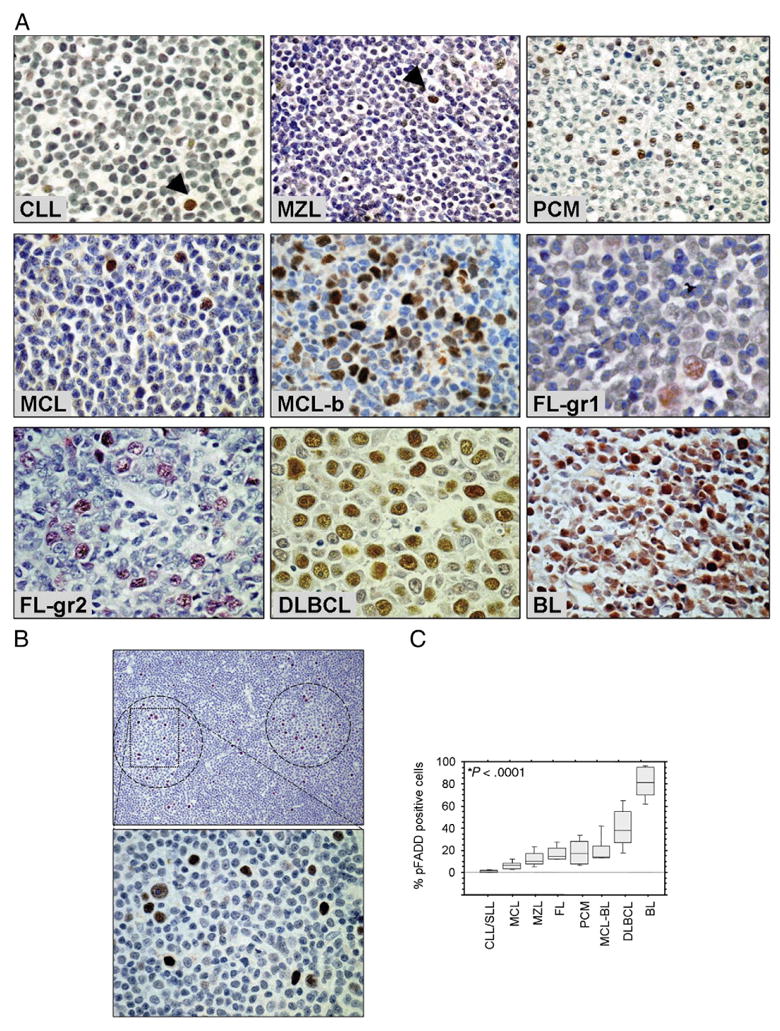
A, Immunohistochemistry showing ser194p-FADD protein expression in tumor cell nuclei from different types of B-cell NHL. The percentage of ser194p-FADD–positive tumor cells is high in highly proliferating lymphoma types such as blastoid/pleomorphic MCL (MCL-b), DLBCL, and BL. Arrowheads indicate single positive cells. B, ser194p-FADD immunostaining identifies proliferation centers (circles) in CLL/SLL (low and high magnifications at the upper and lower panels, respectively). C, Box and whisker plots showing the levels of ser194p-FADD expression (percentage of ser194p-FADD–positive tumor cells) in different types of B-cell NHL (original magnification ×400, hematoxylin as counterstain; *P < .0001, Kruskal-Wallis test). MZL indicates marginal zone B-cell lymphoma.
As a continuous variable, the percentage of ser194p-FADD–positive tumor cells was significantly higher in BL and DLBCL compared with low-grade types of B-cell NHL (P < .0001, Mann-Whitney test; Fig. 3C). In FL, the small number of grade 3 tumors (n = 2) precluded any statistical associations between grade and ser194p-FADD staining. In MCL, ser194p-FADD expression was significantly higher in tumors with blastoid/pleomorphic morphology (mean, 20%; range, 13%-42%) compared with tumors with typical features (mean, 6.5%; range, 3%-13%; P = .003, Mann-Whitney test).
3.4. ser194p-FADD expression correlates significantly with proliferation index in B-cell NHLs
The percentage of ser194p-FADD–positive cells positively correlated with the proliferation index as assessed by Ki-67 expression for the entire study group, and this association was highly significant (Spearman R = 0.9, P < .0001; Fig. 4). Of note, in each tumor the percentage of ser194p-FADD–positive tumor cells was almost always lower than the percentage of Ki-67–positive tumor cells (Fig. 4).
Fig. 4.
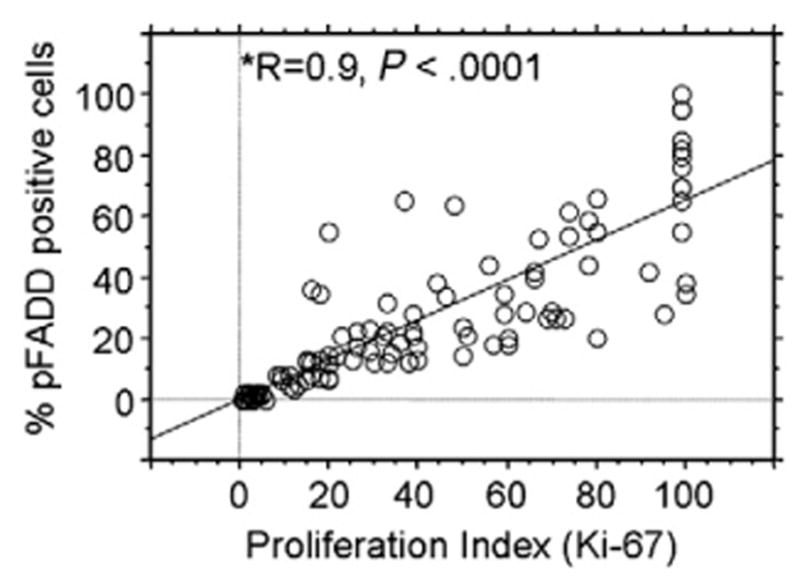
In B-cell NHLs, the levels of ser194p-FADD expression assessed as the percentage of positive neoplastic nuclei (continuous variable) are significantly associated with tumor proliferation index (Ki-67; *Spearman R = +0.9, P < .0001). The bivariate regression plot for the percentage of ser194p-FADD–positive and Ki-67–positive tumor cells as continuous variables is shown.
4. Discussion
This is the first study to provide evidence that FADD phosphorylation at serine 194 is involved in the proliferation of reactive and neoplastic B cells. We further suggest that ser194p-FADD expression has features of a novel proliferation marker in B cells. This suggestion is based on our data showing differential expression patterns of ser194p-FADD in reactive B lymphocytes and various B-NHL types, as well as a highly significant association between ser194p-FADD and the well-established proliferation marker Ki-67.
Previous studies performed in genetically modified mice point to a role for FADD in proliferation of T cells [5,6]. More specifically, T lymphocytes expressing a dominant negative FADD or deficient for FADD showed defective proliferation after T-cell antigen receptor stimulation [22,23]. The assumption that the same holds for B cells after B-cell antigen receptor stimulation, however, was not verified in later studies [24,25]. Nevertheless, B lymphocytes deficient for FADD show defective proliferation after specific innate immune stimuli involving Toll-cell receptor signaling. Furthermore, mice derived from a RAG1-negative chimeric background, deficient for FADD in the hematopoietic compartment, lack B lymphocytes [22,25]. Recent in vitro experiments support the hypothesis that involvement of ser194p-FADD in proliferation is not restricted to lymphocytes but applies to a broader spectrum of mammalian cell types including epithelial and other types of mesenchymal cells [9,26].
Our results that showed ser194p-FADD expression predominantly in reactive germinal centers, mostly in cells within the dark (proliferative) zones, recapitulate the pattern of Ki-67 expression and further support the concept that ser194p-FADD is involved in B-cell proliferation. In accord with these findings, the expression levels of ser194p-FADD in B-NHLs were significantly higher in lymphoma types characterized by a higher proliferation index, such as DLBCL and BL, compared with small B-cell lymphomas (P < .0001, Mann-Whitney test; Fig. 3). In MCL, ser194p-FADD expression levels were significantly higher in blastoid/pleomorphic tumors, known to be characterized by higher proliferation index, than those in typical tumors. In addition, we demonstrated that ser194p-FADD immunostaining identifies the most proliferating component of the lymphoma cell population, such as the proliferation centers in CLL/SLL. In cell lines, ser194p-FADD expression in B-NHL cells after serum starvation and synchronization further supports the hypothesis that ser194p-FADD is involved in proliferation. Recent studies on lung adenocarcinomas that showed that increased ser194p-FADD expression correlated with increased proliferation, tumor progression, and poor prognosis further supports our findings and suggests a broader role for ser194p-FADD in cell proliferation [14,27].
Analysis of ser194p-FADD expression in lymphomas may have prognostic value. Previous studies have provided evidence that the phosphorylation status of FADD at serine 194 can influence the sensitivity of various solid tumors to chemotherapy [28]. Specifically, increased ser194p-FADD levels were reported to enhance the ability of tubulin-disturbing reagents, such as paclicaxel; to induce G2/M cell cycle arrest and to synergize with other chemotherapeutic agents, like etoposide, in carcinomas in vitro [13,28]. Although these studies have not been performed in lymphoma cells, it seems possible that ser194p-FADD expression may similarly enhance chemotherapeutic sensitivity. Furthermore, the correlation between FADD phosphorylation and proliferation index, the latter having prognostic significance in some NHL types [29], suggests that analysis of ser194p-FADD expression may have prognostic value.
Despite the highly significant association between ser194p-FADD and Ki-67 expression (R = +0.9, P < .0001), in individual tumors, the number of ser194p-FADD–positive cells was typically smaller than the number of Ki-67–positive cells. This observation suggests that ser194p-FADD is expressed in a narrower part of the cell cycle. It has been shown that phosphorylation of FADD at serine residue-194 takes place during the G2/M phase of cell cycle, and it is hypothesized that this event modulates a restriction point during the G1-S or G2-M transition of the cycle, or both, depending perhaps on the cell type [5-7]. Our observation that ser194p-FADD is expressed in virtually all cells in mitosis further suggests that ser194p-FADD expression is related to G2/M phase. It is known that Ki-67 stains all phases of proliferating cells including G1, S, G2, and M phases. Thus, the lower numbers of ser194p-FADD–positive cells compared with Ki-67–positive cells is explained. However, the mechanism of action of ser194p-FADD on the cell cycle remains obscure [5,6].
We showed that ser194p-FADD is localized predominantly in the nuclei of normal and neoplastic B lymphocytes. Our findings are in agreement with the results from other studies on ser194p-FADD localization in normal and neoplastic cells [11,13,14]. Notably, the FADD protein contains nuclear localization and export sequences in the DED and, in some recent studies, a large proportion of total FADD protein was found to be localized in the nucleus [11,30,31]. In addition, the nuclear localization of ser194p-FADD is compatible with its role in cell cycle progression. However, ser194p-FADD has been previously detected in both the nuclei and the cytoplasm or shown to have altered (cytoplasmic) localization in some cancer cells [17].
Because of the dual role of FADD in apoptosis and cell cycle regulation, disentangling the biologic effects of FADD alterations using in vivo or in vitro systems remains difficult[5]. In many studies in which tumor progression has been shown to correlate with diminished expression of FADD or ser194p-FADD, the apoptotic role of FADD is stressed, and FADD is regarded as a tumor suppressor gene [32]. It is still unclear whether nuclear ser194p-FADD expression can influence the apoptotic pathways [10]. Some studies have shown that disturbance of FADD phosphorylation at serine residue 194 does not effect the extrinsic apoptotic pathway, whereas other studies support a negative, or, in special circumstances, a positive effect of ser194p-FADD on the apoptotic pathway [8,28,33,34]. It remains possible that nuclear ser194p-FADD expression may play a role in coupling the cell cycle and apoptotic pathways [5]. However, although most influences of FADD on the cell cycle seem to be dependent on phosphorylation of serine 194, by contrast, most apoptotic activities of FADD seem to be independent of serine 194 phosphorylation [5,13].
In conclusion, we show that FADD is highly phosphorylated at serine 194 in proliferating reactive and neoplastic B lymphocytes and that ser194p-FADD expression may represent a novel marker of cell proliferation in B-NHLs.
References
- 1.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 2.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–8. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 3.Reed JC, Doctor KS, Godzik A. The domains of apoptosis: a genomics perspective. Sci STKE. 2004;2004:re9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610–6. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Werner MH, Wu C, Walsh CM. Emerging roles for the death receptor FADD in death receptor avidity and cell cycle regulation. Cell Cycle. 2006;5:2332–8. doi: 10.4161/cc.5.20.3385. [DOI] [PubMed] [Google Scholar]
- 7.Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME. Phosphorylation of FADD/MORT1 at serine 194 and association with a 70-kDa cell cycle–regulated protein kinase. J Immunol. 2000;164:1236–42. doi: 10.4049/jimmunol.164.3.1236. [DOI] [PubMed] [Google Scholar]
- 8.Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity. 2003;18:513–21. doi: 10.1016/s1074-7613(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 9.Alappat EC, Volkland J, Peter ME. Cell cycle effects by C-FADD depend on its C-terminal phosphorylation site. J Biol Chem. 2003;278:41585–8. doi: 10.1074/jbc.C300385200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Zhang D, Hua Z. FADD and its phosphorylation. IUBMB Life. 2004;56:395–401. doi: 10.1080/15216540400008929. [DOI] [PubMed] [Google Scholar]
- 11.Screaton RA, Kiessling S, Sansom OJ, et al. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proc Natl Acad Sci USA. 2003;100:5211–6. doi: 10.1073/pnas.0431215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochat-Steiner V, Becker K, Micheau O, Schneider P, Burns K, Tschopp J. FIST/HIPK3: a Fas/FADD-interacting serine/threonine kinase that induces FADD phosphorylation and inhibits fas-mediated Jun NH(2)-terminal kinase activation. J Exp Med. 2000;192:1165–74. doi: 10.1084/jem.192.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alappat EC, Feig C, Boyerinas B, et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–32. doi: 10.1016/j.molcel.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Bhojani MS, Heaford AC, et al. Phosphorylated FADD induces NF-kappaB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. Proc Natl Acad Sci USA. 2005;102:12507–12. doi: 10.1073/pnas.0500397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuyoshi S, Shimada K, Nakamura M, Ishida E, Konishi N. FADD phosphorylation is critical for cell cycle regulation in breast cancer cells. Br J Cancer. 2006;94:532–9. doi: 10.1038/sj.bjc.6602955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Konishi N. Phosphorylation status of Fas-associated death domain-containing protein (FADD) is associated with prostate cancer progression. J Pathol. 2005;206:423–32. doi: 10.1002/path.1791. [DOI] [PubMed] [Google Scholar]
- 17.Yoo NJ, Lee SH, Jeong EG, et al. Expression of nuclear and cytoplasmic phosphorylated FADD in gastric cancers. Pathol Res Pract. 2007;203:73–8. doi: 10.1016/j.prp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Drakos E, Rassidakis GZ, Lai R, et al. Caspase-3 activation in systemic anaplastic large-cell lymphoma. Mod Pathol. 2004;17:109–16. doi: 10.1038/modpathol.3800039. [DOI] [PubMed] [Google Scholar]
- 19.Oyarzo MP, Medeiros LJ, Atwell C, et al. c-FLIP confers resistance to FAS-mediated apoptosis in anaplastic large-cell lymphoma. Blood. 2006;107:2544–7. doi: 10.1182/blood-2005-06-2601. [DOI] [PubMed] [Google Scholar]
- 20.Rassidakis GZ, Feretzaki M, Atwell C, et al. Inhibition of Akt increases p27Kip1 levels and induces cell cycle arrest in anaplastic large cell lymphoma. Blood. 2005;105:827–9. doi: 10.1182/blood-2004-06-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rassidakis GZ, Jones D, Lai R, et al. BCL-2 family proteins in peripheral T-cell lymphomas: correlation with tumour apoptosis and proliferation. J Pathol. 2003;200:240–8. doi: 10.1002/path.1346. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 23.Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–18. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imtiyaz HZ, Zhou X, Zhang H, Chen D, Hu T, Zhang J. The death domain of FADD is essential for embryogenesis, lymphocyte development, and proliferation. J Biol Chem. 2009;284:9917–26. doi: 10.1074/jbc.M900249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imtiyaz HZ, Rosenberg S, Zhang Y, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hueber AO, Zornig M, Bernard AM, Chautan M, Evan G. A dominant negative Fas-associated death domain protein mutant inhibits proliferation and leads to impaired calcium mobilization in both T-cells and fibroblasts. J Biol Chem. 2000;275:10453–62. doi: 10.1074/jbc.275.14.10453. [DOI] [PubMed] [Google Scholar]
- 27.Bhojani MS, Chen G, Ross BD, Beer DG, Rehemtulla A. Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer. Cell Cycle. 2005;4:1478–81. doi: 10.4161/cc.4.11.2188. [DOI] [PubMed] [Google Scholar]
- 28.Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Kishi M, Konishi N. Phosphorylation of FADD is critical for sensitivity to anticancer drug-induced apoptosis. Carcinogenesis. 2004;25:1089–97. doi: 10.1093/carcin/bgh130. [DOI] [PubMed] [Google Scholar]
- 29.Miller TP, Grogan TM, Dahlberg S, et al. Prognostic significance of the Ki-67–associated proliferative antigen in aggressive non–Hodg-kin's lymphomas: a prospective Southwest Oncology Group trial. Blood. 1994;83:1460–6. [PubMed] [Google Scholar]
- 30.Sheikh MS, Huang Y. The FADD is going nuclear. Cell Cycle. 2003;2:346–7. [PubMed] [Google Scholar]
- 31.Gomez-Angelats M, Cidlowski JA. Molecular evidence for the nuclear localization of FADD. Cell Death Differ. 2003;10:791–7. doi: 10.1038/sj.cdd.4401237. [DOI] [PubMed] [Google Scholar]
- 32.Tourneur L, Delluc S, Levy V, et al. Absence or low expression of fas-associated protein with death domain in acute myeloid leukemia cells predicts resistance to chemotherapy and poor outcome. Cancer Res. 2004;64:8101–8. doi: 10.1158/0008-5472.CAN-04-2361. [DOI] [PubMed] [Google Scholar]
- 33.Curtin JF, Cotter TG. JNK regulates HIPK3 expression and promotes resistance to Fas-mediated apoptosis in DU 145 prostate carcinoma cells. J Biol Chem. 2004;279:17090–100. doi: 10.1074/jbc.M307629200. [DOI] [PubMed] [Google Scholar]
- 34.Meng XW, Chandra J, Loegering D, et al. Central role of Fas-associated death domain protein in apoptosis induction by the mitogen-activated protein kinase kinase inhibitor CI-1040 (PD184352) in acute lymphocytic leukemia cells in vitro. J Biol Chem. 2003;278:47326–39. doi: 10.1074/jbc.M304793200. [DOI] [PubMed] [Google Scholar]


