Abstract
Purpose
To test an evidence-implementation intervention to improve the quality of care in the home health care setting for patients at high risk for fractures.
Methods
We conducted a cluster randomized trial of a multimodal intervention targeted at home care for high-risk patients (prior fracture or physician-diagnosed osteoporosis) receiving care in a statewide home health agency in Alabama. Offices throughout the state were randomized to receive the intervention or to usual care. The primary outcome was the proportion of high-risk home health patients treated with osteoporosis medications. A t-test of difference in proportions was conducted between intervention and control arms and constituted the primary analysis. Secondary analyses included logistic regression estimating the effect of individual patients being treated in an intervention arm office on the likelihood of a patient receiving osteoporosis medications. A follow-on analysis examined the effect of an automated alert built into the electronic medical record that prompted the home health care nurses to deploy the intervention for high risk patients using a pre-post design.
Results
Among the offices in the intervention arm the average proportion of eligible patients receiving osteoporosis medications post-intervention was 19.1%, compared with 15.7% in the usual care arm (difference in proportions 3.4%, 95% CI: −2.6 −9.5%). The overall rates of osteoporosis medication use increased from 14.8% prior to activation of the automated alert to 17.6% afterward, a non-significant difference.
Conclusions
The home health intervention did not result in a significant improvement in use of osteoporosis medications in high risk patients.
Keywords: Osteoporosis, Home Care Services, Quality Improvement, Secondary Prevention
Fragility fractures impose a substantial burden on patients and on society as a whole. They are associated with heightened risks of mortality and morbidity and result in substantial costs for both acute and long-term care [1-3]. Although very effective osteoporosis treatments are available, the rates of use are low, even among individuals who have already experienced a fracture and are thus at very high risk for a subsequent fracture [4]. Numerous evidence implementation and quality improvement efforts have been made to address this care gap, with mixed results. In the UK, fracture liaison services linked to discharge planning were found to be both effective and cost saving to the health system [5, 6]. Other approaches have been tried with more limited success [7, 8].
Patients who are in need of nursing care or rehabilitation services after discharge from inpatient care commonly receive home health services. Becker et al., found that 45.7% of Medicare beneficiaries who experienced a hip fracture received subsequent home health care, with the proportions ranging from 20-47% for other fracture sites [9]. A study by Curtis et al. found that rates of prescription osteoporosis treatment were low (8%) for patients with a fracture history who were receiving home health care, signifying that the home health setting is a promising venue for intervention [10].
To assess the utility of a home care based strategy for osteoporosis quality improvement, we developed a multimodal intervention to improve secondary or tertiary preventive care among patients with a recent fracture who were receiving home health services [11]. Our intervention targeted nurses and physicians involved in home health care and included in-service training for nurses, concise written osteoporosis educational materials and prepared order sets for physicians, and educational materials for patients. A pilot study was conducted to refine the intervention and preliminary results from that study were promising [11]. Here we report the results of the final group randomized trial conducted to evaluate this multi-modal intervention, delivered in the home health care setting, aimed at increasing osteoporosis treatment rates to prevent fractures.
Methods
We conducted a cluster randomized controlled trial to evaluate the effectiveness of our intervention to increase appropriate osteoporosis treatment rates. This study was approved by the Institutional Review Board at the University of Alabama at Birmingham and registered at clinicaltrials.gov (NCT00679198).
Randomization of Home Health Care Field Offices
Alacare Home Health and Hospice is nonprofit home health care agency that provides home health and hospice services throughout the state of Alabama. Using baseline data from the 12 months prior to project initiation, field offices were stratified based on case volume of patients with a fracture history and rates of prescription osteoporosis treatment among these patients and then randomized to either the intervention or usual care arms. Specifically, the offices were stratified into groups based on numbers of fracture patients (at or above and below the median) and the proportion of treated patients (above and at or below the median), and then offices were randomly selected within each of these 4 strata.
Identification of At-Risk Population
Patients were considered at high risk for future fracture and were eligible for the study if they were either admitted to home health care subsequent to a fracture or they had a history of fracture after age 50. Patients were excluded from the analysis if they were receiving hospice care.
Intervention
The development of the intervention and associated materials are described in detail by Outman et al [11]. Briefly, the intervention included: (1) training and development of materials to enhance nurse-patient and nurse-physician risk communication; (2) standard care plan referred to as nursing diagnosis pathway (NDP), incorporated into the home health care agency’s electronic medical record (EMR) system (Homecare Homebase, LP, Dallas, TX); (3) and physician resources including standardized physician order sets (i.e. pre-printed, simple orders to initiate dietary supplements and choose from a list that described all FDA-approved prescription osteoporosis medications) accompanied by a pocket-sized treatment algorithm card [12]. The training for nurses in each home health office was delivered by Dr. Kilgore, who is a Registered Nurse experienced in conducting continuing education activities and was based on slides developed for this purpose.
One field office where the pilot study was conducted was excluded from the trial, as was another site because they provided only hospice care. Training for nurses in the intervention offices was completed in March of 2009 and the primary study period ran through the end of 2009.
The success of the intervention depended on the actions of home health nurses. First, patients needed to be identified as high risk, which was done at the point of care by the nurse providing the home care services. Once a patient was identified as high risk, the EMR programmed care plan (a PDA-based application) needed to be activated. The care plan included delivery of patient education materials and assessment of patient comprehension of teaching goals related to osteoporosis, fractures and treatments, nursing review of patient medication lists, and, for those patients receiving osteoporosis prescription medication, instructions on how to appropriately take the medication. For those patients not receiving any osteoporosis treatment, prepared order sheets had to be transmitted to physicians. The prepared order sheets provided physicians with convenient means to prescribe osteoporosis medications and calcium and vitamin D supplements. The forms were tailored so that the physician needed only to check off the treatment(s) they wished to prescribe and then fax the orders either to the home health agency or directly to the patient’s pharmacy. The final steps required that the patient or patient’s caregiver filled the prescription and the patient take the medication as directed.
An a-priori secondary intervention was planned that would make the patient identification ‘automatic’ by the EMR PDA-based tool. For this analysis, and following completion of the randomized controlled study, we delivered the intervention to the field offices originally randomized to the usual care arm. At that time, we implemented an automatic prompt for nurses in all of the field offices to assist in identifying the patients at high risk for fracture based upon a patient’s prior fracture diagnosis. For these patients, the care plan required the nurse to decide whether to activate the care plan. This allowed us to evaluate the additional effect of the automatic prompt compared with nurse identification alone of high risk patients. This prompting could not be done for the main intervention because the EMR tool did not provide for activation of this automatic prompting selectively at some but not all home care offices.
Outcome Measures
The primary outcome measure was the proportion of eligible patients receiving a prescription for osteoporosis medications (bisphosphonates, teriparatide, calcitonin, or raloxifene). The secondary outcome measure was the proportion of eligible patients for whom the care plan was activated, a process measure. For each field office, deidentified data from the home health care agency EMR were extracted for the 12 months prior to project implementation and each month after intervention initiation indicating how many high risk patients were identified, how many of those patients had the care plan activated as intended, and how many received osteoporosis medications.
Statistical Analyses
The primary analysis involved conducting a t-test of differences between the intervention and usual care arm offices in the post-intervention proportions of patients receiving prescriptions for osteoporosis medications. We also used logistic regression to examine the same outcome, with standard errors adjusted for clustering within offices, to estimate the effect of being treated in an intervention arm office on the likelihood that a patient would receive osteoporosis medication. A secondary analysis examined differences in medication use rates for high risk patients who had the nursing diagnosis pathway activated compared with those who did not.
Since all patients in the pre-planned secondary intervention consisted of a pre-post intervention design, where rates of the same outcomes, care plan activation and use of osteoporosis medications were compared before and after the automatic alert. Analyses were conducted using Stata version 11.0 (StataCorp LLC, College Station, TX).
Results
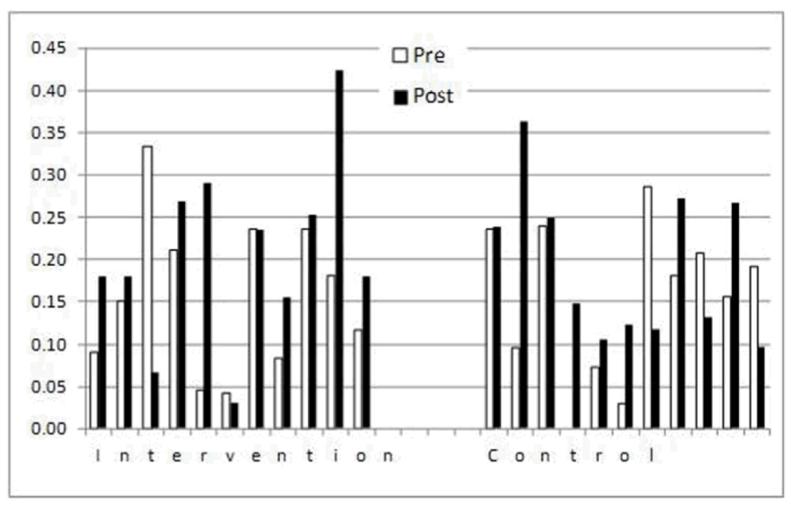
Table 1 shows the number of offices and patients in each of the trial arms. Among the offices in the intervention arm, the average proportion of eligible patients receiving osteoporosis medications post-intervention was 19.1%, compared with 15.7% in the usual care arm (difference in proportions 3.4%, 95% CI: −2.6% −9.5%, p = 0.252). The difference was not statistically significant. Similarly, the results from the logistic regression analysis indicated that patients treated in intervention arm offices were 3.0% more likely to receive osteoporosis medication, but the difference was not significant (95% CI −1.9% −8.0%, p = 0.224). There was considerable variation in the rate of prescription osteoporosis medications by home care office as shown in Figure 1, illustrating prescription rates pre- and post-intervention in the intervention and usual care offices. In some intervention offices, utilization rates increased dramatically, in others there was little or no response.
Table 1.
Enrollment and Drug Treatment Rates in Usual Care & Intervention Arms of the Home Health Care Osteoporosis Trial
| Usual Care Offices |
Intervention Offices |
|||
|---|---|---|---|---|
| Fracture Cases* |
Proportion Treated† |
Fracture Cases* |
Proportion Treated† |
|
| 48 | 17% | 46 | 17% | |
| 42 | 21% | 39 | 18% | |
| 37 | 11% | 38 | 18% | |
| 36 | 25% | 37 | 16% | |
| 36 | 11% | 36 | 28% | |
| 29 | 10% | 31 | 16% | |
| 28 | 11% | 28 | 18% | |
| 25 | 20% | 25 | 12% | |
| 22 | 23% | 21 | 10% | |
| 19 | 11% | 16 | 19% | |
| 15 | 13% | 13 | 38% | |
|
| ||||
| Averages | 31 | 16% | 30 | 19% |
Number of patients with fracture diagnoses who were eligible for the intervention
Proportion of fracture patients receiving prescription osteoporosis medications during follow up after the intervention
Figure 1. Rates of Osteoporosis Medication Use Pre-and Post-Intervention in Treatment and Usual care Offices.
A secondary analysis compared the likelihood of an individual receiving an osteoporosis medication if the care plan was activated. Similar to the treatment rates, the frequency with which the intervention care plan was activated varied across different field offices. Among the 27.5% of eligible patients who had the care plan activated, 37.7% received osteoporosis medications, compared with 11.6% of those who did not have the care plan activated (p < 0.0001).
Once the randomized trial was concluded, the offices in the usual care arm received the in-service training and intervention materials so as to conduct the pre-post secondary intervention. Upon completion of the training, the automatic prompt of the care plan was activated, requiring nurses to activate the care plan or document reasons for not doing so. The rate of care plan activation increased from 27.5% prior to the automatic alert to 72.5% after the automatic alert was initiated (p < 0.0001). The rates of osteoporosis medication use changed much less, from 14.8% to 17.6%, and the change was not significant. Both before and after activating the automatic alert, patients with the care plan activated were significantly more likely to receive osteoporosis treatment, but the magnitude of the difference declined from 26.1% (p < 0.0001) before activation to 21.8% (p < 0.0001) after activation.
Discussion
In our group-randomized controlled trial conducted in a state-wide home health care agency, we failed to find a significant difference in the proportion of patients prescribed osteoporosis medications in the intervention arm of the trial. This was also the case for the before and after comparison of rates with respect to the activation of the automated alert feature in the EMR.
We speculated that an intervention targeting home health providers could replicate facets of the UK fracture liaison services reports by Mitchell et al [6]. Home health providers interact with patients in their own homes and maintain relationships with local primary care physicians, a substantial fraction of nursing time in home care is directed a patient needs assessment and in patient and caregiver teaching. McLellan et al found that when the fracture liaison service increased treatment rates from 20% to 68%, the services were considered to be cost-effective [5]. Thus, even if the results found in our study were statistically significant, they would likely be insufficient to justify the costs of implementation.
The finding that in patients for whom the nursing care plan was activated, irrespective of randomization, were more likely to receive treatment was interesting. It is, however, possible that the difference represents selection bias, with the nurse choosing to activate the care plan for those patients who were perceived to be either at higher risk or who are more likely to benefit from treatment, and this might explain why the overall rates of prescription treatment in the secondary intervention were negative because the automatic identification of patients lessened this selectivity. A follow-up study using telephone surveys of patients receiving home health services is ongoing to examine barriers to the use of osteoporosis medications. Further efforts are also planned to discuss results among nurses in the offices where prescription drug use did increase and in those where the intervention appeared to be ineffective.
Another plausible explanation for why nurses may not have activated the care plan on all patients is offered in the literature comparing cancer survivors to non-cancer patients for use of preventive services [13, 14]. The competing demands model suggests that both health care providers and patients may place lower priority or are too busy to be concerned with osteoporosis treatment compared with treatment for other illnesses. Opportunities for providing optimal care, with the goal of minimizing morbidity and mortality in patients with osteoporosis, may be lost in the home care setting where other concerns are more pressing.
A key limitation of this study was the small sample size and the inability to incorporate the automated alert feature into the randomized trial design. The original intent of the intervention was to include an automated prompt in the EMR system that would alert nurses when patients were at high risk based on diagnosis codes programmed in on admission or discovered during intake assessment. The system would then prompt the nurse to either activate the care plan or indicate reasons why it was not activated. Unfortunately, this feature could not be activated selectively in different agency offices, only throughout the system as a whole. However, the follow-on analysis, pre- and post-activation of the alert, did not suggest that this feature would have produced a much different result. Another limitation of our study is the lack of information on patient characteristics. Access to protected health information was not deemed necessary to the evaluation of this intervention, thus we were only provided with numbers of patients in each office who met the criteria for inclusion and the number of those who had the care plan activated and the number who were treated. If there were substantial numbers of patients who were not expected to survive long enough to benefit from osteoporosis medications, but were not in hospice care, this would bias our results toward the null.
Results from the pilot study done preparatory to the trial suggested that one way to improve performance is to assign one nurse in an office responsibility for coordinating activation of the care plan for high risk patients and communication with physicians about treatment options, much as a fracture liaison services typically identifies a principal responsible party at each site. Future work, building on the lessons learned in this study, should involve a larger system of home health agencies to provide the opportunity to undertake the full intervention, including automated alerts, to many more study sites, and selecting a single individual within each office to be responsible to coordinate implementation of the quality improvement program.
Acknowledgements
This study was supported by a grant from the Agency for Healthcare Research and Quality, U18 HS10389-06S1, Deep South Musculoskeletal Center for Education and Research on Therapeutics. Dr. Curtis is supported by the NIH (AR 05331).
Footnotes
Disclosures:
Meredith Kilgore – Grants/Research support from Amgen, Inc.
Dr. Kenneth Saag – Grants/Research support: NIH; AHRQ; ACR; Amgen; Lilly; Merck Consultant/Honorarium: Amgen; Lilly; Merck
Dr. Jeffrey Curtis – Grants/Research support/Consulting: Amgen, Merck, Lilly
All others – None
Contributor Information
Meredith L. Kilgore, University of Alabama at Birmingham (UAB), Department of Health Care Organization & Policy, 1665 University Blvd, RPHB 330, Birmingham, AL
Ryan Outman, UAB Department of Medicine Ryan.Outman@ccc.uab.edu.
Julie L. Locher, UAB Department of Medicine jlocher@uab.edu
Jeroan J. Allison, University of Massachusetts, Department of Medicine Jeroan.Allison@umassmed.edu
Amy Mudano, UAB Department of Medicine amy.mudano@ccc.uab.edu.
Beth Kitchin, UAB Department of Nutrition Science bkitchin@soph.uab.edu.
Kenneth G. Saag, UAB Department of Medicine Kenneth.Saag@ccc.uab.edu
Jeffrey R. Curtis, UAB Department of Medicine Jeffrey.Curtis@ccc.uab.edu
REFERENCES
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Kilgore ML, Morrisey MA, Becker DJ, Gary LC, Curtis JR, Saag KG, Yun H, Matthews R, Smith W, Taylor A, Arora T, Delzell E. Health Care Expenditures Associated with Skeletal Fractures Among Medicare Beneficiaries, 1999 - 2005. J Bone and Mineral Res. 2009 Dec;24(12):2050–5. doi: 10.1359/jbmr.090523. [DOI] [PubMed] [Google Scholar]
- 3.Curtis J, Arora T, Matthews RS, Taylor A, Becker DJ, Colon-Emeric C, Kilgore ML, Morrisey MA, Saag KG, Safford MM, Warriner A, Delzell E. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc. 2010;11(8):584–91. doi: 10.1016/j.jamda.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, Adekunle F, Roberts D. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011 Jul;22(7):2083–98. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell PJ. Fracture Liaison Services: the UK experience. Osteoporos Int. 2011 Aug;22(Suppl 3):487–94. doi: 10.1007/s00198-011-1702-2. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH. Postfracture interventions disseminated through health care and drug insurers: attempting to integrate fragmented health care delivery. Osteoporos Int. 2011 Aug;22(Suppl 3):465–9. doi: 10.1007/s00198-011-1698-7. [DOI] [PubMed] [Google Scholar]
- 8.Adler RA, Bates DW, Dell RM, LeBoff MS, Majumdar SR, Saag KG, Solomon DH, Suarez-Almazor ME. Systems-based approaches to osteoporosis and fracture care: policy and research recommendations from the workgroups. Osteoporos Int. 2011 Aug;22(Suppl 3):495–500. doi: 10.1007/s00198-011-1708-9. [DOI] [PubMed] [Google Scholar]
- 9.Becker DJ, Yun H, Kilgore ML, Morrisey MA, Gary LC, Curtis JR, Saag KG, Matthews R, Smith W, Taylor A, Arora T, Delzell E. Health services utilization after fractures: recent evidence from Medicare. J Gerontol A Biol Sci Med Sci. 2010;65(9):1012–20. doi: 10.1093/gerona/glq093. PMID: 20530242. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JR, Kim Y, Bryant T, Allison J, Scott D, Saag KG. Osteoporosis in the home health care setting: A window of opportunity? Arthritis Rheum. 2006;55:971–5. doi: 10.1002/art.22349. [DOI] [PubMed] [Google Scholar]
- 11.Outman RC, Curtis JR, Locher JL, Allison JJ, Saag KG, Kilgore ML. Improving osteoporosis care in high-risk home health patients through a high intensity intervention. Contemporary Clinical Trials. 2012 Jan;33(1):206–12. doi: 10.1016/j.cct.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warriner AH, Outman RC, Saag KG, Berry SD, Colón-Emeric C, Flood KL, Lyles KW, Tanner SB, Watts NB, Curtis JR. Management of osteoporosis among home health and long-term care patients with a prior fracture. South Med J. 2009 Apr;102(4):397–404. doi: 10.1097/SMJ.0b013e31819bc1d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBean AM, Yu X, Virnig BA. Screening mammography rate and predictors following treatment for colorectal cancer. J Cancer Surv. 2009;3:12–20. doi: 10.1007/s11764-009-0080-7. [DOI] [PubMed] [Google Scholar]
- 14.Jaen CR, Stange KC, Nutting PA. The use of preventive health services among elderly cancer survivors. J Fam Pract. 1994;38:166–71. [PubMed] [Google Scholar]