Abstract
Abnormal aggregation of α-synuclein and sustained microglial activation are important contributors to the pathogenic processes in Parkinson's disease. However, the relationship between disease-associated protein aggregation and microglia-mediated neuroinflammation remains unknown. Here, using a combination of in silico, in vitro, and in vivo approaches, we show that extracellular α-synuclein released from neuronal cells is an endogenous agonist for toll-like receptor 2 (TLR2), which activates inflammatory responses in microglia. TLR2 ligand activity of α-synuclein is conformation-sensitive; only specific types of oligomer can interact with and activate TLR2. This paracrine interaction between neuron-released oligomeric α-synuclein and TLR2 in microglia suggests that both of these proteins are novel therapeutic targets for modification of neuroinflammation in Parkinson's disease and related neurological diseases.
The role of innate immune responses of microglia in neurodegenerative processes in many central nervous system diseases has become increasingly evident1-3. Abnormal accumulation of specific protein aggregates in neurons and glia is another common pathological feature shared by most neurodegenerative diseases. Whether these two events are related, and, if so, how misfolding and aggregation of specific proteins in neurons might influence the inflammatory milieu in brain parenchyma remains unknown.
A neuronal protein, α-synuclein, has been implicated in many neurodegenerative diseases, including Parkinson's disease (PD), dementia with Lewy bodies, multiple system atrophy, and a Lewy body variant of Alzheimer's disease (AD)4. Although it is a typical cytosolic protein, a small amount of α-synuclein can be released from neurons via brefeldin A-insensitive, unconventional exocytosis5, 6. The structure of the released α-synuclein is unknown. However, there is evidence to suggest that misfolding and aggregation facilitate the release of this protein from neuronal cells5. Released α-synuclein can be transferred to neighboring neurons and astroglia, promoting formation of inclusion bodies and inducing cell death in neurons and proinflammatory responses from astroglia7, 8. In the present study, we attempted to determine the role of neuron-released α-synuclein in microglial activation, the major culprit of inflammation in brain parenchyma, and the mechanism underlying this process.
Results
Cell-released α-synuclein induces microglia activation
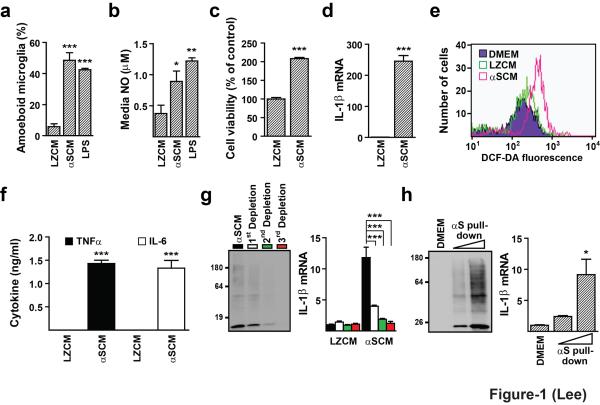
We collected culture media from differentiated SH-SY5Y cells (dSY5Y) overexpressing either human α-synuclein (αSCM) or β-galactosidase (LZCM) (Supplementary Fig. S1a). We chose LacZ gene because it is not a mammalian gene, and thus, it is unlikely to produce unwanted complications, and because among the control genes we tested, only LacZ showed expression levels higher than those of α-synuclein. Ectopic expression of these genes did not affect the viability of dSY5Y cells (Supplementary Fig. S2). The concentrations of α-synuclein in the αSCM were determined by ELISA, and the measurements resulted in an average of 1.06 ± 0.371 μg/ml (Supplementary Fig. S1b). These media were applied to primary rat microglia at a concentration of 0.1 – 5.3 μg/ml α-synuclein. Microglia treated with αSCM, but not with LZCM, underwent a series of changes indicating proinflammatory activation, including increased morphological changes to amoeboid shapes increased, production of nitric oxide and intracellular reactive oxygen species, increased proliferation, and increased production of proinflammatory cytokines at the levels of both mRNA and secreted protein (Fig. 1a-f). Contamination of adenoviral vectors in αSCM has been ruled out (Supplementary Fig. S3). Induction of cytokine production was gradually reduced by serial depletion of α-synuclein proteins from the conditioned medium (Fig. 1g), while α-synuclein proteins purified from αSCM induced cytokine production in a dose-dependent manner (Fig. 1h). From these results, we conclude that α-synuclein released from neuronal cells induces proinflammatory responses from microglia.
Figure 1.
dSY5Y-released α-synuclein activates microglia. Rat primary microglia was treated with LZCM, αSCM, or LPS (1 μg/ml, a positive control) for the indicated hours. LPS is an endotoxin that activates microglial responses that we tested, and therefore, was used as a positive control. (a) Percentage of microglial cells with amoeboid morphology (n = 6). (b) NO produced from microglia (n = 5). (c) Microglial proliferation (n = 6). (d) Relative expression of IL-1μ mRNA. Real-time PCR data were normalized with the average value of LZCM (n = 3). (e) Quantification of intracellular ROS levels using flow cytometry. This is the representative result of three independent experiments. (f) Quantification of cytokines using ELISA in the microglial culture media (n = 3). (g) Depletion of α-synuclein and microglia activation activity from αSCM. Three successive rounds of depletion were performed using an affinity resin. For cytokine induction (n = 3), the amount of α-synuclein in αSCM used was 0.1 μg/ml. (h) Cytokine induction by his-tagged α-synuclein pulled down from his-αSCM. Western blot shows different amounts of pulled-down α-synuclein used in microglia activation (n = 3). Morphology analysis (a), NO production analysis (b), proliferation assay (c), iROS production (e), and cytokine ELISA quantification (f) were performed at 24 hours post treatment. Relative mRNA expression (d,g,h) was determined at 2 hours post treatment. Morphology analysis (a), NO production analysis (b), relative mRNA expression (g,h) data were compared by one-way ANOVA. Proliferation assay (c), cytokine gene expression (d), and cytokine ELISA (f) data were analyzed using unpaired t-test. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001. “n” represents the number of independent experiments and each experiment was performed at least in triplicate.
Signaling in microglia exposed to cell-released α-synuclein
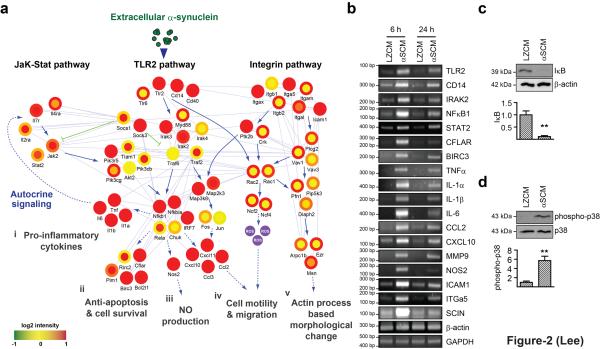
In order to gain a comprehensive understanding of the mechanism of α-synuclein-induced microglial activation, we performed transcriptome analyses using microglial cells treated with either αSCM or LZCM at two different time points, 6 hours and 24 hours. We obtained a total of 2009 differentially expressed genes (DEGs) (877 DEGs at 6 hours only, 797 DEGs at 24 hours only, and 335 DEGs common at both time points) (Supplementary Fig. S4a). KEGG pathway enrichment analyses of upregulated genes showed specific activation of the Jak-STAT signaling pathway during early responses, and leukocyte transendothelial migration, natural killer cell-mediated cytotoxicity, and regulation of actin cytoskeleton during late responses (GSE26532, Supplementary Fig. S4b, Supplementary Table S1 and S2). On the other hand, TLR signaling, cytokine receptor signaling, and other immune receptor signaling pathways were activated with high statistical significance at both early and late time points. Using the DEGs involved in these pathways in concert with protein-protein interaction data from public databases, we have constructed a model for a microglial signaling network activated by exposure to dSY5Y-released α-synuclein. According to the model, the TLR2 and cytokine receptor signaling pathways are activated early, producing various proinflammatory cytokines and chemokines. Later, cell migration and actin cytoskeleton rearrangement pathways are activated, while TLR signaling and cytokine/chemokine production are sustained (Fig. 2a). Validation of gene expression changes in these signaling pathways was provided by RT-PCR (Fig. 2b), and activation of the TLR2 downstream signaling pathway was validated by increased IκB degradation and p38 phosphorylation (Fig. 2c,d).
Figure 2.
Hypothetical signaling network of microglia activated by cell-released α-synuclein. (a) Hypothetical signaling network was constructed by using DEGs from αSCM-exposed microglia. Alterations of gene expression were detected at two different time points (Inner circle; 6 hours and Outer circle; 24 hours). (b) RT-PCR analysis of genes identified in the network. (c, d) IκB degradation (c) and phosphorylation of p38 MAP kinase (d) in microglia exposed to αSCM for 15 minutes (n = 4). All data were analyzed using unpaired t-test. Error bars represent ± s.e.m. **P < 0.01. “n” represents the number of independent experiments.
Role of Tlr2 inα-synuclein-induced microglia activation
Involvement of the TLR2 pathway is one of the striking features of the signaling network model. The role of TLRs as modulators in neurological disorders has received increasing recognition. TLR4 and its interaction with extracellular high-mobility group box-1 contribute to generation of seizures9. In animal models of stroke, TLR2 and 4 induced proinflammatory reactions resulting in aggravated tissue damage10. TLR2 and 4 have been implicated in both clearance of extracellular amyloid β (Aβ) peptide and Aβ-induced microglial activation11-14. Amyloid structure has also been suggested as crucial in triggering of TLR2-mediated inflammatory responses in innate immune cells15, 16. In a rat model, administration of TLR3 agonist into the substantia nigra (SN) induced sustained inflammation and increased susceptibility of dopaminergic neurons to oxidative stress17, suggesting that TLR modulation might have therapeutic implications for PD.
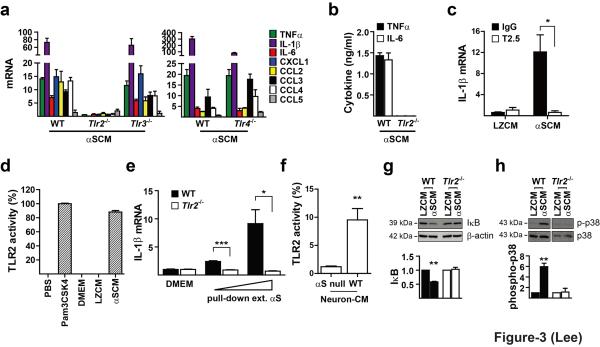
In order to verify involvement of the TLR system, we examined inflammatory responses to dSY5Y-derived α-synuclein in microglia isolated from mice deficient of Tlr2, 3, or 4 gene (Tlr2-/-, Tlr3-/-, and Tlr4-/-). Depletion of Tlr2 gene resulted in complete elimination of cytokine/chemokine gene induction upon exposure to αSCM at both mRNA and protein levels (Fig. 3a,b; Supplementary Fig. S5 and S6). Treatment with blocking antibody against TLR2 also resulted in elimination of αSCM-induced cytokine gene expression (Fig. 3c). Additionally, αSCM activated TLR2 signaling cascade in the HEK293 reporter cells (HEK-Blue-TLR2) ectopically expressing human TLR2 and CD14 (Fig. 3d). dSY5Y-released α-synuclein proteins purified from the conditioned medium also showed Tlr2-dependent cytokine production (Fig. 3e). Conditioned medium obtained from the wild type mouse primary cortical neurons also activated TLR2, whereas that obtained from α-synuclein null mice did not (Fig. 3f, Supplementary Fig. S7). Activation of TLR2 could be observed at concentrations of α-synuclein as low as 1 ng/ml in the HEK293 reporter cells (Supplementary Fig. S8), the level comparable to the levels found in the human interstitial fluid18 (Supplementary Text S1). In contrast, Tlr3 or 4 gene depletion had no effect on α-synuclein-induced gene induction of proinflammatory cytokines/chemokines (Fig. 3a). Significant reduction of κ-synuclein-induced IκB degradation and p38 phosphorylation was observed in Tlr2-/- mouse microglia (Fig. 3g,h), confirming that TLR2 mediates α-synuclein-induced signaling in microglia. These results provide validation for the signaling network model in which the TLR2 signaling pathway is specifically involved in the proinflammatory responses of microglia to α-synuclein released from cells, including neurons.
Figure 3.
Microglia activation by cell-released α-synuclein is mediated by TLR2. (a) Expression of cytokines and chemokines upon treatment of αSCM in wild type (WT), Tlr2-/-, Tlr3-/-, and Tlr4-/- mouse microglia (n = 3). (b) αSCM-induced cytokine production and release in WT and Tlr2-/- microglia (n = 3). (c) Effects of TLR2 blocking antibody (T2.5) on induction of IL-1β mRNA. Microglia were pre-incubated with either T2.5 or control IgG for 30 min before addition of αSCM or LZCM (n = 3). (d) TLR2 activity was determined in the HEK-Blue-TLR2 reporter cells. Pam3CSK4 (10 ng/ml) is a known TLR2 agonist and used as a positive control (n = 3). (e) Induction of IL-1β mRNA by different amounts of α-synuclein purified from αSCM (n = 3). (f) TLR2 activation by the endogenous α-synuclein released from mouse primary cortical neurons. Culture media from wild type and α-synuclein null mice primary neurons were treated to the HEK-Blue-TLR2 reporter cells (n = 3). (g, h) TLR2-dependent IκB degradation (g) and phosphorylation of p38 MAP kinase (h) in microglia exposed to αSCM (n = 3). Relative mRNA expressions (a,c,e) were determined at 2 hours post treatment. Cytokine ELISA (b) was performed at 6 hours post treatment. IκB degradation (g) and p38 phosphorylation (h) analyses were performed at 15 minutes post treatment. All data were analyzed using unpaired t-test. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001. “n” represents the number of independent experiments, and each experiment was performed at least in triplicate.
Interaction of cell-released α-synuclein with TLR2
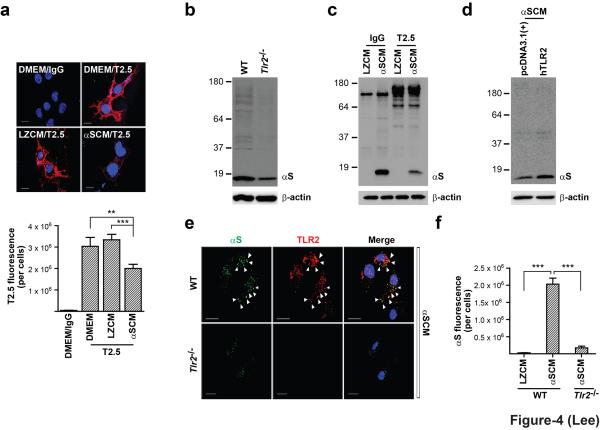
Based on functional significance of TLR2 in α-synuclein-induced microglial activation, we attempted to determine whether TLR2 is the receptor for dSY5Y-released α-synuclein. First, we examined the interaction between dSY5Y-released α-synuclein and TLR2 in the BV2 microglial cell line by assessing the competition between α-synuclein and anti-TLR2 antibody (T2.5). The cells showed robust binding of the TLR2 antibody on the cell surface (Fig. 4a; DMEM control). However, pretreatment with αSCM resulted in significantly reduced binding of the TLR2 antibody, whereas pretreatment with LZCM had little effect. This result suggests a direct interaction between dSY5Y-released α-synuclein and TLR2, which precludes the interaction of the antibody with TLR2 (Supplementary Text S2). Next, we demonstrated that internalization of externally treated dSY5Y-released α-synuclein into microglia was significantly reduced by Tlr2 gene depletion (Fig. 4b, Supplementary Fig. S9) or by co-treatment with TLR2 blocking antibody (Fig. 4c). Internalization was increased upon ectopic expression of TLR2 proteins in COS-7 cells (Fig. 4d). In addition, internalized α-synuclein was found to be co-localized with TLR2 in the same internal compartments (Fig. 4e). These results demonstrate that TLR2 is the receptor for cell-released extracellular α-synuclein, and that it is responsible not only for signaling and inflammatory responses but also for binding and internalization of this protein.
Figure 4.
Properties of TLR2 as the receptor for cell-released α-synuclein. (a) Competition between TLR2 antibody (T2.5) and dSY5Y-released α-synuclein for binding to the surface of BV-2 microglia (n = 3). Scale bars: 10 μm. (b) Uptake of α-synuclein of αSCM by wild type and Tlr2-/- mouse microglia. Cells were treated with αSCM for 30 minutes, and then analyzed. (c) Effect of a TLR2 blocking antibody on the internalization of α-synuclein of αSCM into microglia. Primary mouse microglia was pre-incubated with either TLR2 blocking antibody (T2.5) or control (IgG) for 30 minutes before addition of αSCM to the media for 2 hours. (d) Ectopic TLR2 expression enhanced uptake of α-synuclein of αSCM in COS7 cells. (e) Colocalization of dSY5Y-released α-synuclein with TLR2 in mouse microglia after internalization (top panels). Tlr2-/- mouse microglia show markedly reduced uptake (bottom panels). Scale bars: 10 μm. (f) Fluorescence intensities of internalized α-synuclein in microglia (n = 3). All data were analyzed using one-way ANOVA. Error bars represent ± s.e.m. **P < 0.01; ***P < 0.001. “n” represents the number of independent experiments (see the methods section).
Role of TLR2 in α-synuclein-induced microglia activation in vivo
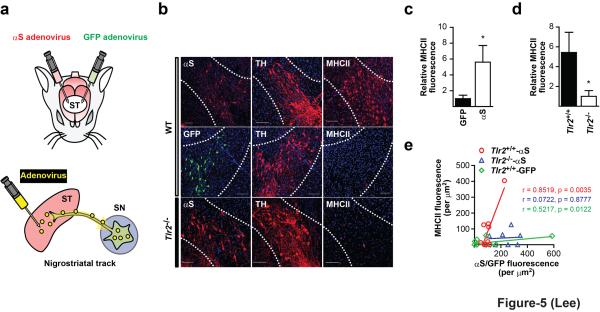
In order to validate the role of TLR2 in vivo, we induced ectopic expression of human α-synuclein in the SN of wild type or Tlr2-/- mice. Adenoviral vectors for expression of human α-synuclein and green fluorescent protein (GFP) were stereotaxically injected into either side of the putamen, respectively, from which viral vectors were transported in a retrograde manner to the cell bodies of dopaminergic neurons in the substantia nigra (SN) (Fig. 5a). The retrograde transport and expression of viral vectors was confirmed by expression of GFP in tyrosine hydroxylase (TH)-positive neuronal cell bodies in the SN (Supplementary Fig. S10). In a control experiment with the GFP vector, about 15% of nigral dopaminergic neurons expressed GFP. In the substantia nigra, all the GFP-positive cells were also TH-positive, verifying that only the dopaminergic neurons expressed human α-synuclein, while microglia did not. Expression of α-synuclein in SN neurons of wild type mice resulted in increased microglial activation, while GFP expression caused little change in microglia (Fig. 5b,c). Significant correlation was observed between the level of α-synuclein expression in SN neurons and the degree of microglial activation (r = 0.8519, P = 0.0035), while the correlation with GFP expression was insignificant (r = 0.5217, P = 0.122) (Fig. 5e). In striking contrast, expression of α-synuclein in SN neurons of Tlr2-/- mice led to significantly lowered microglial activation compared to wild type mice (Fig. 5b,d), and little correlation was observed between neuronal α-synuclein expression and microglial activation (r = 0.0722, P = 0.8777) (Fig. 5e). These results provided verification in vivo that neuronal α-synuclein can activate microglia in a paracrine manner through activation of TLR2.
Figure 5.
TLR2-dependent microglial activation by α-synuclein overexpression in nigral dopaminergic neurons. (a) Experimental scheme. (b) Immunofluorescence images of the SN (dotted lines). Scale bars: 100 μm (c) MHC II fluorescence in the SN of wild type mice that received striatal injections of viral vectors expressing GFP and α-synuclein (n = 5). (d) MHC II fluorescence in the SN of wild type and Tlr2-/- mice after striatal injections of α-synuclein vectors (n = 5). (e) Correlation between MHC II and GFP/α-synuclein fluorescence in SN (WT; n = 9, Tlr2-/-; n = 7, GFP; n = 12). MHC II fluorescence data (c,d) were analyzed using the Mann-Whitney U test. Error bars represent ± s.e.m. *P < 0.05. “n” represents the number of animals.
Cell-releasedα-synuclein is a conformation-sensitive agonist of TLR2
We then compared α-synuclein proteins released from dSY5Y cells and proteins residing in the cytosol after purification of these proteins from culture medium and cell lysate, respectively (Supplementary Fig. S11). The cytosolic α-synuclein is mostly monomeric under the denaturing gel condition, whereas the released protein preparation contains significant amounts of stable oligomers (Supplementary Fig. S11a). The released α-synuclein, but not the cytosolic protein, activated microglia in a TLR2-dependent manner (Supplementary Fig. S11b), and consistently, only the released α-synuclein resulted in TLR2-dependent reporter gene expression, but not the TLR4-reporter gene expression, in a dose-dependent manner in HEK293 reporter cell systems (Supplementary Fig. S11c,d). These results suggest that α-synuclein proteins released from cells possess specific modification(s) and/or type(s) of assembly that are essential for becoming the agonist for TLR2.
Previous studies have shown microglial activation by various forms of bacterially expressed recombinant α-synuclein proteins19-22. In order to assess the molecular forms of α-synuclein that act as the ligand of TLR2, we tested the following forms of recombinant α-synuclein proteins: monomers, fibrils, oligomers induced by incubation with 4-hydroxy-2-nonenal, and mixture of aggregates formed from nitrated α-synuclein (Supplementary Fig. S12a). All of the treated forms of recombinant α-synuclein proteins showed much less potency (>30-fold) in cytokine gene induction than αSCM containing a similar quantity of α-synuclein (Supplementary Fig. S12b). More important, recombinant α-synuclein proteins showed the same degree of cytokine gene induction in Tlr2-/- mouse microglia as that observed in wild type microglia, suggesting that microglial responses triggered by these forms of recombinant α-synuclein proteins do not involve activation of TLR2. Consistently, recombinant α-synuclein fibrils did not require TLR2 for internalization into microglia (Supplementary Fig. S13a), nor did they colocalize with TLR2 after internalization (Supplementary Fig. S13b).
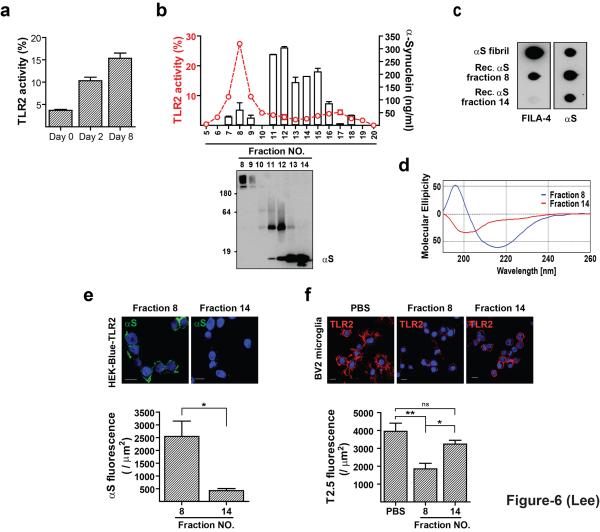
During the fibrillation, various quasi-stable oligomeric intermediates appear. To assess the role of these oligomers in TLR2 activation, we took samples during the oligomerization of recombinant α-synuclein and evaluated the ability to activate TLR2 in the HEK293 reporter cell system. TLR2 activation was increased over time as the protein aged, but before the appearance of fibrils (Fig. 6a, Supplementary Fig. S14), suggesting that oligomerization was required for α-synuclein to become the agonist for TLR2. To directly address the role of oligomerization, the aged α-synuclein preparation was subjected to the size exclusion chromatography (SEC). ELISA and western blotting clearly showed separation of oligomer, dimer, and monomer (Fig. 6b). TLR2 activation was observed only in the oligomer fractions (fraction 8 has the highest activity), but not in the dimer or monomer fractions (Fig. 6b). Depletion of α-synuclein from the fraction 8 resulted in elimination of TLR2 activation (Supplementary Fig. S15). Oligomers in the fraction 8 were stable in the denaturing gel (Fig. 6b), were efficiently recognized by Fila-4 antibody (specific for β-sheet-rich α-synuclein oligomers and fibrils23) (Fig. 6c), and showed a circular dichroism spectrum typical of β-sheet-rich proteins (Fig. 6d). Consistent with the reporter assay data, α-synuclein oligomers in fraction 8 interacted with the HEK-Blue-TLR2 cells, whereas the monomers in fraction 14 did not (Fig. 6e). Furthermore, oligomers in fraction 8, but not the monomers in fraction 14, efficiently competed with the TLR2 antibody for binding to the microglial cell surface receptor (Fig. 6f).
Figure 6.
Conformation-sensitive agonist activity of α-synuclein for TLR2. (a) Development of TLR2 agonist activity with endotoxin-free recombinant α-synuclein by in vitro incubation (n = 3). (b) Separation of “aged” recombinant α-synuclein by SEC. The line trace and the bars indicate TLR2 activity and the α-synuclein levels, respectively. (c) Dot blot analysis of fractions from b. (d) CD spectra of fractions 8 and 14 from b. (e) Binding of pure α-synuclein oligomers (fraction 8) on the surface of HEK293 cells expressing human TLR2/CD14 (n = 3). (f) Competition between TLR2 antibody (T2.5) and pure preparation of oligomeric α-synuclein for the binding to the surface of BV-2 microglia (n = 3). Binding data of α-synuclein oligomers (e) were analyzed by unpaired t-test. TLR2 fluorescence data (f) were analyzed using one-way ANOVA. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01. “n” represents the number of independent experiments (see the methods section).
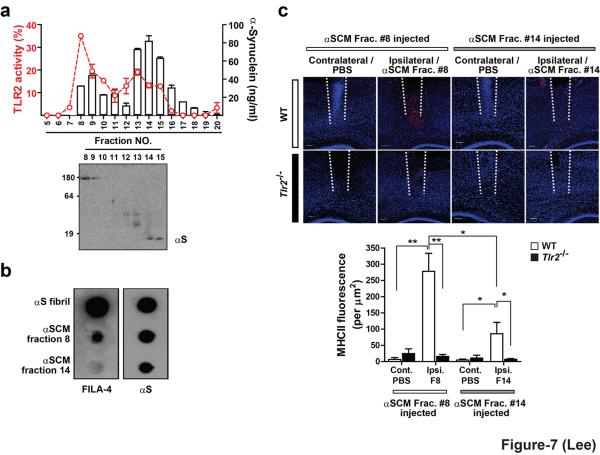
We then asked whether TLR2 activation by dSY5Y-released α-synuclein is mediated by oligomeric forms. Proteins in αSCM were separated with SEC, and each fraction was subjected to α-synuclein ELISA, western blotting, and TLR2 activation assay in the reporter cell system. The highest activity for TLR2 activation was present in the fraction 8, suggesting that dSY5Y-released α-synuclein oligomers are the effective agonist for TLR2 (Fig. 7a). In contrast to recombinant monomers and dimers, dSY5Y-released α-synuclein monomers and dimmers were capable of TLR2 activation, although their activities were weaker than those of oligomers (Fig. 7a). dSY5Y-released α-synuclein oligomers are reactive to Fila-4 antibody, indicating high β-sheet content (Fig. 7b). When injected into the cerebral cortices of wild type mice, these oligomers (fraction 8 of αSCM) induced microglial activation (Fig. 7c). Saline injection into the other hemisphere showed very little microglial activation (Fig. 7c). When the same material was injected into the cerebral cortices of Tlr2-/- mice, neither α-synuclein oligomers nor saline injection resulted in significant activation of microglia (Fig. 7c). Monomers/dimers (fraction 14) derived from αSCM are also capable of activating microglia in the cerebral cortices of wild type mice, albeit to a much weaker extent (Fig. 7c). This activity too disappeared in Tlr2-/- mice (Fig. 7c). This is consistent with the weak activity detected from these monomer/dimer fractions in the TLR2 reporter cells. Therefore, although the majority of TLR2 agonist activity of cell-released α-synuclein is associated with oligomeric forms, a small fraction of monomers/dimers might have either specific modification or conformation that is required for TLR2 activation. Taken together, α-synuclein is a conformation-sensitive endogenous agonist for TLR2; the majority of TLR2 ligand activity resides in β-sheet-rich oligomers among the heterogeneous forms of neuronal cell-released α-synuclein proteins.
Figure 7.
Conformation-sensitive agonist activity of cell-released oligomeric α-synuclein for TLR2. (a) Separation of αSCM by SEC. The line trace and the bars indicate TLR2 activity and the α-synuclein levels, respectively. (b) Dot blot analysis of fractions from a. (c) MHC II fluorescence in the cerebral cortices of wild type and Tlr2-/- mice after injections of fraction 8 and fraction 14 from a (n = 5). Data were analyzed using unpaired t-test. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01. “n” represents the number of animals.
Upregulation of TLR2 in Lewy body diseases and α-synuclein transgenic mice
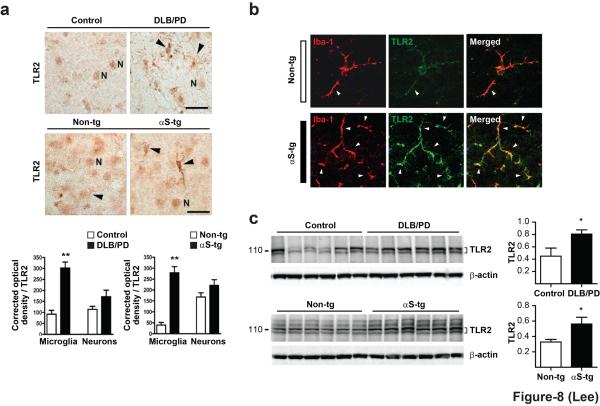
Paraformaldehyde fixed vibratome sections from the temporal cortices of human subjects (DLB patients and non-demented control subjects) and sagital brain sections of mice (α-synuclein transgenic and control mice) were immunolabeled with a TLR2 antibody and analyzed for levels of optical density. In control cases, TLR2 antibody mildly labeled pyramidal neurons in the neocortex in layers 2-3 and 5 and some occasional glial cells with morphological characteristics consistent with microglia (Fig. 8a). In the DLB cases and α-synuclein transgenic mice, there was a significant increase in the levels of TLR2 immunoreactivity in microglial cells. Upregulation of TLR2 in microglial cells was confirmed by double immunofluorescence studies with antibodies against TLR2 and Iba-1, showing colocalization of the two markers (Fig. 8b). This colocalization was seen in most of Iba-1-positive microglial cells, and TLR2 immunofluorescence was stronger in α-synuclein transgenic mice than in control non-transgenic mice. Western analysis also showed significant increases in TLR2 expression in brain homogenates of DLB cases and α-synuclein transgenic mice (Fig. 8c). The molecular weight for the mature TLR2 is between 100 - 110 kDa, and it appears as a doublet. This might represent post-translational modifications such as glycosylation24. For quantification, we analyzed the doublet, which was bracketed in figure 8c.
Figure 8.
TLR2 expression in DLB and α-synuclein transgenic mice. (a) Immunohistochemical analysis of human (top panels, n = 6) and mouse samples (bottom panels, n = 6). The TLR2 antibody immunolabeled neuronal cells (N) as well as microglial cells (arrow heads). (b) Double immunofluorescence studies with antibodies against TLR2 and Iba-1 showing colocalization of the two markers in microglial cells (top panels). These cells usually were close to capillaries (c) that also displayed TLR2 labeling. Double labeling for TLR2 and α-synuclein in the α-synuclein transgenic mice (bottom panels). (c) Western analysis of brain homogenates. All data were analyzed using unpaired t-test. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01. “n” represents the number of patients and animals.
Analysis of transcriptome data from postmortem SN of human PD patients25, collected from the public database (NCBI GEO, GSE7621; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7621), shows significant increases in expression of genes in the TLR2 signaling pathways, including TLR2, CD14, IRAK2, and NFκB (Supplementary Fig. S16), suggesting that the TLR2 signaling pathway is activated in PD.
Discussion
Our work exemplifies the power of a computational approach for identification of receptors and signaling pathways that are relevant to neurodegenerative diseases. The hypothetical signaling network that we constructed proved to be consistent with experimental results, which demonstrate that cell-released α-synuclein is an endogenous agonist for TLR2 through which microglia are activated and become neurotoxic. The fact that microglial activation by neuronal α-synuclein overexpression in the SN was eliminated in Tlr2 deficient mice provided in vivo verification of the role of TLR2 in α-synuclein-induced microglial activation. These results implicate a novel mechanism by which neuronal α-synuclein proteins mediate the pathogenic interaction between neuron and glia as a paracrine factor, thereby cultivating inflammatory milieu in brain parenchyma. Our study suggests that elimination of interaction between neuron-released α-synuclein and TLR2 dampens inflammatory responses in the brain, and therefore may be applicable to therapy (Supplementary Fig. S16). Indeed, our recent study suggested that the clearance of extracellular α-synuclein was the mechanism underlying the protective effects of immunotherapy26.
Recently, a paper by Letiembre et al.27 reported TLR2 upregulation in several animal models of neurodegenerative disease, including models for AD and PD/DLB, and argued that this response is part of a non-specific neuroinflammatory effector phase rather than a disease-specific event. We do not intend to argue that TLR2 activation is a specific event in Lewy body diseases. What is disease-specific might be how this pathway is activated. Novelty of our study is that pathogenic protein aggregates that have previously been thought to be confined within neuron can act as an endogenous agonist for the TLR2 pathway. In a recent signaling network analysis, we have hypothesized that cell-released α-synuclein also activates inflammatory responses in astrocytes, including TLR2 activation8. This needs experimental validation.
We have conducted the uptake and cytokine induction experiments with Tlr2-/- microglia and the TLR2 blocking antibody, and the cytokine induction was always completely inhibited, whereas the uptake was only partially blocked. Our interpretation is the following. When microglia are exposed to α-synuclein that is secreted from neuronal cells, inflammatory activation of microglia is mediated solely by TLR2, whereas the uptake can be mediated not only by TLR2 but also by other cell surface proteins, such as the scavenger receptors28, with the latter being inert in terms of inflammatory signaling.
Most of experiments in our study were conducted at relatively high α-synuclein concentrations (0.1 - 5.3 μg/ml), compared to the concentrations reported in the CSF (45-75 ng/ml)29 and interstitial fluid (up to 8 ng/ml)18. However, as shown in Supplementary figure 7, a cytokine induction can be achieved in microglia as low a concentration as 5 ng/ml, and TLR2 activation in the reporter system at 1 ng/ml (TLR2 reporter system is more sensitive than the assays for microglial responses). At these low concentrations, the responses are very weak, but nevertheless, statistically significant. We do not believe that these weak responses are pathogenic. However, given the fact that α-synuclein secretion is promoted by several factors that are associated with disease, the concentrations used in our study might probably be pathophysiologically relevant. Increased expression of α-synuclein is associated with PD and leads to increased secretion of α-synuclein in neuronal culture6. Oxidative stress, defects in protein degradation systems, and mitochondrial dysfunction are also strongly associated with PD and promote α-synuclein secretion5. Moreover, cytoplasmic calcium can increase the secretion of α-synuclein30, raising the possibility that α-synuclein release is regulated by neural activity. Therefore, the concentration of α-synuclein in the interstitial fluid can vary locally and temporarily, and those used in the current study is likely to be within the range of physiological relevance, especially in pathological situation.
Several studies have suggested that oligomeric aggregates are the toxic culprit of protein aggregation-associated neurodegenerative diseases31. Our study expands the scope of pathogenic functions that oligomeric protein aggregates exert in the pathogenesis of these diseases, by showing that α-synuclein oligomers, through activation of TLR2, are inducers of inflammatory innate immunity in the nervous system. Activation of TLR2 by α-synuclein is highly sensitive to the conformation of the protein; purely monomeric and fibrillar α- synuclein cannot activate TLR2. Oligomerization itself is not sufficient for TLR2 activation; certain types of oligomers, such as the ones induced by HNE, cannot activate TLR2. HNE-induced α-synuclein oligomers generated in our laboratory have little secondary structure32, whereas the oligomer preparation that can activate TLR2 showed α-sheet-rich conformation. In addition, the monomer/dimer fractions from αSCM, but not the same fractions from the recombinant α-synuclein, showed a weak TLR2-activating activity, suggesting that there might be certain modification and/or conformation of monomer/dimer occurring in cells that are required for TLR2 agonist activity. Therefore, elucidation of structure of neuron-released α-synuclein is of great importance in terms of understanding of molecular details underlying the interaction between α-synuclein and TLR2.
One of the central questions in neurodegenerative diseases is whether inflammatory responses promote neurodegeneration or whether insufficient immune responses contribute to disease. Studies assessing this question have yielded conflicting results. Likewise, whether TLR signaling exacerbates neurodegeneration or is protective in disease progression is still largely unknown. In relation to our current study, more specific question would be whether TLR signaling is hyperactivated by protein aggregates or guide the clearance of protein aggregates.
TLRs may mediate the clearance of extracellular protein aggregates. AD mouse models with depletions in TLR genes showed increased cognitive impairments and Aβ deposition12, 14, whereas conflicting results have also been reported; deletion of coreceptor for TLR2/4, CD14 33, and a common adaptor protein, MyD8834, ameliorates Aβ deposition. In mice overexpressing mutant SOD1, transplantation of MyD88-deficient bone marrow cells resulted in earlier disease onset and shorter life-span that of wild type cells35. On the other hand, there is a large body of evidence for the role of TLR-mediated neuroinflammation in the initiation and progression of neurodegenerative diseases. Aβ fibrils activated TLR2 and TLR4 and induced inflammatory responses in cultured microglia and monocytes11, 15. TLR4-dependent neuroinflammation was also demonstrated in mouse models of AD36. TLR4-activated microglia became neurotoxic13 and trigger neurodegeneration in mice37. In models of SOD1-ALS, TLR-dependent microglial activation exacerbated motor neuron degeneration38. TLR3 agonists induced nigral dopaminergic degeneration, which was reversed by systemic administration of an IL-1 receptor antagonist in adult rats17, suggesting that TLR activation and subsequent inflammation is disease-promoting. In contrast to the study in the ALS mouse model, transplantation of MyD88-deficient bone marrow cells reduced inflammatory activation and brain Aβ burden and improved cognitive function in a mouse model of AD39.
A recent study by Stefanova et al.40 suggested that TLR4 is involved in β-synuclein clearance in transgenic mice expressing this protein specifically in oligodendrocytes; the same group, however, suggested in an earlier work that microglial activation mediates neurodegeneration in the same mouse model41. We speculate that these two seemingly opposite effects of TLRs may not be mutually exclusive. Although the primary role of microglia and its receptors for protein aggregates may be to clear the toxic proteins and protect neurons, microglial responses to TLR activation may change in diseased brains. The outcome of TLR activation may depend on various factors, such as the states of microglial cells, the source and assembly state of protein ligand, duration and strength of the stimulation, and repertoire of coreceptors and adaptor proteins that interact with TLR.
The model proposed in the current study (Supplementary Fig. S17) allows the prediction that drugs that act on TLR2 and/or on the extracellular α-synuclein oligomers might modify neuroinflammation in α-synuclein-related neurodegenerative diseases; thus, both these proteins are candidates for mechanism-based drug targets of PD and other related diseases.
Of particular interest, other cytosolic aggregation-prone proteins, such as tau and superoxide dismutase 1, are also released from cells42, 43. Moreover, in human tauopathies and the related animal models, neuronal accumulation of hyperphosphorylated tau was closely associated with microglial activation44. These results along with those of our current study point to an interesting possibility that microglial activation by neuron-originated aggregation-prone proteins might be a general mechanism of neuroinflammation in major neurodegenerative diseases.
Methods
Antibodies and chemicals
Protease inhibitor cocktail, Lipopolysaccharides, and DCF-DA were purchased from Sigma Aldrich (St. Louis, MO). Pam3CSK4 and QUANTI-Blue were obtained from InvivoGen (San Diego, CA). The following antibodies were used: TLR2 (clone T2.5) and MHC class II (eBioscience, San Diego, CA); polyclonal TLR2 antibody (US Biological, Swampscott, MA); IκB, p38 MAPK, phospho-p38 MAPK, and α-synuclein (polyclonal) (Cell Signaling Tenchnology, Beverly, MA); β-actin (Sigma Aldrich); nitrated α-synuclein, α-synuclein (LB509), tyrosine hydroxylase, and TLR2 (Abcam, Cambridge, MA); α-synuclein (Syn-1; BD Bioscience, San Diego, CA); Iba-1 (Wako Laboratories, Richmond, VA); FILA-4 antibody (Dr. Poul Henning Jensen, University of Aarus).
Animals
Sprague-Dawley rats and C57BL/6 mice were purchased from Samtako (Osan, Korea). TLR2-deficient mice were obtained from Oriental Bioservice (Kyoto, Japan)45, TLR3-deficient mice from R. Flavell (Yale University, School of Medicine, New Haven, CT)46, and TLR4-deficient mice from S. Akira (Hyogo College of Medicine, Hyogo, Japan)47. Transgenic mice expressing wild type human α-synuclein under the PGDF-B promoter (line D) were described elsewhere48. Genotypes of mice were analyzed by PCR using specific primers (Supplementary Table S3).
Cell culture
Maintenance and differentiation of SH-SY5Y human neuroblastoma, primary cortical neurons, rat and mouse primary microglia, BV2 murine microglial cell lines, and COS-7 cells have been previously described 49, 50.
Polymerase chain reaction
Total RNAs were reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). For conventional RT-PCR, the cDNA products were amplified using specific primers over the indicated cycles (Supplementary Table S3). For realtime-PCR, target genes were amplified using SYBR Premix ex taq II (Takara, Shiga, Japan) with specific primers (Supplementary Table S3). Amplification of DNA products was measured by the 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA). Relative mRNA levels were calculated according to the 2-ΔΔCT method. All ΔCT values were normalized to GAPDH or β-actin.
Preparation of conditioned media
Differentiated SH-SY5Y cells were infected with adenoviral vectors (serotype Ad5, CMV promoter)51 at a multiplicity of infection of 100. On day two post-infection, media were replaced with serum-free DMEM, then incubated for 18 hours. For conditioned media of cortical neurons, mouse cortical neurons were seeded onto 60 mm culture dish. After a 5-day incubation, media were replaced with B27-free neurobasal media, then incubated for 24 hours. To remove cell debris, the media were centrifuged at 10,000 x g for 10 minutes. The recovered supernatants were concentrated using 10K cut-off centrifugal filters (Millipore, County Cork, Ireland).
Morphological analysis of microglia
Microglia cells were treated with CM or LPS for 24 hours. Cells with amoeboid and ramified morphologies were counted in 10 randomly chosen areas for each experiment, and mean values were obtained.
Nitric oxide and cell viability assays
Microglia cells were treated with CM or LPS. After a 24-hour incubation period, nitric oxide was measured from the microglial culture media using the Griess reagent system (Promega, Madison, WI) according to the manufacturer's instruction. Cell viability was determined from the cells in the same culture using the Cyquant cell proliferation assay kit (Invitrogen, Carlsbad, CA).
Measurement of intracellular ROS
Microglia cells were treated with 2’-7’-dichlorodihydrofluorescence diacetate (DCF-DA) for 20 minutes in the dark. A total of 10,000 cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The oxidized form of DCF-DA was excited at 488 nm and detected at 530 nm.
α-synuclein pull-down
Due to its affinity to metal ions, α-synuclein binds to the Talon metal affinity resins (Clontech, Mountain View, CA) (data not shown). For removal of α-synuclein from αSCM, αSCM was mixed with Talon metal affinity resins and incubated at 4°C for 2 hours with rotation. To isolate cell-released and cytosolic α-synuclein (see Supplementary Fig. S10), His-αSCM and cell extracts obtained from dSY5Y cells expressing His-tagged α-synuclein were pulled down with Talon metal affinity resins.
Preparation of cell and tissue extracts and western blot analysis
Cells were lysed in the RIPA buffer (150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and 50 mM Tris-HCl) with a protease-phosphatase inhibitor mixture. Brain homogenates were prepared in the lysis buffer (1% Triton X-100, 10% glycerol, 50 mM HEPES, pH 7.4, 140 mM NaCl, 1mM EDTA, 1mM Na3VO4, 20 mM β-glycerophosphate, and proteinase inhibitor cocktails), and separated into cytosolic and particulate fractions by centrifugation. Western blot analysis was performed as described previously51.
TNF α and IL-6 measurements
The concentrations of TNFα and IL-6 were determined by the BIOSOURCE Immunoassay kit (Invitrogen).
Microarray experiment and network analysis
Microarray analysis was performed on rat primary microglia
RNA preparation, microarray experiment, and probe annotation were performed as previously described8. Using the normalized intensities, the DEGs (FDR ≤ 0.01) between LZCM and αSCM exposed cells were determined using a previously reported statistical method8. For the gene expression data from 9 normal donors and 16 patients with PD (GSE7621), we used the same method to identify the DEGs between normal and patients (FDR ≤ 0.05). In addition, KEGG pathways enriched by the DEGs were identified (P < 0.01) using DAVID software. For network analysis, we selected a set of DEGs involved in the major pathways and then reconstructed a network describing their interactions and associated pathways based on the interaction data from public databases including BIND, HPRD, BioGRID, and KEGG8.
Analysis of TLR2/α-synuclein interaction
The entire procedure was performed on ice in a cold room. BV2 microglia cells were pre-incubated with conditioned medium or SEC fractions for 30 minutes, then crosslinked by incubation with 2 mM Dithiobis[succinimidylpropionate]. After the incubation, cells were incubated with either T2.5 (20 μg/ml) or with IgG (20 μg/ml). At least 120 cells were analyzed in each independent experiment. For α-synuclein cell surface binding to the HEK293 reporter cells, the cells were incubated with the SEC fractions 8 or 14 for 1 hour. At least 220 cells were analyzed in each independent experiment.
α-synuclein uptake assay
Primary microglia cells were treated with αSCM. After PBS washes, cells were lysed with the Laemmlie sample buffer (LSB). In cases of α-synuclein fibril uptake, microglial cells were treated with α-synuclein fibrils (400 nM) and lysed with 1% TritonX-100/PBS. The Triton-insoluble pellets were resuspended in the LSB. For ectopic expression of Tlr2, COS-7 cells were transfected with pcDNA3.1/Hygro-human Tlr2 or with control pcDNA3.1 using lipofectamine 2000 (Invitrogen). Acid washing was performed with 0.1 M HCl twice before lysis.
Immunofluorescence microscopy for cells
The procedure for immunofluorescence and image analysis have been described elsewhere52.
Stereotaxic injection and analysis of tissue samples
The experimental protocols were approved by Konkuk University's Animal Care and Use Committee. Adenoviral vectors were prepared using the virakit (Virapur, San Diego, CA). Age-matched mice were stereotaxically positioned under deep anesthesia, and 1.33 x 108 infection units of adenoviral vectors were then injected into striatum (AP; 0.8 mm, ML; 1.7 mm, and DV; -3.2 mm). Seven days after injection, the brains were processed for immunofluorescence staining7. Immediately adjacent tissue sections were labeled with different antibodies. Fluorescence intensity of unit area measured in the SN region was normalized with that of the non-SN region. Correlation rates were determined by the Pearson product-moment correlation coefficient method (Pearson's r) using GraphPad Instat software. For the injection of oligomers or monomers, 0.2 ng of αSCM fraction 8 and fraction 14 were injected into the cerebral cortex (AP; 2.0 mm, ML; 1.5 mm, and DV; -1.5 mm). Fluorescence intensity of unit area was measured in the cerebral cortex region around the injection site and normalized with the data obtain from the hippocampus.
Preparation of recombinant α-synuclein and aggregates
Bacterial endotoxins were removed using the Toxineraser endotoxin removal kit (Genscript, Piscotaway, NJ) ( < 0.015 endotoxin unit/1 mg of α-synucein). Fibrils were prepared as described previously53. Nitrated α-synuclein aggregates were generated following the previously described procedure54. For HNE-induced oligomerization, α-synuclein (1 mg/ml) was incubated with 1.4 mM HNE (Cyman chemical, Ann Arbor, MI) for 1 week at 37°C and dialyzed against PBS. For spontaneous oligomerization, lyophilized recombinant α-synuclein was dissolved in PBS (1 mg/ml) and incubated at 4αC. After 2 days of incubation, protein was filtrated through a syringe filter with the pore size of 0.2 μm. One hundred μl of α-synuclein (50 μM) was separated using Superdex 200 HR 10/30 column (GE healthcare Life science) at a flow rate of 0.5 ml/min.
Circular dichroism
Samples (10 μM) were analyzed in a Jasco J-810 spectropolarimeter (Tokyo, Japan) from 190 to 260 nm in a quartz cell at RT. The data used for graphical presentation was an average of six different scans.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as described previously55 with anti-α-synuclein antibodies Ab62 (1 μg/ml) and biotinylated Ab274 (1 μg/ml) as a capture and reporter, respectively.
HEK-Blue TLR reporter assay
HEK293 reporter cells stably expressing human TLR2/CD14 and human TLR4/CD14/MD-2 were purchased from InvivoGen along with matching control cells. HEK-Blue TLR reporter assay was performed according to manufacturer's instructions.
Human specimens, neuropathological evaluation and criteria for diagnosis
Autopsy material (6 non-demented controls, 6 DLB cases) was obtained from patients studied neurologically and psychometrically at the Alzheimer Disease Research Center/University of California, San Diego (Supplementary Table S4). The last neurobehavioral evaluation was performed within 12 months before death and included Blessed score, Mini Mental State Examination and dementia-rating scale56. The diagnosis of DLB was based in the initial clinical presentation with dementia followed by parkinsonism and the presence of α-synuclein and ubiquitin-positive LBs in cortical and subcortical regions57. Procedures for immunocytochemistry and confocal microscopy were described elsewhere7.
Statistical analysis
Statistical significances of data were determined using unpaired t-test, one-way ANOVA, or Mann-Whitney U test.
Supplementary Material
Acknowledgements
We would like to thank S. H. Paek for his initial discussion on the striatal injection experiment and J.-S. Han for the detailed consultation for statistical analysis. This work was supported by the Mid-career Research Program (2010-0015188, to S-JL), by the Bio & Medical Technology Development Program (2007-2004303, to S-JL), and by the Basic Research Promotion Fund (331-2007-1-C00214, to H-JL) through NRF grant funded by the Korean government (MEST). This work was also supported by the NIH grants, AG18440 and AG5131 (to EM).
Footnotes
Author contributions
C.K., D.-H.H., J.-E.S., S.Y, S.M., and J.K. performed experiments. C.K., S.J.L., E.M., D.H., H.-J.L., S.-J.L. designed, analyzed, and interpreted experiments. C.K. and S.-J.L. wrote the paper.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 2.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 3.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C, Lee S-J. Controlling the mass action of alpha-synuclein in Parkinson's disease. J Neurochem. 2008;107:303–316. doi: 10.1111/j.1471-4159.2008.05612.x. [DOI] [PubMed] [Google Scholar]
- 5.Jang A, et al. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee H-J, Patel S, Lee S-J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroso M, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 10.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 11.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter S, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 14.Tahara K, et al. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tukel C, et al. Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deleidi M, Hallett PJ, Koprich JB, Chung CY, Isacson O. The toll-like receptor-3 agonist polyinosinic:polycytidylic acid triggers nigrostriatal dopaminergic degeneration. J Neurosci. 2010;30:16091–16101. doi: 10.1523/JNEUROSCI.2400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emmanouilidou E, et al. Assessment of alpha-synuclein secretion in mouse and human brain parenchyma. PLoS One. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson's disease. Glia. 2008;56:1215–1223. doi: 10.1002/glia.20691. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds AD, et al. Nitrated alpha-synuclein-activated microglial profiling for Parkinson's disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 21.Su X, et al. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 23.Paleologou KE, et al. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- 24.Feng C, et al. Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS One. 2012;7:e32359. doi: 10.1371/journal.pone.0032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papapetropoulos S, et al. Multiregional gene expression profiling identifies MRPS6 as a possible candidate gene for Parkinson's disease. Gene expression. 2006;13:205–215. doi: 10.3727/000000006783991827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae EJ, et al. Antibody-Aided Clearance of Extracellular alpha-Synuclein Prevents Cell-to-Cell Aggregate Transmission. J Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letiembre M, et al. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 29.Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med. 2010;4:683–699. doi: 10.2217/bmm.10.90. [DOI] [PubMed] [Google Scholar]
- 30.Emmanouilidou E, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 32.Bae EJ, et al. Lipid Peroxidation Product 4-Hydroxy-2-Nonenal Promotes Seeding-Capable Oligomer Formation and Cell-to-Cell Transfer of alpha-Synuclein. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed-Geaghan EG, Reed QW, Cramer PE, Landreth GE. Deletion of CD14 attenuates Alzheimer's disease pathology by influencing the brain's inflammatory milieu. J Neurosci. 2010;30:15369–15373. doi: 10.1523/JNEUROSCI.2637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim JE, et al. MyD88 deficiency ameliorates beta-amyloidosis in an animal model of Alzheimer's disease. Am J Pathol. 2011;179:1095–1103. doi: 10.1016/j.ajpath.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J Cell Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao W, et al. Myeloid differentiation factor 88-deficient bone marrow cells improve Alzheimer's disease-related symptoms and pathology. Brain. 2011;134:278–292. doi: 10.1093/brain/awq325. [DOI] [PubMed] [Google Scholar]
- 40.Stefanova N, et al. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefanova N, et al. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- 42.Galasko D. CSF tau and Abeta42: logical biomarkers for Alzheimer's disease? Neurobiol Aging. 1998;19:117–119. doi: 10.1016/s0197-4580(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 43.Urushitani M, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki A, et al. Microglial activation in brain lesions with tau deposits: comparison of human tauopathies and tau transgenic mice TgTauP301L. Brain Res. 2008;1214:159–168. doi: 10.1016/j.brainres.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 46.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 47.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 48.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 49.Lee H-J, Khoshaghideh F, Patel S, Lee S-J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H-J, Suk JE, Bae EJ, Lee S-J. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 51.Lee H-J, Shin SY, Choi C, Lee YH, Lee S-J. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 52.Lee H-J, Lee S-J. Characterization of Cytoplasmic alpha -Synuclein Aggregates. FIBRIL FORMATION IS TIGHTLY LINKED TO THE INCLUSION-FORMING PROCESS IN CELLS. J Biol Chem. 2002;277:48976–48983. doi: 10.1074/jbc.M208192200. [DOI] [PubMed] [Google Scholar]
- 53.Lee H-J, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Thomas MP, et al. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson's disease. J Neurochem. 2007;100:503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee HJ, et al. Enzyme-linked immunosorbent assays for alpha-synuclein with species and multimeric state specificities. J Neurosci Methods. 199:249–257. doi: 10.1016/j.jneumeth.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Salmon DP, Thal LJ, Butters N, Heindel WC. Longitudinal evaluation of dementia of the Alzheimer type: a comparison of 3 standardized mental status examinations. Neurology. 1990;40:1225–1230. doi: 10.1212/wnl.40.8.1225. [DOI] [PubMed] [Google Scholar]
- 57.McKeith IG, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.