Abstract
The hippocampus (HPP) plays a known role in learning novel spatial information. More specifically, the dentate gyrus (DG) hippocampal subregion is thought to support pattern separation, a mechanism for encoding and separating spatially similar events into distinct representations. Several studies have shown that lesions of the dorsal DG (dDG) in rodents result in inefficient spatial pattern separation for working memory; however, it is unclear whether selective dDG lesions disrupt spatial pattern separation for reference memory. Therefore, the current study investigated the role of the dDG in pattern separation using a spatial reference memory paradigm to determine whether the dDG is necessary for acquiring spatial discriminations for adjacent locations. Male Long-Evans rats were randomly assigned to receive bilateral intracranial infusions of colchicine or saline (control) into the dDG. Following recovery from surgery, each rat was pseudo-randomly assigned to an adjacent arm or separate arm condition and subsequently tested on a place-learning task using an eight-arm radial maze. Rats were trained to discriminate between a rewarded arm and a nonrewarded arm that were either adjacent to one another or separated by a distance of two arm positions. Each rat received 10 trials per day and was tested until the animal reached a criterion of nine correct choices out of 10 consecutive trials across 2 consecutive days of testing. Both groups acquired spatial discriminations for the separate condition at similar rates. However, in the adjacent condition, dDG lesioned animals required significantly more trials to reach the learning criterion than controls. The results suggest that dDG lesions decrease efficiency in pattern separation resulting in impairments in the adjacent condition involving greater overlap among the distal cues. Conversely, in the separate condition, there was less overlap among distal cues during encoding and less need for pattern separation. These findings provide further support for a critical role for the dDG in spatial pattern separation by demonstrating the importance of a processing mechanism that is capable of reducing interference among overlapping spatial inputs across a variety of memory demands.
Keywords: Pattern Separation, Spatial, Learning, Memory, Hippocampus, Dentate Gyrus
1. Introduction
The hippocampus (HPP) plays a known role in learning and memory processes. As discussed in detail below, research suggests that a primary mnemonic function of the HPP is to reduce interference among similar inputs during learning, allowing for more accurate encoding and subsequent retrieval. A potential process for reducing interference is referred to as pattern separation, which may serve to encode highly overlapping spatial information into separate representations so that one place can be remembered as distinct from another (Gilbert & Brushfield, 2009; Rolls & Kesner, 2006). Computational models of hippocampal function suggest that the HPP may support pattern separation (Marr, 1971; McNaughton & Nadel, 1989; O’Reilly & McClelland, 1994; O’Reilly & Rudy, 2001; Shapiro & Olton, 1994; Rolls & Kesner, 2006; Myers & Scharfman, 2009; Rolls, 2010). Pattern separation has been suggested to be supported by sparse but powerful connections between dentate gyrus (DG) granule cells and CA3 pyramidal cells coupled with the low probability that the same set of CA3 cells will receive inputs from a similar set of DG granule cells (Jung & McNaughton, 1993; Rolls & Kesner, 2006). The DG receives its major cortical input from the entorhinal cortex (EC) via the perforant pathway. Information is then fed forward to CA3 along the mossy fiber projection system (Amaral & Witter, 1995; Johnston & Amaral, 2004) and there is evidence to suggest that this pathway may play a prominent role during encoding of spatial information, thereby facilitating the formation of distinct memory representations (Eldridge, Engel, Zeineh, Bookheimer, & Knowlton, 2005; Jerman, Kesner, & Hunsaker, 2006; Lee & Kesner, 2004; Rolls, 2010).
Electrophysiological recording data and evidence from behavioral studies provide additional support for hippocampal involvement in pattern separation (Fyhn, Hafting, Treves, Moser, & Moser, 2007; Gilbert, Kesner, & DeCouteau, 1998; Jung & McNaughton, 1993; Leutgeb, Leutgeb, Barnes, Moser, McNaughton, & Moser, 2005; Renaudineau, Poucet, & Save, 2007; Tanila, 1999;). The aforementioned behavioral pattern separation studies have been conducted in both humans and rodents (Bakker, Kirwan, Miller, & Stark, 2008; Gilbert et al., 1998; Kirwan & Stark, 2007; Lacy, Yassa, Stark, Muftuler, & Stark, 2010; McHugh et al., 2007; McTighe, Mar, Romberg; Bussey, & Saksida, 2009). For example, a functional magnetic resonance imaging (fMRI) study conducted by Kirwan and Stark (2007) tested participants on a continuous recognition task that required pattern separation to differentiate between similar visual stimuli. Participants were shown a series of pictures of everyday objects and were asked to make “new, old, or similar” judgments when each visual object was presented. The results showed that HPP activity accurately differentiated between objects that were previously seen (old), and objects that were similar to previously seen objects. Further, there is evidence to suggest that damage to the rodent HPP results in an inability to distinguish between spatial locations with a high degree of similarity among proximal and distal cues (Gilbert et al., 1998). Taken together, findings from these studies suggest that the HPP is important for reducing interference among memory representations with a high degree of similarity.
Subregional accounts of hippocampal function suggest that the DG plays a critical role in pattern separation (Bakker et al., 2008; Clelland et al., 2009; Gilbert et al., 2001; Kesner, 2007; Kesner, Lee, & Gilbert, 2004; Koehl & Abrous, 2011; Lacy et al., 2010; Leutgeb, Leutgeb, Moser, & Moser, 2007; McHugh et al., 2007; Rolls & Kesner, 2006; Sahay et al., 2011; Schmidt, Marrone, & Markus, 2012; Tronel et al., 2010; Yassa & Stark, 2011). In support of this mnemonic processing role, several studies have shown that disruptions of the DG in rats are capable of producing functional alterations in pattern separation on spatial working memory tasks, or tasks that require use of information that is trial unique (Emerich & Walsh, 1989; Gilbert et al., 2001; Olton, 1978; Talpos, McTighe, Dias, Saksida, & Bussey, 2010). For example, Gilbert and colleagues (2001) tested rats with selective dorsal DG (dDG) lesions on a delayed-match-to-sample (DMTS) for spatial location task that was designed to measure the ability to discriminate between spatial locations that varied in spatial similarity. On each trial, animals were given a choice between two identical objects that were separated by one of five spatial separations (15 cm to 105 cm). The results showed that rats with dDG lesions were impaired at short separations (high degree of overlap among distal cues); however, their performance increased as the distance between the two objects increased (lessening degree of overlap among distal cues). Goodrich-Hunsaker et al. (2008) obtained similar results using a spontaneous recognition task and showed that rats with dDG lesions were incapable of detecting a change in metric distance between two objects on a cheeseboard maze as evidenced by a reduction in exploration for the displaced objects compared to control rats. Taken together, results from these studies suggest that the dDG hippocampal subregion is important for reducing interference among representations with a high degree of spatial similarity. The results also indicate that the dDG may be particularly sensitive to manipulations in metric distance (Kesner, 2007).
The HPP was previously thought to support spatial working memory but not spatial reference memory, or memory for information that remains constant across time (Olton, Becker, & Handelman, 1979). However, several studies have shown that HPP damage in rats produces acquisition impairments on spatial reference memory tasks (McDonald & White, 1995; McTighe et al., 2009; Morris, Garrud, Rawlins, & O’Keefe, 1982). For example, McDonald & White (1995) tested rats with fimbria-fornix lesions on an active place-learning paradigm that required animals to distinguish between spatial locations on an eight-arm radial maze with a high degree of similarity among extra-maze cues. The results showed that lesioned animals were impaired in acquiring spatial discriminations when spatial locations were adjacent to each other; however, their performance matched normal control animals when the spatial locations were widely separated. The findings from this study suggest that the HPP is necessary for acquiring spatial discriminations for spatial locations that are close together. In addition, several studies have shown that selective lesions of the DG in rodents disrupt performance on spatial reference memory tasks (McLamb, Mundy, & Tilson, 1988; Nanry, Mundy, & Tilson, 1989; Okada & Okaichi, 2009; Xavier, Oliveira-Filho, & Santos, 1999). There also is evidence to suggest that disruption of neurogenesis in mice disrupts performance on tasks that require animals to discriminate between similar contexts (Tronel et al., 2010). However, the distance between spatial locations was not directly manipulated in these studies and did not assess spatial pattern separation.
Therefore, the present study directly examined the role of the dDG in spatial pattern separation for reference memory using an active place-learning paradigm described by McDonald and White (1995). The primary aim of the study was to determine whether an intact dDG is necessary for spatial discriminations involving adjacent locations, but not locations with a high degree of spatial separation. Impairments on a reference memory task involving close spatial locations in dDG lesion rats would provide further support for a critical role for the dDG in separating spatial memories into distinct representations. The present findings may demonstrate the importance of a processing dDG dependent mechanism that is capable of reducing interference among overlapping inputs across a variety of different memory demands.
2. Materials and Methods
2.1 Subjects
Twenty-four male Long-Evans rats, weighing approximately 250–350 g at the start of the experiment, were used as subjects. Each animal was housed in an individual plastic container located in a colony room. The colony room was maintained on a 12-hour light/dark cycle and all testing was conducted during the light phase. All rats had unlimited access to water but were food restricted to 80–90% of their free-feed weight.
2.2 Surgical Procedures
All planned procedures and animal care were in accordance with the National Institute of Health and Institute for Animal Care and Use Committee guidelines and the Institutional Animal Care and Use Committee at the University of Utah. Each animal was randomly assigned to receive a control lesion (n = 12) or a bilateral dDG lesion (n = 12). Prior to surgery, subjects were deeply anesthetized using isoflurane gas, placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and then maintained with a continuous flow of isoflurane (2–4%) and medical air (1.5–2 L/min) and given atropine sulfate (0.54 mg/kg im). Each subject was prepared for the surgical procedure by applying a surgical drape and betadine antiseptic to the surgical site. An incision was made in the skin above the skull, the skin was retracted, and small burr holes were drilled into the skull. Using a 10 μl Hamilton syringe, intracranial infusions of either a vehicle solution (phosphate-buffered saline [PBS]) or colchicine (2.5 mg/ml, 0.8 μl/site) were slowly infused (2.5 mg/mL, 20.0 uL/hr) into two dorsal DG sites per hemisphere using the following coordinates: dDG: 2.7 mm posterior to bregma, 2.1 mm lateral to midline, 3.4 mm ventral from dura and 3.7 mm posterior to bregma, 2.3 mm lateral to midline, 3.0 mm ventral from dura. All lesion coordinates were based on Paxinos & Watson’s (1997) stereotaxic atlas of the rat brain. For all injections, the injection cannula was left in place for at least 1 minute after the injection to allow for diffusion. Following all surgical procedures, each animal received Children’s Motrin in water as an analgesic and was given a 7–10 day recovery period prior to testing.
2.3 Behavioral Apparatus
Testing was conducted in an eight-arm radial maze. The maze consisted of an octagonal central platform 42 cm in diameter with eight arms radiating from the central platform like the spokes of a wheel. Each arm was 71 cm long and 9.5 cm wide and was attached to the central platform with metal braces. Each arm had 0.3 cm-thick clear Plexiglas sides, which rose 5.7 cm above the surface of the arm. A food-well, 2.5 cm in diameter, was drilled 1.5 cm deep at the distal end of each arm. A 0.3 cm-thick Plexiglas guillotine door was located at the juncture between the platform and the arm. Each door was 10 cm wide and when raised, extended 18 cm above the surface of the platform. The doors were manually raised and lowered by the experimenter to permit entrance to the arms. An opaque cylindrical bucket (38 cm in diameter and 75 cm in height) was positioned directly over the central platform and was manually raised and lowered by the experimenter from a room located directly outside the testing room. The maze was located in the center of a windowless room containing a variety of distal cues.
2.4 Behavioral Procedures
Prior to testing, each animal was allowed to individually explore the test apparatus for 0.25 hr. During the exploration period, Froot Loop cereal (Kellogg, Battle Creek, MI) was distributed across the surface of the apparatus (including each individual food well) and the guillotine doors were lowered to permit the animal to explore each arm of the apparatus and to retrieve the food reward. Once each rat had been acclimated to the apparatus, they were pseudo-randomly assigned to an adjacent condition (dDG n = 6; control n = 6) or a separate condition (dDG n = 6; control n = 6) and subsequently tested on an active place-learning paradigm described by McDonald and White (1995).
For the adjacent condition, one of the eight arms of the radial maze was assigned as the rewarded arm. The arms positioned immediately to the left and right of the rewarded arm were assigned as the nonrewarded arms (see Figure 1). Prior to the beginning of each testing session, the animal was placed on the center platform and an opaque cylindrical bucket was manually lowered over the rat. The experimenter then lowered the doors of the designated rewarded arm and one of the two nonrewarded arms. Two different nonrewarded arms were randomly used to ensure that the rats did not adopt a simple response strategy that could provide an accurate nonspatial solution to the task if only one nonrewarded arm was used. On each trial, the bucket was raised and the rat was allowed to choose between a designated rewarded arm and the nonrewarded arm. If the rat entered the rewarded arm, then the rat received a food reward; however, if the rat entered a nonrewarded arm, then the rat did not receive a food reward and was not allowed to enter the arm containing the food reward. Each of the two nonrewarded arms was used on 5 of the 10 daily trials in a pseudo-randomly determined order. The same arms were used throughout all testing procedures. Each rat received 10 trials per day with a 60 s intertrial interval. Testing was conducted daily and each animal was tested until the animal reached a criterion of nine correct choices out of 10 consecutive trials across two consecutive days of testing.
Figure 1.
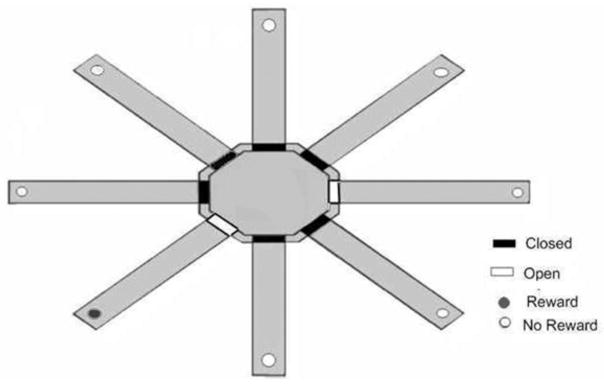
Schematic of eight-arm radial maze configuration for the adjacent condition of the place-learning task.
The separate condition was conducted using an identical procedure and criterion as described for the adjacent condition except that the rewarded arm was separated from the two possible nonrewarded arms by a distance of two arm positions (see Figure 2).
Figure 2.
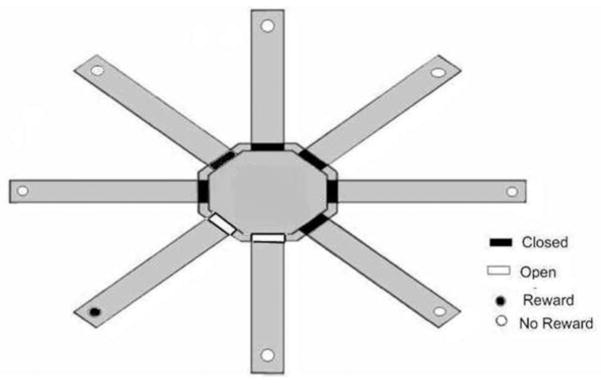
Schematic of eight-arm radial maze configuration for the separate condition of the place-learning task.
2.5 Histological Procedures
At the conclusion of all testing, each animal was deeply anesthetized with an intraperitoneal injection of 1.5 ml sodium pentobarbital (70 mg/kg), and perfused intracardially with normal saline followed by a 10% formalin solution. The brain was removed from the skull and stored in a 10% formalin/30% sucrose solution in a refrigerator (4°C) for 72 hours to equalize the tissue-shrinkage rates across brains. For dDG lesions, a tissue block (Bregma −2.0 through ~ −4.0) containing only the dorsal hippocampus was cut using coronal sections. The block was frozen and cut at 24 μm sections with every third section mounted on a glass slide (the surface-to-surface distance between collected sections = 72 μm). The sections were stained with cresyl violet and examined for histological verification of the lesion placement.
3. Results
3.1 Histological Results
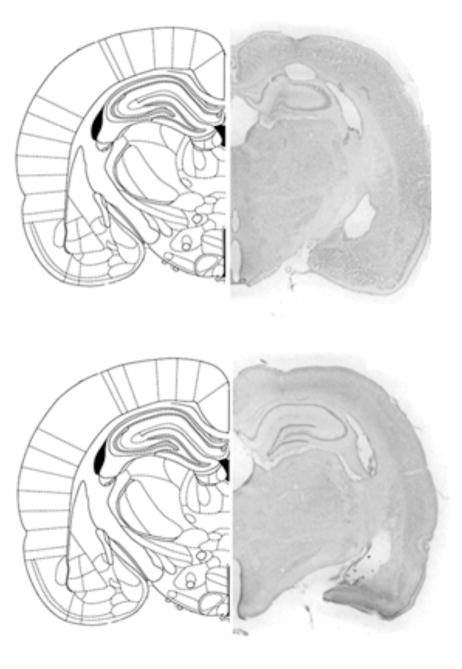
Bilateral lesions of the dDG were generated using colchicine. Anterior/posterior (A/P) coordinates used to target the dDG were 2.7 to 3.7 mm posterior to bregma. A representative dDG lesion is shown along with the corresponding A/P section from the Paxinos and Watson (1997) atlas in Figure 3A. In addition, a representative vehicle-infused control lesion is shown in Figure 3B. Intracranial infusions of the vehicle did not tend to produce any significant damage to any brain region. All colchicine induced lesions tended to be bilateral, complete within the targeted region of the dDG between 2.7 and 3.7 mm posterior to bregma, and selective with minimal damage to surrounding tissue. In addition, we did not observe any significant differences in lesion site or size between groups of animals assigned to the two test conditions, with the exception of one DG lesioned animal (assigned to the adjacent arm condition) that had complete damage to the right DG but less damage to the left DG, limited to the lateral region.
Figure 3.
Figure 3A. Histological representation of a DG lesioned rat brain and schematic drawing of corresponding coronal section (adapted from Paxinos & Watson, 1997).
Figure 3B. Histological representation of a vehicle-infused control rat brain and schematic drawing of corresponding coronal section (adapted from Paxinos & Watson, 1997).
3.2 Behavioral Results
Figure 4 shows the mean (± SE) number of trials required by dDG lesioned rats and control rats to reach the learning criterion on the separate and adjacent conditions of the place-learning task. A 2×2 analysis of variance (ANOVA) with group (dDG, control) and condition (adjacent, separate) as between-group factors was used to analyze the data. The dependent variable was the mean number of trials required to reach the learning criterion of nine correct choices out of 10 consecutive trials across 2 consecutive days of testing. The results revealed a significant main effect of group, F(1, 20) = 4.67, p < .05, indicating that control rats outperformed dDG lesioned rats regardless of task condition. There also was a significant main effect of condition, F(1, 20) = 18.94, p ≤ .001, indicating that rats acquired the spatial discriminations for the separate condition at a faster rate than the adjacent condition. In addition, there was a significant group x condition interaction, F(1, 20) = 10.07, p = .01.
Figure 4.
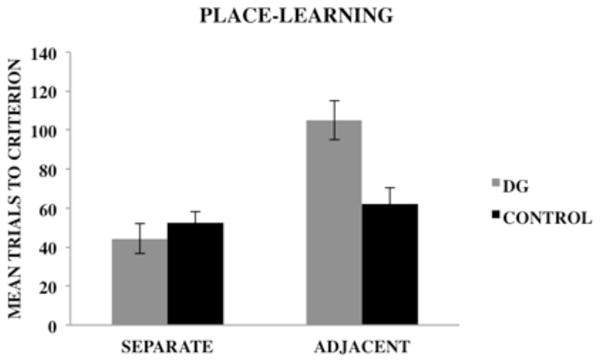
Mean (± SE) trials to criterion for dDG lesioned rats and control rats on the separate and adjacent conditions of the place-learning task.
A Newman-Keuls post hoc comparison test of the group x condition interaction revealed that there were no significant differences in the number of trials required by dDG lesioned and control rats to reach the learning criterion on the separate condition of the task. However, on the adjacent condition, dDG lesioned rats required significantly more trials (p < .05) to reach the learning criterion relative to control rats. In addition, dDG lesioned rats required significantly more trials to reach the learning criterion on the adjacent condition than the separate condition (p < .05). However, there were no significant differences in the number of trials required by control rats to reach learning criterion on the adjacent and separate task conditions.
4. Discussion
The present study investigated the role of the dDG in pattern separation during performance of a spatial reference memory task involving place learning for adjacent and separate locations (McDonald & White, 1995). The results showed that dDG lesioned animals and control animals acquired spatial discriminations for the separate condition at similar rates when overlap among the spatial cues was low and there was less need for spatial pattern separation. However, on the adjacent condition when the overlap among the cues was increased and the need for spatial pattern separation was greater, dDG lesioned animals required significantly more trials to reach the learning criterion than controls. These results suggest that dDG lesions in rats disrupt spatial pattern separation on a reference memory based task involving spatial discriminations for spatially adjacent but not spatially separated locations.
The findings of the present investigation are consistent with results of a study conducted by McDonald and White (1995) showing that damage to the dDG results in inefficient use of place information when animals are required to discriminate between spatial locations defined by a similar set of external cues. The present findings also are consistent with previous research that suggests that the DG plays an important role in pattern separation (Bakker et al., 2009; Clelland et al., 2009; Gilbert et al., 2001; Goodrich-Hunsaker et al., 2008; Lacy et al., 2010; Leutgeb et al., 2007; McHugh et al., 2007; Rolls & Kesner, 2006; Sahay et al., 2011; Schmidt, Marrone, & Markus, 2012; Talpos et al., 2010; Tronel et al., 2010).
Although a number of studies have shown that damage to the HPP or selective DG damage in rodents disrupts acquisition learning for spatial reference memory (McDonald & White, 1995; Morris et al., 1982; Okada & Okaichi, 2009; Xavier et al., 1999), relatively few animal studies have directly examined hippocampal involvement in spatial pattern separation using a reference memory task (Clelland et al., 2009; McTighe et al., 2009). Consistent with our findings, Clelland et al. (2009) showed that mice with ablated hippocampal neurogenesis were impaired on a spatial discrimination task when locations were positioned close together, but not when they were farther apart. Many of the previous studies using selective lesions to investigate DG contributions to pattern separation have included a strong working memory component. Therefore, the present study used an acquisition task that placed minimal demands on working memory (McDonald & White, 1995) to investigate dDG involvement in pattern separation processes for spatial reference memory. To the authors’ knowledge, the present study represents the first direct investigation conducted in rats to show that selective colchicine-induced lesions of the dDG disrupt pattern separation on a spatial reference memory task. The results provide further support for the importance of an intact dDG in spatial pattern separation processes and extend previous findings to include a reference memory component.
It should be mentioned that although animals with dDG lesions were impaired on the adjacent condition, their performance matched controls on the separate condition. This finding suggests that impairments on the spatial reference memory task may be attributable to less efficient spatial pattern separation rather than a direct deficit in spatial reference memory. In support of this hypothesis, a study conducted by Hunsaker and Kesner (2008) found that animals with dDG lesions showed exploration impairments on a temporal order for spatial locations task when the metric distance between spatial locations was reduced. However, the lesioned animals’ performance matched controls when the distance between locations was increased. Taken together with prior reports that DG lesions impair pattern separation on working memory tasks, current results provide further support for dDG involvement in the reduction of interference among overlapping spatial representations across a variety of tasks with different memory demands.
Previous research has shown that DG lesions in rodents impair encoding processes during new learning of spatial information (Jerman et al., 2006; Lee & Kesner, 2004). Consistent with prior investigations, results from the present study show that animals with dDG lesions are impaired in acquiring spatial discriminations for locations with a high degree of spatial similarity. This finding suggests that impairments in pattern separation for spatial reference memory may be attributable to an encoding deficit. More specifically, performance deficits in the ability to distinguish between adjacent locations might be due to impaired pattern separation during encoding of the rewarded arm vs. the adjacent nonrewarded arm and a comparison with the stored representation of the rewarded arm (which may not be very accurate in the first place due to poor pattern separation at the time of encoding). In the adjacent condition, there is high overlap among the cues associated with the rewarded arm and nonrewarded arm thus requiring pattern separation. Conversely, in the separate condition, there is less overlap among distal cues during encoding and less need for pattern separation. Although the present results are consistent with the hypothesis that dDG lesions impair the encoding of spatial discriminations involving locations with a high degree of similarity, it also is possible that the lesions impaired retrieval. Therefore, future investigations using temporary pharmacological manipulations to inactivate the dDG may provide more insight into the nature of the impairment.
Although dDG lesioned rats demonstrated initial impairments in the ability to distinguish between the rewarded arm and adjacent nonrewarded arm in the present study, the animals were able to eventually reach the learning criterion. Improvements in performance on spatial tasks following HPP or selective DG lesions have been reported in numerous studies (Costa, Bueno, & Xavier, 2005; Jarrard, Okaichi, Steward, & Goldschmidt, 1994; Xavier et al., 1999) and have suggested that animals may employ multiple response strategies in order to solve a task. It has been suggested that the HPP supports the use of place strategies to solve spatial tasks, whereas the use of other response strategies may rely on systems outside of the HPP system (O’Keefe & Nadel, 1978; Xavier & Costa, 2009). Based on these observations, one possible explanation for the present finding is that dDG lesioned animals used an egocentric response strategy based on body orientation (e.g. always turn right) in order to correctly identify the rewarded arm and locate the food reward on the adjacent arm condition. However, two different spatial configurations were randomly used to reduce the possibility of successfully using a simple response strategy to provide an accurate nonspatial solution to the task, which would have been possible if only one nonrewarded arm was used. Despite our efforts, the possibility remains that animals were able to utilize a different strategy involving other regions of the brain in order to acquire the task. Consistent with prior investigations (Xavier et al., 1999), findings from the present study suggest that dDG lesions disrupt but do not completely abolish acquisition on a spatial reference memory task.
5. Conclusions
In summary, results from the present study suggest that dDG lesions decrease efficiency in pattern separation resulting in impairments in the ability to discriminate between adjacent spatial locations defined by a similar set of external stimuli. However, when spatial locations are widely separated, there is less overlap among distal cues and less need for pattern separation. The data provide direct evidence for the role of the dorsal DG hippocampal subregion in pattern separation on a spatial reference memory task. Furthermore, these findings provide further support for a critical role for the dDG in spatial pattern separation by demonstrating the importance of a processing mechanism that is capable of reducing interference among overlapping inputs across a variety of different memory demands.
Acknowledgments
The research was supported by NIH grant #AG026505 from NIA to Paul E. Gilbert and by NIH grant #MH065314 to Raymond P. Kesner. The authors would like to thank Nora Ko and Nick Musso for their assistance with data collection and James Taylor for his assistance with histological procedures.
References
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 2. San Diego: Academic Press; 1995. pp. 443–493. [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyer P, et al. A functional role for adult neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VC, Bueno JL, Xavier GF. Dentate gyrus-selective colchicine lesion and performance in temporal and spatial tasks. Behavioural Brain Research. 2005;160(2):286–303. doi: 10.1016/j.bbr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. The Journal of Neuroscience. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Walsh TJ. Selective working memory impairments following intradentate injection of colchicine: attenuation of the behavioral but not the neuropathological effects by gangliosides GM1 and AGF2. Physiology Behavior. 1989;45(1):93–101. doi: 10.1016/0031-9384(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446(7132):190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(5):774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. The Journal of Neuroscience. 1998;18(2):804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18(9):955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behavioral Neuroscience. 1984;98(6):946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jerman JT, Kesner RP, Hunsaker MR. Disconnection analysis of CA3 and DG in mediating encoding but not retrieval in a spatial maze learning task. Learning and Memory. 2006;13(4):458–464. doi: 10.1101/lm.246906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shephard GM, editor. The synaptic organization of the brain. 5. Oxford University Press; Oxford: 2004. pp. 455–498. [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Progress in Brain Research. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the Neurosciences. 2004;15(5):333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory. 2007;14(9):625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Abrous DN. A new chapter in the field of memory: Adult hippocampal neurogenesis. European Journal of Neuroscience. 2011;33:1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning and Memory. 2010;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14(1):66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EL, McNaughton BL, Moser EI. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309(5734):619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philosophical transactions of the Royal Society of London. Series B Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behavioral Neuroscience. 1995;109(4):579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McLamb RL, Mundy WR, Tilson HA. Intradentate colchicine disrupts the acquisition and performance of a working memory task in the radial arm maze. Neurotoxicology. 1988;9(3):521–528. [PubMed] [Google Scholar]
- McNaughton BL, Nadel L. Hebb-Marr networks and the neurobiological representation of action in space. In: Gluck MA, Rumelhart DE, editors. Neuroscience and connectionist theory. Hillsdale, NJ: Erlbaum; 1990. pp. 1–63. [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;29(9):881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: A computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanry KP, Mundy WR, Tilson HA. Colchicine-induced alterations of reference memory in rats: Role of spatial versus non-spatial task components. Behavioural Brain Research. 1989;35(1):45–53. doi: 10.1016/s0166-4328(89)80007-5. [DOI] [PubMed] [Google Scholar]
- Okada K, Okaichi H. Functional differentiation and cooperation among the hippocampal subregions in rats to effect spatial memory processes. Behavioural Brain Research. 2009;200(1):181–191. doi: 10.1016/j.bbr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Olton DS. Characteristics of spatial memory. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale: Erlbaum; 1978. pp. 327–342. [Google Scholar]
- Olton DS, Becker JT, Handelman GE. Hippocampus, space, and memory. Behavioral and Brain Sciences. 1979;2:313–22. [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychological Review. 2001;108(2):311–45. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [Google Scholar]
- Renaudineau S, Poucet B, Save E. Flexible use of proximal objects and distal cues by hippocampal place cells. Hippocampus. 2007;17(5):381–395. doi: 10.1002/hipo.20277. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A computational theory of episodic memory formation in the hippocampus. Behavioural Brain Research. 2010;215(2):1265–1274. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, et al. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: The dentate gyrus and pattern separation. Behavioural Brain Research. 2012;226:56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory systems. London: MIT Press; 1994. [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): A novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiology of Learning and Memory. 2010;94(3):341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H. Hippocampal place cells can develop distinct representations of two visually identical environments. Hippocampus. 1999;9(3):235–246. doi: 10.1002/(SICI)1098-1063(1999)9:3<235::AID-HIPO4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Xavier GF, Costa VC. Dentate gyrus and spatial behaviour. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33(5):762–773. doi: 10.1016/j.pnpbp.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: Difficulties in “place strategy” because of lack of flexibility in the use of environmental cues? Hippocampus. 1999;9(6):668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]