Abstract
Background
Since United States Food and Drug Administration approval in 2005, the short-term safety and efficacy of thoracic endovascular aortic repair (TEVAR) have been established. However, longer-term follow-up data remain lacking. The objective of this study is to report 6-year outcomes of TEVAR in clinical practice.
Methods
A prospective cohort review was performed of all patients undergoing TEVAR at a single referral institution between March 2005 and May 2011. Rates of reintervention were noted. Overall and aortic-specific survival were determined using Kaplan-Meier methods. Log-rank tests were used to compare survival between groups.
Results
During the study interval, 332 TEVAR procedures were performed in 297 patients. Reintervention was required after 12% of procedures at a mean of 8 ± 14 months after initial TEVAR and was higher in the initial tercile of patients (15.0% vs 9.9%). The 6-year freedom from reintervention was 84%. Type I endoleak was the most common cause of reintervention (5%). Six-year overall survival was 54%, and aorta-specific survival was 92%. Long-term survival was significantly lower than that of an age- and sex-matched United States population (p < 0.001). Survival was similar between patients requiring a reintervention vs those not (p = 0.26). Survival was different based on indication for TEVAR (p = 0.007), and patients with degenerative aneurysms had the lowest survival (47% at 6 years). Cardiopulmonary pathologies were the most common cause of death (27 of 93 total deaths).
Conclusions
Long-term aortic-related survival after TEVAR is high, and the need for reintervention is infrequent. However, overall long-term survival is low, particularly for patients with degenerative aneurysms, and additional work is needed to identify patients unlikely to derive a survival benefit from TEVAR.
The proven short-term safety and efficacy of thoracic endovascular aortic repair (TEVAR) have led to its expanding application to a wide variety of thoracic aortic pathologies [1–3]. However, given its relatively recent United States Food and Drug Administration approval in 2005, longer-term outcome data are limited. Data available from single-center series have demonstrated high rates of reintervention after TEVAR of 17% to 22% [4, 5]. Moreover, reintervention rates after endovascular abdominal aortic aneurysm repair, for which there is longer follow-up given earlier Food and Drug Administration approval, are significant, approaching 30% [6]. Of additional concern are reports of high rates of late death after TEVAR (40% mortality 3 years after the procedure), even at experienced, high-volume centers [4]. Lastly, recent observational data from Medicare patients with descending thoracic aortic aneurysms have shown lower survival in patients treated with TEVAR compared with open repair, even after risk adjustment [7].
Given the rapidly expanding use of TEVAR [1] and paucity of long-term outcome results, additional work investigating long-term safety and efficacy are needed. The objective of this study was to describe 6-year outcomes of TEVAR in clinical practice at an academic referral institution, focusing on rates of reintervention and survival.
Patients and Methods
This study was reviewed and approved by the Institutional Review Board of Duke University, and the need for individual patient consent was waived.
Data Source
A retrospective review was performed on prospectively collected data from all patients undergoing TEVAR between March 2005 and May 2011 at one referral institution. Preoperative, intraoperative, and postoperative variables were abstracted from the Duke Thoracic Aortic Surgery Database, a prospectively maintained clinical registry of all patients undergoing thoracic aortic operations at Duke University Medical Center (Durham, NC).
TEVAR Procedures
All patient procedures and management were part of routine clinical care as determined by the clinical care team. Patient selection for TEVAR, techniques of device delivery and deployment, and postoperative surveillance have been previously described [2, 3, 8]. The 5 currently approved Food and Drug Administration thoracic stent grafts, as well as the investigational Zenith TX2-LP (Cook Medical, Bloomington, IN) device, were used. Indications for each TEVAR procedure were classified as degenerative aneurysm (fusiform and saccular including penetrating atherosclerotic ulcers), acute and chronic dissection, or acute blunt traumatic aortic injury (transection). Procedures were further classified as descending-only repair [9], hybrid arch repair (aortic arch debranching with endograft coverage) [2], or hybrid thoracoabdominal repair (visceral abdominal debranching with aortic endograft coverage) [3, 10].
Variables, Outcomes, and Statistics
The primary outcome was overall survival. Survival was estimated using the Kaplan-Meier method. For patients with multiple procedures, the first procedure was used to calculate survival. Cause of death was determined by autopsy, medical record, or family interview. The Social Security Death Index (http://ssdi.rootsweb.com/) was used to confirm all deaths.
Survival was compared between groups based on need for reintervention, procedure status, and indication for TEVAR using log-rank tests. A one-sample log-rank test was used to compare the survival of the study cohort with an age-matched and sex-matched sample from the United States 2008 Census [11]. Death due to an aortic pathology or death during initial hospitalization or within 30 days of initial TEVAR procedure were considered aortic-related deaths for the calculation of aortic-specific survival.
Aortic reintervention was defined as the need to perform an additional aortic surgical procedure to treat a complication of the initial TEVAR procedure or the need to reintervene on the same aortic pathology. Angiography and treatment of type II endoleaks were also included in this definition. Primary technical success was defined according to the Society for Vascular Surgery reporting standards [12].
Continuous variables are presented as mean ± standard deviation. Categoric variables described in the Tables are presented as percentage (raw number). Categoric variables were compared with χ2 and Fisher exact tests.
Results
During the study interval, 332 TEVAR procedures were performed in 297 patients (Table 1). Procedures included descending only (66%), hybrid arch (19%), and hybrid thoracoabdominal (15%) repair. The 30-day/in-hospital mortality rate was 6%. The rates of postoperative permanent paraparesis/paraplegia and stroke were 1.8% and 1.8%, respectively. Acute kidney injury requiring any postoperative dialysis occurred after 3.3% of procedures. Operative characteristics are presented in Table 2. Primary technical success occurred in 96% of procedures. Vascular access site complications occurred in 6.6%.
Table 1. Preoperative Patient Characteristics.
| Variables | Mean ± SD or % (No.) |
|---|---|
| Age, age | 64 ± 14 |
| Male sex | 59 (196) |
| White race | 64 (214) |
| Hypertension | 86 (287) |
| Diabetes | 13 (44) |
| Body mass index, kg/m2 | 28 ± 6 |
| Smoker | 61 (204) |
| COPD | 29 (96) |
| History of stroke/TIA | 12 (39) |
| Coronary artery disease | 31 (104) |
| Peripheral vascular disease | 29 (96) |
| Ejection fraction | 0.54 ± 0.04 |
| Preoperative hemoglobin, g/dL | 12 ± 2 |
| Baseline creatinine > 1.5 mg/dL | 28 (94) |
| Previous aortic operation | 46 (153) |
| Connective tissue disease | 3 (11) |
| ASA class 4 | 46 (154) |
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; SD = standard deviation; TIA = transient ischemic attack.
Table 2. Operative Characteristics.
| Variable | % (No.) or Mean ± SD |
|---|---|
| Indication for TEVAR | |
| Degenerative Aneurysm | 63 (208) |
| Acute or chronic dissection | 27 (89) |
| Acute Blunt Aortic Injury | 5 (18) |
| Other | 5 (17) |
| Procedure status | |
| Elective | 64 (212) |
| Urgent or emergency | 36 (120) |
| Access vessel | |
| Femoral | 71 (236) |
| Iliac | 21 (71) |
| Infra-renal aorta | 2 (8) |
| Other | 10 (33) |
| Required conduit for access | 31 (104) |
| Left subclavian coverage | 51 (169) |
| Maximum aortic diameter, cm | 5.8 ± 1.6 |
| Type of endograft | |
| TAG/C-TAGa | 60 (200) |
| Talent/Valiantb | 12 (40) |
| Zenith TX2/TX2-LPc | 19 (63) |
| Other | 2 (5) |
| Combination | 7 (24) |
| Endografts used, No. | 1.8 ± 1.0 |
| Intraoperative CSF drainage | 22 (74) |
W.L. Gore and Associates, Flagstaff, AZ.
Medtronic, Minneapolis, MN.
Cook, Bloomington, IN.
CSF = cerebrospinal fluid; SD = standard deviation; TEVAR = thoracic endovascular aortic repair.
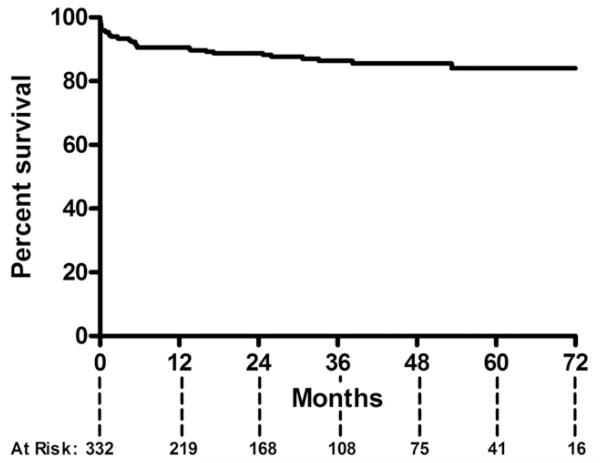
Mean follow-up for living patients was 37 ± 21 months. Aortic reintervention was required after 12% of TEVAR procedures (42 reinterventions in 39 patients) at a mean of 8 ± 13 months after the initial TEVAR (Table 3). Type I endoleak was the most common cause of reintervention (5.1%). Reinterventions were performed using conventional endovascular means in 69%, followed by hybrid (17%) or open (14%) procedures. Reintervention for type II endoleak was required in 3.6%. The rate of reintervention in the initial tercile (N = 110) of TEVAR procedures in the series was 15% vs 9.9% for the subsequent two-thirds (p = 0.14). The reintervention rate for patients undergoing TEVAR limited to the descending thoracic aorta was 13.3% compared with 8.8% for those undergoing hybrid repairs (p = 0.30); rates did not vary by device manufacturer (p = 0.52). Six-year freedom from reintervention was 84% (Fig 1).
Table 3. Indications for Major Reintervention.
| Variable | % (No.) (N = 42) |
|---|---|
| Endoleak | |
| Type I | 5.1 (17) |
| Type Ia | 3.9 (13) |
| Type Ib | 1.2 (4) |
| Type Ib and III | 0.3 (1) |
| Type II | 3.6 (12) |
| Type III | 0.3 (1) |
| Graft collapse | 1.5 (5) |
| Retrograde type A dissection | 1.5 (5) |
| Aortoesophageal fistula | 0.3 (1) |
Fig 1.
Kaplan-Meier curve demonstrates an 84% freedom from reintervention at 6 years after thoracic endovascular aortic repair.
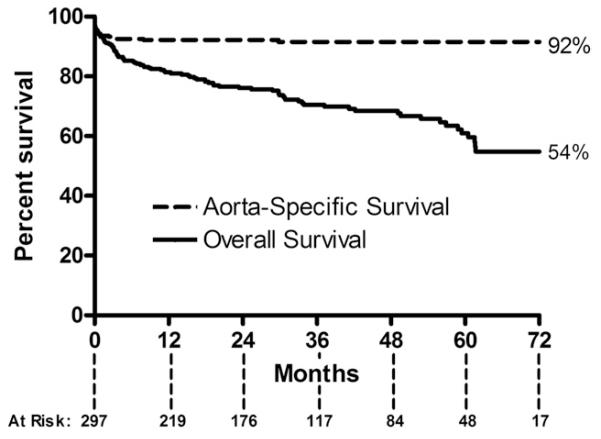
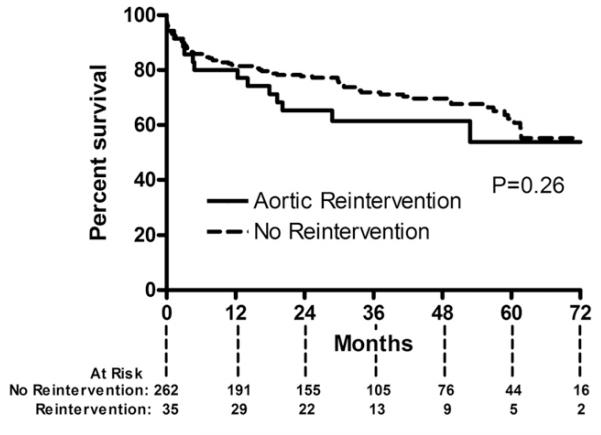
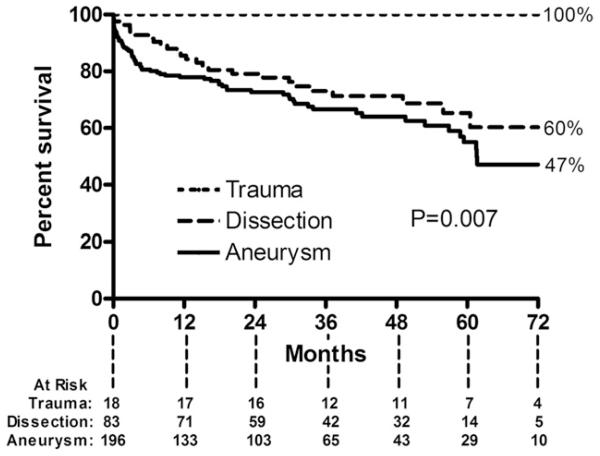
Overall survival for the entire cohort at 6 years was 54%, whereas the aorta-specific survival at 6 years was 92% (Fig 2). Long-term survival of the entire cohort was lower than that of an age-matched and sex-matched United States population (p < 0.001). Long-term survival was similar between patients who did and did not require reintervention (p = 0.26; Fig 3). Long-term survival was also similar between patients undergoing elective vs urgent or emergency procedures (p = 0.57). Survival was different based on surgical indication (p = 0.007; Fig 4). Patients with degenerative aneurysms had the lowest long-term survival of 47% at 6 years; however, survival did not differ (p = 0.75) between those with fusiform and saccular aneurysms, including penetrating atherosclerotic ulcers. Cardiopulmonary pathologies were the most common cause of death (27 of 93 total deaths, Table 4). Only 5 patients died of an aortic-related cause after the perioperative period.
Fig 2.
Kaplan-Meier curve demonstrates a 54% overall (solid line) and 92% aorta-specific survival (dashed line) after thoracic endovascular aortic repair.
Fig 3.
Kaplan-Meier curve demonstrates survival after thoracic endovascular aortic repair according to those who did (solid line) and did not (dashed line) need aortic reintervention.
Fig 4.
Kaplan-Meier curve demonstrates survival after thoracic endovascular aortic repair based on indication for procedure. Six-year survival for patients with degenerative aneurysms (solid line) was 47% compared with 60% for patients with acute or chronic dissection (dashed line) and 100% for patients with acute blunt aortic injury (dotted-dashed line).
Table 4. Causes of Death.
| Cause | Deaths, No. (N = 93) |
|---|---|
| Cardiopulmonary | 27 |
| Perioperative | 19 |
| Sepsis | 5 |
| Failure to thrive | 5 |
| Aortic-related | 5 |
| Neurologic | 4 |
| Cancer | 4 |
| Other | 5 |
| Unknown | 19 |
Comment
Recent reports of sobering rates of reintervention and late death [4, 5] have raised questions about the durability and appropriate use of TEVAR. In this large, single-center series, we demonstrate that TEVAR can be performed with a low rate of reintervention (12%) and high rate of aorta-specific long-term survival (92% at 6 years). Further, reintervention rates decreased by 50% after the initial tercile of patients treated, likely related to the availability of newer-generation devices over this interval and the presence of a learning curve for the technology. However, overall long-term survival remains low, with many patients dying of cardiopulmonary causes.
During a mean follow-up of greater than 3 years after TEVAR, the reintervention rate in the current series was 12%. This is lower than that reported in other large series of TEVAR use in real-world clinical practice, even though one-third of the TEVAR procedures were complex, off-label, hybrid repairs. For example, Geisbusch and colleagues [5] reported a reintervention rate of 22% over 31 months of follow-up in 264 patients, whereas Lee and colleagues [4] performed secondary procedures in 17% of their 400-patient cohort during a median of 299 days.
Reasons to explain this difference in reintervention rates are unknown and may include differences in patient and procedure selection, operative technique, or other as-yet-unidentified variables. For example, one could speculate that the aggressive use of hybrid techniques to create adequate landing zones might explain some of the differences. This is supported by the lower reintervention rates observed in patients who underwent hybrid procedures in the series. Further, as highlighted above, there appears to be a learning curve related to application of the technology, including more appropriate patient selection, which, although difficult to quantitate, almost certainly must also play a role.
More than 75% of the reinterventions in the present series occurred during the first year after TEVAR, which agrees with other reported studies [4]. In addition, type I endoleak was the most common cause of reintervention (5.1%), which also concurs with current published data [5, 13]. Finally, 69% of reinterventions were performed using endovascular techniques, similar to other studies [13].
Overall survival of the present cohort was 71% at 3 years and 54% at 6 years after TEVAR. These numbers are similar to the 3-year survival of 60% [4] and 9.8-year survival of 51% [14] reported by other large, tertiary referral centers. The long-term survival data are quite humbling, and it is evident from this and other studies, including modern series of open aortic repair from centers of excellence [15–18], that patients with descending thoracic and thoracoabdominal aortic disease represent a unique subgroup of patients at notable risk for late death, despite correction of their aortic pathology (Table 5). For comparison, survival rates after coronary artery bypass grafting are approximately 92% at 3 years and 84% at 6 years [19], which are substantially higher than that reported after endovascular or open descending and thoracoabdominal repair. Long-term survival after TEVAR in the current series was highest for patients with blunt aortic injury, which may be expected given that these patients are often younger with fewer comorbidities. Survival of patients who required reinterventions was similar to those who did not, which has previously been shown [5].
Table 5. Comparison of Modern Results of Open Aortic Repair in Centers of Excellence.
| Patients (No.) |
Mean Age (years) |
Elective (%) |
Peri-op Mortality (%) |
Survival (%) |
||||
|---|---|---|---|---|---|---|---|---|
| First Author | Year | Procedure | 3-Year | 5-Year | ||||
| Estrera [15] | 2005 | Open descending | 300 | 67 | 8 | … | 64 | |
| Conrad [17] | 2007 | Open TAAA | 445 | 71 | 76 | 8.3 | … | 54 |
| Fehrenbacher [18] | 2010 | Open descending and TAAA |
343 | 66 | 87 | 5 | 79 | 69 |
| Wong [16] | 2011 | Open TAAA | 509 | 64 | 80 | 7.9 | 74 | |
| Current series | 2012 | TEVAR | 297 | 64 | 64 | 6 | 71 | 61 |
TAAA = thoracoabdominal aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
The notable long-term mortality rate after TEVAR, as well as data in the abdominal aortic aneurysm literature, have raised questions about the durability of TEVAR devices and the appropriate selection of patients for the procedure. Recent data on the treatment of descending thoracic aortic aneurysms in the Medicare population has shown that patients undergoing TEVAR have lower 5-year survival than patients undergoing open repair, even after risk adjustment [7]. The authors of that study proposed the lower survival might be due to device failures, or more likely, patient-level variables that were unadjusted for, because higher-risk patients are now being offered TEVAR [7]. The data presented here would support the latter hypothesis given that aortic-related survival is high—92% at 6 years—and device failure highly infrequent. Most deaths with a documented cause were due to cardiopulmonary pathologies (29%), which is consistent with other studies [4].
Limitations of the current study are worth noting. There is no control group of medically managed patients or patients treated with open surgical repair with which to compare the TEVAR cohort. The study is also retrospective, and a selection bias may be present. In addition, the subject population is heterogeneous in the diseases treated and the procedures performed. Lastly, the cause of death was unknown in 19 patients, which may under-estimate the frequency of certain causes of death. However, this unknown percentage is substantially lower than that in other published studies [4] and is a known obstacle given the number of unwitnessed deaths at home. Finally, continued and ongoing improvement in the available TEVAR technology makes any reported results something of a “moving target,” which should be considered when comparison is made with earlier published results.
In conclusion, in this large, single-center series, we demonstrate that long-term aortic-related survival after TEVAR is high and reintervention is infrequent. However, overall long-term survival is low, particularly for patients with degenerative aneurysms, and many patients die of cardiopulmonary causes after the perioperative period. Given its short-term safety and efficacy, TEVAR is perhaps being offered to higher-risk patients who may not obtain a survival or quality of life benefit from the procedure. In their recent series of 400 consecutive TEVAR procedures performed at a tertiary referral center, Lee and colleagues [4] highlight that the high late mortality rate suggests that patients with thoracic aortic disease may represent a subset in whom underlying comorbidities may pose a greater risk of death than the aortic disease itself. We certainly agree with this conclusion and also concur with their suggestion that, going forward, improved patient selection is needed to draw the line between utility and futility. Additional work is ongoing at our institution to preoperatively identify these patients [20], and we believe that doing so may assist with the proper application of TEVAR in the future.
Biographies
DISCUSSION
DR THOMAS E. MACGILLIVRAY (Boston, MA): I know it was the smallest group in your series, but the patients who were treated with thoracic endovascular aortic repair (TEVAR) for trauma had a pretty remarkable result. Oftentimes, those patients’ survival is limited not because of their aortic disease but because of their comorbid injuries. What was the timing when these patients received their stent graft compared with their injury?
DR SHAH: We generally perform TEVAR for acute blunt aortic injury within 12 to 24 hours. But we do look at each patient individually and delay operative management in patients with severe pulmonary injury requiring significant mechanical ventilatory support, such as an oscillating ventilator, and in patients with severe neurologic injury precluding survival.
DR JAHANZAIB IDREES (Cleveland, OH): I am interested in knowing whether, in your study, you looked closely if there was a correlation between the rate of reintervention and extent of distal aortic disease?
DR SHAH: We did not look at that specifically, but we did not see a difference in survival based on extent of the TEVAR procedure performed.
DR LEONARD N. GIRARDI (New York, NY): Did you break down your subgroups by age? We had seen two previous papers that had shown fairly good survival in younger patients. Did you have an opportunity to look at your data in any more detail?
DR SHAH: We did not break down overall survival by age, but we have looked at the outcome of 1-year mortality to try to sort out who may or may not derive a survival benefit from TEVAR. We have found that age older than 75 was significantly associated with death within 1 year of operation, as was American Society of Anesthesiologists class 4 and aortic diameter exceeding 6.5 cm.
DR JOHN FEHRENBACHER (Indianapolis, IN): When comparing your 6-year TEVAR survival and long-term survival with the retrospective groups, I think some of these groups are from older literature, with a 61% average 5-year survival. We published a 74% 5-year and a 54% 10-year survival using open repair. My question is: Are you truly comparing TEVAR vs open repair in chronologic studies?
DR SHAH: We looked at series from the late 1990s and early 2000s because we tried to include large series from centers of excellence over the last 10 to 15 years. I believe Dr Estrera’s was from 2005. I agree that adding more recent series, such as the one from your group, could be helpful for comparison, and we can certainly add that to the manuscript.
DR ERIC ROSELLI (Cleveland, OH): Great paper. It really brings up the most important question we face every day when we see these older people with aneurysms: How can we predict the future? We want to do a prophylactic operation on their aneurysm, but we have the competing risk of all their other comorbidities. I was wondering if you have any data available on the patients with aneurysms that you evaluate—but don’t operate on—to provide some understanding of what their survival is in comparison with the operated-on patients. Also, if you have data on those patients, how frequently was their cause of death related to cardiopulmonary disease?
DR SHAH: After examining this data, particularly the 1-year survival data, for patients with extensive comorbidities, home oxygen, very large aneurysms, or who are older than 75 years and probably have a low chance of living for another year, we have started to look at them very closely and even withhold performing TEVAR because it would likely not provide a survival benefit and have significant associated costs.
DR ROSELLI: Do you have the data on those patients to include in the manuscript?
DR SHAH: We do not have follow-up data on the patients who we deny TEVAR to, unfortunately.
Footnotes
Presented at the Forty-eighth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 28–Feb 1, 2012.
Dr Hughes discloses that he has a financial relationship with Medtronic, W.L. Gore and Associates, and Vascutek Terumo.
References
- 1.Scali ST, Goodney PP, Walsh DB, et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53:1499–505. doi: 10.1016/j.jvs.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes GC, Daneshmand MA, Balsara KR, et al. “Hybrid” repair of aneurysms of the transverse aortic arch: midterm results. Ann Thorac Surg. 2009;88:1882–7. doi: 10.1016/j.athoracsur.2009.07.027. discussion 1887–8. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008;136:21–8. 28.e1–6. doi: 10.1016/j.jtcvs.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 4.Lee WA, Daniels MJ, Beaver TM, Klodell CT, Raghinaru DE, Hess PJ., Jr Late outcomes of a single-center experience of 400 consecutive thoracic endovascular aortic repairs. Circulation. 2011;123:2938–45. doi: 10.1161/CIRCULATIONAHA.110.965756. [DOI] [PubMed] [Google Scholar]
- 5.Geisbusch P, Hoffmann S, Kotelis D, Able T, Hyhlik-Dürr A, Böckler D. Reinterventions during midterm follow-up after endovascular treatment of thoracic aortic disease. J Vasc Surg. 2011;53:1528–33. doi: 10.1016/j.jvs.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 6.De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1881–9. doi: 10.1056/NEJMoa0909499. [DOI] [PubMed] [Google Scholar]
- 7.Goodney PP, Travis L, Lucas FL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the medicare population. Circulation. 2011;124:2661–9. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes GC, Daneshmand MA, Swaminathan M, et al. “Real world” thoracic endografting: results with the Gore TAG device 2 years following U.S. FDA approval. Ann Thorac Surg. 2008;86:1530–7. doi: 10.1016/j.athoracsur.2008.07.089. discussion 1537–8. [DOI] [PubMed] [Google Scholar]
- 9.Hughes GC, Lee SM, Daneshmand MA, et al. Endovascular repair of descending thoracic aneurysms: results with “on-label” application in the post Food and Drug Administration approval era. Ann Thorac Surg. 2010;90:83–9. doi: 10.1016/j.athoracsur.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes GC, McCann RL. Hybrid thoracoabdominal aortic aneurysm repair: concomitant visceral revascularization and endovascular aneurysm exclusion. Semin Thorac Cardiovasc Surg. 2009;21:355–62. doi: 10.1053/j.semtcvs.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention [Accessed: Dec 28, 2011];National vital statistics reports. Deaths: final data for 2008. http://www.cdc.gov/nchs/nvss.htm. [PubMed]
- 12.Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52:1022–33. 1033.e15. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Almeida RM, Leal JC, Saadi EK, et al. Thoracic endovascular aortic repair—a Brazilian experience in 255 patients over a period of 112 months. Interact Cardiovasc Thorac Surg. 2009;8:524–8. doi: 10.1510/icvts.2008.192062. [DOI] [PubMed] [Google Scholar]
- 14.Desai ND, Pochettino A, Szeto WY, et al. Thoracic endovascular aortic repair: evolution of therapy, patterns of use, and results in a 10-year experience. J Thorac Cardiovasc Surg. 2011;142:587–94. doi: 10.1016/j.jtcvs.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Estrera AL, Miller CC, III, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80:1290–6. doi: 10.1016/j.athoracsur.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg. 2011;212:569–79. doi: 10.1016/j.jamcollsurg.2010.12.041. discussion 579–81. [DOI] [PubMed] [Google Scholar]
- 17.Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg. 2007;83:S856–61. doi: 10.1016/j.athoracsur.2006.10.096. discussion S890–2. [DOI] [PubMed] [Google Scholar]
- 18.Fehrenbacher JW, Siderys H, Terry C, Kuhn J, Corvera JS. Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S154–60. doi: 10.1016/j.jtcvs.2010.08.054. discussion S185–90. [DOI] [PubMed] [Google Scholar]
- 19.Malenka DJ, Leavitt BJ, Hearne MJ, et al. Comparing long-term survival of patients with multivessel coronary disease after CABG or PCI: analysis of BARI-like patients in northern New England. Circulation. 2005;112(9 Suppl):I371–6. doi: 10.1161/CIRCULATIONAHA.104.526392. [DOI] [PubMed] [Google Scholar]
- 20.Shah AA, Craig DM, Andersen ND, et al. Risk factors for one-year mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.05.005. in press. [DOI] [PubMed] [Google Scholar]