Abstract
Cardiac hypertrophy is an independent predictor of adverse outcomes in patients with heart failure, and thus represents an attractive target for novel therapeutic intervention. JQ1, a small molecule inhibitor of bromodomain and extraterminal (BET) acetyl-lysine reader proteins, was identified in a high throughput screen designed to discover novel small molecule regulators of cardiomyocyte hypertrophy. JQ1 dose-dependently blocked agonist-dependent hypertrophy of cultured neonatal rat ventricular myocytes (NRVMs) and reversed the prototypical gene program associated with pathological cardiac hypertrophy. JQ1 also blocked left ventricular hypertrophy (LVH) and improved cardiac function in adult mice subjected to transverse aortic constriction (TAC). The BET family consists of BRD2, BRD3, BRD4 and BRDT. BRD4 protein expression was increased during cardiac hypertrophy, and hypertrophic stimuli promoted recruitment of BRD4 to the transcriptional start site (TSS) of the gene encoding atrial natriuretic factor (ANF). Binding of BRD4 to the ANF TSS was associated with increased phosphorylation of local RNA polymerase II. These findings define a novel function for BET proteins as signal-responsive regulators of cardiac hypertrophy, and suggest that small molecule inhibitors of these epigenetic reader proteins have potential as therapeutics for heart failure.
Keywords: Cardiac hypertrophy, BET protein, acetyl-lysine
1. Introduction
Approximately 6 million Americans suffer from heart failure, placing an economic burden on the United States that is projected to rise to nearly $100 billion annually by 2030 [1]. The 5-year mortality rate following first admission for heart failure is 42.3%, further highlighting an urgent need for novel therapeutic approaches [2]. A hallmark of heart failure is cardiomyocyte hypertrophy. Cardiac hypertrophy has historically been viewed as a compensatory mechanism that normalizes wall stress and enhances cardiac performance. However, long-term suppression of left ventricular hypertrophy (LVH) is associated with reduced morbidity and mortality in patients with cardiovascular disease [3, 4], and thus cardiac hypertrophy is now recognized as an attractive target for novel therapeutic intervention [5].
Acetylation of lysine residues in core histones within chromatin provides an important post-translational mechanism for regulating gene expression, and enzymes that govern lysine acetylation have emerged as critical regulators of cardiac hypertrophy. Genetic and pharmacological manipulation of histone deacetylases (HDACs) and histone acetyltransferases (HATs) in rodent models of heart failure has revealed several key roles for these enzymes as positive and negative regulators of pathological cardiac growth [6]. However, it is not known whether acetyl-lysine ‘reader’ proteins, which recruit multi-protein complexes to chromatin via bromodomains, function in the control of cardiac hypertrophy. Here, we demonstrate that selective small molecule inhibition of bromodomain and extraterminal (BET) family proteins blocks agonist-dependent hypertrophy of cultured cardiac myocytes, and is efficacious in a mouse model of pressure overload-induced LVH. These findings establish a novel role for acetyl-lysine binding proteins in the control of pathological cardiac remodeling, and suggest potential for BET protein inhibitors for the treatment of heart failure.
2. Materials and Methods
A detailed description of methods is provided in the supplemental section.
3. Results
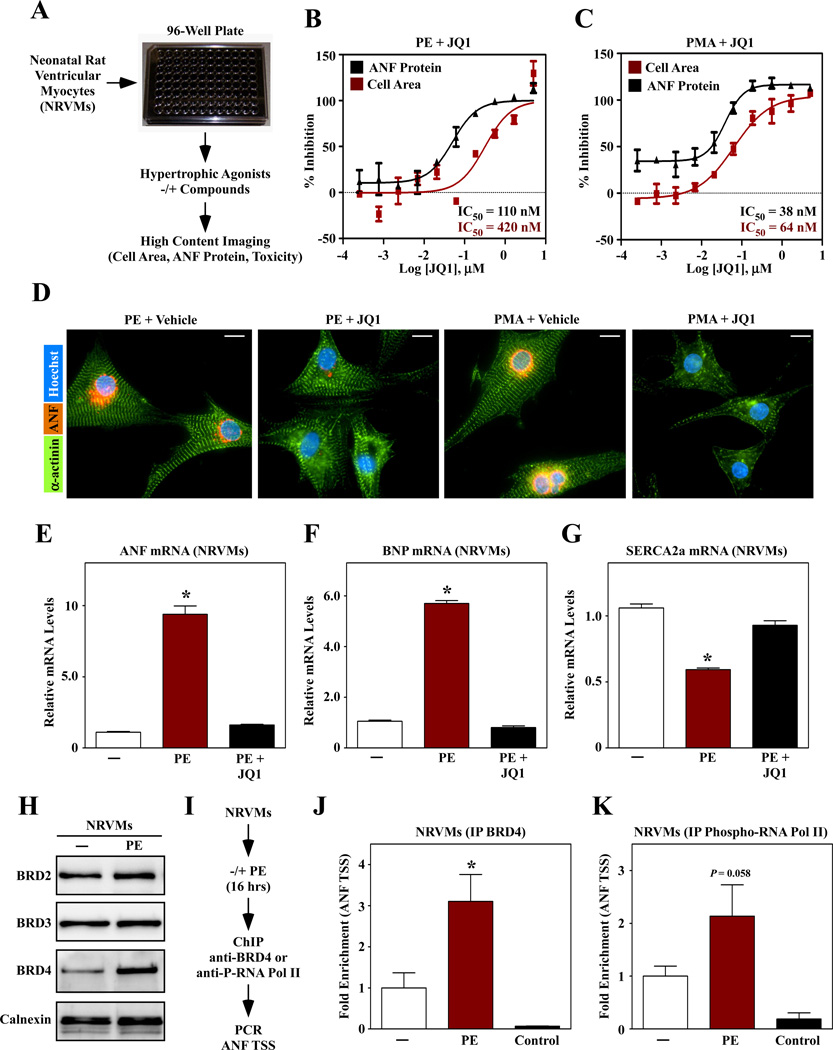
To facilitate discovery of novel therapeutics for heart failure, we developed a high throughput assay of cardiomyocyte hypertrophy. The assay is based on the use of primary neonatal rat ventricular myocytes (NRVMs) and a high content imaging platform that enables simultaneous quantification of cell size and expression of atrial natriuretic factor (ANF) protein as a biomarker of pathological cardiac hypertrophy (Fig. 1A). Screening of small molecule libraries yielded several ‘hit’ compounds, which will be described in detail elsewhere. JQ1, a compound that blocks binding of bromodomain and extraterminal (BET) proteins to acetyl-lysine [7], was identified as a potent inhibitor of NRVM hypertrophy. JQ1 dose-dependently suppressed cardiac hypertrophy mediated by phenylephrine (PE), which stimulates the α1-adrenergic receptor, and phorbol-12-myristate-13-acetate (PMA), which stimulates hypertrophy intracellularly by activating protein kinase C (PKC) (Fig. 1B and C). JQ1 was well tolerated by NRVMs; representative images of cells exposed to PE and PMA in the absence or presence of JQ1 are shown (Fig. 1D). Pathological cardiac hypertrophy is associated with a prototypical gene program that includes induction of ANF and brain natriuretic peptide mRNA expression, downregulation of sarcoendoplasmic reticulum calcium ATPase (SERCA2a), and β- and α-myosin heavy chain isoform switching. JQ1 completely blocked induction of this gene program in PE-treated NRVMs (Fig. 1E – G; sFig. 1A and B).
Fig. 1.
A small molecule BET protein inhibitor blocks cardiac hypertrophy. (A) A high throughput assay of cardiomyocyte hypertrophy based on culture of primary neonatal rat ventricular myocytes (NRVMs) on 96-well plates and high content imaging to quantify cell size and expression of atrial natriuretic factor (ANF) protein as a biomarker of pathological cardiac hypertrophy. (B, C) A hit compound, JQ1, dose-dependently blocked hypertrophy in response to treatment with phenylephrine (PE; 10 µM) or phorbol myristate acetate (PMA; 50 nM). (D) Images of ANF and α-actinin staining in NRVMs treated with agonists in the absence or presence of JQ1 (1 µM). Scale bar = 10 µm. (E – G) NRVMs were stimulated for 48 hrs with PE in the absence or presence of JQ1 and quantitative PCR was performed to assess expression of ANF, brain natriuretic peptide (BNP) and sarcoendoplasmic reticulum Ca2+ ATPase 2a (SERCA2a). N = 3 plates of cells per condition; *P<0.05 vs. unstimulated controls. (H) Immunoblot analysis of BET proteins in NRVMs treated with PE for 48 hrs. Calnexin is a loading control. (I) Chromatin immunoprecipitation (ChIP) strategy. (J, K) BRD4 and phospho-RNA Pol II association with the ANF transcription start site. IP with normal IgG was used as a negative control. N = 3 plates of cells per condition (4 pooled plates per N); *P<0.05 vs. unstimulated controls.
There are 47 bromodomain-containing proteins, and JQ1 has been shown to selectively inhibit binding of BET family bromodomains to acetyl-lysine [7]. Quantitative PCR and immunoblotting was performed to determine whether BET family members (BRD2, BRD3, BRD4 and BRDT) are expressed in the heart. Messenger RNA transcripts for BRD2, BRD3 and BRD4 were detected in isolated neonatal and adult rat cardiac myocytes and fibroblasts; BRDT mRNA was downregulated in adult cells (sFig. 2). BRD2, BRD3 and BRD4 protein was present in homogenates from cultured NRVMs, and BRD4 protein expression was upregulated following PE treatment (Fig. 1H).
Given the increased expression of BRD4 in response to a hypertrophic signal, this BET family member was chosen for further investigation. BRD4 has previously been shown to recruit the positive transcriptional elongation factor b (P-TEFb) complex to transcriptional start sites (TSSs) [8]. P-TEFb is a complex containing cyclin-dependent kinase 9 (CDK9) and cyclin T, which is able to phosphorylate the carboxy-terminal domain (CTD) of RNA polymerase II (RNA pol II) and thereby stimulate transcription elongation. P-TEFb and its negative regulators, 7SK RNA and Hexim/CLP-1, have all been shown to regulate cardiac hypertrophy [9–12]. Using the ANF promoter as a prototype, we tested the hypothesis that BRD4 is recruited to target genes and regulates RNA pol II phosphorylation in response to hypertrophic stimuli. Cultured NRVMs were stimulated with PE for 16 hours and harvested for chromatin immunoprecipitation (ChIP) analysis using antibodies directed against BRD4 or phosphorylated RNA pol II (Fig. 1I). PE treatment led to increased abundance of BRD4 at the ANF TSS (Fig. 1J), and this correlated with enhanced phosphorylation of the CTD of RNA pol II (Fig. 1K). Together, these data support the notion that BET proteins integrate upstream signals with the transcriptional program for cardiac growth.
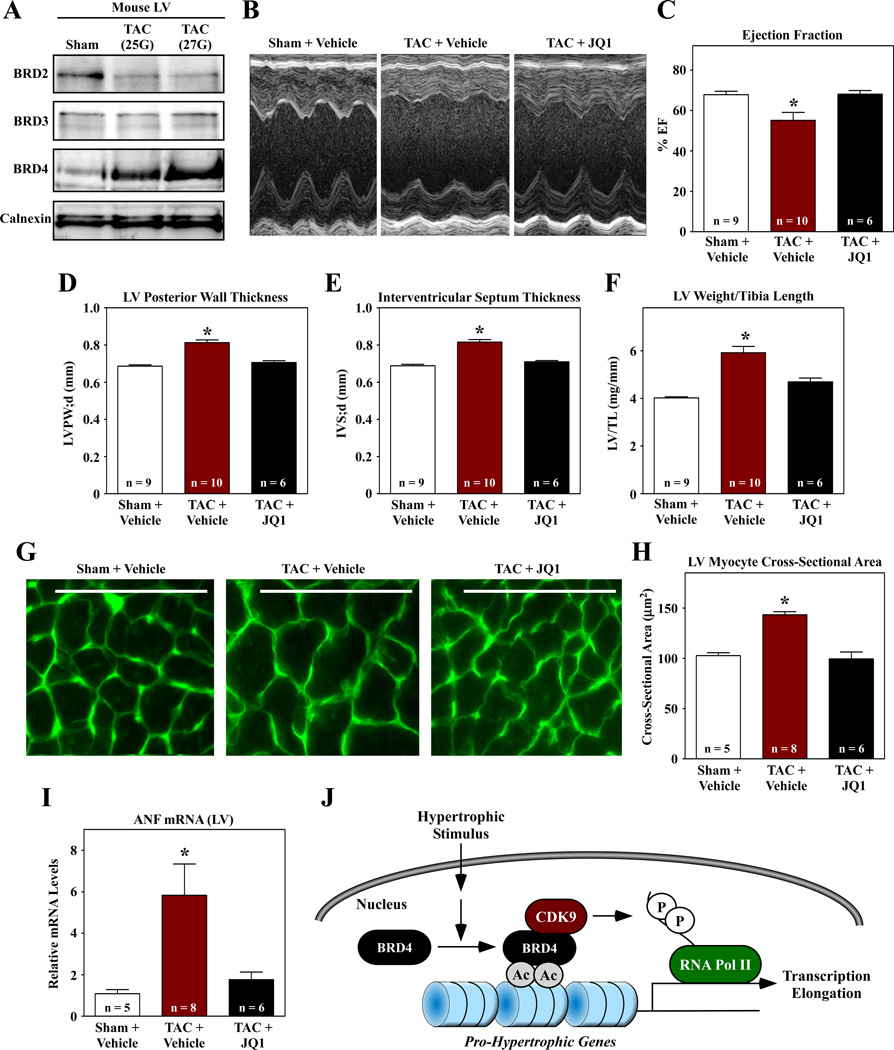
To address the role of BET proteins in cardiac remodeling in vivo, mice were subjected to transverse aortic constriction (TAC) and administered JQ1 or vehicle control every other day for four weeks. Consistent with results obtained with cultured cardiac myocytes (Fig. 1H), pressure overload stimulated BRD4 protein expression in the LV (Fig. 2A); full images of the BRD2, BRD3 and BRD4 immunoblots are shown in sFig. 3. TAC promoted LV systolic dysfunction, which was rescued by JQ1, as determined by echocardiographic analysis (Fig. 2B and C). Echocardiography also revealed that JQ1 normalized LV and septal wall thickness in mice subjected to TAC, suggesting that the compound reduced cardiac hypertrophy (Fig. 2D and E). Consistent with this, JQ1 normalized LV weight-to-tibia length ratio, myocyte cross-sectional area and expression of ANF in the LV (Fig. 2F – I). JQ1 also blunted pathological LV interstitial fibrosis in animals subjected to TAC (sFig. 4).
Fig. 2.
JQ1 suppresses left ventricular hypertrophy in response to pressure overload. (A) Immunoblot analysis of BET proteins in LVs of mice subjected to TAC for four weeks using a 25 gauge or 27 gauge needle to establish suture diameter. Calnexin is a loading control. Mice were subjected to TAC or sham surgery and were dosed IP with JQ1 (50 mg/kg) every other day for four weeks. (B – E) M-mode echocardiographic images were used to quantify ejection fraction, LV posterior wall thickness, and interventricular septum thickness. (F) LV weight-to-tibia length ratios were determined at necropsy. (G) LV sections were stained with fluorescein-conjugated peanut agglutinin to assess myocyte cross-sectional area. Scale bar = 10µm. (H) Quantification of myocyte cross-sectional area (µm2); >100 myocytes were quantified per the indicated number of LVs. (I) Quantitative PCR analysis of ANF mRNA expression. N = 3 plates of cells per condition; *P<0.05 vs. sham or unstimulated controls. (J) Model for the regulation of cardiac hypertrophy by BRD4.
4. Discussion
The findings of this study define a novel function for BET acetyl-lysine binding proteins in the control of cardiac hypertrophy. We propose a model in which BET proteins serve as nodal regulators of the transcriptional program for cardiac hypertrophy. According to this model, pro-hypertrophic stimuli stimulate gene transcription by promoting recruitment of BET proteins (e.g., BRD4) to regulatory regions of downstream target genes. Since BRD4 associates with the P-TEFb complex, recruitment of BRD4 to these regulatory elements results in CDK9-mediated phosphorylation of RNA pol II and enhanced transcriptional elongation (Fig. 2J).
Of the four BET proteins, only BRD2, BRD3 and BRD4 are expressed in adult cardiac myocytes. Increased expression of BRD4 (Figs. 1H and 2A) and recruitment of BRD4 to the ANF promoter (Fig. 1J) in response to a hypertrophic signal suggests a prominent role for this BET family member in the control of cardiac remodeling. However, the lack of induction of BRD2 and BRD3 expression during cardiac hypertrophy does not exclude the possibility that these proteins also control heart muscle growth. For example, BRD2/3 activity, interacting partners or subcellular localization could be affected by hypertrophic stimuli.
Regulation of ANF expression by BRD4 is an example of how BET proteins can affect gene expression during cardiac hypertrophy. However, it should be noted that ANF itself does not promote hypertrophy. We propose that BET proteins are required for induction of critical pro-hypertrophic genes. BET protein-focused ChIP-sequencing should lead to the discovery of these target genes and will provide further mechanistic insight into the involvement of BET acetyl-lysine binding proteins in the regulation of cardiac growth.
BET inhibitors are in clinical development for the treatment of cancer and atherosclerosis and have shown promise in pre-clinical models of inflammation and HIV latency [13]. Our data suggest novel potential for small molecule BET inhibitors for the treatment of pathological cardiac remodeling and heart failure.
Supplementary Material
Acknowledgements
We thank J. Mahaffey, M. Jeong and B. Ferguson for cardiac cell cultures, S. Bowers for high content imaging, C. J. Phiel and members of the D. Bentley and lab for advice regarding ChIP, and P.C. Simpson and P.M. Swigart (UCSF) for guidance on performing TAC surgeries.
Sources of Funding
J.I.S. was supported by a predoctoral T32 training grand from the NIH (5T32GM007635-34). M.S.S. was supported by a postdoctoral T32 training grant from the NIH (5T32HL007822-12). Other support for the work was provided by start-up funds to T.A.M. from the University of Colorado Denver.
Footnotes
Disclosures
No conflicts of interest exist for the authors.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, de SG, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 4.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004;292:2396–2398. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 5.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 6.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 7.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Espinoza-Derout J, Wagner M, Shahmiri K, Mascareno E, Chaqour B, Siddiqui MA. Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy. Cardiovasc Res. 2007;75:129–138. doi: 10.1016/j.cardiores.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinoza-Derout J, Wagner M, Salciccioli L, Lazar JM, Bhaduri S, Mascareno E, et al. Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1. Circ Res. 2009;104:1347–1354. doi: 10.1161/CIRCRESAHA.108.191726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, et al. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat Med. 2002;8:1310–1317. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 12.Sano M, Schneider MD. Cyclin-dependent kinase-9: an RNAPII kinase at the nexus of cardiac growth and death cascades. Circ Res. 2004;95:867–876. doi: 10.1161/01.RES.0000146675.88354.04. [DOI] [PubMed] [Google Scholar]
- 13.Prinjha RK, Witherington J, Lee K. Place your BETs: the therapeutic potential of bromodomains. Trends Pharmacol Sci. 2012;33:146–153. doi: 10.1016/j.tips.2011.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.