Abstract
Previously, we have pioneered Drosophila melanogaster as a reductionist model to show that 1-octen-3-ol, a musty-smelling volatile compound emitted by fungi and other organisms, causes loss of dopaminergic neurons and Parkinson’s disease-like symptoms in flies. Using our in vivo Drosophila system, the modulatory roles of important signaling pathways—JNK, Akt and the caspase-3-dependent apoptotic pathway were investigated in the context of 1-octen-3-ol-induced dopamine neurotoxicity. When heterozygous flies carrying mutant alleles for these proteins were exposed to 0.5 ppm of 1-octen-3-ol, they had shorter survival times than wild-type Drosophila. The overexpressed levels of wild-type JNK and Akt, (UAS-bsk and UAS-Akt) with TH-GAL4 and elav-GAL4 drivers improved the survival duration of exposed flies compared with controls. Thus, we found that Akt and JNK both protect against loss of dopamine activity associated with 1-octen-3-ol exposure, indicating the pro-survival role of these signaling pathways. Further, 1-octen-3-ol exposure was associated with activation of caspase 3, a hallmark for apoptosis.
Keywords: 1-Octen-3-ol, Dopamine, Drosophila melanogaster, Parkinson’s disease, Volatile organic compounds (VOCs)
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by loss of dopaminergic neurons and concomitant clinical features that include resting tremor, postural instability and rigidity, and cognitive problems. It is the second most common chronic neurodegenerative disease (Ebadi and Pfeifer 2005). Approximately 5–10 % of PD has a genetic etiology, but most cases are sporadic and of unknown cause. Nevertheless, there is considerable evidence that exposure to a variety of environmental agents may contribute to death of dopaminergic neurons in PD (Shaw and Höglinger 2008; Cicchetti et al. 2009). The list of the possible environmental toxins such as paraquat, rotenone, and MPTP as risk factors for neurodegenerative diseases is increasing (Tanner 1989; McCormack et al. 2002; Di Monte 2003; Salama and Arias-Carrión 2011).
Gene–environment interactions have been known to modify the susceptibility of organisms to environmental toxins in various mammalian and non-mammalian studies. Non-mammalian models, especially Drosophila PD models, are useful for understanding the consequences of genetic variation in the signal transduction pathways associated with disease processes and to identify the genes which confer sensitivity or resistance to particular environmental agents (Chaudhuri et al. 2007; Lu and Vogel 2009; Inamdar et al. 2012a). Drosophila also serves as a powerful model for understanding pathogenic mechanisms as well as for drug discovery for PD and other chronic neurodegenerative diseases (Bilen and Bonini 2005; Nichols 2006).
We previously reported that exposure to fungal VOCs causes neurotoxicity in a Drosophila model (Inamdar et al. 2010). In particular, low concentrations of the vapor form of “mushroom alcohol” (1-octen-3-ol) selectively affects dopaminergic neurons in adult Drosophila brain and induces Parkinson’s like behavioral alteration in adult flies (Inamdar et al. 2010).
In this report, we have further exploited the genetic versatility of Drosophila as an in vivo genetic model to identify the possible signaling pathways implemented in 1-octen-3-ol-induced dopamine neurotoxicity. The pathogenesis of PD and associated cognitive dysfunction involve many complex molecular mechanisms, and several signaling pathways associated with inflammation and apoptosis are known to be altered during the various stages of PD (Zhang et al. 2011; Xu et al. 2012; Pan et al. 2013). We focused on two basic signaling pathways reported to play roles in neuronal apoptosis and survival. The first signaling pathway we targeted was the c-Jun N terminal kinases (JNK), which belongs to the mitogen-activated protein kinase family. Activation of the JNK pathway is critical for apoptosis in normal development and has been implicated with neuronal death in PD. Specifically, when PD is induced in mammalian models using MPTP and paraquat, activation of JNK via phosphorylation of c-Jun, is associated with apoptosis of dopaminergic neurons (Peng et al. 2004; Borsello and Forloni 2007; Klintworth et al. 2007). Furthermore, other signaling pathways such as PI3K and caspase 3 also are associated with dopaminergic neuron death and pharmacological agents which are able to attenuate caspase 3 levels provide neuroprotection in PD models (Xu et al. 2013). Caspase-3 functions as an effector agent for apoptosis to cause neuronal death during several acute and neurodegenerative disorders including PD in human patients (Hartmann et al. 2001). The second signaling pathway we targeted was Akt, a serine–threonine protein kinase. Akt, also known as protein kinase B, was originally identified as an oncogene. Activated AKT is involved in the regulation of cell survival and growth (Kockel et al. 2010).
Drosophila JNK is a homolog of mammalian JNK and is encoded by the gene basket on the left arm of the second chromosome (Nüsslein-Volhard et al. 1984). The Drosophila JNK pathway includes downstream signaling components homologous to mammalian counterparts and plays important roles in morphogenesis and mobility, wound healing, and both immune and stress responses in flies (Glise et al. 1995; Stronach and Perrimon 2002). Drosophila also has evolutionarily conserved mechanisms for programmed cell death and possesses homologs for several caspases (Kumar and Doumanis 2000; Vernooy et al. 2000). Similarly, the Drosophila genome contains a single Akt1 gene encoding a protein that is 76.5 % similar to the mammalian Akt protein (Franke et al. 1994). All known components of the mammalian Akt signaling pathway are implicated in the Drosophila Akt signaling pathway and function as anti-apoptotic genes (Scanga et al. 2000). Several studies have suggested that inactivation of Akt results in apoptotic death of DA neurons (Yang et al. 2005; Timmons et al. 2009).
We first tested heterozygous loss-of-function mutant strains for basket and Akt1 against 0.5 ppm 1-octen-3-ol. The heterozygous loss-of-function mutants for bsk and Akt1 exhibit extreme sensitivity to 1-octen-3-ol. This sensitivity was reversed upon overexpression of JNK and Akt in dopaminergic neurons indicating their pro-survival function in 1-octen-3-ol-induced DA toxicity. We further measured the protein levels of JNK and Akt in head extracts and found differential effects on these signaling pathways. Upon exposure to 0.5 ppm 1-octen-3-ol, Akt, JNK and phospho- Akt levels were decreased while phospho-JNK was elevated in head extracts. Moreover, caspase-3 assay showed increased levels in 1-octen-3-ol-exposed head extracts indicating the recapitulation of mechanisms involved with pathogenesis of PD in our model upon exposure of 1-octen-3-ol. Therefore, our Drosophila model provides an exceptionally flexible system in which to screen for the relevant signaling pathways involved in 1-octen-3-ol- and other fungal VOCs-mediated toxicity.
Materials and Methods
Exposure of 1-Octen-3-ol to Flies
The chemical standard for 1-octen-3-ol (98 %) was purchased from Sigma-Aldrich and comes in liquid form. Using undiluted aliquots of the liquid form at 0.5 ppm (volume:volume) adult flies were exposed to 1-octen-3-ol using the method published in Inamdar et al. (2012b) with minor modifications. Briefly, the flies were fed on 2 % agar media supplemented with 5 % sucrose in 250 ml glass flasks with 0.5 ppm of 1-octen-3-ol. The control and experimental flasks were kept on an orbital shaker at 50 rpm for even distribution of the 1-octen-3-ol vapors.
Drosophila Strains
48 h old adult wild-type flies y1 w1118 were used for testing toxicity of 1-octen-3-ol. Mutants and transgenic flies for JNK were loss-of-function allele of the gene encoding JNK, bsk1/CyO and bsk2/CyO (Sluss et al. 1996) and y1 w1118; P{UAS-bsk.A-Y}1 (deposited by Takashi Adachi-Yamada, Kobe University at the Bloomington Stock Center [BSC]) while for Akt were ry506 P{PZ}Akt104226/TM3, ryRK Sb1 Ser1 and y1 w67c23; P{w+mCy+mDint2 = EPgy2}Akt1EY10012/TM3 Sb1 Ser1 (Bellen et al. 2004) and y1 w1118; P{w[+mC] = UAS-Akt1.Exel}2 (deposited by Park A from Exelixis Inc. CA at the BSC), respectively. Flies were reared on Ward’s Instant Drosophila medium (blue) and all experiments were performed at 25 °C. All mutants and transgenic strains were obtained from the BSC except for TH-GAL4; UAS-eGFP, and elav-GAL and UAS-eGFP which were obtained from Dr. Janis O′Donnell (University of Alabama) and Dr. Venugopal Reddy (Rutgers, The State University of NJ), respectively and were described previously (Inamdar et al. 2010).
Confocal Microscopy
The transgenic lines, TH-Gal4; UAS-eGFP; TH-GAL4; UAS-eGFP/UAS-bskWT and TH-GAL4; UAS-eGFPUAS-AktWT were used to assess the number and status of dopaminergic neurons by visualizing GFP-expressing neurons. The brains from age matched controls and 1-octen-3-ol-exposed flies were dissected and mounted on slides. Each brain was scanned to include 5–8 sections to obtain the final image as an average of all the scanned sections using a Zeiss LSM 710 confocal microscope (Oberkochen, Germany). The quantitative data on the DA neurons was calculated for the average DA neurons for each subgroup by directly visualizing the GFP-expressing neurons from the mounted control and exposed adult brains under the microscope.
Western Blot Analysis
Frozen (−80 °C) control and experimental flies that had been exposed to 0.5 ppm of 1-octen-3-ol for 24 h were vortexed to separate the heads from the bodies. The fly heads were homogenized in ice-cold lysis buffer (1 % Triton X-100, 150 mM Nacl, 50 mM Tris, 1 mM EDTA, protease inhibitor (Roche), pH 7.2). The samples were centrifuged at 14,000 rpm for 10 min at 4 °C, and the supernatant was quantified for protein concentration using a bicinchonic acid (BCA) assay kit (Pierce, Rockford, IL, USA). 50 μg (for Akt, phospho-Akt, JNK, and phospho-JNK) of protein samples from control and 1-octen-3-ol-exposed flies were loaded per lane on 4–12 % NuPAGE Novex Bis–Tris Mini Gels (Invitrogen). The separated proteins were blotted to polyvinylidene difluoride membranes (Invitrogen) upon electrophoresis. The membranes were incubated in 7.5 % nonfat milk in TTBS buffer (Tris buffered saline with 0.1 % Tween 20) for 1 h at room temperature to block nonspecific protein binding sites. The membranes were then incubated overnight at 4 °C with the following primary antibodies: anti-Akt, anti-JNK, anti-phospho-Akt, and anti-phospho-JNK (1:1000) (Cell Signaling: catalog numbers: 4691, 9252, 4054, 9251, respectively). The membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature upon washes with TTBS. Protein bands were detected by SuperSignal West Dura Extended Duration Substrate (Thermo Scientific Pierce) using the Alpha Innotech Fluorochem (San Leandro, CA) imaging system and processed in Adobe Photoshop CS5. The stripping was performed at room temperature with Pierce Stripping Buffer and re-probing with a monoclonal alpha-tubulin antibody was done to confirm equal protein concentrations in each lane.
Caspase Assay
Caspase-3 was determined according to Hossain and Richardson (2011) with some modifications. In brief, after treatment, flies brains (50–100) were homogenized with 0.2 ml Tris buffer (50 mM Tris–HCl, 1 mM EDTA, 10 mM EGTA, and 10 μM digitonin, pH 7.4). Homogenates were then centrifuged at 10,000 rpm for 15 min and supernatants were incubated with 50 mM Ac-DEVD-AMC at 37 °C for 60 min. Caspase-3 activity was then determined by measuring the levels of released 7-amino-4-methylcoumarin (AMC) using a Spectramax spectrofluorometer with excitation at 380 nm and emission at 460 nm. The activity was calculated as fluorescence units (FU)/mg protein/h and expressed as percentage relative to control.
Statistical Analysis
All the graphs were plotted using Prism GraphPad 5 and data were analyzed using one-tail Student’s t test or one-way ANOVA (Dunnett’s post-test or Bonferroni’s correction post-test) wherever appropriate. The details of the analyses are described in the figure legends.
Results
1-Octen-ol Exposure Truncates Survival Span of JNK and Akt Mutants Flies
We recently reported the regulatory role of JNK and Akt signal transduction pathways in dopaminergic neurodegeneration employing our in vivo Drosophila PD model (Inamdar et al. 2012a). Our earlier data (Inamdar et al. 2010) on 1-octen-3-ol-induced dopaminergic neurodegeneration led us to assess the role of JNK and Akt in 1-octen-3-ol-mediated toxicity in the same model system. Flies carrying heterozygous mutant alleles for JNK, bsk1 and bsk2, and for Akt, Akt1 and Akt2 were exposed to 0.5 ppm of 1-octen-3-ol and their survival time was compared with similarly exposed wild-type flies. The average survival spans were about 14 and 12 days for bsk and Akt mutants, respectively; these survival times were significantly lower than the 18-day-long-survival span observed for exposed wild-type flies (Fig. 1).
Fig. 1.
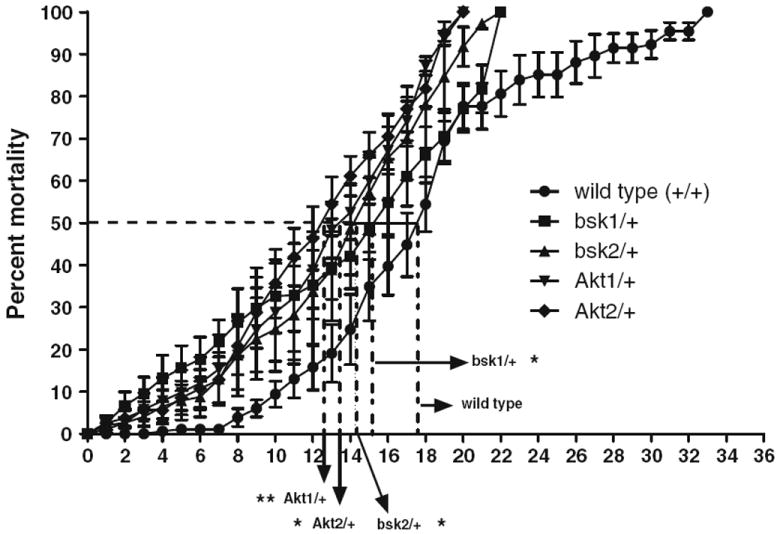
Exposure of 1-octen-3-ol to JNK and Akt mutants causes truncation of life span. Flies carrying two heterozygous alleles of JNK and Akt: bsk1, bsk2; and Akt1, Akt2 were exposed to 0.5 ppm of 1-octen-3-ol, and percentage mortality was calculated until all flies were dead in each group. The average times to obtain 50 % mortality for the respective groups group are 12.4, 13.2, 14.0, 14.9, and 17.9 days for Akt1, Akt2, bsk1, bsk2, and wild-type flies, respectively. Error bars represent the standard error of mean and *represents a significant difference between wild-type flies, and each JNK and Akt mutant strain calculated at a point when 50 % mortality was achieved, where *P < 0.05 and **P < 0.005. (N = 70–100 for each group and the data was collected from three independent experiments)
Overexpression of Wild-Type JNK and Akt Pan-Neuronally and in Dopaminergic Neurons Prevents 1-Octen-3-ol-induced Truncation of Survival Duration
The sensitivity of loss-of-function JNK and Akt heterozygous mutants toward 1-octen-3-ol suggests the protective function of wild-type JNK and Akt genes. In order to evaluate the protective function of JNK and Akt, we hypothesized that overexpression of JNK and Akt would reverse the 1-octen-3-ol-mediated toxicity. In order to test this hypothesis, we used the transgenic lines for JNK and Akt, UAS-bskWT and UAS-AktWT and overexpressed them pan-neuronally and in dopaminergic neurons using the elav-GAL4 and TH-GAL4 transgenic drivers, respectively. We then exposed these crossed transgenic lines to 0.5 ppm of 1-octen-3-ol and compared the survival rates with control flies. The overexpression of JNK and Akt pan-neuronally and in dopaminergic neurons lead to an increase in survival duration by about 5 and 7 days, respectively (Fig. 2).
Fig. 2.
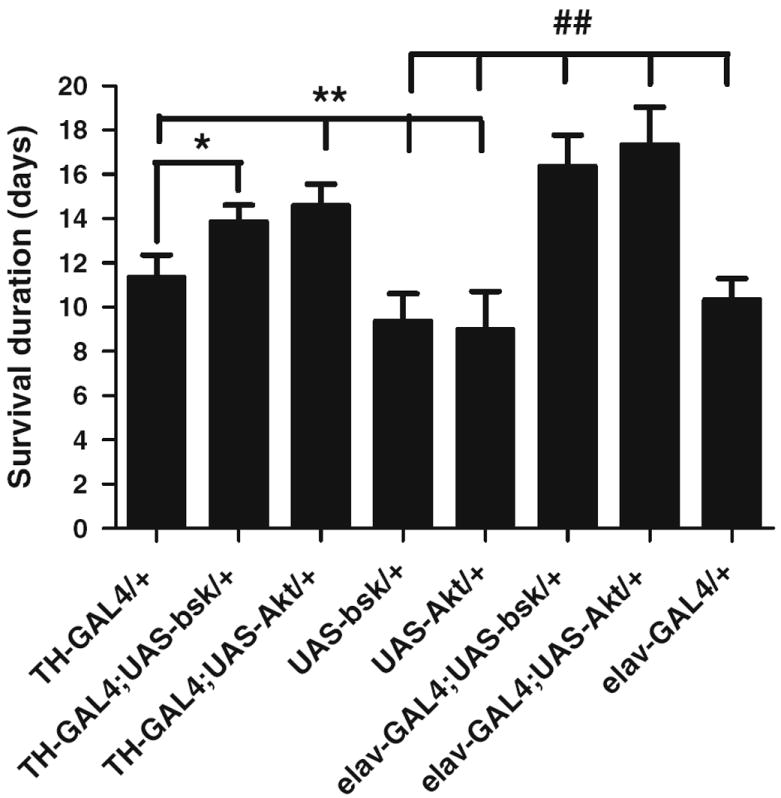
JNK and Akt function as pro-survival genes and prevent1-octen-3-ol-induced truncation of survival duration. The overexpression of wild-type UAS-bsk and UAS-Akt with TH-GAL4 and elav-GAL4 drivers improved the survival duration when compared to control flies. Error bars represent the standard error of mean, and *and #represent a significant difference between wild-type flies, TH-GAL4 and elav-GAL4, and other transgenic JNK and Akt strains as shown in the graph where *P < 0.05 and **/##P < 0.005. (N = 120 for each group and the data were collected from two independent experiments)
Overexpression of Wild-Type JNK and Akt in Dopaminergic Neurons Delays 1-Octen-3-ol-induced Loss of Dopaminergic Neurons
The improvement in the survival span of flies when wild-type JNK and Akt were overexpressed in dopaminergic neurons led us to evaluate the status of dopaminergic neurons in these transgenic flies. The transgenic flies: TH-GAL4; UAS-eGFP/UAS-bskWT, TH-GAL4; UAS-eGFP/UAS-AktWT and TH-GAL4; UAS-eGFP were exposed to 0.5 ppm of 1-octen-3-ol for 24 h. The number and morphology of dopaminergic neurons were restored in dissected adult brains of TH-GAL4; UAS-eGFP/UAS-bskWT, TH-GAL4; and UAS-eGFP/UAS-AktWT flies when compared with those of TH-GAL4; UAS-eGFP flies suggesting a protective function of JNK and Akt in dopaminergic neurons (Fig. 3).
Fig. 3.
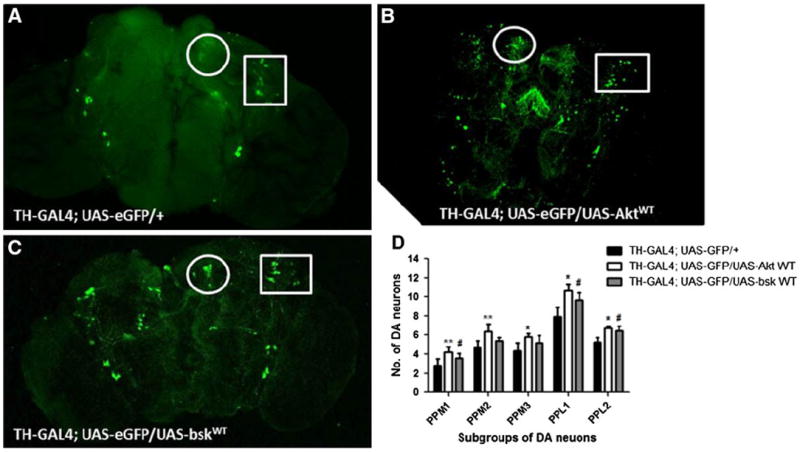
JNK and Akt function as protective genes and prevent 1-octen-3-ol-induced loss of dopaminergic neurons. The exposure of TH-GAL4; UAS-eGFP adult brains to 0.5 ppm of 1-octen-3-ol for 24 h led to a decrease of GFP-expressing TH neurons (a) while TH-GAL4; UAS-eGFP/UAS-AktWT and TH-GAL4; UAS-eGFP/UAS-bskWT (b, c) prevented the decrease in DA neurons. The circle and rectangle in each image show the morphology and number of PPM2 and PPL1 subgroups of DA neurons, respectively. d The scoring for average number of subgroups of dopaminergic neurons for 0.5 ppm 1-octen-3-ol exposed to TH-GAL4; UAS-eGFP, TH-GAL4; UAS-eGFP/UAS-AktWT, and TH-GAL4; UAS-eGFP/UAS-bskWT adult brains (n = 7–10). Error bars represent the standard error of mean where *represents a significant difference between TH-GAL4; UAS-eGFP, and TH-GAL4; UAS-eGFP/UAS-AktWT while #represents a significant difference between TH-GAL4; UAS-eGFP, and TH-GAL4; UAS-eGFP/UAS-bskWT-exposed brains where */#P < 0.05, **P < 0.005
1-Octen-3-ol Induces Alteration in Protein Expressions of JNK, Akt, Phospho-JNK, and Phospho-Akt
To assess the effect of 1-octen-3-ol on the protein levels of JNK and Akt in adult brain, we performed Western Blot analysis. The quantification of the ratio of expression of the phosphorylated and the un-phosphorylated form was performed. Interestingly, the head extract from wild-type flies exposed for 24 h to 0.5 ppm of 1-octen-3-ol demonstrated an increase in the ratio p-JNK/JNK levels by 32 % while a decrease in ratio of p-Akt/Akt protein levels by 19 % was observed (Fig. 4a, b, c).
Fig. 4.
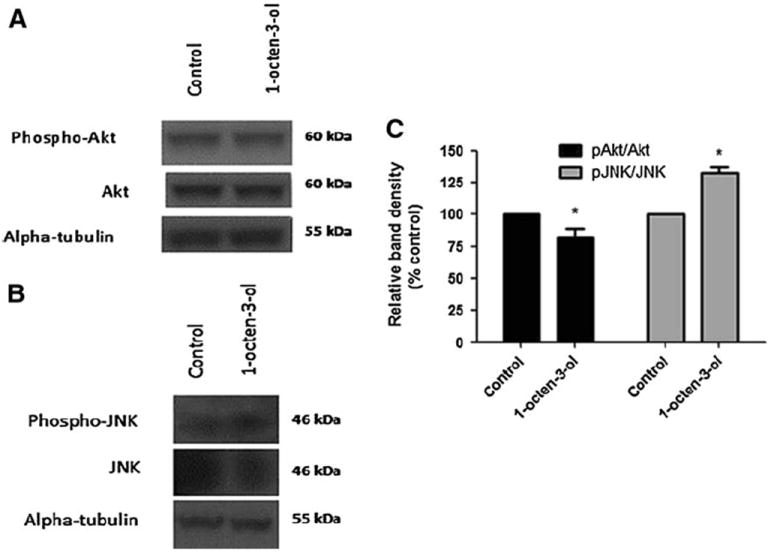
Exposure to 1-octen-3-ol causes alteration in phosphorylation activity levels ofAkt and JNK. The western blots of head extracts of adult flies exposed to 0.5 ppm of 1-octen-3-ol for 24 h show a decrease in p-Akt and Akt protein expression levels (a), and an increase in p-JNK, but a decrease in JNK protein levels (b). c The quantification of ratio of p-Akt/Akt and p-JNK/JNK for unexposed (control) and exposed flies shows a significant decrease in protein levels for phospho-Akt and an increase in phospho-JNK. Data were calculated as relative pixel density from three independent samples. Error bars represent the standard error of mean, and *represents a significant difference between control and 1-octen-3-ol-exposed brains where *P < 0.05
Exposure to 1-Octen-3-ol Increases Caspase-3 Activity in Drosophila Head Extracts
Exposure to 1-octen-3-ol causes a significant decrease in the survival duration. To determine if exposure to 1-octen-3-ol caused activation of an apoptotic pathway, we assessed the activity of caspase-3 in control and 1-octen-3-ol-exposed head extracts. Upon 24-h exposure to 0.5 ppm of 1-octen-3-ol, there was a 33 % increase in the caspase-3 activity (Fig. 5).
Fig. 5.
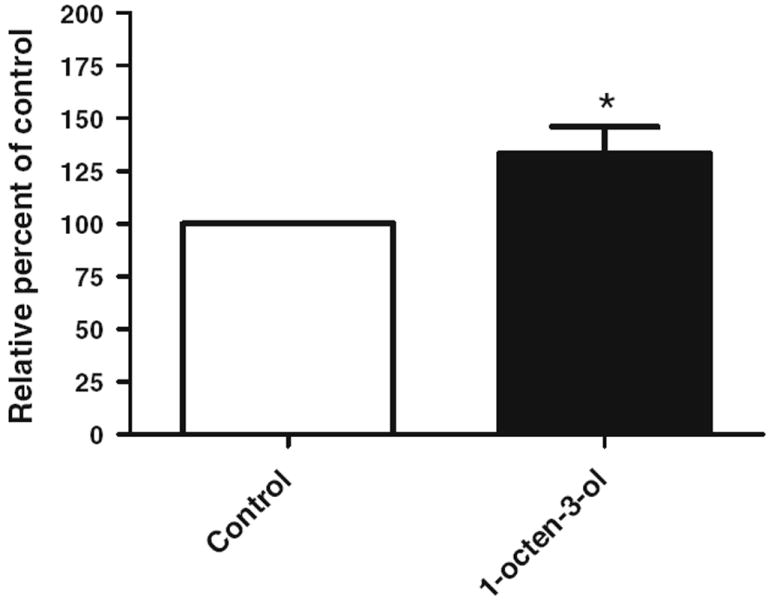
Exposure to 1-octen-3-ol increases caspase-3 activity. 1-octen-3-ol exposure for 24 h led to a 33 % increase in caspase 3 activity in head extracts. Error bars represent the standard error of mean, and *represents a significant difference between control and 1-octen-3-ol exposed groups, where *P < 0.05. (N = 200 flies for each group and data collected from two independent assays)
Discussion
Epidemiological studies on the impact of damp indoor environments on human health have found that exposure to molds and their metabolites may be correlated with a variety of neurological and neuropsychological symptoms (Kilburn 2003, 2004; Empting 2009). The neurological symptoms include headache, difficulties with concentration, movement disorders, delirium, dementia, and disorders of balance and coordination. Data from other epidemiological studies have led to the hypothesis that fungal volatile organic compounds may be responsible for some of the pathological impact of exposure to moldcontaminated indoor environments (Mølhave 2009). In the context of these concerns about the health impact of molds and their metabolites, we have pioneered the use of Drosophila melanogaster as an in vivo and in vitro model for studying the possible neurotoxicity of fungal VOCs (Inamdar et al. 2010; Inamdar et al. 2012b).
Fungi produce numerous, low molecular weight volatile compounds, one of the most abundant of which is 1-octen-3-ol or “mushroom alcohol” which is responsible for much of the distinctive odor of mushrooms (Combet et al. 2006). It is produced as a break down product of linoleic acid by fungi and other organisms (Wurzenberger and Grosch 1984); is used commercially in food technology as a flavoring agent and as an ingredient of many perfumes (Fraatz and Zorn 2010; Bennett et al. 2012); and functions as a semiochemical in attracting insects (Luntz 2003; Hooper and Pickett 2004). In addition, our earlier studies have shown that low concentrations (1–3 ppm) of 1-octen-3-ol and certain other eight carbon hydrocarbons function as neurotoxicants in Drosophila melanogaster and cause Parkinsonian-like symptoms and selective loss of dopaminergic neurons (Inamdar et al. 2010).
In this report, we used genetic and expression studies to better understand the role of JNK and Akt signal transduction pathways in 1-octen-3-ol-induced dopaminergic neurodegeneration. Mammalian PD models have identified the pro-apoptotic function of JNK. In some studies, activation of JNK via phosphorylation of c-Jun is associated with apoptosis of dopaminergic neurons, while in other cases, the presence of neurotoxins causes an increase in superoxide and related reactive oxygen species, subsequently activating JNK, and thereby triggering up-regulation of caspase-3 and contributing to the apoptotic response (Peng et al. 2004; Borsello and Forloni 2007). Pharmacological and genetic inhibition of JNK signaling pathways alleviate Parkinson’s symptoms in experimental models and improve survival of the dopaminergic neurons (Wang et al. 2003; Jimenez-Del-Rio et al. 2008). Moreover, Cha et al. (2005) detected the up-regulation of JNK in loss-of-function of parkin mutants.
The Drosophila JNK signaling pathway has been well studied, and is evolutionarily conserved (Davis 2000; Chang and Karin 2001). Drosophila JNK plays crucial roles in morphogenesis and mobility, immune response, wound healing, and stress response in flies. In Drosophila, the functional roles of JNK have been studied in the context of neuronal connectivity and in response to oxidative and pathogenic stress. JNK is essential for longevity and combating paraquat-induced oxidative stress in flies (Wang et al. 2003; Inamdar et al. 2012a).
In this report, we found that JNK serves a specific prosurvival role in Drosophila against 1-octen-3-ol-induced loss of dopaminergic neurons. The loss-of-function heterozygous JNK mutants showed greater sensitivity to 0.5 ppm 1-octen-3-ol. In contrast, the overexpression of wild-type JNK, pan-neuronally or in dopaminergic neurons, was able to extend the survival span and delayed 1-octen-3-ol-mediated loss of dopaminergic neurons. Western blot analysis of head extracts showed the activation of phospho-JNK suggesting the up-regulating of JNK signaling by 1-octen-3-ol. Our earlier studies demonstrated that exposure of 1-octen-3-ol causes an increase in the lipid peroxidation levels in head extracts suggesting that 1-octen-3-ol exposure potentiates induction of reactive oxygen species (Inamdar et al. 2010). Furthermore, up-regulation of caspase 3 was detected in the head extracts of 1-octen-3-ol-exposed flies thereby indicating the role of programmed cell death-related pathways in 1-octen-3-ol-mediated neurotoxicity. The activation of caspase 3 is associated with dopamine neurotoxicity in toxic models of PD (Turmel et al. 2001; Ramachandiran et al. 2007).
Akt is a serine/threonine protein kinase acting as a cellular homolog of the viral oncogene v-Akt. In mammals, there are three known isoforms of the Akt kinase, Akt1, Akt2, and Akt3, each activated by various growth and survival factors. In general, Akt promotes cell survival through various distinct pathways and also has a role in regulating cell growth proliferation, migration, glucose metabolism, transcription, protein synthesis, and angiogenesis (Brazil and Hemmings 2001). The Drosophila genome contains a single Akt1 gene encoding a protein that is 76.5 % similar to mammalian Akt protein (Franke et al. 1994). There is compelling evidence supporting dysregulation of the mammalian Akt signaling pathway in genetic and toxin models for PD (Yang et al. 2005; Timmons et al. 2009; Inamdar et al. 2012a).
In this report, we showed that exposure to 1-octen-3-ol leads to a decrease in phospho-Akt indicating that 1-octen-3-ol exposure is associated with inhibition of the Akt signaling pathway. Furthermore, we showed that transgenic overexpression of Akt pan-neuronally, as well as in dopaminergic neurons, rescued 1-octen-3-ol-mediated loss of dopaminergic neurons. These data support a pro-survival function for Akt in 1-octen-3-ol-mediated neurotoxicity.
Drosophila melanogaster, long a powerful model for genetic analysis, has emerged as a valuable model for the study of Alzheimer’s disease, PD, and other neurodevelopment diseases (Rand 2010). In particular, toxins-induced Drosophila modeling has been shown to successfully recapitulate the pathophysiology of PD and to enhance our understanding of the pathogenic mechanism of PD (Coulom and Birman 2004; Bayersdorfer et al. 2010; Whitworth 2011). Toxicity can be measured by end points such as survival duration and behavioral aberrations. Using the wealth of available genetic resources, appropriate biochemical assays and GFP-enabled imaging technologies, the fly system can be used to dissect the molecular interactions of associated signaling pathways underlying neurodevelopmental vulnerabilities. We here examined the functional role of JNK and Akt in 1-octen-3-ol-mediated neurotoxicity and show that flies are well adapted for studying the signaling pathways that impact neurotoxicity.
In summary, we have explored the modulatory function of kinase-related signal transduction pathways on 1-octen-3-ol-mediated toxicity in flies. Our study demonstrates that both JNK and Akt signaling pathways play important roles in 1-octen-3-ol-mediated dopaminergic toxicity. JNK and Akt act as pro-survival pathways for dopamine neuronal protection against 1-octen-3-ol. Overall, we found that exposure to 1-octen-3-ol activates the JNK and caspase-3 dependent signaling pathways while inhibiting Akt in Drosophila head extracts. We advocate Drosophila melanogaster as an inexpensive, reliable model system to provide both a screening procedure for assessing numerous chemicals for their neurotoxicity as well as an experimental system that can be used to dissect the impact of neurotoxins on signaling pathways.
Acknowledgments
This study was supported by the Rutgers University Research Funds. The authors are thankful to Richard Hung, Samantha Lee, Shannon Morath, and Sally Padhi for helpful discussions concerning fungal volatile organic compounds, and to Priya Sudheer for her help in maintaining the Drosophila stocks.
Contributor Information
Arati A. Inamdar, Email: inamdar@rci.rutgers.edu, Department of Plant Biology and Pathology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Prakash Masurekar, Department of Plant Biology and Pathology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Muhammad Hossain, Environmental and Occupational Health Sciences Institute, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA.
Jason R. Richardson, Environmental and Occupational Health Sciences Institute, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA
Joan W. Bennett, Department of Plant Biology and Pathology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA
References
- Bayersdorfer F, Voigt A, Schneuwly S, Botella JA. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol Dis. 2010;40(1):113–119. doi: 10.1016/j.nbd.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC. The BDGP gene disruption project: single transposon insertions associated with 40 % of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JW, Hung R, Lee S, Padhi S. Fungal and bacterial volatile organic compounds: an overview and their role as ecological signaling agents. Mycota. 2012;9:373–393. [Google Scholar]
- Bilen J, Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Cha GH, Kim S, Park J, Lee E, Kim M, Lee SB, Kim JM, Chung J, Cho KS. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci USA. 2005;102:10345–10350. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM. Interaction of genetic and environmental factors in a Drosophila Parkinsonism model. J Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Combet E, Henderson J, Eastwood DC, Burton KS. Eight-carbon volatiles in mushrooms and fungi: properties, analysis, and biosynthesis. Mycoscience. 2006;47:317–326. [Google Scholar]
- Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci. 2004;24(48):10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson’s disease: Is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Pfeifer R. Parkinson’s disease. CRC Press; Boca Raton: 2005. [Google Scholar]
- Empting LD. Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health. 2009;25:577–581. doi: 10.1177/0748233709348393. [DOI] [PubMed] [Google Scholar]
- Fraatz MA, Zorn H. Fungal flavours. In: Hofrichter M, editor. The Mycota X: industrial applications. 2. Springer-Verlag; Berlin: 2010. pp. 249–264. [Google Scholar]
- Franke TF, Tartof KD, Tsichlis PN. The SH2-like Akt homology (AH) domain of c-akt is present in multiple copies in the genome of vertebrate and invertebrate eukaryotes. Cloning and characterization of the Drosophila melanogaster c-akt homolog Dakt1. Oncogene. 1994;9:141–148. [PubMed] [Google Scholar]
- Glise B, Bourbon H, Noselli S. Hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Troadec JD, Hunot S, Kikly K, Faucheux BA, Mouatt-Prigent A, Ruberg M, Agid Y, Hirsch EC. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson’s disease, but pathway inhibition results in neuronal necrosis. J Neurosci. 2001;21:2247–2255. doi: 10.1523/JNEUROSCI.21-07-02247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AM, Pickett JA. Semiochemistry. In: Atwood JL, Steed JW, editors. Encyclopedia of supramolecular chemistry. Vol. 2. Marcel Dekker; New York: 2004. pp. 1270–1277. [Google Scholar]
- Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci. 2011;122:512–525. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar AA, Masurekar P, Bennett JW. Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. Toxicol Sci. 2010;117:418–426. doi: 10.1093/toxsci/kfq222. [DOI] [PubMed] [Google Scholar]
- Inamdar AA, Chaudhuri A, O’Donnell J. The protective effect of minocycline in a paraquat-induced Parkinson’s disease model in Drosophila is modified in altered genetic backgrounds Parkinson’s disease. Parkinsons Dis. 2012a;2012:938528. doi: 10.1155/2012/938528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar AA, Zaman T, Morath SU, Pu DC, Bennett JW. Drosophila melanogaster as a model to characterize fungal volatile organic compounds. Environ Toxicol. 2012b doi: 10.1002/tox.21825. [DOI] [PubMed] [Google Scholar]
- Jimenez-Del-Rio M, Daza-Restrepo A, Velez-Pardo C. The cannabinoid CP55, 940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: implications in Parkinson’s disease. Neurosci Res. 2008;61:404–411. doi: 10.1016/j.neures.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Kilburn KH. Indoor mold exposure associated with neurobehavioral and pulmonary impairment: a preliminary report. Arch Environ Health. 2003;58:390–398. doi: 10.1080/00039896.2003.11879139. [DOI] [PubMed] [Google Scholar]
- Kilburn KH. Role of molds and mycotoxins in being sick in buildings: neurobehavioral and pulmonary impairment. Adv Appl Microbiol. 2004;55:339–359. doi: 10.1016/S0065-2164(04)55013-X. [DOI] [PubMed] [Google Scholar]
- Klintworth H, Newhouse K, Li T, Choi WS, Faigle R, Xia Z. Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat-and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 2007;97:149–162. doi: 10.1093/toxsci/kfm029. [DOI] [PubMed] [Google Scholar]
- Kockel L, Kerr KS, Milnick M, Bruckner K, Hebrok M, Perrion N. Dynamic switch of negative feedback regulation in Drosophila AKT-TOR signaling. PLoS Genet. 2010;6:1–17. doi: 10.1371/journal.pgen.1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Doumanis J. The fly caspases. Cell Death Differ. 2000;7:1039–1044. doi: 10.1038/sj.cdd.4400756. [DOI] [PubMed] [Google Scholar]
- Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz AJ. Arthropod semiochemicals: mosquitoes, midges and sealice. Biochem Soc Trans. 2003;31:128–133. doi: 10.1042/bst0310128. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Coryslechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Mølhave L. Volatile organic compounds and the sick building syndrome. In: Lippmann M, editor. Toxicants: human exposures and their health effects. 3. Wiley-Interscience; New York: 2009. pp. 241–256. [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux’s Arch Dev Biol. 1984;193:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- Pan M, Gao H, Long L, Xu Y, Liu M, Zou J, Wu A, Wei X, Chen X, Tang B, Wang Q. Serum uric acid in patients with Parkinson’s disease and vascular parkinsonism: a cross-sectional study. NeuroImmunoModulation. 2013;20:19–28. doi: 10.1159/000342483. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XA, Stevenson FF, Hsu M, Anderson JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95(1):163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- Rand MD. Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol. 2010;32:74–83. doi: 10.1016/j.ntt.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, Arias-Carrión O. Natural toxins implicated in the development of Parkinson’s disease. Ther Adv Neurol Disord. 2011;4:361–373. doi: 10.1177/1756285611413004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga SE, Ruel L, Binari RC, Snow B, Stambolic V, Bouchard D, Peters M, Calvieri B, Mak TW, Woodgett JR, Manoukian AS. The conserved PI3′K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene. 2000;19:3971–3977. doi: 10.1038/sj.onc.1203739. [DOI] [PubMed] [Google Scholar]
- Shaw CA, Höglinger GU. Neurodegenerative diseases: neurotoxins as sufficient etiologic agents? Neuromolecular Med. 2008;10:1–9. doi: 10.1007/s12017-007-8016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss HK, Han Z, Barrett T, Goberdhan DC, Wilson C, Davis RJ, Ip YT. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- Stronach B, Perrimon N. Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev. 2002;16:377–387. doi: 10.1101/gad.953002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM. The role of environmental toxins in the etiology of Parkinson’s disease. Trends Neurosci. 1989;12:49–54. doi: 10.1016/0166-2236(89)90135-5. [DOI] [PubMed] [Google Scholar]
- Timmons S, Coakley MF, Moloney AM, O’Neill C. Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett. 2009;467:30–35. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, Agid Y, Hirsch EC. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord. 2001;16:185–189. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- Vernooy SY, Copeland J, Ghaboosi N, Griffin EE, Yoo SJ, Hay BA. Cell death regulation in Drosophila: conservation of mechanism and unique insights. J Cell Biol. 2000;150:F69–F76. doi: 10.1083/jcb.150.2.f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ. Drosophila models of Parkinson’s disease. Adv Genet. 2011;73:1–50. doi: 10.1016/B978-0-12-380860-8.00001-X. [DOI] [PubMed] [Google Scholar]
- Wurzenberger M, Grosch W. The formation of 1-octen-3-ol from the 10-hydroperoxide isomer of linoleic acid by a hydroperoxide lyase in mushrooms (Psalliota bispora) Biochim Biophys Acta. 1984;794:25–30. [Google Scholar]
- Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y, Wang Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2012;97:1–13. doi: 10.1016/j.pneurobio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Long L, Yan JQ, Wei L, Pan MQ, Gao HM, Zhou P, Liu M, Zhu CS, Tang BS, Wang Q. Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci Ther. 2013;19:170–177. doi: 10.1111/cns.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yan J, Xu Y, Long L, Zhu C, Chen X, Jiang Y, Yang L, Bian L, Wang Q. The combination of homocysteine and C-reactive protein predicts the outcomes of Chinese patients with Parkinson’s disease and vascular parkinsonism. PLoS ONE. 2011;6:e19333. doi: 10.1371/journal.pone.0019333. [DOI] [PMC free article] [PubMed] [Google Scholar]


