Abstract
Abnormal serotonergic pathways are implicated in numerous neuropsychiatric disorders including alcohol and drug dependence (abuse). The human 5-hydroxytryptamine (serotonin) receptor 1B, encoded by the HTR1B (5-HT1B) gene, is a presynaptic serotonin autoreceptor that plays an important role in regulating serotonin synthesis and release. Although there was evidence of associations of the HTR1B gene variants in the etiologies of substance use disorders, negative findings were also reported. To clarify the roles of commonly-reported single nucleotide polymorphisms (SNPs) of the HTR1B gene underlying alcohol and drug dependence (abuse), we performed a meta-analysis based on the available genotype data from individual candidate gene-based association studies. Evidence of association was found between the functional SNP -161A>T (rs130058) and alcohol, cocaine, and heroin dependence (e.g., P = 0.03 and odds ratio = 1.2 (1.02, 1.42) in the combined European, Asian, African, and Hispanic populations). SNP -261T>G (rs11568817) also showed evidence of association but with different directions in Europeans and non-Europeans (e.g., P = 0.0018 with odds ratio = 1.42 (1.14, 1.76) and P = 0.01 with odds ratio = 0.5 (0.3, 0.85), respectively). This meta-analysis supports the associations of HTR1B -261T>G and -161A>T with alcohol and drug abuse and further investigations are warranted in larger samples.
Keywords: Substance Use Disorder, Addiction, Meta-analysis, Association, Linkage Disequilibrium
Introduction
Substance abuse is one of the major worldwide public health and social problems. The annual financial costs of illicit drug use disorders and alcohol abuse in the United States were estimated to be $181 billion and $185 billion, respectively, according to the National Institute on Drug Abuse. Fifty-three percent of the state and 45% of the federal prisoners met the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for substance use disorders (Mumola and Karberg 2006). According to a National Survey on Drug Use and Health, 35.3 million Americans aged 12 and older were reported to have used cocaine, 8.5 million reported having used crack, and approximate 2.4 million Americans were current users of cocaine. Substance abuse is characterized by compulsive alcohol or drug seeking or physical dependence on the substance in question. Family, twin, and adoption studies have shown that genetic factors play an important role in the etiology of substance abuse (Devor and Cloninger 1989), e.g., the heritable influences account for 40–70% of the variation in liability to alcoholism (Gelernter and Kranzler 2009; Kendler and others 2007; Uhl and others 2008). Serotonin reuptake inhibitors have shown effectiveness for the treatment of alcoholism (Sellers and others 1992).
The 5-hydroxytryptamine (serotonin) (5-HT or HTR) receptors are categorized into seven main classes (HTR1-7), and have shown important roles in substance abuse and related mental disorders. Recently, abnormal HTR patterns were observed in suicidal post modem brains (Zahodne and others 2012). A novel and highly selective HTR6 receptor antagonist reduced nicotine self-administration and reinstatement in mice with nicotine seeking (de Bruin and others 2012), and the HTR6 receptors may impact cocaine reinforcement via facilitatory interaction with dopamine projections to the nucleus accumbens shell, which could open new avenues for cocaine dependence treatment (Valentini and others 2012). Function of HTR3A was implicated in alcohol addiction (Shorter and Kosten 2011) and the association of the HTR1B gene variant was found with heroin dependence among Han Chinese (Gao and others 2011). Indirect-acting HTR receptor agonists can enhance the antinociceptive effects of morphine although the specific subtype(s) mediating this enhancement is not established (Li and others 2012). Many drugs target the serotonin system, including Vilazodone that was approved by the United States Food and Drug Administration (informally USFDA) in 2011 for depression (Bach and Arango 2012), Methoxectamine, a HTR2 agonist currently being explored as a fast-acting antidepressant, and Rotundine, currently used in China to treat cocaine addiction (Hagan and others 2012).
The HTR1B (5-HT1B) receptor is one of the important serotonin receptor family that includes over 20 subtypes based on their pharmacologic and biochemical properties. The HTR1B receptors, which are widely distributed in the basal ganglia, hippocampus, and other regions of the cortex, are located on presynaptic and postsynaptic terminals and mediate the release of both serotonin and nonserotonin neurotransmitters (Moret and Briley 2000). Genetic, pharmacological, and animal studies implied the involvement of HTR1B in brain neuropsychiatric functions and in locomotion, feeding and thermoregulation (Barnes and Sharp 1999). Knockout mice that lacked the HTR1B receptor had increased impulsive aggression (Brunner and Hen 1997), special memory performance (Malleret and others 1999), and exploratory activity (Malleret and others 1999), and higher preference for alcohol (Groenink and others 2003), but decreased anxiety (Zhuang and others 1999). Decreased HTR1B activity enhanced locomotor response to cocaine self-administration (Rocha and others 1998) and alcohol consumption (Crabbe and others 1996) in the HTR1B receptor knockout mice while increased HTR1B function reduced cocaine self-administration in rats with HTR1B agonists (Parsons and others 1998). Recent studies indicated HTR1B in migraine medication (Pytliak and others 2011), emotional memory processing via the hippocampus (Eriksson and others 2012), regulation of serotonin transporter activity (Hagan and others 2012), and an altered relationship between childhood aggression and adulthood hostility (Hakulinen and others 2012). HTR1B appeared to differentially modulate cocaine abuse-related behaviors, with a facilitative influence during periods of active drug use in contrast to an inhibitory influence during protracted withdrawal, suggesting value in stage-based cocaine dependence treatment (Pentkowski and others 2012). HTR1B receptor antagonists showed promise for treating cocaine (Miszkiel and others 2011) and methamphetamine addictions (Miszkiel and others 2012) based on attenuated self-administration in rat studies.
The HTR1B gene has one single exon. The two single nucleotide polymorphisms (SNPs) -261T/G (rs11568817) and -161A/T (rs130058) are located in the 5′ untranslated region; 129C/T (rs6298) in N-terminal domain (synonymous); 861G/C (rs6296) in intracellular loop III (synonymous); 1180G/A (rs6297) in the 3′ untranslated region. The 129C/T and 861G/C have been reported to be in complete linkage disequilibrium (Cigler and others 2001; Huang and others 1999). The schematic representation of the HTR1B variants is shown in Figure 1, including the four SNPs analyzed in this meta-analysis. The HTR1B SNPs were reported to play important roles in alcohol and drug dependence and psychiatric disorders. For example, for -161A>T, three studies (Cao and others 2011; Cigler and others 2001; Sun and others 2002) showed significant associations while no evidence was found in other studies (Cigler and others 2001; Contini and others 2012; Lee and others 2009; Proudnikov and others 2006; Ujike and others 2011); for 1180G>A, the G allele was reported to be associated with heroin addiction with a protective effect in Caucasians (Proudnikov and others 2006), however, other studies (Gao and others 2011; Sun and others 2002; Ujike and others 2011) implied inconsistent results.
Figure 1.

Solid rectangle represents the coding region (single exon) of the HTR1B gene from the start codon ATG to the stop codon TGA. Transparently-filled rectangles represent 5′ and 3′ untranslated regions. The arrows indicate the relative positions of the polymorphisms or start/stop codons. The polymorphisms analyzed in our meta-analysis are indicated with solid arrows and others with broken arrows.
The inconsistency prompted us to examine the genetic associations in an attempt to better understand alcohol and drug abuse as complex traits. In particular, the sample size in an individual study may give rise to spurious association. Alcohol, cocaine, and heroin abuse are part of a group of addictions, and may share some common genetic risks (Fu and others 2002; True and others 1999; Xian and others 2008). In this study, to facilitate the investigation of the pathogenesis of substance use disorder, we analyzed whether the genetic associations existed in the combined samples of European, Asian, African, and Hispanic populations from the published candidate gene association studies for HTR1B -261T>G, -161A>T, 861G>C, and 1180G>A.
Methods
Literature search
The publications included in the meta-analysis were selected from Scopus, PubMed, and the database of Chinese Academic Journals with keywords ‘serotonergic receptor’, ‘HTR1B’, ‘5-HT1B’, ‘serotonin receptor’, ‘association’, ‘associated’, ‘drug’, ‘substance’, ‘alcoholism’, ‘alcohol’, ‘alcoholics’, ‘heroin’, ‘cocaine’, ‘opiate’, and ‘opioid’. The references cited in these studies were examined in order to identify additional works. The analyzed data cover the available English and Chinese publications up to August 2012.
Inclusion criteria
Eligible studies met the following criteria: they (i) were published in peer-reviewed journals and contained original data; (ii) presented genotype data to calculate the odds ratio (OR) with confidence interval (CI) and P value; (iii) were association studies investigating one or more of the four SNPs using either case-control or family-based approaches; (iv) diagnosed the patients according to the World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD)(World Health Organization), American Psychiatric Association’s DSM(American Psychiatric Association) or Chinese classification of mental disorders (CCMD)(Chen 2002) systems; and (v) used random population or healthy individuals as controls in case-control studies. Authors were contacted in cases where it would be necessary to have additional information regarding their studies.
Statistical analyses
Studies were divided among those dealing with samples with European ancestries, those with Asian ancestries, those with African ancestries, and those with Hispanic ancestries. A study that contained data from multiple populations, each was considered effectively as an independent study. Data from the case-control and haplotype relative risk (HRR) studies were summarized by two-by-two tables. The studies were statistically combined by the method described in our previous studies (Li and others 2006a; Li and He 2007; Li and others 2006b) into a single meta-analysis.
From each table a log-odds ratio and its sampling variance were calculated (Li and others 2011). The Cochran’s χ2-based Q statistic test was performed in order to assess heterogeneity to ensure that each group of studies was suitable for meta-analysis. A test for funnel plot asymmetry (Egger and others 1997) was used to assess evidence for publication bias. The test used a linear regression approach to measure funnel plot asymmetry on the natural logarithm of the OR. The significance of the intercept was evaluated using the t test. When publication bias is found, the “Duval and Tweedie’s Trim and Fill” procedure (Duval and Tweedie 2000) is used to impute the number of potentially-missing studies. The Trim and Fill procedure imputes these missing studies, adds them to the meta-analysis, and then re-computes the adjusted overall effect size.
ORs were pooled using the method of DerSimonian and Laird (DerSimonian and Laird 1986), and 95% CIs were constructed using Woolf’s method (Woolf 1955). The significance of the overall odds ratio (OR) was determined using the Z-test. To measure sensitivity of our analysis results, each study was removed in turn from the total, and the remainder then reanalyzed. This procedure was used to ensure that no individual study was entirely responsible for the combined results. In addition, different combinations of the populations (e.g., European, Asian, African American, and Hispanic) and different combinations of the traits (e.g., alcohol, cocaine, and heroin dependence) were also analyzed. Genotypic analyses were carried out when data were available using the dominant and recessive models. Retrospective analysis was performed to better understand the potential effect of year of publication upon the meta-analysis. The type I error rate was set at 0.05. The tests were two-tailed. Haplotype construction, counting, and linkage disequilibrium (LD) block defining were performed separately for European and Asian populations (Barrett and others 2005). The details were also described in our previous studies (Li and others 2011).
Results
The literature search yielded 2,405 references. The overlapping references and those which clearly did not meet the criteria were discarded. These studies were then filtered to ensure conformity with the inclusion criteria. One study (Himei and others 2000) was excluded because it was investigating the 294-Rsa1 polymorphism, rather than the polymorphisms analyzed in this meta-analysis. In the end, 18 studies, composed of 17 case-control (Cao and others 2011; Cigler and others 2001; Contini and others 2012; Gao and others 2011; Hasegawa and others 2002; Lee and others 2009; Proudnikov and others 2006; Sander and others 2000; Sun and others 2002; Ujike and others 2011; Wang and others 2012; Yuan and others 2005) and one HRR study (Hill and others 2002), met our criteria for inclusion. These studies included four studies for European populations (Cigler and others 2001; Hill and others 2002; Proudnikov and others 2006; Sander and others 2000), three studies for Hispanic subjects (Cigler and others 2001; Contini and others 2012; Proudnikov and others 2006), two studies for African Americans (Cigler and others 2001; Proudnikov and others 2006), and nine for Asian populations (Cao and others 2011; Gao and others 2011; Hasegawa and others 2002; Lee and others 2009; Proudnikov and others 2006; Sun and others 2002; Ujike and others 2011; Wang and others 2012; Yuan and others 2005). One study (Cigler and others 2001) included data from three populations and one study (Proudnikov and others 2006) included data from four populations. These patients were diagnosed as alcohol, cocaine, methamphetamine, and (or) heroin dependence (or abuse). Among the 18 studies, six studies (Gao and others 2011; Proudnikov and others 2006; Yuan and others 2005) investigated heroin dependence or abuse; one study (Ujike and others 2011) investigated methamphetamine dependence; three studies (Cigler and others 2001) investigated cocaine and (or) alcohol dependence or abuse, or both; and the other eight studies (Cao and others 2011; Contini and others 2012; Hasegawa and others 2002; Hill and others 2002; Lee and others 2009; Sander and others 2000; Sun and others 2002; Wang and others 2012) investigated alcohol dependence or abuse. The demography of the combined studies is shown in supplementary Table 1. The results for each polymorphism are detailed below.
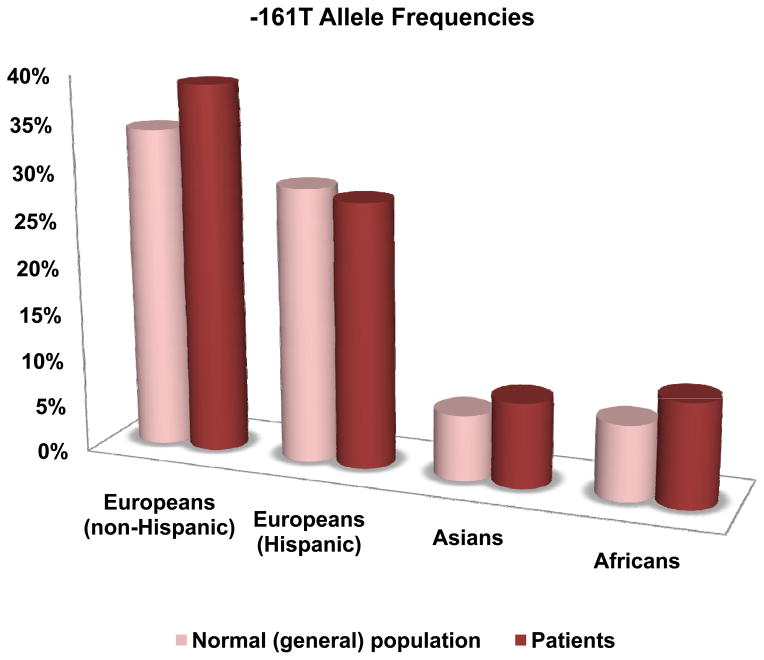
The -161A>T Polymorphism
The minor -161T allele frequency varied widely across the normal controls, being abundant in Europeans (34 – 35%) and Hispanic samples (26 – 31%), but low in Africans (6 – 7%) and Asians (6 – 10%). Figure 2 shows the average frequencies of the -161T allele in these populations. In the 12 case-control studies, 9 studies showed higher frequency in cases than in controls. The meta-analysis of all the combined studies produced a significant overall P value of 0.03 (OR = 1.2, 95% CI 1.02 – 1.42) with no evidence for heterogeneity between studies. Furthermore, evidence of association was found in the combined African and Asian studies and the combined Asian studies with P values of 0.01 (OR = 1.37 (1.08, 1.74)) and 0.016 (OR = 1.36 (1.06, 1.75)), respectively. In addition, the combined studies of cocaine and alcohol dependent (or abuse) showed evidence of significant association with P value of 0.027 (OR = 1.77 (1.07, 2.93)). The results are shown in Table 1, and the forest plots are shown in Figure 3.
Figure 2.
Average frequencies of the -161T allele.
Table 1.
Results of the overall and sub-grouped studies based on ethnicities and traits
| SNPs / Groups | Studiesa | Samplesb | LnOR (95% CI) | OR (95% CI) | P(Z) | P(Q) |
|---|---|---|---|---|---|---|
| -161A>T | ||||||
| All the combined studiesc | 12 | 1,325/1,230 | 0.18 (0.02,0.35) | 1.2 (1.02,1.42) | 0.0304 | 0.1242 |
| Europeans (including Hispanic) | 5 | 367/394 | 0.06 (−0.17,0.29) | 1.06 (0.85,1.34) | 0.5922 | 0.2765 |
| Africans & Asians | 7 | 958/836 | 0.32 (0.07,0.56) | 1.37 (1.08,1.74) | 0.0102 | 0.1644 |
| Asians | 5 | 847/760 | 0.31 (0.06,0.56) | 1.36 (1.06,1.75) | 0.0165 | 0.0667 |
| Alcohol & cocaine dependence (abuse) | 7 | 859/821 | 0.37 (0.04,0.69) | 1.44 (1.04,2) | 0.0275 | 0.0413 |
| Cocaine (mixed alcohol) dependence (abuse) | 3 | 244/329 | 0.57 (0.07,1.07) | 1.77 (1.07,2.93) | 0.0267 | 0.5737 |
| -261T>G | ||||||
| All the combined studiesc | 8 | 479/490 | 0 (−0.38,0.37) | 1 (0.69,1.45) | 0.9956 | 0.0166 |
| Europeans (including Hispanic) | 5 | 367/394 | 0.35 (0.13,0.57) | 1.42 (1.14,1.76) | 0.0018 | 0.4169 |
| Africans & Asians | 3 | 112/96 | −0.69 (−1.21,−0.16) | 0.5 (0.3,0.85) | 0.0109 | 0.7312 |
| Heroin & alcohol dependence (abuse) | 5 | 371/398 | 0.3 (0.08,0.52) | 1.35 (1.08,1.68) | 0.0083 | 0.0600 |
the number of studies included in the meta-analysis are indicated.
the number of cases and controls.
all the studies investigating addictions on any substance.
P(Z): Z test used to determine the significance of the overall OR. P values < 0.05 are indicated in boldfaces. P(Q): Cochran’s X2-based Q statistic test used to assess the heterogeneity.
The random effect model was used when the heterogeneity test was significant (P(Q) < 0.05); otherwise, the fixed effect model was applied.
The 5-HT1B 861G>C were reported to be in complete linkage disequilibrium with 129C>T in some studies (Cigler and others 2001; Proudnikov and others 2006)
The results are shown in Supplementary Table 2 for 861G>C and 1180G>A.
P values > 0.05 are indicated in boldfaces.
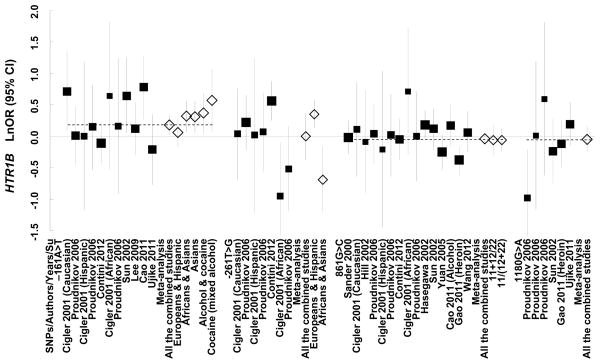
Figure 3.
Forest plots of ln (OR) and overall ln (OR) with 95%CI for the four SNPs. Black squares indicate the ln (OR), with the size of the square inversely proportional to its variance, and horizontal lines represent the 95% CIs. The overall ln (OR) are indicated by the unshaded black diamond. The study of Asian samples (Proudnikov et al. 2006) was not shown for each SNP due to small sample size of the cases.
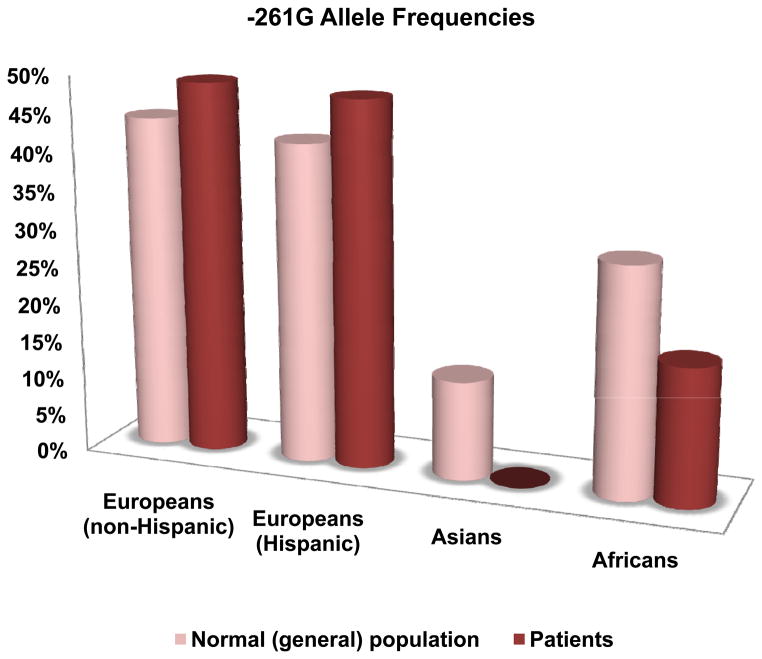
The -261T>G Polymorphism
The minor -261G allele frequency also varied widely across normal populations, being abundant in Europeans (43–45%), Hispanic subjects (34–44%), and African Americans (29–32%), but low in Asians (13%). The European and Hispanic studies showed higher allele frequency in cases than in controls while the African and Asian studies showed opposite direction, i.e., lower frequency in cases than in controls. Figure 4 shows the average frequencies of the -261G allele in these populations. No evidence of significant association was found when all the studies were combined. However, the results showed evidence of significant association in the combined European and Hispanic populations (P = 0.0018 and OR = 1.42 (1.14, 1.76)), and in the combined African and Asian populations (P = 0.01 and OR = 0.5 (0.3, 0.85)). The results are shown in Table 1 and Figure 3.
Figure 4.
Average frequencies of the -261G allele.
The 861G>C and 1180G>A Polymorphisms
The 861G>C polymorphism was reported to be in complete LD with 129C>T. The 861C allele frequency varied widely across normal populations, being abundant in Asians (43–54%), but low in Europeans (26 – 34%), Hispanic samples (29 – 35%), and African Americans (12 – 21%). In the 16 studies, nine studies showed higher frequency in cases than in controls (or more allele transmissions in families). No evidence of significant association was found in all the combined studies, any individual population or sub-group analysis for allelic or genotypic analysis (supplementary Table 2). For the 1180G>A polymorphism, the minor 1180G allele frequency varied across normal populations (5–15%). In the seven case-control studies, four studies showed higher frequency in cases than in controls. No evidence of significant association was observed in the overall or sub-group analysis (supplementary Table 2).
Publication Bias and Fail-safe Analyses
In the present meta-analysis, no evidence of significant publication bias was found except a marginal bias was observed for the -261T>G polymorphism (Egger’s regression P values (1-tailed) = 0.053 in all the combined studies or P = 0.041 in the combined European and Hispanic studies). However, the Duval and Tweedie’s trim and fill analysis showed that for the meta-analysis of all the combined studies, the adjusted effect size was 1.43 (0.98, 1.93) while the original effect size was 1 (0.69, 1.45); and for the combined European and Hispanic studies, the adjusted effect size was 1.56 (1.28, 1.90), which was larger than the original value of 1.42 (1.14, 1.76). The funnel plots of precision (1 / standard error) by log odds ratio are shown for all the combined studies of -161A>T and -261T>G, and the combined European and Hispanic studies of -261T>G in supplementary Figures 1–3, respectively. Solid black indicates the trimmed missing studies and imputed effect size for -261T>G.
For the -261T>G polymorphism, the classic fail-safe analysis showed that at least two assumed non-significant association studies would be required to bring the P values to > 0.05 for the meta-analysis of combined European and Hispanic studies; for the -161A>T polymorphism, at least eight assumed non-significant studies would be required to bring the P value to > 0.05 for the meta-analysis of all the combined studies. The findings supported the associations of the two polymorphisms.
Sensitivity Analyses
For the -161A>T polymorphism, sensitivity analysis showed that the P value was > 0.05 when any of the three studies (Cao and others 2011; Cigler and others 2001; Sun and others 2002) was excluded from the analysis, and the smallest observed P value was 0.0047 (when the study by Contini et al.(Contini and others 2012) was excluded). For the -261T>G polymorphism, the smallest P value was 0.001 but the association was dominated by the two studies (Cigler and others 2001; Contini and others 2012). The results are shown for -161A>T and -261T>G in supplementary Table 3.
Retrospective Analyses
The meta-analyses based on publication years showed that the cumulative results (i.e., effect size), as represented by the asymptote lines on the plots, have not reached stable. It implied more replication studies are necessary for a better understanding of the associations of the polymorphisms. The plots are shown for the -161A>T allelic analysis of all the combined populations in supplementary Figure 4.
Discussion
In this meta-analysis, we examined four HTR1B polymorphisms with substance abuse and found evidence of significant association with -161A>T and -261T>G, which might contribute to the vulnerability to alcohol, cocaine, and heroin dependence in the combined populations (e.g., P = 0.0018 in the European populations for -261T>G). These individual association studies produced contradictory results, and several factors can explain the discrepancy. First, the inconsistent findings could be explained by population heterogeneity. The second might be the heterogeneity of the patient samples or the differences in the diagnostic classification and in the ascertainment. In addition, different control sampling methods differentiated the results; and addictions are heterogeneous in nature and can be differentiated by age of onset (Chen and others 2011) and by the presence or absence of comorbid mental disorders.
As far as the LD structure is concerned, the HTR1B gene was within a large and strong haplotype block of 80 kilo base pairs on the chromosome. The European samples (supplementary Figure 5) showed stronger LD than the Asian samples (supplementary Figure 6). Based on our meta-analysis, the -261T>G, -161A>T, 861G>C, and 1180G>A polymorphisms might not be the “disease” variants, however, they might be in LD with other variants that regulate the gene expression or function of gene products, leading to the association observed in our study. It might be interesting to also examine other variants, e.g., −511, −184/−183, and −182/−181 (two base pair deletions) that are in LD with −261 and −161 in the promoter region.
Previous study showed that the -161T variant was expressed in higher level than -161A in both BeWo and COLO 320 DM cell lines (Sun and others 2002). The haplotype -261G-161A was found to enhance transcriptional activity 2.3-fold compared to -261T-161A; the substitution of A in position −161 with T reverses this effect, making transcriptional activity of -261G-161T equal to the major haplotype -261T-161A(Duan and others 2003a). The synonymous SNPs, such as, 129C>T, 772A>G, and 861G>C, for which we found no evidence of significant association, may have less selection pressure (e.g., than non-synonymous polymorphisms). However, previous studies have shown that synonymous polymorphisms, while not altering protein structure, may influence gene expression levels (Duan and others 2003b). Study also showed that synonymous mutations affected the stability of mRNA secondary structure (Chamary and Hurst 2005), and were important for correct splicing (Chamary and others 2006). Some synonymous codons are more often used than others to increase the efficiency of protein synthesis, which are regulated by tRNA abundances (Akashi and Eyre-Walker 1998; Duret 2002; Ikemura 1985). Thus, the assumption that synonymous mutations are all neutral no longer seems safe as some of them may display functional significance and predispose an individual to genetic diseases (Chamary and others 2006).
The limitations of this study include: firstly, the sample size was small, in particular, for subgroup analyses, and therefore, the results should be interpreted cautiously; secondly, some less significant associations might not survive if Bonferroni correction was applied although the subgroup analyses were not independent. Taken together, this meta-analysis supports the associations of HTR1B -161A>T and -261T>G with alcohol, cocaine, and heroin abuse and implies that the HTR1B gene might confer susceptibility to substance use disorders, however, further investigations are warranted in larger samples.
Supplementary Material
Acknowledgments
This work was supported by the start-up fund from the University of Vermont, and by the research grants DA12849, DA12690, AA017535, AA12870, and AA11330 from the National Institutes of Health, U.S.A. We thank the authors who provided the data related to their individual association studies for this meta-analysis, and thank Drs. Joel Gelernter and Hongyu Zhao for their comments on the manuscript. We also thank the reviewers for very helpful suggestions to improve the manuscript.
Footnotes
Conflict of Interest
None
Electronic-database information
Accession Numbers and URLs for data in this article are as follows:
GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ for genomic structure of HTR1B;
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim for HTR1B;
Genotype data, http://www.hapmap.org/ for HTR1B;
Genome data, http://genome.ucsc.edu/ for HTR1B.
References
- Akashi H, Eyre-Walker A. Translational selection and molecular evolution. Curr Opin Genet Dev. 1998;8(6):688–93. doi: 10.1016/s0959-437x(98)80038-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM) Washington, DC: American Psychiatric Press; [Google Scholar]
- Bach H, Arango V. Neuroanatomy of Serotonergic Abnormalities in Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Cao JX, Hu J, Ye XM, Xia Y, Haile CA, Kosten TR, Zhang XY. Association between the 5-HTR1B gene polymorphisms and alcohol dependence in a Han Chinese population. Brain Res. 2011;1376:1–9. doi: 10.1016/j.brainres.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Chamary JV, Hurst LD. Evidence for selection on synonymous mutations affecting stability of mRNA secondary structure in mammals. Genome Biol. 2005;6(9):R75. doi: 10.1186/gb-2005-6-9-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamary JV, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat Rev Genet. 2006;7(2):98–108. doi: 10.1038/nrg1770. [DOI] [PubMed] [Google Scholar]
- Chen YC, Prescott CA, Walsh D, Patterson DG, Riley BP, Kendler KS, Kuo PH. Different phenotypic and genotypic presentations in alcohol dependence: age at onset matters. J Stud Alcohol Drugs. 2011;72(5):752–62. doi: 10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF. Chinese classification of mental disorders (CCMD-3): towards integration in international classification. Psychopathology. 2002;35(2–3):171–5. doi: 10.1159/000065140. [DOI] [PubMed] [Google Scholar]
- Cigler T, LaForge KS, McHugh PF, Kapadia SU, Leal SM, Kreek MJ. Novel and previously reported single-nucleotide polymorphisms in the human 5-HT(1B) receptor gene: no association with cocaine or alcohol abuse or dependence. Am J Med Genet. 2001;105(6):489–97. doi: 10.1002/ajmg.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini V, Bertuzzi GP, Polina ER, Hunemeier T, Hendler EM, Hutz MH, Bau CH. A haplotype analysis is consistent with the role of functional HTR1B variants in alcohol dependence. Drug Alcohol Depend. 2012;122(1–2):100–4. doi: 10.1016/j.drugalcdep.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14(1):98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, McCreary AC, van Loevezijn A, de Vries TJ, Venhorst J, van Drimmelen M, Kruse CG. A novel highly selective 5-HT(6) receptor antagonist attenuates ethanol and nicotine seeking but does not affect inhibitory response control in Wistar rats. Behav Brain Res. 2012;236C:157–165. doi: 10.1016/j.bbr.2012.08.048. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Devor EJ, Cloninger CR. Genetics of alcoholism. Annu Rev Genet. 1989;23:19–36. doi: 10.1146/annurev.ge.23.120189.000315. [DOI] [PubMed] [Google Scholar]
- Duan J, Sanders AR, Molen JE, Martinolich L, Mowry BJ, Levinson DF, Crowe RR, Silverman JM, Gejman PV. Polymorphisms in the 5′-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression. Mol Psychiatry. 2003a;8(11):901–10. doi: 10.1038/sj.mp.4001403. [DOI] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003b;12(3):205–16. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Duret L. Evolution of synonymous codon usage in metazoans. Curr Opin Genet Dev. 2002;12(6):640–9. doi: 10.1016/s0959-437x(02)00353-2. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Alvarsson A, Stan TL, Zhang X, Hascup KN, Hascup ER, Kehr J, Gerhardt GA, Warner-Schmidt J, Arango-Lievano M, et al. Bidirectional regulation of emotional memory by 5-HT(1B) receptors involves hippocampal p11. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59(12):1125–32. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhu YS, Wei SG, Li SB, Lai JH. Polymorphism G861C of 5-HT receptor subtype 1B is associated with heroin dependence in Han Chinese. Biochem Biophys Res Commun. 2011;412(3):450–3. doi: 10.1016/j.bbrc.2011.07.114. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126(1):91–9. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14(5–6):369–83. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Hagan CE, McDevitt RA, Liu Y, Furay AR, Neumaier JF. 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes. Synapse. 2012 doi: 10.1002/syn.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Jokela M, Hintsanen M, Merjonen P, Pulkki-Raback L, Seppala I, Lyytikainen LP, Lehtimaki T, Kahonen M, Viikari J, et al. Serotonin receptor 1B genotype and hostility, anger and aggressive behavior through the lifespan: the Young Finns study. J Behav Med. 2012 doi: 10.1007/s10865-012-9452-y. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm. 2002;109(4):513–21. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- Hill EM, Stoltenberg SF, Bullard KH, Li S, Zucker RA, Burmeister M. Antisocial alcoholism and serotonin-related polymorphisms: association tests. Psychiatr Genet. 2002;12(3):143–53. doi: 10.1097/00041444-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Himei A, Kono Y, Yoneda H, Sakai T, Koh J, Sakai J, Inada Y, Imamichi H. An association study between alcoholism and the serotonergic receptor genes. Alcohol Clin Exp Res. 2000;24(3):341–2. [PubMed] [Google Scholar]
- Huang YY, Grailhe R, Arango V, Hen R, Mann JJ. Relationship of psychopathology to the human serotonin1B genotype and receptor binding kinetics in postmortem brain tissue. Neuropsychopharmacology. 1999;21(2):238–46. doi: 10.1016/S0893-133X(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64(11):1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lin WW, Huang SY, Kuo PH, Wang CL, Wu PL, Chen SL, Wu JY, Ko HC, Lu RB. The relationship between serotonin receptor 1B polymorphisms A-161T and alcohol dependence. Alcohol Clin Exp Res. 2009;33(9):1589–95. doi: 10.1111/j.1530-0277.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006a;15(12):1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatry. 2007;12(1):47–54. doi: 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006b;15(14):2276–84. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70(6):504–12. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Shah AP, Patel SK, Rice KC, France CP. Modification of the behavioral effects of morphine in rats by serotonin (5-HT)(1A) and 5-HT (2A) receptor agonists: antinociception, drug discrimination, and locomotor activity. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19(14):6157–68. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszkiel J, Adamczyk P, Filip M, Przegalinski E. The effect of serotonin 5HT1B receptor ligands on amphetamine self-administration in rats. Eur J Pharmacol. 2012;677(1–3):111–5. doi: 10.1016/j.ejphar.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Filip M, Przegalinski E. Role of serotonin (5-HT)1B receptors in psychostimulant addiction. Pharmacol Rep. 2011;63(6):1310–5. doi: 10.1016/s1734-1140(11)70695-8. [DOI] [PubMed] [Google Scholar]
- Moret C, Briley M. The possible role of 5-HT(1B/D) receptors in psychiatric disorders and their potential as a target for therapy. Eur J Pharmacol. 2000;404(1–2):1–12. doi: 10.1016/s0014-2999(00)00581-1. [DOI] [PubMed] [Google Scholar]
- Mumola CJ, Karberg JC. Use and Dependence, State and Federal Prisoners, 2004. Washington: Bureau of Justice Statistics; 2006. [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18(23):10078–89. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL. Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry. 2012;72(5):396–404. doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, Ott J, Kreek MJ. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenet Genomics. 2006;16(1):25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, Felsoci M. Serotonin receptors - from molecular biology to clinical applications. Physiol Res. 2011;60(1):15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393(6681):175–8. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Sander T, Ostapowicz A, Samochowiec J, Smolka M, Rommelspacher H, Winterer G, LGS Evaluation of an allelic association of the serotonin 5-HT1B G681C polymorphism with antisocial alcoholism in the German population. Addict Biology. 2000;5(2):167–172. doi: 10.1080/13556210050003757. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Higgins GA, Sobell MB. 5-HT and alcohol abuse. Trends Pharmacol Sci. 1992;13(2):69–75. doi: 10.1016/0165-6147(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, Yu WY, Cheng AT. Association study of novel human serotonin 5-HT(1B) polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51(11):896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56(7):655–61. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–81. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Kishimoto M, Okahisa Y, Kodama M, Takaki M, Inada T, Uchimura N, Yamada M, Iwata N, Iyo M, et al. Association Between 5HT1b Receptor Gene and Methamphetamine Dependence. Curr Neuropharmacol. 2011;9(1):163–8. doi: 10.2174/157015911795017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini V, Piras G, De Luca MA, Perra V, Bordi F, Borsini F, Frau R, Di Chiara G. Evidence for a role of a dopamine/5-HT6 receptor interaction in cocaine reinforcement. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Wang TY, Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, Tzeng NS, Wang CL, Lee IH, et al. Interaction between Serotonin Transporter and Serotonin Receptor 1 B genes polymorphisms may be associated with antisocial alcoholism. Behav Brain Funct. 2012;8:18. doi: 10.1186/1744-9081-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19(4):251–3. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD) Geneva: WHO; [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103(8):1391–8. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- Yuan C, Zhao M, Fang Y, Zhang Y, Jiang S, Zhang M. Association between heroin dependence and the polymorphism of 5-hydroxytryptamine receptor gene. Chin J Behav Med Sci. 2005;14(3):229–231. [Google Scholar]
- Zahodne LB, Bernal-Pacheco O, Bowers D, Ward H, Oyama G, Limotai N, Velez-Lago F, Rodriguez RL, Malaty I, McFarland NR, et al. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson’s disease? J Neuropsychiatry Clin Neurosci. 2012;24(3):326–30. doi: 10.1176/appi.neuropsych.11090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21(2 Suppl):52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.