Abstract
In vitro exposure of neural progenitor cell (NPC) populations to reduced O2 (e.g. 3% versus 20%) can increase their proliferation, survival and neuronal differentiation. Our objective was to determine if an acute (<1 hr), in vivo exposure to intermittent hypoxia (AIH) alters expansion and/or differentiation of subsequent in vitro cultures of NPC from the subventricular zone (SVZ). Neonatal C57BL/6 mice (postnatal day 4) were exposed to an AIH paradigm (20×1 minute; alternating 21% and 10% O2). Immediately after AIH, SVZ tissue was isolated and NPC populations were cultured and assayed either as neurospheres (NS) or as adherent monolayer cells (MASC). AIH markedly increased the capacity for expansion of cultured NS and MASC, and this was accompanied by increases in a proliferation maker (Ki67), MTT activity and hypoxia-inducible factor-1α (HIF-1α) signaling in NS cultures. Peptide blockade experiments confirmed that proteins downstream of HIF-1α are important for both proliferation and morphological changes associated with terminal differentiation in NS cultures. Finally, immunocytochemistry and Western blotting experiments demonstrated that AIH increased expression of the neuronal fate determination transcription factor Pax6 in SVZ tissue, and this was associated with increased neuronal differentiation in cultured NS and MASC. We conclude that in vivo AIH exposure can enhance the viability of subsequent in vitro SVZ-derived NPC cultures. AIH protocols may therefore provide a means to “prime” NPC prior to transplantation into the injured central nervous system.
Keywords: neural stem cell, neurosphere, intermittent hypoxia, population expansion, differentiation, Ki67, Pax6, HIF-1α
Introduction
The postnatal brain contains neural progenitor cell (NPC) populations within the subventricular zone (SVZ) of the lateral ventricles and in the subgranular zone of the hippocampal dentate gyrus. These NPC pools are mitotically active, multipotent (i.e., generate different cell types), and self-renewing (Gage, et al., 1995, Reynolds and Weiss, 1992, Temple, 1989). NPC from these regions are candidates for cell-based therapies following neurological insult (Sohur, et al., 2006). For example, potential NPC based therapies include targeted recruitment of endogenous populations (Teng, et al., 2006), or replacing dead or damaged cells through auto- or allograft transplantation procedures (Eftekharpour, et al., 2008). Fundamental to the success of NPC transplant is the ability to increase the overall cell yield, cell robustness (e.g. for subsequent graft survival) and/or generation of neuronal phenotypes [e.g. for the goal of gray matter replacement (Reier, 2004)] in cell cultures. Manipulation of O2 levels may provide a relatively simple means to achieve these goals. For instance, culture of NPC in a sustained 3% O2 environment [i.e. approximating physiological brain O2 levels (Studer, et al., 2000)] as compared to standard culture conditions of 20% O2 yields populations that are both more proliferative and less susceptible to programmed cell death (Chen, et al., 2007, Morrison, et al., 2000, Pistollato, et al., 2007, Studer, et al., 2000, Theus, et al., 2008). Reducing the O2 content in cell culture also impacts cell fate choice (Chen, et al., 2007) and enhances neuronal yield (Studer, et al., 2000) in progeny cells.
The aforementioned in vitro results raise the possibility of using in vivo hypoxia to achieve the same goals. For example, chronic exposure to intermittent hypoxia (CIH) can trigger proliferation in CNS postnatal neurogenic niches including the dentate gyrus of the hippocampus and the SVZ of the lateral ventricle (Zhu, et al., 2005). The Zhu et al. study establishes the proof-of-principle that in vivo CIH protocols can affect CNS progenitors. However, that study employed extended periods of IH (i.e., days to weeks), and it is unknown how rapidly NPC can respond to in vivo IH. Further, to our knowledge the impact of in vivo IH on the ex vivo expansion and differentiation of cultured NPC has not been explored. This potential application of AIH is intriguing when considering that pre-harvest in vivo hypoxia could “prime” NPC for increased in vitro expansion prior to transplant into the injured CNS.
In the present study we hypothesized that acute in vivo exposure to AIH can alter the proliferation and neuronal differentiation of NPC isolated and cultured from the SVZ of postnatal mice. We further hypothesized that AIH would activate known hypoxia-driven signaling pathways and neuronal fate choice pathways immediately following hypoxia exposures. We focused on HIF-1α, which has previously been shown to be activated in neurogenic niche sites following hypoxia exposure (Cunningham, et al., 2011, Nanduri, et al., 2008). In addition, we examined NF-κß since it is associated with NPC proliferation after hypoxia (Widera, et al., 2006). Our results demonstrate that in vivo AIH profoundly increases NPC population expansion, and that this effect is mediated through increased proliferation as evidenced by increased Ki67 expression and MTT activity. We further show evidence that HIF-1α and related downstream signaling may be involved with AIH-mediated effects on NPC proliferation and differentiation.
Materials and Methods
Animals
Neonatal C57BL/6 mice (P4) were housed in the animal care facilities at the University of Florida and bred under University of Florida in house breeding protocols. All procedures were in compliance with the regulations of the Institutional Animal Care and Use Committee.
AIH Protocol
Exposure to AIH was accomplished using a commercially available whole-body plethysmograph (Buxco Inc.) placed in a 37°C incubator. Gas influx was maintained at 1 L/min and the protocol consisted of 1 minute exposures alternating between 21% O2 and 10% O2 for 20 cycles (40 minute duration overall). Control animals were maintained at 37°C and exposed to 21% O2.
Neurosphere (NS) and Monolayer Astrocytic Stem/progenitor Cells (MASC) Culture and Growth/Differentiation Analysis
All cells were generated from the subventricular zone (SVZ) of neonatal (P4) mouse pups and dissociated within 30 minutes following AIH. NS and MASC cell cultures were generated and characterized as previously described (Marshall, et al., 2008, Ross, et al., 2008, Scheffler, et al., 2005). Briefly, tissue blocks containing the SVZ were dissected, trypsinized (0.05%), dissociated and plated overnight in growth media (Mouse Neurocult/Proliferation Supplement/10 ng/μl bFGF/EGF, Stem Cell Technologies). Non-adherent neurosphere forming cells (NFC) were incubated in trypsin, triturated, washed and plated in non-adherent flasks at clonal density (10,000 cells/cm2). Resultant NS were passaged every 5-7 days. NS plated at equal densities were assessed for total number (i.e. yield) and size as a measure of proliferative capacity (Ross, et al., 2008). Yield was determined by counting NS in random fields from experiments plated in triplicate while using a 10X microscope objective. NS size was determined by measuring diameters of NS in random fields from experiments plated in triplicate using calibrated measuring probes and digital image capture software (Leica Application Software, Version 3.50). For experiments necessitating serial passage, NS were plated in triplicate, the total number of dissociated single cells were counted with trypan blue at each passage point, and remaining cultures re-plated at 10,000cells/cm2. To assess multipotency, NS were propagated for 7 days, transferred to poly-L-ornithine-coated coverslips, and allowed to attach for 2-3 days to poly-L-ornithine coated coverslips (in triplicate) in the absence of growth factors. These conditions were used because they are known to stimulate terminal differentiation (Scheffler, et al., 2005). For morphological assessment of plated NS (Figure 5), those that had attached by 24 hours post-plating were rated for the presence or absence of significant process extension surrounding the plated NS (typical of differentiating cells under these conditions with no bias to any particular terminal cell type). For assessment of ß-III tubulin+ cells, NS were photographed from five random fields on each slide. DAPI+ only cells (negative) and DAPI/ ß-III tubulin+ cells (positive) were counted and quantified as a ratio to generate the proportion of percent beta-3-tubulin+ cells. In parallel cultures from the same primary cell slurries, MASC were defined as those cells that attached to standard tissue culture-treated flasks during the initial plating step. These cells were cultured to confluence and then passaged 2-3 times prior to assay. For growth curves, MASC were plated at equal density (50,000 cells in a T25 flask) and counted three days after plating using Trypan Blue exclusion. For, assessment ß-III tubulin+ cells, MASC plated on poly-L-ornithine coated coverslips were counted in the same manner as NS.
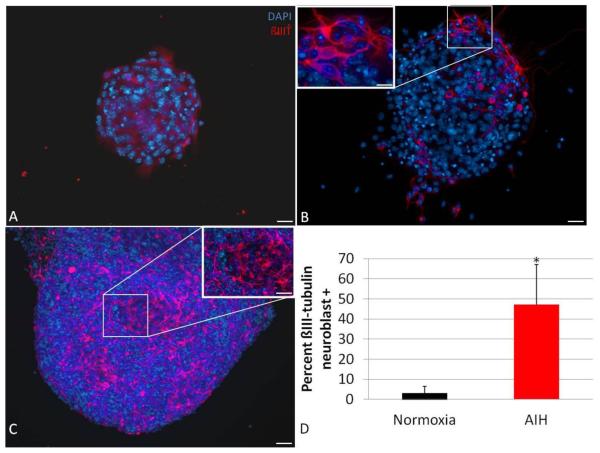
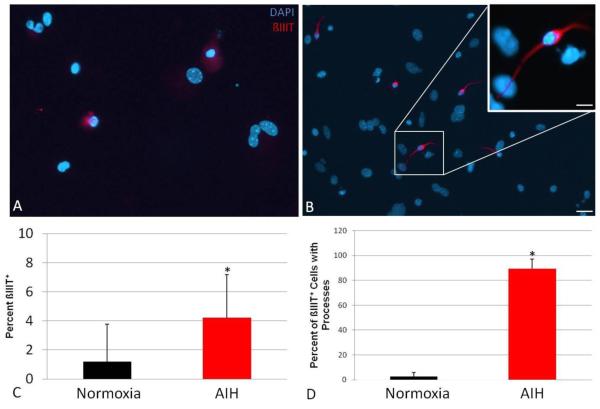
Figure 5. In vivo AIH increases neuronal differentiation in cultured NS progeny.
NS from control or AIH-treated animals were plated without growth factors for 2-3 days and assessed for expression of a neuronal marker via immunocytochemistry. Compared to controls (A, scale bar = 50μm), fully formed NS from IH-treated animals (B, scale bar = 50μm) yielded larger with more distinct ß-III tubulin+ neuroblast progeny (red: ß-III tubulin, blue: DAPI). The inset in panel B is 60X image of boxed area, scale bar = 10μm. Panel C shows a representative image of particularly large ß-III tubulin+ sphere-derived projections obtained from cultures from an AIH-treated animal; scale bar = 100μm. The inset in C is a 40X image of the boxed area; scale bar = 50μm. ß-III tubulin positivity is quantified in panel D as percent of cells that were ß-III tubulin positive (*: p<0.05).
MTT assay and EPO/VEGF antibody blockade
For the MTT 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Promega, #G4001), single cells (5000) from dissociated, second passage NS were plated in triplicate in 96 well plates. Cells were exposed to vehicle control, erythropoietin (EPO) neutralizing antibody (R&D Systems, AF959, [1.25 μg/ml]) or vascular endothelial growth factor (VEGF) neutralizing antibody (R&D Systems, AF493NA, [0.6 μg/ml]) for 72 hours in the presence of mitogenic growth factors (see growth media, above). At 72 hours, cells were exposed to MTT dye and then incubated at 37°C for 4 hours. MTT Solubilization/Stop solution (solubilizes the reaction product, formazan) was added, and one hour later, absorbance was read at 570 nm (PowerWave XS2, Biotek). Background absorbance was read at 630 nm and subtracted from final readings.
Immunocytochemistry
Cells were prepared for immunocytochemistry as previously described (Ross, et al., 2008). Briefly, cells were fixed for 30 minutes (4% paraformaldehyde) and blocked for 1 hour (PBS/0.01% TritonX-100/10% FBS). Primary antibody (mouse anti-ß-III tubulin 1:1000, Promega #G7121; rabbit anti-Ki67 1:2000, Santa Cruz #sc-15402) was applied overnight at 4°C. Coverslips were washed and incubated with fluorescence-conjugated secondary antibody (HRP-conjugated goat anti-mouse or goat anti-rabbit 1:500, Rockland Immunochemicals). Slips were washed and mounted on positively charged glass slides in Vectashield containing DAPI counterstain. Fluorescence micrographs were obtained with a Leica DM5000B epifluorescence microscope (Spot CCD digital camera) or an Olympus IX81-DSU spinning disc confocal microscope (Hammamatsu CCD digital camera). Image analysis was conducted using Leica Application Suite Version 3.50 or 3i SlideBook software.
Western Blots
Protein was subjected to Western blot analysis as previously described (Ross and Fillmore, 2007, Ross, et al., 2008). Briefly, SVZ tissue blocks were harvested from animals 30 minutes following AIH protocol termination. Tissue was homogenized in RIPA buffer with a motorized mortar and pestle (DAKO). Protein concentration was determined with a standard assay (DC Protein Assay, Biorad) and equal amounts of protein lysate (15-20 μg) were subjected to electrophoresis and transferred to nitrocellulose (NuPage System, Invitrogen). Membranes were blocked for 1 hour (TBS-Tween 20/0.05% milk). Primary antibody (rabbit anti-Pax6 1:200, abcam #ab5790; mouse anti-cyclophilin A 1:2000, Santa Cruz #sc-133494; rabbit anti-hypoxia-inducible factor-1alpha, HIF-1α 1:100, santa cruz sc-10790; anti-p65 1:400, abcam ab7970; anti-p50 1:400, abcam ab-7971) was applied overnight at 4°C. Membranes were washed and incubated in secondary antibody (HRP-conjugated goat-anti-rabbit or –anti-mouse, 1:2000, Rockland) at room temperature for 1 hour. Membranes were exposed to ECL detection (HyGlo, Denville Scientific) and developed for 1-5 minutes. Image J software was employed for densitometric quantitation protein bands (Ross and Fillmore, 2007, Ross, et al., 2008).
Statistical Analysis
Results are expressed as the mean ± standard deviation, where indicated. Hypoxia treatment effects were examined using two-way repeated measures ANOVA with Student-Newman-Keuls post-hoc analysis (factor 1 = time; factor 2 = treatment), Student’s t-test, or a Chi-square test as appropriate (SPSS Statistics Software Version 17.0 or StigmaStat Version 2.03).
Results
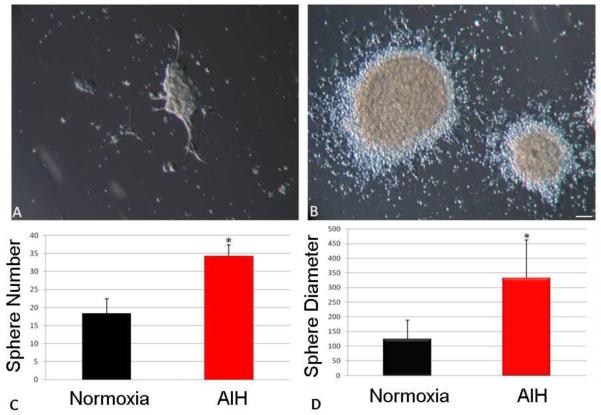
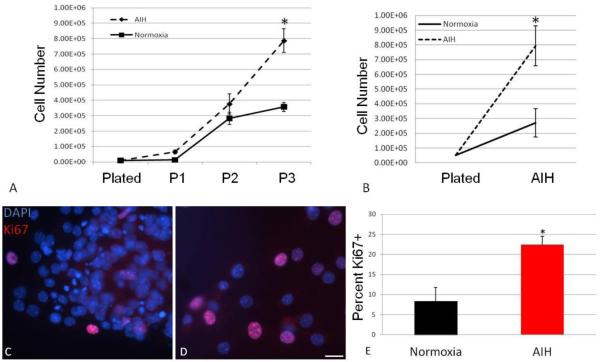
To assess the effect of AIH on the expansion of NPC cultures, growth parameters of neurosphere forming cells, the resultant neurospheres (NS), and also monolayer astrocytic stem/progenitor cell (MASC) populations were studied. Neurosphere forming cells were plated in triplicate with each replicate generated from an individual animal. The overall NS yield was compared between cultures generated from control and AIH-treated animals (Fig. 1A-B). The NS yield from AIH-treated animals was approximately two-fold greater than in control cultures (Fig. 1C, p<0.05). NS derived from AIH-treated pups were also considerably larger than controls (Fig. 1D, p<0.0001). In a second set of experiments, NS were serially passaged every 4-5 days to enable assessment of longer-term expansion rates. Over three successive passages, NS populations from AIH-treated animals expanded at rates greater than control (Fig. 2A). In parallel experiments, MASC cultures were established, with replicates again representing individual animals. Over a three day period, MASC from AIH-treated animals demonstrated more rapid expansion (doubling time of 0.75 days) compared to control cells (doubling time of 1.23 days). Thus, by 72 hours post-plating there were substantially more MASC from AIH than control mice (p<0.001, Fig. 2B). These results suggest increased proliferation in NPC populations, and thus immunocytochemistry was performed for expression of a proliferation marker (Ki67). At 4 days post-plating, NS from AIH-treated animals demonstrated a more than two-fold increase in the number of Ki67-positive cells (Fig. 2C-E, p<0.005 vs. control).
Figure. 1. In vivo AIH increases in vitro NFC survival and expansion of NFC progeny cells.
Single-cell NFC from control or treated animals were plated at clonal density (10,000 cells/cm2) with each replicate representing an individual animal, and cultured into full NS. Representative examples of NFC derived from the SVZ are provided for control (A) and AIH-treated pups (B, scale bar = 100μm). On average, AIH treated animals yielded a greater number of NS (C), reflecting increased survival of NFC. Individual NS from AIH-treated animals were also significantly larger than those from control animals (D), reflecting increased expansion within individual NS.
Figure. 2. In vivo AIH increases in vitro NS and MASC expansion and proliferation.
NS were serially passaged every 4-5 days and assessed for population expansion by counting single, dissociated cells with a Trypan Blue exclusion assay. Compared to normoxic (control) NS, those from AIH-treated animals exhibited progressive population expansion (A, *: P<0.001 interaction between time and treatment). In addition, three days after plating, more MASC could be detected in cultures from AIH-treated animals compared to controls (B*: P<0.001 interaction between time and treatment). In parallel wells, NS progeny were assessed for expression of the proliferation marker, Ki67. Representative images showing Ki67 (red) and DAPI immunostaining (blue) are shown in panels C (AIH-treated) and D (control) (scale bar = 25μm). Compared to control, cells from AIH-treated animals exhibited a significantly greater number of Ki67 positive cells (E, *: P<0.005).
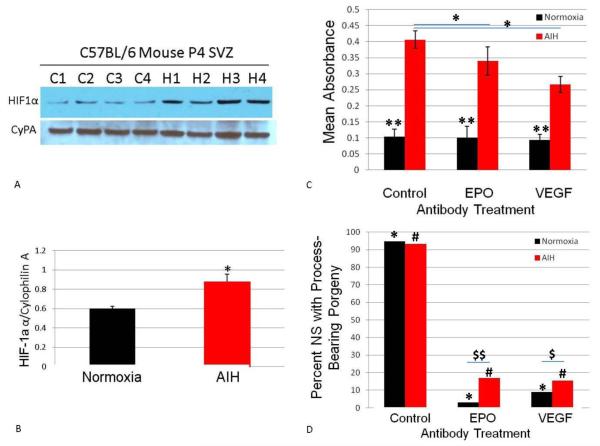
To begin to identify signaling pathways triggered by AIH, we first assessed HIF-1α protein levels obtained from SVZ tissue blocks. Prior work has shown HIF-1α activation in CNS neurogenic niches following sustained hypoxia or chronic intermittent hypoxia (Cunningham, et al., 2011, Nanduri, et al., 2008). Compared to controls, HIF-1α was upregulated in AIH tissues (Fig. 3A-B, p<0.05). To determine if pathways downstream of HIF-1α signaling contribute to NPC proliferation, we next conducted an MTT cell proliferation assay. In these experiments, NS were cultured with or without neutralizing antibodies for two downstream gene targets of HIF-1α —EPO or VEGF. In control NS cultures, inclusion of the neutralizing antibodies did not impact proliferation (Fig. 3C, black series). In contrast, neutralizing antibodies partially reduced proliferation in AIH cultures (Fig. 3C, * p<0.05). Importantly, both with and without the neutralizing antibodies, MTT activity was markedly higher in AIH cells (Fig. 3C, red series, **p=0.01).
Fig. 3. HIF-1α signaling downstream of AIH affects NPC proliferation and differentiation.
Freshly dissociated tissue from normoxic or AIH-treated animals was flash frozen, protein isolated, and subjected to Western blot analysis. HIF-1α protein was increased immediately following AIH as shown in the representative protein bands in panel A. Bands are shown from multiple animals with “C” indicating control tissue and “H” indicating AIH treated tissue. Densitometry results are expressed as a ratio of HIF-1α to Cyclophilin A and are depicted in panel B (*, p<0.05 vs. normoxia). Panel C shows the results of experiments in which cultured NS from normoxic or AIH-treated animals were dissociated to single-cells and plated for MTT assay with or without EPO or VEGF neutralizing antibodies. NS cultures from normoxic animals (black bars) exhibited less MTT activity than those from AIH-treated animals across all conditions (red bars). AIH-treated NS exhibit only a partial reduction in MTT activity under the same antibody blockade conditions (black series). Panel D depicts second passage NS plated under conditions for terminal differentiation (i.e. in the absence of mitogens on poly-L-ornithine coated coverslips) in the presence or absence of neutralizing antibodies for EPO or VEGF. Inclusion of EPO or VEGF neutralizing antibodies reduced the presence of process-bearing cells radiating away from the NS perimeter (indicative of differentiating progeny cells) in both control (normoxic) and AIH cells (*, #, p<0.0001). This reduction was less severe in AIH cells ($$, p<0.005; $, p<0.05).
The neutralizing antibodies also altered morphology of plated NS (Fig. 3D). Under control conditions, cultures from both normoxic and AIH-treated animals produced NS with process-bearing progeny cells at the periphery (Fig. 3D, “control” pair). However, inclusion of EPO or VEGF neutralizing antibodies in resulted in a near-truncation of this morphological quality (Fig. 3D, p<0.0001 for both control cells and AIH treated cells). Nevertheless, cultures from AIH-treated cells maintained a greater percentage of process-bearing NS periphery cells in the presence of neutralizing antibodies (Fig. 5C, p<0.005 for control cells and p<0.05 for AIH-treated cells).
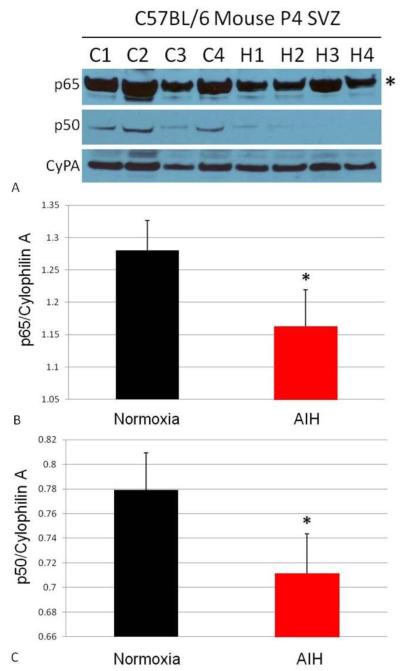
NPC proliferation following sustained hypoxia is associated with NF-κß pathway activation in the neurogenic niche (Hu, et al., 2005, Nanduri, et al., 2008). Accordingly, the impact of AIH on expression of the NF-κß pathway was examined in SVZ tissue blocks (Fig. 4). Both the p65 and p50 subunits of NF-κß were significantly downregulated immediately following AIH (Fig. 4A-C, p65, p<0.05; p50, p<0.05). Taken together, our data demonstrate HIF-1α pathway activation, activity of proteins downstream of HIF-1α signaling, and a decrease in NF-κß protein levels immediately following AIH protocol administration.
Figure 4. In vivo AIH affects NF-κß signaling.
The same tissue from Fig. 3 was also interrogated for NF-κß protein expression via Western blot (A). C1-C4 indicates replicates of control (normoxic) animals; H1-H4 indicates replicates of AIH-treated animals. Expression of the NF-κß p65 subunit (A, top panel, band indicated with asterisk; quantitated in B) and p50 subunit (A, middle panel, quantitated in C) were downregulated in SVZ tissue immediately following AIH. In panels B and C the “*” indicates p<0.05 vs. normoxia tissues. Densitometry results are expressed as a ratio of the protein of interest to Cyclophilin A.
We next assessed neuronal differentiation in NS progeny cells. (Fig. 5) Fully grown NS were plated in the absence of mitogens for 2-3 days and then assessed for neuroblast morphology and expression of a neuronal marker (ß-III tubulin). NS from both control and AIH animals had diffuse ß-III tubulin staining over non-differentiated progeny cells with a “flat” morphology (Fig. 5A-B). However, cultures from AIH-treated animals had a higher percentage of NS containing ß-III tubulin-positive cells with a characteristic neuroblast morphology—i.e. having a distinct, compact soma and one or more distinct processes (Fig. 5B-D, p<0.05). We also noted that a subset of NS from AIH-treated animals were extremely large and/or multilobular. These larger NS appeared to be highly neurogenic as evidenced by particularly robust ß-III tubulin staining (Fig. 5C). We qualitatively noted that ß-III tubulin+ neuroblasts from AIH animals had longer and more ramified processes as compared those present in control NS (Fig. 5B,C insets).
MASC populations were also assessed for neuronal differentiation (Fig. 6). Coordinate with the NS results, AIH was associated with an unexpected increase in the number of spontaneous ß-III tubulin+ MASC progeny cells in the presence of growth factor—the formation of which is typically dependent upon growth factor withdrawal (Fig. 6A-C, p<0.05). Furthermore, the majority of ß-III tubulin+ cells in cultures from AIH-treated animals exhibited neuroblast morphology, characterized (as above) by a distinct soma and ramification of processes extending from the soma (Fig. 6B inset). Quantitatively, a greater percentage of ß-III tubulin+ cells showed processes extending from the soma in cells from AIH vs. control pups (Fig. 6D, p<0.0001). Collectively, these results show that MASC from AIH-treated animals contain a higher percentage of spontaneously-forming neuroblasts.
Figure 6. In vivo AIH increases neuronal differentiation in cultured MASC progeny.
MASC from control or treated animals were plated in triplicate on poly-L-ornithine coverslips and analyzed for ß-III tubulin positivity, with each replicate representing an individual animal. Compared to controls (A), cultures from AIH-treated animals (B) were observed to contain a greater number of spontaneously-formed neuroblasts prior to growth factor withdrawal. The scale bars in panel B indicate 25μm and 5 μm, respectively. Panel C shows the percentage of cells which were positive for ß-III tubulin (*, p<0.05). Panel D shows the percent of beta-3 tubulin+ cells that showed distinct processes extending from the cell body (*, p<0.0001).
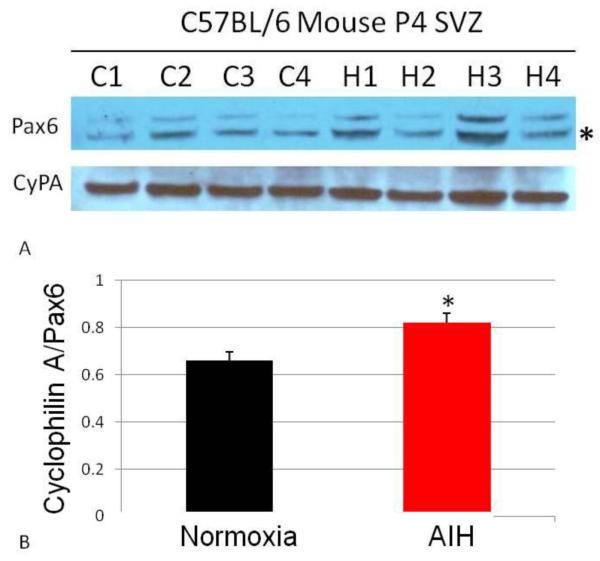
Additional experiments further examined the molecular responses that could be responsible for altered neurogenesis following AIH. We started with Pax6, a highly conserved transcription factor which is a potential key regulator of neuronal cell fate choice (Kallur, et al., 2008). Western blots were used to quantify Pax6 protein levels in SVZ tissues isolated immediately following AIH treatment (Fig. 7A). Densitometry analyses indicated that Pax6 protein was moderately elevated after AIH (Fig. 7B, p<0.05), suggesting that Pax6 could be part of the mechanism whereby AIH influences SVZ-derived cells.
Figure 7. In vivo AIH increases Pax6 expression in SVZ tissue.
SVZ tissue was harvested immediately following AIH treatment and analyzed for Pax6 expression via Western blot (panel A). C1-C4 indicates replicates of control (normoxic) animals; H1-H4 indicates replicates of AIH-treated animals. Densitometry data shown in panel B and are expressed as ratio of Pax6 expression to Cyclophilin A expression (*, p<0.05).
Discussion
The present study is the first to describe the ex vivo behavior of neural progenitor cell (NPC) cultures following in vivo exposure to brief intermittent hypoxia. We have demonstrated that in vivo acute intermittent hypoxia (AIH) significantly increases the expansion of subventricular zone (SVZ)-derived progenitor populations and their progeny. These data suggest that AIH merits further study as an in vivo tool to “prime” NPC populations intended for use in neural transplantation paradigms.
AIH increases proliferation in SVZ-derived NPC cultures
The enhanced growth of monolayer astrocytic stem/progenitor cells (MASC) and serially passaged neurospheres (NS) suggest that AIH initiates processes that influence subsequent in vitro cell proliferation and/or cell death (Fig. 1-2). The robust increase in Ki67 following AIH (Fig. 2C-E) is consistent with the former possibility. Ki67 is an antigen associated with the nucleus during interphase and mitosis phase of the cell cycle, and is strictly associated with cell proliferation (Starborg, et al., 1996). Further, MTT activity assay results demonstrate a significant increase in cellular metabolism, indicative of greater population expansion (Fig. 3C).
AIH increases neuronal differentiation in SVZ-derived NPC cultures
The diffuse staining of NS with a “flat cell” morphology that was observed in control tissues is consistent with prior observations that some progeny in cultured NS can possess this morphology and co-express both neuronal and glial markers (Laywell, et al., 2005, Rosser, et al., 1997) (Fig. 5A-B). However, AIH appears to increase the proportion of NS progeny cells possessing a more specific neuroblast morphology and immunophenotype. Specifically, we observed that AIH was associated with an increase in the neuronal yield of cultured NPC populations (Fig. 5-6). SVZ tissue from control neonates yielded fewer NS with distinct ß-III tubulin+ foci as compared to NS from AIH-treated pups (Fig. 5D). One potential caveat is that the overall percentage of ß-III tubulin+ foci in cultured NS can show considerable variability (H. H. Ross, unpublished observation). Nevertheless, direct comparison between control and AIH tissues revealed robust differences in the appearance and number of neuroblasts generated from NS. This relationship remains consistent over repeated trials, and in preliminary studies in rat tissues, suggesting conservation between mammalian species (H. H. Ross, unpublished observation).
Our data are generally consistent with prior studies of the response of cultured NPCs to in vitro relative hypoxia. This work has established that reducing O2 levels can increase proliferation, reduce apoptosis and enhance neuronal yield (Chen, et al., 2007, Morrison, et al., 2000, Pistollato, et al., 2007, Studer, et al., 2000, Theus, et al., 2008). It should be noted that reduced oxygen in cell culture (e.g., 3% O2) can be considered “relative normoxia” as compared to standard culture condition (e.g., 20% O2), and may approximate more closely to brain tissue oxygen levels. In contrast to these prior studies, the current experiments were conducted on cells exposed to hypoxia in vivo, and subsequently cultured in standard in vitro conditions of 20% O2. The effects of AIH reported here were observed following several days of culture and therefore several rounds of replication, suggesting that in vivo AIH could affect a wide range of cellular properties, including but not limited to epigenetic cell programming. In contrast to the prior in vitro work, the sustained exposure to lowered O2 in culture conditions was not required for the effects to manifest after in vivo AIH.
Molecular mechanisms associated with AIH-mediated changes in SVZ-derived NPC cultures
Hypoxia is a potent stimulator of multiple pathways that mediate cell or tissue repair in the injured CNS, and is proposed to be an important factor in in vivo stem cell maintenance (Harms, et al., 2010, Mohyeldin, et al., 2010). A complete understanding of signaling pathways activated in SVZ-derived NPC populations following in vivo hypoxia has not been achieved, in particular related to how hypoxia-driven signaling results in increased neurogenesis. Two inter-related candidate signaling pathways of particular interest are the hypoxia-inducible factor-1 alpha (HIF-1α) pathway and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κß) (Culver, et al., 2010, Gorlach and Bonello, 2008). In an immediate response to hypoxia, HIF-1α protein already present in the cell is post-translationally regulated though stabilization, dimerization, and nuclear translocation. Upon translocation, HIF-1 heterodimers transcriptionally regulate several downstream gene targets. This has been shown to occur in CNS neurogenic niches following sustained hypoxia or chronic intermittent hypoxia (Cunningham, et al., 2011, Nanduri, et al., 2008). A recent study demonstrated that transplanted NPC following stroke mediate neuroprotection though activation of the HIF-1α-VEGF pathway (Harms, et al., 2010). In the current experiments, HIF-1α protein levels were significantly upregulated in the SVZ of AIH-treated animals as compared to controls (Fig. 3A-B). Our MTT assays established that AIH-treated NS demonstrated significantly greater MTT activity, associated with a greater rate of proliferation. AIH-treated NS that were cultured in the presence of neutralizing antibodies for proteins downstream of HIF-1α signaling, EPO or VEGF, exhibited a partial reduction of MTT activity, suggesting their involvement in AIH-mediated increases in NS proliferation (Fig. 3C, red series).
The majority of NS in cultures that were grown in the absence of neutralizing antibodies for EPO or VEGF produced cells with morphological changes associated with terminal differentiation into CNS cell types [i.e. process-bearing morphology at the periphery (Fig. 5D, control pair)]. This was true of both control and AIH-treated NS. It should be noted that these periphery cells are typically non-neuronal in phenotype, and do not adopt a neuroblast morphology or immunophenotype. This observation could indicate that the effects of IH may be specific to neurons in these cultures, although further study will be required to evaluate this possibility. Contrary to our expectations, the inclusion of EPO or VEGF neutralizing antibodies resulted in a near-truncation of this morphological quality of plated NS (Fig. 3D, EPO and VEGF pairs). Therefore, these pathways may affect cell differentiation in a hypoxia-independent manner. However, the morphological changes associated with blocking the EPO and VEGF pathways observed in these experiments were partially attenuated in AIH cultures (Fig. 3D, red series). This observation could reflect activation of other HIF-1α target genes or of unknown pathways following AIH. Alternatively HIF-1α-driven increases in EPO and VEGF protein levels could necessitate a higher titer of neutralizing antibody to produce the same reduction in process extension. It should be emphasized that HIF-1α signaling can affect expression of a very large number of genes (Cunningham, et al., 2011), and this fact should be kept in mind when interpreting the present results. Study of a wider panel of responsive genes is likely to reveal additional candidate genes related to the physiological effects of AIH. However, these initial results suggest a novel role for the HIF-1α, EPO and VEGF signaling pathways in SVZ-derived NPC population dynamics, including proliferation rate and neurogenesis.
NF-κß is another hypoxia-regulated transcription factor. Sustained hypoxia results in phosphorylation of inhibitor of NF-κß (Iκß) proteins and subsequently increased transcription of the NF-κß gene (Gorlach and Bonello, 2008). The NF-κß pathway is activated in the neurogenic niche following hypoxia (Hu, et al., 2005) and has been shown to be involved in increased proliferation of NPC proliferation following sustained hypoxia (Widera, et al., 2006). Chronic IH can also activate NF-κß, but through unknown mechanisms (Greenberg, et al., 2006). The immediate response of NF-κß pools have not been studied following acute IH. Unexpectedly, our results show that protein levels of both p50 and p65 NF-κß subunits were downregulated in SVZ tissues immediately following AIH protocols (Fig. 4A-C). Further study is required to determine if the transcriptional pathway is activated at later time points following AIH treatment, as well as to directly study any link between HIF-1α and NF-κß signaling pathways following this stimulus.
Preliminary protein studies of SVZ tissue extracts immediately following AIH suggested no increases in the neuronal markers MAP2 or ß-III tubulin (data not shown). We therefore sought to look at an upstream neuronal fate control gene that could be activated by AIH. One candidate gene is paired box gene 6 (Pax6). Pax6 is a well-conserved transcription factor with an established role in sensory organ development, and is also a potential key regulator of neuronal cell fate choice (Kallur, et al., 2008). Western blotting results showed a moderate elevation of Pax6 protein levels in SVZ tissues isolated immediately following AIH treatments (Figure 7). This result suggests that the Pax6 pathway could be important for neuronal programming in SVZ-derived NPC following AIH. However, the relative elevation in Pax6 was modest, and further study is required to determine if additional pathways involved with neuronal cell fate choice are activated following AIH.
Conclusion
This work is the first to establish that in vivo exposure to a brief period during which O2 oscillates between normoxia and moderate hypoxia can influence the behavior and biology of subsequently cultured NPC. However, several key questions remain. For example, further study of apoptosis/necrosis pathways will be required to determine if AIH pre-conditioning is also associated with reduced in vitro apoptosis or other cell death pathways. Additionally, it is unclear if the effects of AIH are due to the oscillations in O2 levels, or if it is the stimulus of hypoxia that is the essential factor. Further study of gene expression and protein levels in NS/MASC cultures following in vivo AIH as compared to a sustained stimulus are required to gain a better understanding of proliferation, apoptosis, and/or cell fate choice following AIH.
We suggest that AIH could provide a powerful “priming tool” for NPC that could be employed for therapeutic manipulation. For example, AIH pre-treatment could enhance the yield and neuronal potency of NPC cultures, and could therefore improve the functional integration of these cells when placed into the injured CNS. The advantage of this approach is the relative ease of application and mild nature of the stimulus to the subject. Finally, as similar AIH protocols are currently being evaluated as a rehabilitation tool in pre-clinical animal studies (Dale-Nagle, et al., Vinit, et al., 2009) and human trials (Trumbower, et al., 2011), AIH represents a translationally-relevant paradigm that merits further study in the context of NPC harvest and transplantation.
Highlights.
in vitro hypoxia has been shown to enhance stem/progenitor cell proliferation and neuronal differentiation.
We examine the ability of in vivo acute intermittent hypoxia to elicit similar effects.
Only a brief exposure to systemic intermittent hypoxia results in increased expansion and neuronal progeny from subventricular zone progenitors.
This finding supports the use of intermittent hypoxia as a priming tool for progenitor cells and their downstream applications.
Acknowledgements
This work is supported by the following grants: NICHD Training Grant 5K12HD055929 (HHR) NIH 1 R21 HL104294-01 (DDF). The authors wish to thank Dr. Dennis Steindler, Daniel J. Silver and the University of Florida Cell and Tissue Analysis Core (CTAC) for assistance with spinning disk image acquisition. The authors also wish to thank Dr. Nadeem Shafi for thoughtful discussions regarding Pax6 and Dr. Andrew Judge for NF-κß antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- 2.Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol. 2010;30:4901–4921. doi: 10.1128/MCB.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham LA, Candelario K, Li L. Roles for HIF-1alpha in neural stem cell function and the regenerative response to stroke. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci. 1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008;24:E19. doi: 10.3171/FOC/2008/24/3-4/E18. [DOI] [PubMed] [Google Scholar]
- 6.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 7.Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. Biochem J. 2008;412:e17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Nesic-Taylor O, Qiu J, Rea HC, Fabian R, Rassin DK, Perez-Polo JR. Activation of nuclear factor-kappaB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J Neurochem. 2005;93:26–37. doi: 10.1111/j.1471-4159.2004.02968.x. [DOI] [PubMed] [Google Scholar]
- 11.Kallur T, Gisler R, Lindvall O, Kokaia Z. Pax6 promotes neurogenesis in human neural stem cells. Mol Cell Neurosci. 2008;38:616–628. doi: 10.1016/j.mcn.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Laywell ED, Kearns SM, Zheng T, Chen KA, Deng J, Chen HX, Roper SN, Steindler DA. Neuron-to-astrocyte transition: phenotypic fluidity and the formation of hybrid asterons in differentiating neurospheres. J Comp Neurol. 2005;493:321–333. doi: 10.1002/cne.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall GP, 2nd, Ross HH, Suslov O, Zheng T, Steindler DA, Laywell ED. Production of neurospheres from CNS tissue. Methods Mol Biol. 2008;438:135–150. doi: 10.1007/978-1-59745-133-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci. 2007;35:424–435. doi: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 20.Ross HH, Fillmore HL. Identification of a novel human MT5-MMP transcript variant in multipotent NT2 cells. FEBS Lett. 2007;581:5923–5928. doi: 10.1016/j.febslet.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 21.Ross HH, Levkoff LH, Marshall GP, 2nd, Caldeira M, Steindler DA, Reynolds BA, Laywell ED. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells. 2008;26:3218–3227. doi: 10.1634/stemcells.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosser AE, Tyers P, ter Borg M, Dunnett SB, Svendsen CN. Co-expression of MAP-2 and GFAP in cells developing from rat EGF responsive precursor cells. Brain Res Dev Brain Res. 1997;98:291–295. doi: 10.1016/s0165-3806(96)00189-7. [DOI] [PubMed] [Google Scholar]
- 23.Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohur US, Emsley JG, Mitchell BD, Macklis JD. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361:1477–1497. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starborg M, Gell K, Brundell E, Hoog C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci. 1996;109(Pt 1):143–153. doi: 10.1242/jcs.109.1.143. [DOI] [PubMed] [Google Scholar]
- 26.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- 28.Teng YD, Liao WL, Choi H, Konya D, Sabharwal S, Langer R, Sidman RL, Snyder EY, Frontera WR. Physical activity-mediated functional recovery after spinal cord injury: potential roles of neural stem cells. Regen Med. 2006;1:763–776. doi: 10.2217/17460751.1.6.763. [DOI] [PubMed] [Google Scholar]
- 29.Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to Acute Intermittent Hypoxia Augments Somatic Motor Function in Humans With Incomplete Spinal Cord Injury. Neurorehabil Neural Repair. 2011 doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 31.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widera D, Mikenberg I, Kaltschmidt B, Kaltschmidt C. Potential role of NF-kappaB in adult neural stem cells: the underrated steersman? Int J Dev Neurosci. 2006;24:91–102. doi: 10.1016/j.ijdevneu.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res. 2005;1055:1–6. doi: 10.1016/j.brainres.2005.04.075. [DOI] [PubMed] [Google Scholar]