Abstract
The development of positron emission tomography (PET) radioligands for the non-invasive imaging of amyloid-β plaque burden has been the focus of intense research efforts over the last decade. A variety of structural backbones have been investigated and several radiolabeled molecules have been evaluated in phase I (and later) clinical studies. These efforts have been driven by the desire not only to develop a suitable diagnostic imaging agent but also to develop a means to evaluate potential therapies for Alzheimer’s disease. This review focuses on the development of these ligands, as well as the radiochemistry and current regulatory status of these PET radioligands. Particular attention is given to those ligands that have progressed to the later stages of drug development (phase II/III clinical trial studies) or approved New Drug Application status.
Keywords: Aβ plaques, amyloid, PET, florbetaben, flutemetamol, florbetapir
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that was first described by Alois Alzheimer in 1907.1 The primary pathological hallmarks of AD are the presence of extracellular neuritic plaques (aggregates of amyloid-β (Aβ) protein) and intracellular neurofibrillary tangles (NFTs) composed of phosphorylated tau protein.2 To date, the ‘gold-standard’ diagnosis for AD continues to be post-mortem histopathological staining studies utilizing dyes (such as thioflavin-S, thioflavin-T, and Congo red), silver stains (such as Bielschowsky) and antibodies that stain β-amyloid (Aβ) deposits.3,4 While the specific pathological sequencing of events related to AD is still unknown, the deposition of extracellular neuritic plaques (comprised of Aβ protein) is thought to be an early event.5,6
The development of a positron emission tomography (PET) imaging agent (or agents) suitable for the in vivo delineation and quantification of Aβ plaque burden has been the focus of intense research efforts by a multitude of research groups over the last decade. The development of these agents might provide the means for early non-invasive detection of Aβ plaques in AD and pre-AD subjects (which might provide an early diagnostic tool for AD) and also might provide support for the amyloidcascade hypothesis of AD7,8 and help solidify our understanding of the underlying pathological basis for this disease. In addition, the development of suitable in vivo PET imaging agents for AD provide a means for the evaluation of novel AD therapies designed to modify or decrease Aβ plaque load in AD subjects.9
The general criteria for an in vivo PET imaging agent intended for neuroimaging applications are primarily related to the following: (i) the ability of the compound to rapidly cross the blood-brain-barrier (BBB) in amounts suitable for non-invasive imaging; (ii) selective, high-affinity binding to the target of interest; and (iii) rapid clearance from non-target brain regions. In general, a compound must have a molecular weight less than 600 and be a neutral molecular species in order to cross the BBB via passive diffusion.10 In addition, the target range for lipophilicity is generally accepted to be somewhere between 1.0 and 3.5 log P in order for a compound to passively cross the BBB.11 The target ligand should display relatively high initial brain uptake (>4% dose/g at 2-min post-injection (i.v.) in mice) and rapidly clear from normal brain (<1% dose/g at 30-min post-injection (i.v.) in mice).12,13 Ideally, the candidate compound will not be metabolized in brain and either (i) will be resistant to peripheral metabolism in plasma or (ii) the resultant peripheral radiolabeled metabolite(s) will be polar enough to prevent its (their) accumulation in the brain.
A generic binding model based upon the interaction of a planar aromatic structure with negatively charged functional groups that interact with specific amino acid residues present in the β-sheet conformation of the fibrillar amyloid aggregates has been proposed.14 This generic model was utilized to explain the binding interaction of Congo red to amyloid aggregates. The development of PET imaging agents for Aβ plaque deposits in AD has primarily been based upon structural modifications of pathology dyes (such as Congo red and thioflavin-T) that have been used for post-mortem histopathology staining of Aβ plaques and NFTs in brain sections as confirmation of an AD diagnosis. Structural modifications have been primarily focused on elimination of charged structural characteristics that would limit BBB penetration and modifications that increase normal brain clearance and binding affinity to the target: specifically fibrillar Aβ plaques.
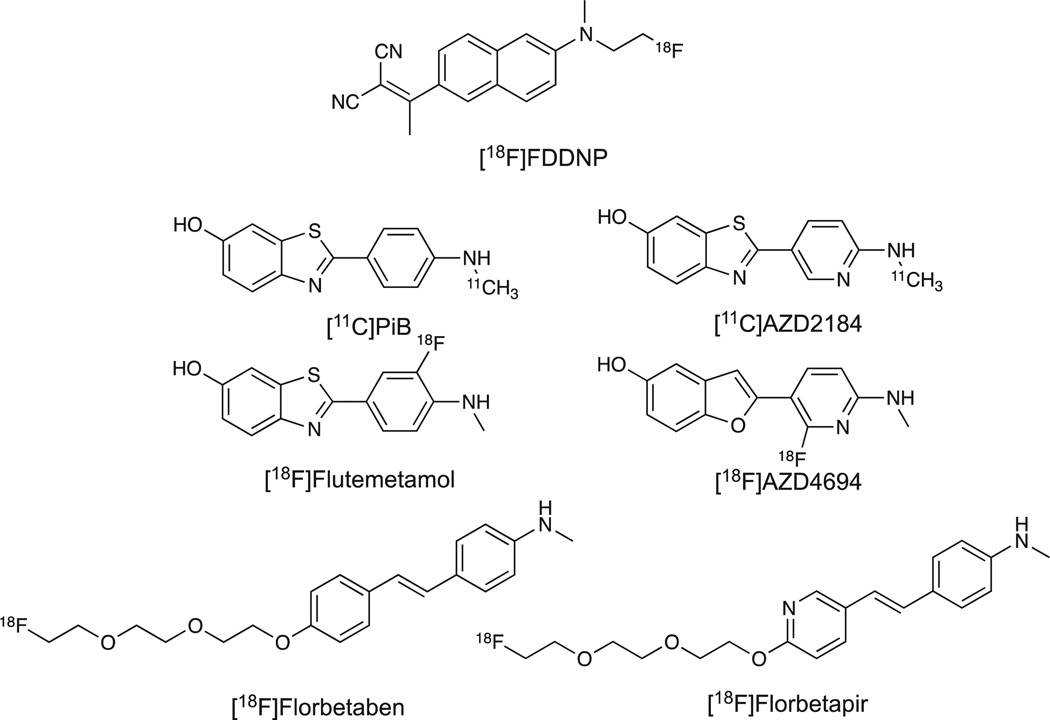
The focus of this review will be limited to PET imaging agents labeled with F-18 or C-11 designed to bind in vivo to Aβ plaques and that have demonstrated some utility in in vivo human imaging applications (See Figure 1). The design and radiochemistry of these selected agents will be discussed as well as the current regulatory status of the selected agents. There have been several reviews of a wider scope published and readers are directed to these for additional information regarding in vitro applications and the design of radiolabeled and/or fluorescent probes for use in in vitro or in vivo imaging of Aβ plaques.15–24 The PET imaging agents included in this review comprise three broad structural classes: naphthalene analogs, planar heteroaromatic compounds (benzothiazoles, benzoxazole analogs, and benzofurans), and diaryl alkene analogs.
Figure 1.
Chemical structures of selected Aβ plaque positron emission tomography imaging agents.
Naphthalene analogs
[18F]FDDNP
Although the first attempt to image amyloid in vivo utilized an antibody fragment,25 the earliest small molecule PET imaging agent proposed for use in Aβ plaque imaging applications in AD was 2-(1-[6-[(2-[18F]fluoroethyl)methylamino]-2-naphthyl]-ethylidene)malonitrile ([18F]FDDNP).26 This PET imaging agent was a fluoroethyl modification of the lipophilic fluorescent molecule DDNP. The radiosynthesis of [18F]FDDNP was based upon the standard nucleophilic displacement radiochemistry using a tosyloxy-derivative as the precursor material. Reported radiosyntheses utilize the standard nucleophilic fluorination cryptand, Kryptofix 222 in acetonitrile at elevated temperatures followed by semi-preparative HPLC purification in reported radiochemical yields of 30–60%.27,28 The reported radiochemical purities of [18F]FDDNP were >98% with end of synthesis (EOS) specific activities of 102 ± 56 GBq/µmol27 and 148–296 GBq/µmol.28 The two radiosynthetic processes describe radiolytic stability concerns and non-specific retention of [18F]FDDNP to tubing and filters. The radiolytic decay concerns were addressed by the use of ascorbic acid, and the non-specific retention to tubing and filters were addressed by the use of human serum albumin or polysorbatum-80 as a component of the final product formulation.
A potential drawback to the use of [18F]FDDNP in in vivo imaging applications is its relatively small dynamic imaging range. While PET imaging utilizing [18F]FDDNP has been reported to provide a means for separating healthy controls from AD subjects,29 there was only a 9% higher cortical uptake in AD subjects compared with controls (compared with a 70% increase in cortical binding in AD subjects when using [11C]PiB).30,31
Planar heteroaromatic analogs
[11C]PiB ([11C]6-OH-BTA-1)
Thioflavin-T has been utilized as the pharmacophore for a variety of proposed Aβ plaque imaging agents.24,32,33 The small neutral 2-phenylbenzothiazole derivative, 2-(4-[11C]-(methylamino)phenyl) benzo[d]thiazol-6-ol or [11C]6-OH-BTA-1, also known as [11C] PiB, was developed as an analog of thioflavin-T designed to be able to cross the BBB and retain (or improve upon) the ability of thioflavin-T to label Aβ plaques.13 [11C]PiB is among the most widely utilized and is the most well-characterized PET imaging agent for the delineation of Aβ plaques in the brain. [11C]PiB has been utilized in PET imaging studies of thousands of subjects and has been utilized as a component of the non-invasive imaging studies that comprised a component of the AD Neuroimaging Initiative.34–36 In addition, [11C]PiB has been utilized as a non-invasive imaging marker in investigational studies evaluating the efficacy of two potential therapies for AD.9,37
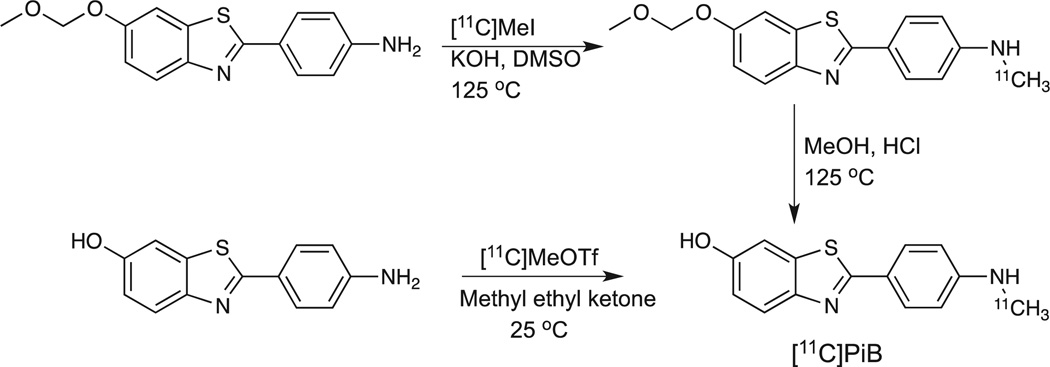
The initial radiochemistry approach utilized the corresponding methoxymethoxy (MOM)-protected aniline derivative (6-MOMOBTA-0) as precursor (Scheme 1). The use of a strong base (KOH) in dimethyl sulfoxide with [11C]CH3I at elevated temperature provided the desired labeled intermediate. The MOM-protecting group was removed under acidic conditions (MeOH/HCl) at 125 °C for 5minutes. The desired [11C]PiB was purified by reverse-phase semi-preparative HPLC to yield the final product in average yields of 12.1% (EOS, based upon [11C]MeI). Radiochemical and chemical purities were >95% and the average specific activity was 85 GBq/µmol at EOS.13
Scheme 1.
Radiosynthetic routes for [11C]PiB.
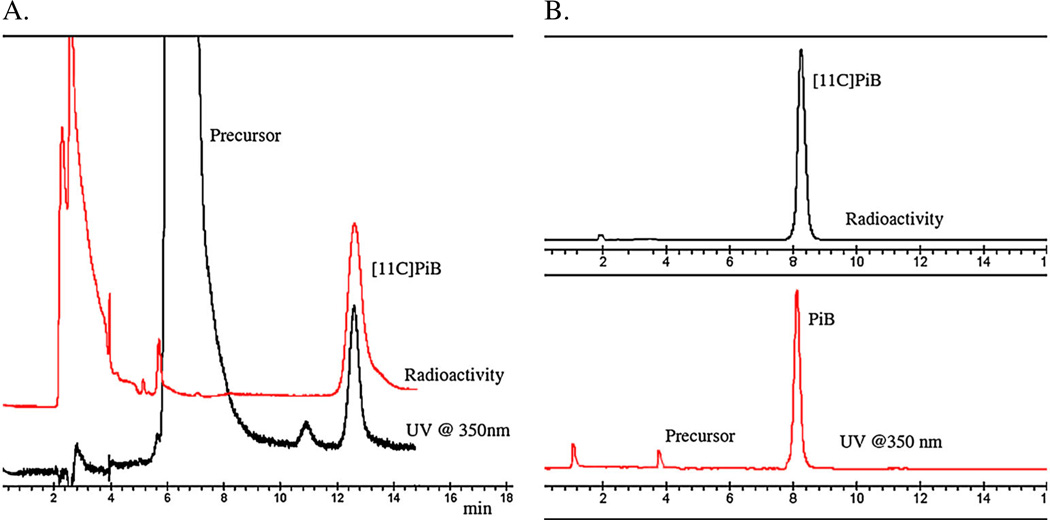
The utilization of the more reactive synthon [11C]methyl triflate under neutral conditions with the free phenolic precursor, 2-(4-aminophenyl)benzo[d]thiazol-6-ol, in conjunction with the application of the thin-film captive solvent technique (so-called ‘loop’ chemistry) has provided a direct one-step radiosynthetic approach to the synthesis of [11C]PiB (Scheme 1).38 This approach has been utilized at the University of Pittsburgh PET facility for the last 8 years for over 1000 productions of [11C]PiB and has been demonstrated to be reliable (failure rates <1%) and routinely provides [11C]PiB in a manner consistent with the United States Pharmacopoeia <823>(Radiopharmaceuticals for PET-Compounding). This radiosynthetic process provides average radiochemical yields of 19–28.5% (decay-corrected based upon [11C]CO2) in total synthesis times of 40–45min (including quality control) with chemical and radiochemical purities >95% (Figure 2) and specific activities of 120 ± 45 GBq/µmol (3.23 ± 1.31 Ci/µmol) at EOS. Recently, a fully automated production process for [11C]PiB has been described based upon the Tracerlab™ FXC-Pro system (GE Healthcare).39 The automated process is modeled on the thin-film captive solvent technique and produces [11C]PiB in yields of 1.6% (non-decay corrected) based upon starting [11C]CO2.
Figure 2.
A. Semi-preparative HPLC traces for [11C]PiB radiosynthesis and B. corresponding analytical quality control HPLC traces.
[18F]Flutemetamol ([18F]3′F-PiB or [18F]GE067)
The successful use of [11C]PiB for imaging Aβ plaque deposition in vivo led to efforts to develop an F-18 labeled analog that would behave in a similar manner to [11C]PiB. A variety of structural analogs have been evaluated in vitro and in preclinical models, and several compounds have been evaluated in human research subjects (under IND or eIND mechanisms). One of these compounds, 2-(3-[18F]-fluoro-4-(methylamino)phenyl)benzo[d]thiazol-6-ol ([18F] 3′F-PiB, [18F]GE067, [18F]Flutemetamol) has been licensed by GE Healthcare and is currently under clinical development.
Initial human studies comparing [18F]3′F-PiB and [11C]PiB in AD and control subjects demonstrated the [18F]3′F-PiB that possesses uptake and specific binding characteristics that were very similar to PiB.40 Further studies regarding the in vivo whole-body distribution and subsequent calculation of radiation dosimetry of [18F]GE067 yielded dosimetry values comparable with other F-18 labeled radiopharmaceuticals.41 Subsequent phase I studies provided support for the continued evaluation of [18F]Flutemetamol as a potential in vivo marker of amyloid Aβ plaque deposition in humans.42 Phase II study results provided additional evidence that [18F]Flutemetamol performs similarly to [11C]PiB and provides high test–retest reproducibility.43 Other studies, including the ACRIN-PA 4004 study have provided additional data supporting the in vivo similarity between [11C]PiB and [18F]Flutemetamol44 as well as the concordance between in vivo [18F]Flutemetamol imaging and histopathology.45
The completion of phase III clinical trials for [18F]Flutemetamol have recently been reported by GE Healthcare and the US Food and Drug Administration New Drug Application (NDA) filing is expected late 2012 or early 2013.46
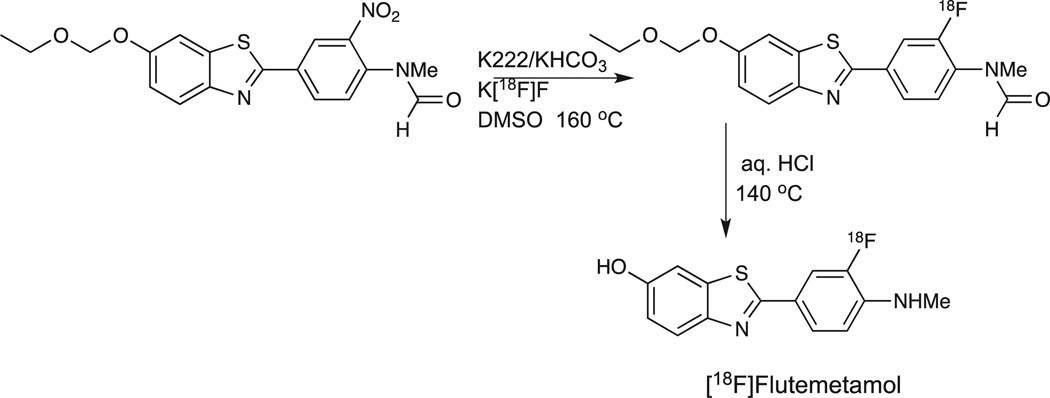
The radiosynthesis of [18F]Flutemetamol follows standard aromatic nucleophilic radiochemistry methodology. The initially optimized precursor (N-methyl-N-[4-(6-ethoxymethoxy-2-benzo [d]thiazolyl)-2-nitrophenyl]formamide) was allowed to react with Kryptofix K222/potassium bicarbonate/K[18F]F in DMSO at elevated temperatures. Subsequent acidic hydrolysis followed by reverse-phase HPLC purification yielded the desired [18F]Flutemetamol in average yields of 15% (EOB) with specific activities of >74 GBq/µmol(>2.0 Ci/µmol) (Scheme 2).47 Subsequently, this synthesis method was modified and optimized for the GE FASTlab™ synthesizer in order to allow this synthesis platform to be utilized in support of phases II and III studies in Europe and the USA. The optimized process provides [18F]Flutemetamol in 15–25% yield (EOS) and data from 10 manufacturing sites in the USA and Europe demonstrates that the automated system provides a robust process with the added advantage of solid-phase purification process without the need for HPLC purification.48,49
Scheme 2.
Radiosynthetic route for [18F]Flutemetamol.
[11C]AZD2184
2-(6-[11C]-methylamino)pyridine-3-yl)benzothiazol-6-ol ([11C] AZD2184) is a PiB analog where the 2-phenyl substituent has been replaced by pyridine moiety. Initial preclinical evaluation of [3H] AZD2184 demonstrated that this ligand bounded to Aβ with high affinity and demonstrated lower non-specific binding in autoradiograhy studies when compared with [3H]PiB.50 This pyridine for phenyl substitution lowers the lipophilicity compared with [11C]PiB and may decrease the non-specific binding attributed to white matter retention. In human subject studies (AD and control), specific binding peaked at 30-min post-injection with standardized uptake value ratios of 1.19–2.57.51 Several different methods were examined for the radiosynthesis of [11C]AZD2184. Initially, the standard [11C] methyl triflate conditions (no base) reported for the synthesis of [11C]PiB were examined.52 Under these conditions, none of the desired compound was produced. The addition of base (0.5M NaOH) yielded almost exclusively (>70%) the O-methylation product. An alternative approach utilized the ethoxymethoxyprotecting group for the phenolic position. The use of this protected precursor for methylation under basic (KOH) conditions utilizing [11C]MeI in DMSO yielded the desired radiolabeled intermediate in 50% yield. Subsequent acidic hydrolysis was reported to be quantitative, yielding the desired [11C] AZD2184. In addition tert-butyl-dimethylsilyl was evaluated as a phenolic hydroxyl-protecting group. The initial C-11 methylation chemistry was identical to the conditions for the EOM protected precursor; however, the deprotection required only the addition of water to the crude reaction mixture. The radiosynthesis utilizing the TBDMS-protected precursor provided [11C]AZD2184 in 4–13% radiochemical yield (decay-corrected based upon [11C]CH4) in a total synthesis time of 30–33 min. The radiochemical purity was >99% with a specific activity at EOS of 1600–4000 GBq/µmol.
[18F]AZD4694
2-(2-[18F]fluoro-6-(methylamino)pyridine-3-yl)benzofuran-5-ol ([18F] AZD4694) is an aromatic fluoro-substituted pyridinyl-benzofuran that was designed to address the inherent shortcomings of [11C] AZD2184 (20min half-life and necessity of on-site cyclotron for production). Initial characterization of the tritium analog demonstrated that AZD4694 was compared favorably with PiB.53 The radiosynthetic approach for [18F]AZD4694 was based upon aromatic nucleophilic substitution of the corresponding nitropyridine analog where the aminomethyl substituent was protected utilizing the standard BOC-protection chemistry, and the phenolic hydroxyl group was protected using an ethoxymethoxy-protecting group. The protected nitro-precursor was allowed to react at elevated temperature in DMSO with anhydrous Kryptofix/potassium carbonate/K[18F]F, followed by acidic hydrolysis (6M HCl) and purification utilizing semi-preparative reverse-phase HPLC. [18F]AZD4694 was isolated in 6% radiochemical yield (decay-corrected) with a specific activity of 16–290GBq/µmol (at time of injection) (Patent WO 2010/ 024769). The results of an early clinical validation study of [18F] AZD4694 have recently been reported.54
Diaryl alkene analogs
The rigid, conjugated aromatic structural core found in diaryl alkenes (stilbenes and styrylpyridines) have provided the basis for a variety of Aβ plaque PET imaging agents.12,55–57 Two of these compounds [(E)-4-(4-(2-(2-(2-[18F]fluorethoxy)-ethoxy) ethoxy)styryl)-N-methylaniline, also known as [18F]AV-1, [18F] BAY94-9172, or [18F]Florbetaben and 4-(2-(6-(2-(2-(2-[18F]fluoroethoxy) ethoxy)ethoxy)pyridine-3-yl)vinyl-N-methylbenzamide, also known as [18F]AV-45, Florpiramine, [18F]Florbetapir F 18 Injection (Amyvid™)] have a significant history of use in humans and will be discussed in more detail below.
[18F]Florbetaben ([18F]AV-1 or [18F]BAY94-9172)
[18F]Florbetaben has been produced utilizing mesylate-substituted precursors both with BOC-protected N-methylamino substituent and the unprotected N-methylamino substituent. The original radiosynthetic approach utilized the BOC-protected N-methylaniline mesyloxy precursor. In this two-step radiosynthetic process (nucleophilic displacement reaction with Kryptofix K222 and potassium carbonate in DMSO at elevated temperatures followed by aqueous hydrogen chloride mediated hydrolysis), the desired [18F] Florbetaben was produced in 30% decay-corrected yields (following reverse-phase semi-preparative HPLC purification) in a total synthesis time of 90min. The radiochemical purity of the final product was reported to be >99% and the measured specific activity was 48.2–55.6GBq/µmol (1.3–1.5 Ci/µmol).55 This radiosynthetic approach has been utilized to support the majority of human imaging studies carried out to date, including a multicenter phase II diagnostic study (funded by Bayer Schering Pharma AG).58–61 A recent report describes the use of an unprotected N-methylaniline mesyloxy precursor for the radiosynthesis of [18F]BAY94-9172. A solid-phase supported purification method was reported (C18 Sep-Pak Plus cartridge, Waters Corporation) that leads to significantly shorter total synthesis times (30min). Radiochemical yields of 23 ± 3% (non-decay corrected) were reported. However, the chemical purity of the obtained radiolabeled product was not satisfactory when only the solid-phase purification method was utilized.62
The results of phase III clinical trial study for [18F]Florbetaben were presented at the 64th annual meeting of the American Academy of Neurology (April 2012). Recently, Piramal Imaging (a subsidiary of Piramal Healthcare (India)) agreed to purchase a portfolio of molecular imaging agents from Bayer Schering Pharma AG, including [18F]Florbetaben. Subsequent to this purchase, Piramal Imaging has announced that it hopes to submit an NDA to the US Food and Drug Administration for approval of [18F]Florbetaben in the third quarter of 2012.
Florbetapir F 18 Injection ([18F]AV-45 or Amyvid™)
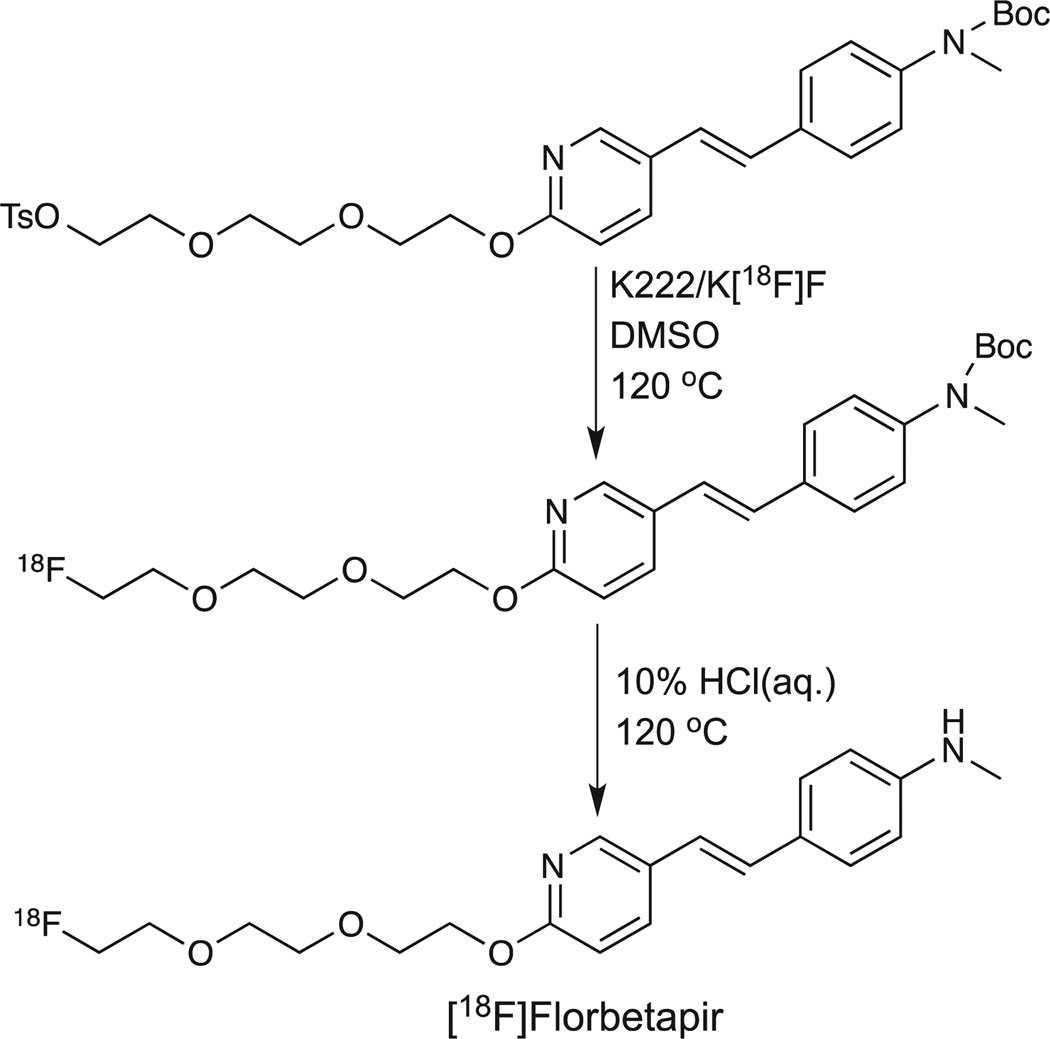
Modification of the stilbene core present in [18F]Florbetaben with a styrylpyridine moiety provides the molecular core of Florbetapir F18 Injection (Amyvid™). This structural modification reduced the lipophilicity of the molecule and led to faster brain kinetics compared with [18F]Florbetaben. The initial radiosynthetic process reported by Choi, et al.63 is very similar to the two-step process utilized for [18F]Florbetaben. The primary difference is in the use of the N-BOC-protected tosyloxy precursor in the radiosynthetic process. In this two-step radiosynthetic process (nucleophilic displacement reaction with Kryptofix K222 and potassium carbonate in DMSO at elevated temperatures followed by aqueous hydrogen chloride mediated hydrolysis), the desired [18F]Florbetapir ([18F]AV-45) was produced in 10–30% decay-corrected yields (following reverse-phase semi-preparative HPLC purification). The radiochemical purity of the final product was reported to be >99% and the measured specific activity was 37–185 GBq/µmol (1–5Ci/µmol).63 A good manufacturing practice (GMP)-compliant radiosynthetic process for [18F]Florbetapir has recently been reported. This process was based upon an automated multipurpose fluorination synthesismodule (F121R, Sumitomo) and provided radiochemical yields at EOS of 25.4 ± 7.7% in an overall synthesis time of approximately 104 min. The radiochemical purity of the isolated radiolabeled product was 95.3 ± 2.2% and the EOS specific activity was 470 ± 135GBq/µmol.64 Interestingly, an acetonitrile adduct of precursor was determined to be present as a cold impurity in the final product formulation of [18F]Florbetapir produced utilizing this GMP method. (Scheme 3)
Scheme 3.
Radiosynthetic route for [18F]Florbetapir.
On April 6, 2012, Florbetapir F 18 Injection (Amyvid™) became the first F-18 labeled PET tracer approved for clinical use by the US Food and Drug Administration since Fludeoxyglucose F-18 Injection (New Drug Application #202008). The labeling indication states that [18F]Florbetapir is a radioactive diagnostic agent for PET imaging of the brain to estimate amyloid-β neuritic plaque density in adult patients with cognitive impairment who are being evaluated for AD and other causes of cognitive decline. [18F]Florbetapir is an adjunct to other diagnostic evaluations and is not a test for predicting the development of AD-associated dementia and is not for monitoring patient responses to AD therapy.
Conclusion
Evidence is growing that the in vivo presence of Aβ plaque deposits are necessary but not sufficient for the presentation of cognitive dysfunction associated with AD.65,66 Nevertheless, the relatively recent research history regarding the design, development, evaluation, and now commercialization of PET imaging agents designed to non-invasively provide information regarding in vivo Aβ plaque deposition has highlighted the potential of molecular imaging with PET and its utility in areas outside of previously accepted clinical indications. These Aβ imaging agents have shown the impact a biomarker can have in the study of disease pathophysiology as well as diagnosis and therapy development. The impact of this and related biomarkers is already clear in recently revised criteria for the diagnosis of AD.67,68 The impact of Aβ imaging is beginning to be apparent in drug development as well.9,37 The success achieved with Aβ imaging has spawned a quest for the development of molecular imaging agents for other markers of neurodegenerative disease pathology, such as NFTs (tau protein) and Lewy bodies (α-synuclein). While molecular imaging with PET had historically been directed at normal physiological processes (e.g., blood flow, glucose metabolism, and neuroreceptors) that may have subtle abnormalities in disease states, Aβ imaging showed that pathology associated almost exclusively with disease states can be an informative target for molecular imaging.
Biographies

Scott Mason was born in 1962 in Richmond, Virginia, USA and is today a Research Associate Professor of Radiology at the University of Pittsburgh. He received a B.S. in Chemistry in 1984 from King College and a Ph.D. in Organic Chemistry in 1991 from Vanderbilt University. Following the completion of his degree he joined the Vanderbilt University Medical Center PET Facility as a post-doctoral researcher focused on organic and radiochemistry synthetic efforts in dopamine imaging. His current research interests include the application of microfluidic techniques to the production of radiolabeled tracers and the development and adaptation of radiosynthetic methods for PET radiopharmaceuticals in neuroreceptor and oncology applications. He also provides radiochemistry and regulatory support for investigator-initiated studies utilizing PET imaging both in preclinical animal applications and in human subject clinical research.

William E. Klunk was born in 1956 in Pennsylvania, USA. He is a Distinguished Professor of Psychiatry and Neurology and Co-Director of the Alzheimer Disease Research Center at the University of Pittsburgh. He is Vice-Chair of the Medical and Scientific Advisory Council of the National Alzheimer’s Association. Dr. Klunk completed an MD/PhD degree at Washington University in St. Louis focusing on neuropharmacology and medicinal chemistry. Dr. Klunk then completed a general psychiatry residency at the University of Pittsburgh, followed by a Fellowship in Geriatric Neuropsychopharmacology at the same institution. He has published well over 150 journal articles and book chapters and is Principal Investigator of several NIH and Foundation grants and has received a MERIT Award from the NIA. Dr. Klunk, along with colleague Chester A. Mathis, is a pioneer in the field of in vivo amyloid imaging in humans. His work spans from basic synthetic chemistry and neuropharmacological evaluation of amyloid imaging tracers to human PET studies of these tracers

Chester A. Mathis was born in 1949 in Poplar Bluff, Missouri, USA. He is Distinguished Professor of Radiology and Pharmaceutical Sciences at the University of Pittsburgh, Director of the University of Pittsburgh Positron Emission Tomography (PET) Facility, and UPMC Endowed Chair of PET Research. He received a PhD in physical chemistry in 1979 from the University of California, Davis and has a long-standing interest in applying synthetic radiochemistry techniques to develop PET radiopharmaceuticals to study brain function in vivo. Approximately 18 years ago, he joined efforts with William E. Klunk of the Department of Psychiatry at the University of Pittsburgh to devise a PET radiotracer capable of imaging amyloid-beta plaques in living human brain. This work led to the development of carbon-11-labeled Pittsburgh Compound-B ([11C]PiB) to non-invasively assess amyloid-beta plaque load in the brains of humans using PET imaging. Drs. Mathis and Klunk devised a longer-lived analog of PiB, [F-18]flutemetamol, which GE Healthcare has licensed and has in Phase III clinical trials as a PET radiopharmaceutical for the detection of amyloid-beta deposits.
Footnotes
Conflict of Interest
CAM and WEK claim a conflict of interest resulting from a license agreement between the University of Pittsburgh and GE Healthcare related to PiB technolgy.
References
- 1.Alzheimer A. Allg. Z. Psychiat. 1907;64:146. [Google Scholar]
- 2.Trojanowski JQ, Clark CM, Schmidt ML, Arnold SE, Lee VM. Neurobiol. Aging. 1997;18:S75. doi: 10.1016/s0197-4580(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 3.Bacskai BJ, Hickey GA, Skoch J, Kajdasz ST, Wang Y, Huang GF, Mathis CA, Klunk WE, Hyman BT. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12462. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delacourte A. Ann. Biol. Clin. (Paris) 1998;56:133. [PubMed] [Google Scholar]
- 5.Selkoe DJ. Ann. N. Y. Acad. Sci. 2000;924:17. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 6.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Mol. Med. 2008;14:451. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberson ED, Mucke L. Science. 2006;314:781. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakob-Roetne R, Jacobsen H. Angew. Chem. Int. Ed Engl. 2009;48:3030. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 9.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. Lancet Neurol. 2010;9:363. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 10.Dischino DD, Welch MJ, Kilbourn MR, Raichle ME. J. Nucl. Med. 1983;24:1030. [PubMed] [Google Scholar]
- 11.Waterhouse RN. Mol. Imaging Biol. 2003;5:376. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Kung MP, Oya S, Hou C, Kung HF. Nucl. Med. Biol. 2007;34:89. doi: 10.1016/j.nucmedbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. J. Med. Chem. 2003;46:2740. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 14.Klunk WE, Pettegrew JW, Abraham DJ. J. Histochem. Cytochem. 1989;37:1273. doi: 10.1177/37.8.2666510. [DOI] [PubMed] [Google Scholar]
- 15.Cai L, Innis RB, Pike VW. Curr. Med. Chem. 2007;14:19. doi: 10.2174/092986707779313471. [DOI] [PubMed] [Google Scholar]
- 16.Mathis CA, Lopresti BJ, Klunk WE. Nucl. Med. Biol. 2007;34:809. doi: 10.1016/j.nucmedbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kung HF, Choi SR, Qu W, Zhang W, Skovronsky D. J. Med. Chem. 2010;53:933. doi: 10.1021/jm901039z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M. Chem. Pharm. Bull. (Tokyo) 2009;57:1029. doi: 10.1248/cpb.57.1029. [DOI] [PubMed] [Google Scholar]
- 19.Nordberg A. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(Suppl 1):S46. doi: 10.1007/s00259-007-0700-2. [DOI] [PubMed] [Google Scholar]
- 20.Nordberg A. J. Nutr. Health Aging. 2009;13:346. doi: 10.1007/s12603-009-0038-5. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro Morais G, Paulo A, Santos I. Eur. J. Org. Chem. 2012;2012(7):1279. [Google Scholar]
- 22.Furumoto S, Okamura N, Iwata R, Yanai K, Arai H, Kudo Y. Curr. Top. Med. Chem. 2007;7:1773. doi: 10.2174/156802607782507402. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Pike VW, Wang Y. Curr. Top. Dev. Biol. 2005;70:171. doi: 10.1016/S0070-2153(05)70008-9. [DOI] [PubMed] [Google Scholar]
- 24.Mathis CA, Wang Y, Klunk WE. Curr. Pharm. Des. 2004;10:1469. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- 25.Friedland RP, Kalaria R, Berridge M, Miraldi F, Hedera P, Reno J, Lyle L, Marotta CA. Ann. N. Y. Acad. Sci. 1997;826:242. doi: 10.1111/j.1749-6632.1997.tb48475.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrio JR, Huang SC, Cole G, Satyamurthy N, Petric A, Phelps ME, Small GW, Label J. Compd. Radiopharm. 1999;42:S194. [Google Scholar]
- 27.Klok RP, Klein PJ, van Berckel BN, Tolboom N, Lammertsma AA, Windhorst AD. Appl. Radiat. Isot. 2008;66:203. doi: 10.1016/j.apradiso.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Kepe V, Zabjek A, Petric A, Padgett HC, Satyamurthy N, Barrio JR. Mol. Imaging Biol. 2007;9:6. doi: 10.1007/s11307-006-0061-4. [DOI] [PubMed] [Google Scholar]
- 29.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. N. Engl. J. Med. 2006;355:2652. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 30.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Neurology. 2007;68:1718. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 31.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Ann. Neurol. 2004;55:306. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 32.Henriksen G, Yousefi BH, Drzezga A, Wester HJ. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(Suppl 1):S75. doi: 10.1007/s00259-007-0705-x. [DOI] [PubMed] [Google Scholar]
- 33.Barrio JR, Satyamurthy N, Huang SC, Petric A, Small GW, Kepe V. Acc. Chem. Res. 2009;42:842. doi: 10.1021/ar800189x. [DOI] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Brain. 2009;132:1355. [Google Scholar]
- 35.Ewers M, Insel P, Jagust WJ, Shaw L, Trojanowski JJ, Aisen P, Petersen RC, Schuff N, Weiner MW. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Brain. 2009;132:1310. [Google Scholar]
- 37.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu ZX, Loetscher H, Santarelli L. Arch. Neurol. 2012;69:198. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 38.Wilson AA, Garcia A, Chestakova A, Kung H, Houle S, Label J. Compd. Radiopharm. 2004;47:679. [Google Scholar]
- 39.Shao X, Hoareau R, Runkle AC, Tluczek LJM, Hockley BG, Henderson BD, Scott PJH. J. Label. Compd. Radiopharm. 2011;54:819. doi: 10.1002/jlcr.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathis CA, Lopresti B, Mason N, Price J, Flatt N, Bi W, Zioloko S, DeKosky S, Klunk W. J. Nucl. Med. 2007;48:56P. [Google Scholar]
- 41.Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, Vandenberghe R, Van Laere K. J. Nucl. Med. 2009;50:818. doi: 10.2967/jnumed.108.060756. [DOI] [PubMed] [Google Scholar]
- 42.Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, Bormans G, Brooks DJ, Vandenberghe R. J. Nucl. Med. 2009;50:1251. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 43.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ. Ann. Neurol. 2010;68:319. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 44.Mountz J, Zhang Z, Laymon C, Price J, Boudhar S, Newberg A, Mathis C. J. Nucl. Med. 2011;52:116. [Google Scholar]
- 45.Wolk DA, Grachev ID, Buckley C, Kazi H, Grady MS, Trojanowski JQ, Hamilton RH, Sherwin P, McLain R, Arnold SE. Arch. Neurol. 2011;68:1398. doi: 10.1001/archneurol.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rattue P. Medical News Today. MediLexicon, Intl.; 2012. [13 April 2012]. [Google Scholar]
- 47.Mason NS, Klunk WE, Debnath M, Flatt N, Huang G, Shao L, Mathis CA. J. Label. Compd. Radiopharm. 2007;50:S87. [Google Scholar]
- 48.Dyrstad KR, Powell N. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:S208. [Google Scholar]
- 49.Horn E. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:S208. [Google Scholar]
- 50.Johnson AE, Jeppsson F, Sandell J, Wensbo D, Neelissen JA, Jureus A, Strom P, Norman H, Farde L, Svensson SP. J. Neurochem. 2009;108:1177. doi: 10.1111/j.1471-4159.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- 51.Nyberg S, Jonhagen ME, Cselenyi Z, Halldin C, Julin P, Olsson H, Freund-Levi Y, Andersson J, Varnas K, Svensson S, Farde L. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1859. doi: 10.1007/s00259-009-1182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson JD, Varnas K, Cselenyi Z, Gulyas B, Wensbo D, Finnema SJ, Swahn BM, Svensson S, Nyberg S, Farde L, Halldin C. Synapse. 2010;64:733. doi: 10.1002/syn.20782. [DOI] [PubMed] [Google Scholar]
- 53.Jureus A, Swahn BM, Sandell J, Jeppsson F, Johnson AE, Johnström P, Neelissen JA, Sunnemark D, Farde L, Svensson SP. J. Neurochem. 2010;114:784. doi: 10.1111/j.1471-4159.2010.06812.x. [DOI] [PubMed] [Google Scholar]
- 54.Cselenyi Z, Jonhagen ME, Forsberg A, Halldin C, Julin P, Schou M, Johnström P, Varnas K, Svensson S, Farde L. J. Nucl. Med. 2012;53:415. doi: 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. Nucl. Med. Biol. 2005;32:799. doi: 10.1016/j.nucmedbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. J. Med. Chem. 2005;48:5980. doi: 10.1021/jm050166g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono M, Haratake M, Nakayama M, Kaneko Y, Kawabata K, Mori H, Kung MP, Kung HF. Nucl. Med. Biol. 2005;32:329. doi: 10.1016/j.nucmedbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, O’Keefe G, Ackerman U, Tochon-Danguy H, Chan JG, Reininger CB, Fels L, Putz B, Rohde B, Masters CL, Rowe CC. J. Nucl. Med. 2011;52:1210. doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- 59.Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, Tochon-Danguy H, Chan G, Berlangieri SU, Jones G, Dickinson-Rowe KL, Kung HP, Zhang W, Kung MP, Skovronsky D, Dyrks T, Holl G, Krause S, Friebe M, Lehman L, Lindemann S, Dinkelborg LM, Masters CL, Villemagne VL. Lancet Neurol. 2008;7:129. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 60.Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, Hiemeyer F, Wittemer-Rump SM, Seibyl J, Reininger C, Sabri O. Lancet Neurol. 2011;10:424. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- 61.Barthel H, Sabri O. J. Alzheimers Dis. 2011;26(Suppl 3):117. doi: 10.3233/JAD-2011-0068. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Shi H, Yu H, Jiang S, Tang G. Nucl. Med. Biol. 2011;38:121. doi: 10.1016/j.nucmedbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, Benedum TE, Kilbourn MR, Skovronsky D, Kung HF. J. Nucl. Med. 2009;50:1887. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao CH, Lin KJ, Weng CC, Hsiao IT, Ting YS, Yen TC, Jan TR, Skovronsky D, Kung MP, Wey SP. Appl. Radiat. Isot. 2010;68:2293. doi: 10.1016/j.apradiso.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Sojkova J, Zhou Y, An Y, Kraut MA, Ferrucci L, Wong DF, Resnick SM. Arch. Neurol. 2011;68:644. doi: 10.1001/archneurol.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Ann. Neurol. 2011;69:181. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Lancet Neurol. 2007;6:734. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 68.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. Alzheimer’s Dement. 2011;7:263. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]