Abstract
The N-methyl-d-aspartate (NMDA) receptor antagonist ketamine has rapid and potent antidepressant effects in treatment-resistant major depressive disorder and bipolar depression. These effects are in direct contrast to the more modest effects seen after weeks of treatment with classic monoaminergic antidepressants. Numerous open-label and case studies similarly validate ketamine’s antidepressant properties. These clinical findings have been reverse-translated into preclinical models in an effort to elucidate ketamine’s antidepressant mechanism of action, and three important targets have been identified: mammalian target of rapamycin (mTOR), eukaryotic elongation factor 2 (eEF2), and glycogen synthase kinase-3 (GSK-3). Current clinical and preclinical research is focused on (a) prolonging/maintaining ketamine’s antidepressant effects, (b) developing more selective NMDA receptor antagonists free of ketamine’s adverse effects, and (c) identifying predictor, mediator/moderator, and treatment response biomarkers of ketamine’s antidepressant effects.
Keywords: NMDA receptor antagonist, rapid-acting antidepressants, major depressive disorder, bipolar depression, preclinical models of depression, mammalian target of rapamycin, mTOR
INTRODUCTION
The treatment of major depressive disorder (MDD) and bipolar depression (BDep) has historically focused on monoamine modulation, largely owing to the initially unexpected discovery that tricyclic antidepressants and monoamine oxidase inhibitors preferentially inhibited the reuptake and breakdown, respectively, of dopamine, norepinephrine, and serotonin. Indeed, monoamine research in depression dominated most of the twentieth century owing to the wide popularity of second-generation antidepressants, e.g., the selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and atypical antidepressants that more selectively modulate monoamine reuptake and receptor activation/inhibition. Although not more effective than earlier antidepressants, these medications had much better adverse-effect and toxicological profiles with rare lethality in overdose. However, several large real-world effectiveness trials conducted over the past 20 years—including the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) (1) and Combining Medications to Enhance Depression Outcomes (CO-MED) (2) trials—found that first-line antidepressants and combinations thereof are unfortunately ineffective for many patients (3). In addition, all currently approved monoaminergic antidepressants require weeks to months of daily administration to obtain maximum efficacy, placing a considerable personal, psychosocial, and professional burden on affected individuals and the health-care system (4). Typical interventions that alleviate symptoms of MDD and BDep differ greatly from interventions for other medical conditions in that the latter are often more rapid and robust (e.g., loop diuretics in congestive heart failure and aerosolized sympathomimetics/anticholinergics in asthma).
Due to the widespread treatment resistance to standard antidepressants and mood stabilizers in individuals with mood disorders, as well as the slow onset of action of these agents, there is a critical need for rapid-acting antidepressants with alternative mechanisms of action (5). Initial research and development in this area focused on neuropeptide-based strategies (6) such as corticotropin-releasing hormone receptor antagonists and neurokinin 1 receptor antagonists. Although these agents were effective in preclinical models of despair, they unfortunately lacked clinical efficacy. Interestingly, both monoamines and neuropeptides modulate neurotransmission, unlike the primary central nervous system (CNS) neurotransmitters glutamate and γ-aminobutyric acid (GABA). Glutamate N-methyl-d-aspartate (NMDA) receptor antagonists were initially studied for the prevention of glutamate-based (excitotoxic) cell death in chronic neurodegenerative disorders and stroke. Although NMDA receptor antagonists were not successful in treating these disorders, continued preclinical research found that the NMDA receptor antagonists AP-7 and MK-801 had antidepressant-like effects (7). These antagonists were not available for testing in humans; however, the anesthetic ketamine, which had been safely used for decades (8), was a reasonable alternative for translational studies. In slightly more than a decade, the emergence of ketamine’s rapid antidepressant effects has been viewed by some experts in the field as arguably the most important psychiatric discovery in half a century (9).
This article discusses the pharmacokinetics and pharmacodynamics of ketamine and reviews the evidence in support of ketamine’s rapid antidepressant effects drawn from randomized, placebo-controlled studies; open-label trials; and case series/reports. We also explore biomarkers of ketamine’s antidepressant properties and recent data regarding ketamine’s mechanism of action in preclinical models of depression. We conclude by reviewing the emerging literature describing other NMDA receptor antagonists in depressive disorders (10) and specify future directions based on the outstanding needs and questions in the field.
THE PHARMACOLOGY OF KETAMINE
Ketamine is a voltage-dependent NMDA receptor antagonist that inhibits the flux of cations (calcium, sodium) in the presence of the coagonists glutamate and glycine. The S(+) and R(−) enantiomers have differential affinity for the NMDA receptor, with the S(+) enantiomer having greater biological potency as evidenced by decreased mean plasma concentration at the termination of successful anesthesia (11). Ketamine also affects other neurotransmitters including norepinephrine, acetylcholine, and serotonin. For instance, ketamine metabolites (norketamine enantiomers) were recently found to inhibit whole-cell patch-clamp currents by antagonizing α7 nicotinic acetylcholine receptors (12). These off-target sites mediate ketamine’s acute but transient cardiovascular, respiratory, and sympathomimetic effects (13).
Ketamine also inhibits voltage-gated sodium and calcium channels as well as the production of nitric oxide by stimulating the l-arginine/nitric oxide/cyclic guanosine monophosphate pathway; this stimulation is accomplished by neuronal nitric oxide synthase to produce analgesic and anesthetic effects (14). For the past several decades, ketamine has been used as an FDA-approved dissociative anesthetic. It is typically used as surgical premedication owing to its potent anesthetic properties, relatively minor effects on vital signs, and low risk of respiratory depression. Because of this safety profile, ketamine has also been used as a research tool to transiently induce or exacerbate multidimensional schizophrenia-like symptoms (8, 15, 16).
Ketamine can be administered intravenously, intramuscularly, intranasally, sublingually, rectally, orally, and topically. With oral administration, ketamine undergoes extensive first-pass metabolism in the liver by CYP3A4 (major), CYP2C9 (minor), and CYP2B6 (minor) to norketamine (via N-demethylation). It is believed to have lower biological potency than unmetabolized ketamine but threefold higher plasma levels. The oral preparation has relatively poor gastrointestinal absorption (20% bioavailability), but nonparenteral preparations have much higher bioavailability: intranasal, 25–50%; intramuscular, 93%; and intravenous, ~99–100%. Maximal plasma concentrations take less than 1 min to peak with intravenous ketamine and take only slightly longer with other preparations (roughly 5–15 min for intramuscular preparations and 30–60 min for oral preparations). Ketamine’s plasma half-life is 1 to 3 h (17), and norketamine’s plasma half-life is 12 h. Although ketamine is still detectable in serum, its anesthetic and psychotomimetic effects often wear off within 30 min to 1 h after administration.
KETAMINE IN CLINICAL DEPRESSION
Rapid and Robust Antidepressant Efficacy
Berman and colleagues (18) were the first to report that a single subanesthetic (0.5 mg/kg) dose of ketamine rapidly reduced standardized depression scores in patients with MDD. Although small (n = 7 completers), this initial study revealed a moderate-to-large effect size and paved the way for future studies. The finding was replicated in several large samples with single (19–21) and repeated (22, 23) ketamine administration. Ketamine also has rapid antidepressant effects in MDD patients resistant to electroconvulsive therapy (ECT) (24) and rapidly resolves suicidal ideation (25), including in acutely suicidal patients in the emergency department (26). Ketamine has also been shown to reduce both explicit and implicit measures of suicidal cognition (27).
Table 1 summarizes the three randomized, placebo (saline)-controlled trials of ketamine in treatment-resistant MDD and the two randomized, placebo (saline)-controlled trials in refractory BDep. In patients with MDD, ketamine maintained a moderate-to-large effect with response rates of ~70% and remission rates of ~30% at 24 h postinfusion; effects were sustained for up to one week. In patients with treatment-resistant BDep, ketamine was an effective add-on therapy to lithium or divalproex (28, 29). The antidepressant effect size in patients with BDep remained large one day after infusion in both published studies. This result is particularly important because our BDep studies were the first to demonstrate therapeutic success associated with direct engagement of a specific CNS target (specifically, the NMDA receptor complex). Indeed, all prior treatment trials in BDep with demonstrable efficacy were based on repurposed antiepileptic or antipsychotic medications that had elusive CNS targets for mood stabilization.
Table 1.
Published randomized, controlled trials of ketamine for major depression
| Reference | Diagnosis | n | Comorbidity | Dose/route | Adjunctive medications |
Effect size at 24 h postinfusion |
Effect size at 1 week postinfusion |
Response rate at 24 h postinfusion |
Response rate at 1 week postinfusion |
Remission rate at 24 h postinfusion |
Remission rate at 1 week postinfusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | MDD and BDep (TRD not reported) | 8 | Current anxiety disorder: 13% | 0.5 mg/kg racemic/IV | None | d = 0.90 (95% CI: 0.69–1.11) | N/A (final endpoint at 72 h) | 25% (2/8) | N/A (final endpoint at 72 h) | 0% (0/8) (HDRS ≤7) | N/A (final endpoint at 72 hours) |
| 19 | MDD/TRD | 17 | Lifetime anxiety disorder: 65% | 0.5 mg/kg racemic/IV | None | d = 1.46 (95% CI: 0.91–2.01 | d = 0.68 (95% CI: 0.13–1.23) | 71% (12/17) | 38% (6/16) | 29% (5/17) (HDRS ≤7) | 31% (5/16) (HDRS ≤7) |
| 28 | BDep/TRD | 16 | Lifetime anxiety disorder: 35% | 0.5 mg/kg racemic/IV | Li or VPA | d = 0.67 (95% CI: 0.42–0.91) | d = 0.16 (95% CI: −0.09–0.41) | 44% (7/16) | 29% (4/14) | 31% (5/16) (MADRS <10) | 14% (2/14) (MADRS <10) |
| 20 | MDD (TRD not reported) | 10 | Current anxiety disorder: 20% | 0.5 mg/kg racemic/IV | None | d = 0.80 (95% CI: 0.55–1.05) | d = 0.53 (95% CI: 0.22–0.84) | 20% (2/10) | 20% (2/10) | 20% (2/10) (HDRS ≤7) | 30% (3/10) (HDRS ≤7) |
| 29 | BDep/TRD | 14 | Lifetime anxiety disorder: 73% | 0.5 mg/kg racemic/IV | Li or VPA | d = 0.70 (95% CI: 0.42–0.98) | d = 0.13 (95% CI: −0.14–0.41) | 43% (6/14) | 8% (1/12) | 29% (4/14) (MADRS <10) | 0% (0/12) (MADRS <10) |
Abbreviations: BDep, bipolar depression; CI, confidence interval; HDRS, Hamilton Depression Rating Scale; IV, intravenous; Li, lithium; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; TRD, treatment-resistant depression; VPA, valproic acid.
Nonintravenous ketamine preparations such as oral (30, 31) and intramuscular (32, 33) have also shown antidepressant efficacy, and intranasal ketamine is of interest owing to its high CNS penetrance and ease of administration. Furthermore, several of the clinical trials described above found that ketamine had anxiolytic properties; as a result, its use in other psychiatric disorders is being explored. A small open-label clinical trial at Yale of patients with treatment-resistant obsessive-compulsive disorder (OCD) found that ketamine had antidepressant effects in patients with comorbid depression (34). Since that initial trial, a placebo-controlled ketamine trial in OCD at Columbia has been completed; it revealed rapid and sustained anti-OCD effects up to one week postinfusion (35). Also, Mount Sinai has completed an active placebo (midazolam) trial of ketamine in posttraumatic stress disorder; those results await publication (J.W. Murrough & D.S. Charney, unpublished results; ClinicalTrials.gov identifier: NCT00749203).
Maintaining Antidepressant Response
In the randomized, controlled trials cited above, the antidepressant effects of ketamine lasted, on average, one to two weeks. In the only randomized, placebo-controlled extension trial with a single subanesthetic infusion, approximately 27% of ketamine responders maintained efficacy during the 28-day follow-up period (average time to relapse = 13.2 days; standard error of the mean = 2.2) (36). Given the growing evidence for ketamine’s robust—although transient—antidepressant effects, interest in sustaining these effects has naturally increased. The most obvious method of maintaining response involves multiple infusions, which have proven efficacious in several reports (37–39). These results have led some groups to propose ketamine maintenance therapy and/or “boosters” upon early detection of clinical deterioration, which have proven efficacious in ECT responders.
Several case series and small clinical studies have also revealed protracted antidepressant effects (including sustained remission) with repeat-dose ketamine (22, 39, 40). In the largest repeat-infusion trial, 24 patients with treatment-resistant MDD received up to six intravenous infusions of 0.5 mg/kg ketamine over 12 days. At the end of the trial, 70.8% of the patients remained in remission up to 83 days after treatment began; mean time to relapse was 18 days after the last infusion (38). Dissociative side effects did not worsen, and tolerance did not develop with subsequent infusions (38). Another repeat-dose ketamine protocol in patients with treatment-resistant MDD used twice-weekly 0.5 mg/kg open-label ketamine infusions for 100 min total for two weeks or until remission was obtained (41). In that study, 5 of 10 patients achieved remission within two weeks, and 2 patients sustained remission for four weeks. As in the previous repeat-dose study, no protracted adverse effects were observed. The effects of repeat-dose ketamine may last even longer in certain patients. Blier and colleagues (39) described a 44-year-old woman with treatment-resistant MDD who received approximately 40 ketamine infusions; she exhibited sustained antidepressant effects but no long-term cognitive or other adverse sequelae.
Riluzole, an FDA-approved medication for the treatment of amyotrophic lateral sclerosis, has also been used in two ketamine extension studies (21, 36). Riluzole is a glutamate modulator (42) with preliminary efficacy as monotherapy (43) and adjunctive therapy (44–46) in patients with treatment-resistant MDD. Both studies found a main antidepressant effect at the end of the four-week protocol, which is substantial in a treatment-resistant population; however, no statistically significant difference between riluzole and placebo was observed (21, 36). In the first study (21), 14 patients who met response criteria 72 h after subanesthetic ketamine infusion participated in a 32-day, randomized, double-blind, placebo-controlled, flexible-dose (100–200 mg/day) continuation trial. An interim analysis found no significant differences in the main outcome measure, time to depressive relapse (80% of patients relapsed on riluzole versus 50% on placebo); as a result, the trial was stopped for futility. In the second study (36), 42 subjects with treatment-resistant MDD were randomized four to six hours after ketamine infusion to double-blind treatment with either riluzole (100–200 mg/day; n = 21) or placebo (n = 21) for four weeks; again, no difference between the riluzole and placebo groups was found. Nevertheless, 27% of the ketamine responders randomized to either riluzole or placebo did not relapse at the end of the four-week protocol. This result is clinically significant in this treatment-refractory population.
ECT has also been proposed as an augmentation and/or maintenance strategy for ketamine’s acute antidepressant effects, and an initial case report (47), a retrospective cohort study (48), and a single-blind longitudinal study (49) obtained promising initial results. Wang and colleagues (50) randomized depressed subjects to one of three groups for anesthesia (n = 16 in each group): propofol, ketamine (0.8 mg/kg), or ketamine + propofol. All subjects then received a single ECT treatment and were followed for one week. More rapid and lasting antidepressant effects were seen in both the ketamine and ketamine + propofol groups than in the group receiving propofol alone. However, because the seizure energy index and duration were greater in the ketamine groups, and because ketamine decreases seizure threshold (51), the effects may be confounded by seizure strength and duration instead of a specific effect of ketamine. In another intriguing study, Abdallah and colleagues (52) randomized treatment-resistant MDD and BDep patients to either thiopental or thiopental + ketamine (0.5 mg/kg) prior to a six-treatment ECT course. Although ECT was effective, ketamine had no group or group-by-time augmenting antidepressant effect; in addition, thiopental anesthesia and/or ECT impaired ketamine’s rapid and robust antidepressant properties (52). In the most recent studies, a Finnish group indicated that an S-ketamine bolus (0.4 mg/kg) did not improve the speed or magnitude of antidepressant response to ECT in severe or psychotic treatment-resistant MDD (53), whereas in a sample of treatment-resistant depressed patients in Australia, racemic ketamine slightly improved efficacy in the first two weeks over placebo when combined with thiopentone in ultrabrief pulse-width right unilateral ECT (54). Given the small size and varied design of these studies, no definitive conclusions may be drawn regarding ketamine and ECT. On the basis of the inverted-U dose response curve observed for ketamine’s antidepressant-like effects in preclinical studies (55, 56), one might not expect antidepressant effects with the higher anesthetic doses used in some of the aforementioned studies. The forthcoming results of ketamine/ECT trials may elucidate these outstanding issues (http://www.clinicaltrials.gov/ct2/results?term=ketamine+AND+depression+AND+ECT&Search=Search).
Future studies will continue to explore novel, postketamine pharmacological relapse prevention strategies. Indeed, numerous alternative treatment strategies to extend antidepressant efficacy are currently under investigation. These include traditional antidepressants, mood stabilizers [because both ketamine and lithium are glycogen synthase kinase-3 (GSK-3) inhibitors], antipsychotics [because of their efficacy in BDep (57) and as augmenting agents in MDD (58)], and evidence-based psychotherapy.
Limitations and Concerns
Several limitations and clinical caveats require discussion. First, randomized, controlled trials involving intravenous ketamine have used 0.5 mg/kg for 40 min and, given that no dose-finding studies have been reported, whether this is indeed the optimal antidepressant dose is presently unclear. A second issue of concern is the adequacy of investigator and patient blinding with the use of an inert placebo (normal saline). However, a dual-site active placebo (intravenous midazolam) parallel arm study was recently completed (59; see also Reference 10). Although intravenous midazolam does not fully mimic the pharmacological effects of ketamine, the acute sedative/intoxicating effects of both drugs provide better blinding and confirmed previous controlled ketamine studies in which saline was used. Third, the long-term sequelae of repeat-dose ketamine remains unclear. Ketamine is recreationally abused in the United States (60–64), in the United Kingdom, and in Asia (65–67). Chronic recreational use of NMDA receptor antagonists such as ketamine and phencyclidine is correlated with working memory deficits and other psychopathological effects analogous to those observed in schizophrenia (68–70). In addition, chronic NMDA receptor antagonist administration correlates with neurotoxicity and white matter dysfunction in preclinical (71, 72) and human (22, 73–75) studies. As a result, several authors have expressed concerns about sensitization to repeated intermittent administration of ketamine (76).Many ketamine abusers, however, use in excess of several hundred milligrams per week, a dose far greater than the 0.5 mg/kg infusion dose used in most depression studies (77). Also, as a street drug, ketamine may be adulterated with other substances of abuse, making it difficult to infer any causal relationship between ketamine and neuropathological sequelae. Notably, no deleterious long-term neuropsychiatric or medical consequences have been observed in the numerous studies of single or repeated infusions of subanesthetic-dose ketamine in either healthy volunteers or depressed patients. For instance, Perry and colleagues (8) noted that over the course of many research studies at Yale (415 patients who received at least one active ketamine infusion), only 10 adverse effects were noted in nine subjects (2% of subjects and 1.5% of all infusions). Murrough and colleagues (38) similarly observed no serious adverse events with repeated ketamine infusions. Despite this safety profile, ketamine use must be preceded by careful screening of past and present substance use disorders to mitigate the risk of addiction.
Biomarkers of Ketamine’s Treatment Response
The identification of baseline predictor and treatment response biomarkers has been a major focus of investigation by our group and others. Although all of the biomarkers described below require validation and qualification before any firm conclusions are drawn, several promising avenues (summarized in Table 2) have emerged.
Table 2.
Biomarkers of ketamine’s antidepressant response
| Reference | Finding(s) |
|---|---|
| Genetics/BDNF | |
| 78 | No change in peripheral BDNF levels without enrichment |
| 81 | Increase in SWA from baseline + increase in peripheral BDNF→>antidepressant response |
| 82 | Greater antidepressant response in BDNF val66val66 >met haplotype |
| 27, 86 | Family history of alcohol dependence→>antidepressant response |
| Functional neuroimaging/electrophysiology | |
| 91 | ↑ Pretreatment rostral ACC activity with fearful faces (MEG)→>antidepressant response |
| 92 | Pretreatment pregenual ACC–amygdala desynchronization (MEG)→ >antidepressant response |
| 92 | ↓ Baseline pregenual ACC activity (MEG)→>antidepressant response |
| 94 | ↑ Baseline slow-wave delta sleep activity (EEG)→>antidepressant response |
| 95 | ↑ Baseline-to-post-treatment somatosensory evoked potential (MEG)→ >antidepressant response |
| 20 | Baseline-to-post-treatment GABA and glutamate in occipital cortex (1H-MRS) not correlated with antidepressant response |
| 103 | Pretreatment PFC GABA and glutamate (1H-MRS) not correlated with antidepressant response |
| 103 | ↓ Baseline PFC Glx/glutamate (1H-MRS)→>antidepressant response |
| Other | |
| 105 | ↑ [Norketamine] metabolite within 230 min postinfusion→<antidepressant response in BDep |
| 107 | Antidepressant response correlates with ↑ peripheral mTOR expression (single patient) |
Abbreviations: ACC, anterior cingulate cortex; BDep, bipolar depression; BDNF, brain-derived neurotrophic factor; EEG, electroencephalography; GABA, γ-aminobutyric acid; Glx, MRS-detectable glutamine + glutamate; MEG, magnetoencephalography; MRS, magnetic resonance spectroscopy; mTOR, mammalian target of rapamycin; PFC, prefrontal cortex; SWA, slow-wave activity.
Several major positive findings have involved brain-derived neurotrophic factor (BDNF). This is hardly surprising because the discovery that low peripheral BDNF levels respond to effective antidepressant treatment is one of the best-replicated findings in MDD, which is especially impressive considering the clinical heterogeneity of MDD (78, 79). In an admittedly small sample, our group did not observe a correlation between peripheral BDNF levels and ketamine’s antidepressant response (80). Subsequent studies found that changes in peripheral BDNF (a major inducer of synaptic plasticity) were directly proportional to change from baseline slow-wave sleep electroencephalography (a surrogate marker of synaptic plasticity) in ketamine responders (81). Another study (82) identified the BDNF val66met rs6265 single-nucleotide polymorphism as a predictor of ketamine response; in that study, BDNF val/val homozygotes (n = 41) had a better antidepressant response to ketamine than the met haplotype (n = 21). Smaller hippocampal volumes were observed in BDep patients with the met allele (83), and knockin of the BDNF met allele in rodents led to basal prefrontal cortex (PFC) synaptic deficits and decreased synaptic strengthening in response to subanesthetic ketamine (84).
Several studies have shown that a key predictor biomarker of ketamine’s antidepressant efficacy is a first-degree relative with a history of an alcohol-use disorder. In both MDD (85) and BDep (86), subjects with a positive family history of alcoholism had a more robust and sustained antidepressant response, which is consistent with the differential effects of ketamine in recently detoxified alcoholics (87) and in healthy volunteers with a positive family history of alcohol dependence (88). Genetic polymorphisms in glutamate genes may have contributed to this effect. For instance, the BDNF val66met rs6265 A haplotype, which is overrepresented in subjects with comorbid depression and alcoholism, had a better antidepressant response to sertraline (89).
Noninvasive neuroimaging and neurophysiological techniques have also been used to probe the neural correlates of ketamine’s antidepressant response (90). For instance, treatment-resistant MDD subjects with increased pretreatment rostral anterior cingulate cortex reactivity to fearful faces had an augmented antidepressant response to ketamine (91). A magnetoencephalography study administered a spatial working memory task at baseline, preketamine, and postketamine and found that performance on this task predicted antidepressant response (92). Increased baseline slow-wave sleep activity—particularly the delta sleep ratio as defined by the delta wave intensity between the first nonrapid eye movement and second nonrapid eye movement periods (decreased in many patients with MDD; improvement correlates with maintenance of remission) (93)—positively correlated with ketamine’s antidepressant effects (94). Next, increased tactile stimulus-evoked somatosensory cortical response from baseline to post–ketamine infusion was also correlated with increased antidepressant effects (95). Finally, a positron emission tomography study of 20 patients with treatment-resistant MDD measured regional cerebral glucose metabolism at baseline and following ketamine infusion. Although whole-brain metabolism did not change significantly following ketamine, regional metabolism decreased significantly in the habenula, insula, and ventrolateral and dorsolateral prefrontal cortices of the right hemisphere. Metabolism increased postketamine in bilateral occipital, right sensorimotor, left parahippocampal, and left inferior parietal cortices. Improvement in depression ratings correlated directly with change in metabolism in right superior and middle temporal gyri. Conversely, clinical improvement correlated inversely with metabolic changes in the right parahippocampal gyrus and temporoparietal cortex (96).
Because ketamine increases glutamate in the rodent PFC, acute and/or chronic changes in glutamate have been hypothesized to contribute to ketamine’s antidepressant response. One study of MDD patients found that changes in glutamate, its precursor glutamine, and GABA did not correlate with ketamine’s antidepressant efficacy in the occipital cortex (20). Notably, baseline glutamine + glutamate and GABA levels are increased (97) and decreased (98), respectively, in the occipital cortex and are corrected with standard antidepressant treatments in MDD (99–102). In the MDD PFC, pretreatment glutamate and GABA did not correlate with antidepressant response to ketamine (20, 103); however, in one of the studies (103), the baseline Glx [combination of magnetic resonance imaging (MRS)-detectable glutamine + glutamate]/glutamate ratio was lower in patients with greater antidepressant response, and pretreatment glutamate levels were increased in patients whose anxiety symptoms improved with ketamine. In a healthy volunteer study, subanesthetic-dose ketamine caused no acute changes in Glx or glutamate (104); however, that study used a 3-tesla magnet, and many authors believe that reliably measuring glutamate at this magnetic strength by 1H-MRS is not possible (97). Although this sample was small, several groups are concurrently investigating ketamine’s effect on amino acid neurotransmitters in both healthy volunteers and depressed patients, so additional data are forthcoming.
Finally, we performed a correlational analysis of ketamine metabolite levels in MDD and BDep with nosology, antidepressant response, and acute adverse effects (105). Interestingly, higher levels of (2S,5S;2R,5R)-norketamine—a norketamine enantiomer—were associated with nonresponse to ketamine in patients with BDep within 230 min. Increased levels of several hydroxylated ketamine metabolites were also associated with lower psychotomimetic side effects. No association was found between several cytochrome P450 genes (responsible for ketamine metabolism) and antidepressant efficacy.
Taken together, the studies described indicate that ketamine likely increases neural connectivity by enhancing synaptic plasticity in several brain regions implicated in depression (e.g., PFC, anterior cingulate cortex, amygdala, and hippocampus). These neuromodulatory effects may improve our existing descriptive and clinically heterogeneous nosology, allow quantification of pretreatment antidepressant probability of efficacy, and provide more reliable measures than those provided by clinical assessments alone. Nevertheless, these promising biomarkers of treatment response require replication/validation in larger studies to advance to surrogate endpoint status (106).
KETAMINE IN PRECLINICAL RESEARCH
mTOR, p70S6K, 4E-BP1
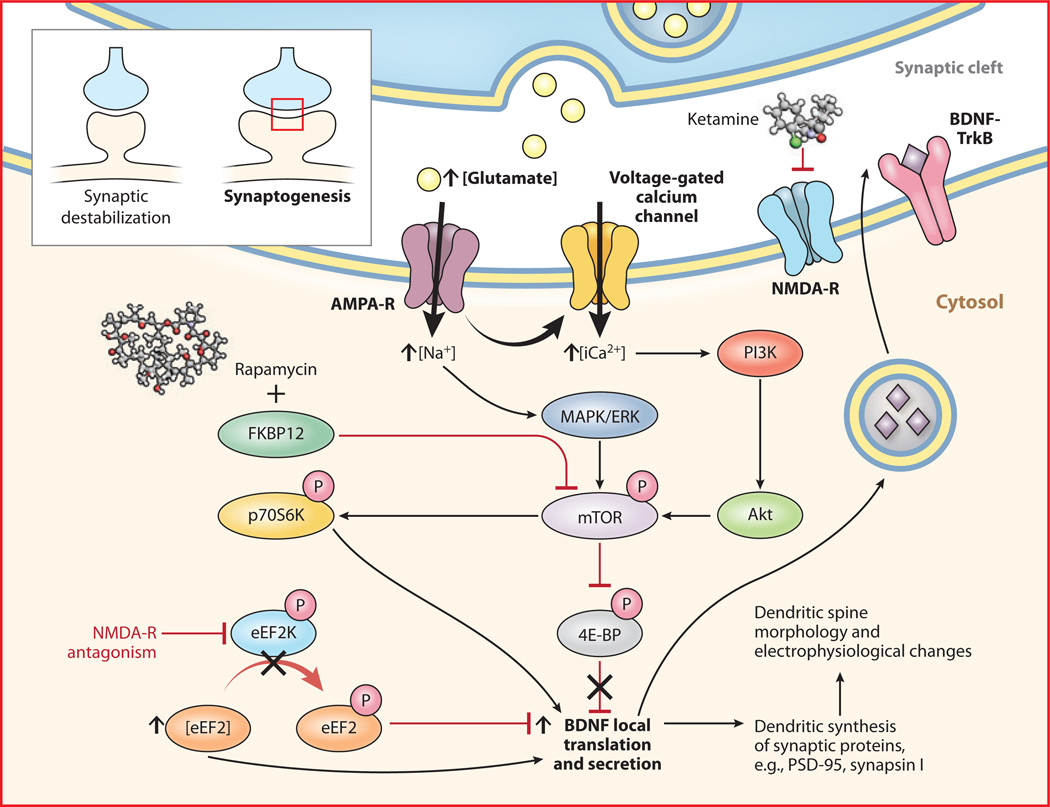
The numerous clinical studies demonstrating ketamine’s rapid antidepressant properties generated preclinical interest in elucidating its mechanism of action in ways presently inaccessible in clinical populations. Ketamine and other NMDA receptor antagonists increase mature synaptic protein expression; promote synaptogenesis; increase the number, morphology, and activity of spine synapses; and reduce depressive-like behaviors in rodent models of depression (Figure 1) (55, 56). In one of two recent mechanistic landmark studies (56), ketamine increased expression of several postsynaptic density proteins (Arc, PSD-95, GluR1, and synapsin I) in PFC synaptosomal preparations approximately 1 to 2 h postinjection. Protein levels remained elevated for up to 72 h, consistent with the time course for the induction of new spine synapses. Induction of synaptosomal PSD-95, GluR1, and synapsin I was abrogated by pretreatment with rapamycin, which selectively inhibits mammalian target of rapamycin (mTOR). Ketamine also increased mTOR, p70 S6 kinase (p70S6K), and 4E-binding protein 1 (4E-BP1) phosphorylation within 1 h of infusion; interestingly, all these proteins stimulate the transcription of target genes involved in synaptogenesis. At 24 h postinfusion, ketamine increased the number of mature-appearing “mushroom” spines and increased the frequency and amplitude of excitatory postsynaptic currents induced by serotonin (from cortico-cortical synapses) and hypocretin (from apical thalamo-cortical synapses) in PFC pyramidal neurons. Again, these effects were prevented by rapamycin pretreatment. Low-dose (10–20 mg/kg) but not high-dose (80 mg/kg, which is an anesthetic dose) ketamine rapidly reversed despair-like phenotypes, i.e., impairments on the learned helplessness, forced swimming, and novelty-suppressed feeding tests, all of which were blocked by rapamycin pretreatment. Intracerebroventricular pretreatment with the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) inhibitor U0126 and the phosphoinositide-3 kinase (PI3K)/Akt inhibitor LY294002 also negated ketamine’s antidepressant effects. These biochemical, electrophysiological, and behavioral antidepressant-like effects were replicated in a rodent model of chronic 21-day) unpredictable stress. Taken together, the results suggest that mTOR activation via known intracellular second messenger/signal transduction mediators, e.g., MAPK/ERK and PI3K, is necessary for ketamine’s rapid antidepressant-like effects in rodents (56). Although these exciting preclinical results await translation, one case report found that peripheral mTOR phosphorylation correlated with ketamine’s antidepressant response in a single patient with treatment-resistant MDD (107).
Figure 1.
Synaptic and intracellular processes activated by the rapid-acting antidepressant ketamine. Preliminary preclinical and unpublished clinical data suggest that postsynaptic NMDA receptor antagonism increases presynaptic glutamate release (i.e., glutamate “surge”). Glutamate is then hypothesized to increase AMPA/NMDA receptor flux. AMPA channel opening in the CNS increases sodium and, indirectly, calcium, stimulating the PI3K cascade to phosphorylate mTOR through Akt. Activated mTOR then phosphorylates p70S6K, increasing translation of downstream postsynaptic targets (notably, mTOR activity can be inhibited by rapamycin through the formation of an inhibitory complex with FKBP12). Activated mTOR also inhibits 4E-BP to relieve inhibition upon translation. NMDA receptor activation also inhibits eEF2K, which increases levels of dephosphorylated eEF2. Dephosphorylated eEF2 relieves inhibition upon BDNF translation in dendritic spines and promotes local secretion, which, in turn, binds to cognate TrkB receptors to activate intracellular mTOR and its downstream targets. In sum, the translational activation induced by acute NMDA receptor blockade increases the expression of several neuromodulatory proteins involved in, among other effects, postsynaptic scaffolding, neurotransmitter dynamics, and dendritic spine morphogenesis from synaptically unstable filopodia to synaptically dynamic mushroom-shaped spines (see inset), which form the morphological substrate for antidepressant-like behavioral effects. Through release of inhibition upon local translation of BDNF, ketamine increases excitatory postsynaptic currents in prefrontal cortical and hippocampal neurons. Abbreviations: 4E-BP, 4E-binding protein; AMPA, 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid; AMPA-R, AMPA receptor; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; eEF2, eukaryotic elongation factor 2; eEF2K, eEF2 kinase; ERK, extracellular signal-regulated kinase; FKBP, FK506 binding protein; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NMDA, N-methyl-d-aspartate; NMDA-R, NMDA receptor; p70S6K, p70 S6 kinase; PI3K, phosphoinositide-3 kinase; TrkB, neurotrophic tyrosine kinase, type 2.
Interestingly, the antidepressant effects of ketamine may depend on a rapid (within 30 min of administration) presynaptic glutamate “surge” in critical brain regions such as the PFC and hippocampus. In 13C-MRS ex vivo studies of rats, subanesthetic (30 mg/kg) but not anesthetic (80 mg/kg) ketamine significantly increased the percentage of 13C-detectable glutamate, glutamine, and GABA in the medial PFC (108). As detected by microdialysis, amedial PFC glutamate surge occurs with infusion of subanesthetic (10–30 mg/kg) but not higher-dose (50 and 200 mg/kg) ketamine on a time course consistent with mTOR activation (peaks within 30–60 min and returns to baseline at approximately 2 h) (109). As mentioned above, no difference was observed in occipital cortical Glx at 3 and 24 h after ketamine infusion (20), which might have missed the window to detect this rapid glutamate surge.
eEF2K/eEF2/BDNF
In a second landmark mechanistic study,Monteggia and colleagues (110) reported that ketamine’s rapid antidepressant effects depended on desuppression of BDNF translation in the hippocampus. On the forced swim test, an inducible BDNF knockout mouse was minimally responsive to the antidepressant effects of ketamine (110). These effects were reduced by the translational inhibitor anisomycin but not by the RNA polymerase/transcriptional inhibitor actinomycin D. Consistent with prior studies (55, 56), the antidepressant effects of ketamine could be prevented by pretreatment with NBQX, a 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid (AMPA) receptor antagonist. Ketamine decreased the activity of eukaryotic elongation factor 2 kinase (eEF2K) [or calcium-calmodulin dependent protein kinase III (CaMKIII)], thereby reducing eEF2 phosphorylation and desuppressing BDNF translation (110). These results were replicated using another NMDA receptor antagonist, MK-801, as well as more specific eEF2K inhibitors; interestingly, BDNF conditional knockout mice were insensitive to eEF2K inhibition (110). In this study, ketamine-induced mTOR phosphorylation was not increased, and the antidepressant-like effects were not blocked with rapamycin. [Potential explanations for the differences in these two important mechanistic studies are presented in the excellent review by Duman et al. (111).]
GSK-3
Like lithium, ketamine inhibits GSK-3 by increasing its phosphorylation. Phospho-GSK-3 interacts with a “destruction complex” of other proteins that target it for degradation in lysosomes, thereby allowing the transcription factor β-catenin to translocate from the cytosol to the nucleus and transcribe target genes (112). Conversely, a recent rodent study found that intracerebroventricular infusion of GSK-3 attenuated ketamine’s psychotomimetic-like effects (113). The GSK-3 inhibitor lithium, as well as more selective GSK-3β inhibitors, potentiated mTOR signaling, synaptogenesis, and antidepressant-like effects of lower-dose ketamine (<10 mg/kg) (114, 115). Because GSK-3 inhibition reduces both depressive and psychotic symptoms, it is possible that small-molecule inhibitors may ultimately be effective mood stabilizers in cases of BDep and MDD that include psychotic features. Indeed, psychotically depressed patients have been excluded from all ketamine depression trials to date over concerns of worsening psychosis, which has been observed in healthy volunteers (116) and individuals with schizophrenia (16, 117) exposed to subanesthetic doses of ketamine.
OTHER NMDA RECEPTOR ANTAGONISTS
Owing to ketamine’s dissociative side effects and its potential for long-term toxicity, researchers have sought more selective NMDA receptor antagonists that replicate ketamine’s antidepressant effects but not its psychotomimetic and other adverse effects. Toward this end, the NMDA receptor antagonist memantine, which is approved for the treatment of moderate-to-severe Alzheimer’s-type dementia, has been studied in mood disorders. Several preclinical reports in rodent models of depression have noted memantine’s antidepressant-like effects (118, 119). However, the first clinical report in MDD was an 8-week, placebo-controlled trial that demonstrated a lack of efficacy of memantine monotherapy (5–20 mg/day) (120). Another small, placebo-controlled, 12-week trial in depressed older patients who had recently suffered a disabling medical event confirmed memantine’s lack of efficacy with regard to depressive and functional outcomes (121). In contrast, memantine does show some success in treating BDep. In a randomized, placebo-controlled, 8-week trial of individuals with BDep inadequately treated with stable-dose lamotrigine, escalating-dose memantine was superior to placebo after 4 weeks but not at trial endpoint (122). In another study, an increased rate of response was seen with adjunctive memantine, although no statistically significant difference versus placebo was observed after 8 weeks (123). Subsequently, a case report explored the antidepressant efficacy of repeat-dose ketamine followed by memantine; although this patient was eventually on seven psychotropic medications, she remained in remission for at least 13 weeks (124). Echoing the previous link between NMDA receptor antagonists and ketamine response, another randomized, non-placebo-controlled trial of memantine (20 mg/day) versus escitalopram (20 mg/day) in patients with MDD comorbid with alcohol dependence found that memantine had antidepressant and anxiolytic effects, improved psychosocial functioning, and decreased alcohol consumption (125, 126).
Other NR2B-specific inhibitors have demonstrated preclinical efficacy in depression. Maeng and colleagues (127) found that the NR2B-selective NMDA receptor antagonist Ro(25)-6981 had antidepressant-like effects similar to those of MK-801, a nonselective NMDA receptor antagonist. These effects were eliminated by pretreatment with NBQX, implicating AMPA receptor activation as a primary mediator of NMDA receptor antagonists’ antidepressant effects (127). In the study by Li and colleagues (55), Ro(25)-6981 increased the expression of postsynaptic and second messenger/signal transduction cascade intermediaries (including mTOR) on a similar time course to that identified above. It also had antidepressant-like effects in the same behavioral models of depression, and these effects were reversed by rapamycin pretreatment (55). Ro(25)-6981 had analogous biochemical and behavioral effects in rodents exposed to chronic unpredictable stress (56). Notably, several preclinical studies have been conducted with compounds that increase glutamatergic signaling in depression—e.g., metabotropic glutamate receptor (mGluR) 2/3 antagonists (128) and AMPA receptor agonists (also known as AMPA potentiators or AMPAkines) (129, 130)—even though their effects are not instigated by glutamate receptor antagonism.
Other NMDA receptor antagonists have demonstrated initial clinical efficacy in MDD. A single intravenous infusion of the NR2B-selective NMDA receptor antagonist CP-101,606 had rapid antidepressant effects (131). Unfortunately, further development of this compound was stopped because of cardiovascular adverse effects (QTc prolongation). The oral NR2B-selective antagonist MK-0657, when administered daily to patients with treatment-resistant MDD, had antidepressant effects as assessed by the clinician-administered Hamilton Depression Rating Scale and the self-reported Beck Depression Inventory but not the Montgomery-Åsberg Depression Rating Scale; no serious or dissociative adverse effects were noted (132). Next, a single infusion of the low-trapping, nonselective NMDA receptor antagonist AZD6765 had rapid antidepressant effects in treatment-resistant depression (133). Although these antidepressant effects occurred on a time scale similar to those of ketamine, no psychotomimetic effects were observed with the novel compound, likely because of AZD6765’s rapid association/dissociation at the NMDA receptor (memantine is likewise believed not to cause psychotomimetic side effects owing to similar low-trapping potential). The results of a multi-infusion AZD6765 trial in treatment-resistant MDD were presented at the 2012 American College of Neuropsychopharmacology annual meeting and, although discussed elsewhere (10), await publication at the time of writing.
CONCLUSIONS AND FUTURE DIRECTIONS
In this review, we have provided a broad overview of NMDA receptor antagonists as experimental therapies for depressive disorders. One author recently observed that the discovery of NMDA receptor antagonists as rapid and robust antidepressants has revolutionized the field of mood disorders research, noting that this discovery is “arguably the most important discovery [in depression research] in half a century [since the discovery of monoamine antidepressants]” (9, p. 73). Whereas history will no doubt confirm or deny this promising claim, several fundamental avenues of pursuit are critical needs in the field (Table 3). First, we need safe, tolerable, effective strategies to maintain the robust antidepressant efficacy of NMDA receptor antagonists such as ketamine. To date, the most effective strategy has been repeated ketamine infusions, but no effective prolongation strategies with demonstrable efficacy in randomized, psychoactive placebo-controlled, i.e., well-blinded, studies exist. Moreover, if these multiple-dosing approaches ultimately prove to be the most effective maintenance strategy, we need to be especially cautious of addiction and long-term toxicity. Second, we need to develop psychotomimetic-free and/or oral NMDA receptor modulators that can eventually be used in routine clinical practice. Third, although significant advances in identifying predictor and moderator/mediator biomarkers of ketamine’s antidepressant response have been made, many of these discoveries have resulted from pilot/exploratory projects conducted with the express purpose of facilitating drug development. Clearly, more work must be conducted to make this class of agents available to more individuals suffering from MDD and BDep. In conclusion, the initial ketamine trials have spurred worldwide interest from the pharmaceutical industry, academia, and government in developing the next generation of antidepressant medications. Results from several additional randomized, well-controlled trials with ketamine and other NMDA receptor antagonists are forthcoming in the next several years, thereby moving this exciting and burgeoning field closer to clinical practice.
Table 3.
Future directions for NMDA receptor antagonists in clinical depression research
| Antidepressant dose-response testing |
| Further testing of strategies to augment and/or maintain antidepressant response |
| Continued validation and qualification of biomarkers of antidepressant response with goal of surrogate endpoints |
| Alternative means of administration, e.g., oral, intramuscular, and intranasal administration |
| Drug development/testing of more specific compounds, e.g., NR2B-selective antagonists, for improved efficacy and/or decreased psychotomimetic and other adverse effects |
| Assessment of the long-term risks of repeated NMDA receptor antagonist administration in supervised settings |
| Psychoeducation of psychiatric clinicians, patients, and society about the potential risks of NMDA receptor antagonists in nonsupervised settings |
ACKNOWLEDGMENTS
This work was funded by the Intramural Research Program of the National Institute of Mental Health/National Institutes of Health (to C.A.Z., M.J.N., I.D.H., and D.A.L.) and Extramural Research Program of the National Institute of Mental Health/National Institutes of Health (to D.S.C.), by a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (to C.A.Z. and D.S.C.), by a Brain and Behavior Foundation Bipolar Research Award (to C.A.Z.), and by the United States Army Medical Research Acquisition Activity (to D.S.C.). The authors thank the 7SE Inpatient Mood and Anxiety Disorders Research Unit of the National Institute of Mental Health/National Institutes of Health for their support.
Footnotes
DISCLOSURE STATEMENT
M.J.N., I.D.H., and D.A.L. are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. C.A.Z. is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. C.A.Z. has assigned his rights in the patent to the US Government but will share a percentage of any royalties that may be received. D.S.C. and Mount Sinai School of Medicine have been named on a use patent application of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and were to receive approval from the US Food and Drug Administration (FDA) for this indication, D.S.C. and Mount Sinai School of Medicine could benefit financially.
LITERATURE CITED
- 1.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, et al. Combining Medications to Enhance Depression Outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am. J. Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr. Serv. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 4.Olchanski N, McInnis Myers M, Halseth M, Cyr PL, Bockstedt L, et al. The economic burden of treatment-resistant depression. Clin. Ther. 2013;35:512–522. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J. Clin. Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paschos KA, Veletza S, Chatzaki E. Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs. 2009;23:755–772. doi: 10.2165/11310830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 8.Perry EB, Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology. 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 9.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolgin E. Rapid antidepressant effects of ketamine ignite drug discovery. Nat. Med. 2013;19:8. doi: 10.1038/nm0113-8. [DOI] [PubMed] [Google Scholar]
- 11.White PF, Ham J, Way WL, Trevor AJ. Pharmacology of ketamine isomers in surgical patients. Anesthesiology. 1980;52:231–239. doi: 10.1097/00000542-198003000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 14.Romero TR, Galdino GS, Silva GC, Resende LC, Perez AC, et al. Ketamine activates the l-arginine/nitric oxide/cyclic guanosine monophosphate pathway to induce peripheral antinociception in rats. Anesth. Analg. 2011;113:1254–1259. doi: 10.1213/ANE.0b013e3182285dda. [DOI] [PubMed] [Google Scholar]
- 15.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc. Natl. Acad. Sci. USA. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 17.Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J. Pharm. Sci. 1982;71:539–542. doi: 10.1002/jps.2600710516. [DOI] [PubMed] [Google Scholar]
- 18.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 19.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 20.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int. J. Neuropsychopharmacol. 2010;13(1):71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J. Neuropsychiatry Clin. Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diazgranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-d-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int. J. Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 27.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, et al. A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J. Palliat. Med. 2010;13:903–908. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNulty JP, Hahn K. Compounded oral ketamine. Int. J. Pharm. Compd. 2012;16:364–368. [PubMed] [Google Scholar]
- 32.Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J. Palliat. Med. 2012;15:400–403. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- 33.Cusin C, Hilton GQ, Nierenberg AA, Fava M. Long-term maintenance with intramuscular ketamine for treatment-resistant bipolar II depression. Am. J. Psychiatry. 2012;169:868–869. doi: 10.1176/appi.ajp.2012.12020219. [DOI] [PubMed] [Google Scholar]
- 34.Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol. Psychiatry. 2012;72:964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine versus add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J. Clin. Psychiatry. 2011;72:414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 38.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry. 2013;74(4):250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blier P, Zigman D, Blier J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol. Psychiatry. 2012;72:e11–e12. doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 40.Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J. Biol. Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, et al. Serial infusions of low-dose ketamine for major depression. J. Psychopharmacol. 2013;27(5):444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 42.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012;100:656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am. J. Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 44.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol. Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. Am. J. Psychiatry. 2004;161:2132. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]
- 46.Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol. Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostroff R, Gonzales M, Sanacora G. Antidepressant effect of ketamine during ECT. Am. J. Psychiatry. 2005;162:1385–1386. doi: 10.1176/appi.ajp.162.7.1385. [DOI] [PubMed] [Google Scholar]
- 48.Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261:575–582. doi: 10.1007/s00406-011-0205-7. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J. ECT. 2010;26:223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J. ECT. 2012;28:128–132. doi: 10.1097/YCT.0b013e31824d1d02. [DOI] [PubMed] [Google Scholar]
- 51.Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, III, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J. Neuropsychiatry Clin. Neurosci. 2003;15:27–34. doi: 10.1176/jnp.15.1.27. [DOI] [PubMed] [Google Scholar]
- 52.Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J. ECT. 2012;28:157–161. doi: 10.1097/YCT.0b013e31824f8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Järventausta K, Chrape kW, Kampman O, Tuohimaa K, Björkgvist M, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J. ECT. 2013;29:158–161. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- 54.Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J. Affect. Disord. 2012;142:233–240. doi: 10.1016/j.jad.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, et al. Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frye MA. Clinical practice. Bipolar disorder—a focus on depression. N. Engl. J. Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- 58.Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am. J. Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 59.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clatts MC, Goldsamt LA, Yi H. Club drug use among young men who have sex with men in NYC: a preliminary epidemiological profile. Subst. Use Misuse. 2005;40:1317–1330. doi: 10.1081/JA-200066898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelly BC, Parsons JT, Wells BE. Prevalence and predictors of club drug use among club-going young adults in New York City. J. Urban Health. 2006;83:884–895. doi: 10.1007/s11524-006-9057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grov C, Bimbi DS, Nanin JE, Parsons JT. Exploring racial and ethnic differences in recreational drug use among gay and bisexual men in New York City and Los Angeles. J. Drug Educ. 2006;36:105–123. doi: 10.2190/1G84-ENA1-UAD5-U8VJ. [DOI] [PubMed] [Google Scholar]
- 63.Halkitis PN, Palamar JJ, Mukherjee PP. Poly-club-drug use among gay and bisexual men: a longitudinal analysis. Drug Alcohol Depend. 2007;89:153–160. doi: 10.1016/j.drugalcdep.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pantalone DW, Bimbi DS, Holder CA, Golub SA, Parsons JT. Consistency and change in club drug use by sexual minority men in New York City, 2002 to 2007. Am. J. Public Health. 2010;100:1892–1895. doi: 10.2105/AJPH.2009.175232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review of 233 cases. Hong Kong Med. J. 2010;16:6–11. [PubMed] [Google Scholar]
- 66.Chen WJ, Fu TC, Ting TT, Huang WL, Tang GM, et al. Use of ecstasy and other psychoactive substances among school-attending adolescents in Taiwan: national surveys 2004–2006. BMC Public Health. 2009;9:27. doi: 10.1186/1471-2458-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leong HS, Tan NL, Lui CP, Lee TK. Evaluation of ketamine abuse using hair analysis: concentration trends in a Singapore population. J. Anal. Toxicol. 2005;29:314–318. doi: 10.1093/jat/29.5.314. [DOI] [PubMed] [Google Scholar]
- 68.Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001;96:749–760. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- 69.Morgan CJA, Monaghan L, Curran HV. Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction. 2004;99:1450–1461. doi: 10.1111/j.1360-0443.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 70.Morgan CJA, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 71.Majewski-Tiedeken CR, Rabin CR, Siegel SJ. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 2008;92:217–227. doi: 10.1016/j.drugalcdep.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L, Lam WP, Wong YW, Lam LH, Tang HC, et al. Permanent deficits in brain functions caused by long-term ketamine treatment in mice. Hum. Exp. Toxicol. 2011;30:1287–1296. doi: 10.1177/0960327110388958. [DOI] [PubMed] [Google Scholar]
- 73.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liao Y, Tang J, Ma M, Wu Z, Yang M, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133:2115–2122. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- 75.Liao Y, Tang J, Corlett PR, Wang X, Yang M, et al. Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol. Psychiatry. 2011;69:42–48. doi: 10.1016/j.biopsych.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 76.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol. Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Morgan CJA, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 79.Kim YK, Lee HP, Won SD, Park EY, Lee HY, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 80.Machado-Vieira R, Yuan P, Brutsche N, Diazgranados N, Luckenbaugh D, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-d-aspartate antagonist. J. Clin. Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry. 2012;72:e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, et al. Effects of the brain-derived neurotrophic growth factor Val66Met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-d-aspartate antagonist. Biol. Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, et al. Family history of alcohol dependence and antidepressant response to an N-methyl-d-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- 88.Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am. J. Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- 89.Su N, Zhang L, Fei F, Hu H, Wang K, et al. The brain-derived neurotrophic factor is associated with alcohol dependence–related depression and antidepressant response. Brain Res. 2011;1415:119–126. doi: 10.1016/j.brainres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Stahl SM. Psychiatric stress testing: novel strategy for translational psychopharmacology. Neuropsychopharmacology. 2010;35:1413–1414. doi: 10.1038/npp.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol. Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kupfer DJ, Frank E, McEachran A, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch. Gen. Psychiatry. 1990;47:1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 94.Duncan WC, Jr, Selter J, Brutsche N, Sarasso S, Zarate CA., Jr Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J. Affect. Disord. 2013;145:115–119. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol. Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol. Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol. Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, et al. Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 99.Taylor M, Murphy SE, Selvaraj S, Wylezinkska M, Jezzard P, et al. Differential effects of citalopram and reboxetine on cortical Glx measured with proton MR spectroscopy. J. Psychopharmacol. 2008;22:473–476. doi: 10.1177/0269881107081510. [DOI] [PubMed] [Google Scholar]
- 100.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 101.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 102.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol. Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 103.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int. J. Neuropsychopharmacol. 2012;15:1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J. Psychopharmacol. 2012;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol. Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck CW. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am. J. Psychiatry. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- 108.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. 1H-[13C]- nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol. Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2012;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chan MH, Chiu PH, Lin CY, Chen HH. Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr. Res. 2012;136:96–103. doi: 10.1016/j.schres.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 114.Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J. Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- 115.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 117.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. NeuroReport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 118.Reus GZ, Stringari RB, Kirsch TR, Fries GR, Kapczinski F, et al. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res. Bull. 2010;81:585–589. doi: 10.1016/j.brainresbull.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Quan MN, Zhang N, Wang YY, Zhang T, Yang Z. Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience. 2011;182:88–97. doi: 10.1016/j.neuroscience.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 120.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 121.Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int. J. Geriatr. Psychiatry. 2012;27:974–980. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, et al. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012;14:64–70. doi: 10.1111/j.1399-5618.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 123.Stevens J, Bies RR, Shekhar A, Anand A. Bayesian model of HDRS with memantine augmentation in bipolar depression. Br. J. Clin. Pharmacol. 2013;75:791–798. doi: 10.1111/j.1365-2125.2012.04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kollmar R, Markovic K, Thurauf N, Schmitt H, Kornhuber J. Ketamine followed by memantine for the treatment of major depression. Aust. N. Z. J. Psychiatry. 2008;42:170. doi: 10.1080/00048670701787628. [DOI] [PubMed] [Google Scholar]
- 125.Muhonen LH, Lonnqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J. Clin. Psychiatry. 2008;69:392–399. doi: 10.4088/jcp.v69n0308. [DOI] [PubMed] [Google Scholar]
- 126.Muhonen LH, Lahti J, Sinclair D, Lonnqvist J, Alho H. Treatment of alcohol dependence in patients with co-morbid major depressive disorder—predictors for the outcomes with memantine and escitalopram medication. Subst. Abuse Treat. Prev. Policy. 2008;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 128.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int. J. Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Farley S, Apazoglou K, Witkin JM, Giros B, Tzavara ET. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int. J. Neuropsychopharmacol. 2010;13:1207–1218. doi: 10.1017/S1461145709991076. [DOI] [PubMed] [Google Scholar]
- 130.Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf+/− heterozygous null mice. Neuropharmacology. 2012;62:391–397. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 131.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 132.Ibrahim L, Diazgranados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonistMK-0657 in patients with treatment-resistant major depressive disorder. J. Clin. Psychopharmacol. 2012;32:551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, et al. A randomized trial of a low-trapping nonselective N-methyl-d-aspartate channel blocker in major depression. Biol. Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]