Abstract
Low intraoperative Bispectral Index (BIS) values may be associated with increased mortality. In a previously reported trial to prevent delirium, we randomized patients undergoing hip fracture repair under spinal anesthesia to light (BIS>80) or deep (BIS~50) sedation. We analyzed survival of patients in the original trial. Among all patients, mortality was equivalent across sedation groups. However, among patients with serious comorbidities (Charlson score >4), 1-year mortality was reduced in the light (22.2%) versus deep (43.6%) sedation group (Hazard Ratio [HR], 0.43; 95% CI, 0.19–0.97; P=0.04) during spinal anesthesia. Similarly, among patients with Charlson score >6, 1-year mortality was reduced in the light (28.6%) versus deep (52.6%) sedation group (HR 0.33; 95%CI 0.12–0.94; P=0.04) during spinal anesthesia. Further research on reduced mortality after light sedation during spinal anesthesia is needed.
Introduction
Low intraoperative Bispectral Index (BIS) values have been associated with increased mortality in several studies,1–4 although this finding has not been confirmed universally.5 Because few randomized trials of BIS-targeted anesthesia have been reported, it is unclear whether low BIS values are simply a marker of poor prognosis, or whether targeting anesthetic management based on BIS monitoring could reduce mortality.
Between 2005–2008, we randomized patients undergoing surgical repair of a hip fracture under spinal anesthesia to light sedation (BIS>80) versus deep sedation (BIS~50). We reported results of this trial previously,6 which demonstrated a 50% reduction in postoperative delirium in the light versus deep sedation group. Subsequent to this trial, conflicting evidence emerged that low intraoperative BIS values might be associated with increased long-term mortality. In order to address this question, we conducted a follow-up survival analysis of patients enrolled in the original trial, with the hypothesis that light sedation compared to deep sedation would reduce 1-year and long-term mortality, particularly among patients with serious comorbidity. We also hypothesized that the association between light sedation during spinal anesthesia and decreased mortality would be mediated by reduced delirium incidence.
Methods
The Johns Hopkins IRB approved this study, and subjects provided written informed consent. The results of this study are based on a randomized trial (NCT00590707) to prevent postoperative delirium originally conducted between 2005–2008, with results reported previously,6 including a full description of the methods. This study examines long-term survival among patients in the original trial.
Patients
In the original trial, 114 patients > 65 years old admitted to the hip fracture service at Johns Hopkins Bayview Hospital underwent surgical repair under spinal anesthesia. Patients were randomized (using block randomization with variably-sized blocks) to light intraoperative sedation (BIS>80, generally responsive to voice) or deep intraoperative sedation (BIS~50, generally not arousable to deep stimulation), using a propofol infusion or midazolam.
Outcome
The outcomes were 1-year mortality (primary outcome) and overall mortality (secondary outcome), in all patients and in patients with serious comorbidity. Patient status was determined from medical records, Social Security Death Index, National Death Index, and obituaries.
Statistical analysis
Baseline characteristics were compared using t-tests for continuous variables, chi-squared tests for binary variables, and Mann-Whitney tests for ordinal variables. The Charlson Comorbidity index7 is a validated comorbidity-based scoring system to predict mortality. We primarily defined patients with serious comorbidity as a Charlson score >4, which was the 25th percentile of scores and has been associated with increased mortality after hip fracture surgery.8,9 Using a validated conversion of cognitive scoring,10 we also estimated the Nottingham Hip Fracture Score, which has been validated to predict mortality after hip fracture,11 with a score >4 being associated with increased 1-year mortality.12 Furthermore, for each scale, we used a cutoff of >6 (approximately the median value) to define patients with even more serious comorbidity and higher risk for mortality based on evidence that patients with higher comorbidity scores have higher mortality risk.12,13 We estimated survival functions using the Kaplan-Meier estimator and hazard ratios with Cox proportional hazards models. Living independently was included as a covariate in all models because of post-randomization group differences. We tested the proportional hazards assumptions through visual inspection of log-log survival plots and by testing the significance of the interaction of time with randomization. We also explored a series of parametric survival models in the generalized gamma family14 and selected the best model using likelihood ratio tests. Our sample has a power of 0.8 with an alpha of 0.05 to detect an overall hazard ratio of 0.58. The analysis was performed in accordance with intention-to-treat principles and P-values <0.05 were considered significant.
Results
Demographic, comorbidity, and operative characteristics in patients with Charlson score >4 (Table 1) and in all patients (Tables 1 and 2 previously reported6) were generally similar between the light sedation and deep sedation groups, although patients in the light sedation group received less propofol (P <0.001) and were more likely to live independently (P =0.02). Duration of hypotension was similar between the two groups (P =0.76). Mean follow-up time to death or censoring was 2.7 years with a range of 3 days to 5.8 years. There were 28 deaths at 1 year and 51 deaths overall. In patients with Charlson score >4, there were 27 deaths at 1 year and 46 deaths overall.
Table 1.
Characteristics of patients with Charlson Comorbidity Index >4
| Characteristics | Light sedation (n=45) |
Deep sedation (n=39) |
Total (n=84) |
P value |
|---|---|---|---|---|
| General | ||||
| Age (mean, SD) | 81.2±7.6 | 82.3±6.8 | 81.7±7.2 | 0.47 |
| Male (%) | 33.3 | 30.8 | 32.1 | 0.80 |
| Race (% Caucasian) | 95.6 | 94.9 | 95.3 | 0.88 |
| ASA score (median, IQR) | 3 (3–3) | 3 (3–3) | 3 (3–3) | 0.77 |
| ADL (median, IQR) | 5 (5–6) | 5 (4–6) | 5 (4–6) | 0.31 |
| IADL (median, IQR) | 7 (4–8) | 5 (2–8) | 6 (3–8) | 0.16 |
| Living independently (%) | 82 | 59 | 71 | 0.02 |
| Comorbidities | ||||
| Charlson Score (median, IQR) | 6 (5–7) | 6 (6–8) | 6 (5.5–8) | 0.42 |
| Cerebrovascular diseasea (%) | 26.7 | 18.0 | 22.6 | 0.34 |
| CABG/PCI/MI/CAD (%) | 57.8 | 59.0 | 58.3 | 0.91 |
| Atrial fibrillation (%) | 20.0 | 30.8 | 25.0 | 0.26 |
| Chronic renal insufficiencyb (%) | 33.3 | 28.2 | 31.0 | 0.62 |
| Peripheral vascular diseasec (%) | 15.6 | 25.6 | 20.2 | 0.25 |
| Anemia (%) | 22.2 | 25.6 | 23.8 | 0.71 |
| Surgical characteristics | ||||
| Minutes of surgery (mean, SD) | 78.6±36.0 | 87.0±41.8 | 82.5±38.8 | 0.33 |
| Number of ICU days (median, IQR) | 0 (0–0) | 0 (0–2) | 0 (0–0) | 0.06 |
| Average BIS (mean, SD) | 85.7±12.4 | 50.3±13.4 | 68.9±21.9 | <0.001 |
| Average BIS < 50 in minutes (median, IQR) | 0 (0–0) | 40 (15.5–63) | 2 (0–40) | <0.001 |
| Anesthesia Characteristics | ||||
| Conversion to general anesthesia (n, %) | 6 (13.3) | 3 (7.7) | 9 (10.7) | 0.40 |
| Received propofol (n, %) | 40 (88.9) | 39 (100) | 79 (94.1) | 0.03 |
| Propofol dose in mg/kg (median, IQR) | 1.3 (0.6–3.2) |
8.1 (6.4–12.7) |
4.7 (1.2–8.2) |
<0.001 |
| Received midazolam (n, %) | 10 (22.2) | 2 (5.1) | 12 (14.3) | 0.03 |
| Midazolam dose in mg/kg (median, range) | 0 (0–0.06) | 0 (0–0.04) | 0 (0–0.06) | 0.03 |
| Duration of hypotensiond in minutes (median, IQR) | 5 (0–12) | 0 (0–15) | 5 (0–13.5) | 0.76 |
SD = standard deviation; ASA = American Society of Anesthesiologists; IQR = interquartile range; ADL = activities of daily living; IADL = instrumental activities of daily living; CABG/PCI/MI/CAD = coronary artery bypass graft surgery/percutaneous coronary intervention/myocardial infarction/coronary artery disease; ICU = intensive care unit; BIS = Bispectral Index.
Cerebrovascular disease was defined as history of stroke, transient ischemic attack, or carotid endarterectomy.
Chronic renal insufficiency was defined as serum creatinine >1.5 mg/dL in males or >1.3 mg/dL in females.
Peripheral vascular disease was defined as any one of the following: history of peripheral vascular disease, history of surgical procedure for peripheral vascular disease, claudication, inter-arm asymmetry in blood pressure measurement.
Hypotension was defined as intraoperative systolic blood pressure decrease greater than 30% from preoperative values and/or systolic blood pressure less than 90 mm Hg.
Table 2.
Hazard ratio for death and relative time to death comparing light intraoperative sedation to deep intraoperative sedation
| Patient groups (n) | Hazard ratioa |
95% CI |
P value |
Relative time to deatha, b |
95% CI |
P value |
|---|---|---|---|---|---|---|
| 1-year follow-up | ||||||
| All patients (114) | 0.61 | 0.28–1.33 | 0.21 | 2.68 | 0.70–10.23 | 0.15 |
| Charlson > 4 (84) | 0.43 | 0.19–0.97 | 0.04 | 4.47 | 1.16–17.31 | 0.03 |
| Charlson >6 (40) | 0.33 | 0.12–0.94 | 0.04 | 8.9 | 1.42–56.0 | 0.02 |
| Nottingham Hip Fracture Score >4 (97) |
0.44 | 0.21–0.96 | 0.04 | 4.37 | 1.16–16.41 | 0.03 |
| Nottingham Hip Fracture Score >6 (50) |
0.32 | 0.11–0.90 | 0.03 | 10.90 | 1.41–84.0 | 0.02 |
| Overall follow-up | ||||||
| All patients (114) | N/Ac | N/Ac | N/Ac | 1.40 | 0.47–4.2 | 0.54 |
| Charlson > 4 (84) | N/Ac | N/Ac | N/Ac | 2.97 | 0.94–9.35 | 0.06 |
| Charlson >6 (40) | N/Ac | N/Ac | N/Ac | 6.95 | 1.43–33.72 | 0.02 |
| Nottingham Hip Fracture Score >4 (97) |
N/Ac | N/Ac | N/Ac | 2.69 | 0.93–7.77 | 0.07 |
| Nottingham Hip Fracture Score >6 (50) |
N/Ac | N/Ac | N/Ac | 5.02 | 1.16–21.64 | 0.03 |
CI = confidence interval.
Models were adjusted for baseline living independently
The relative time to death was calculated by using parametric models in which the failure times followed a log-normal distribution.
Cox proportional hazards assumption of constant proportional hazards over time were not fully supported for overall follow-up in all models; therefore a hazard ratio using the Cox model is not presented.
After 1 year of follow-up in all patients, mortality did not differ between patients in the light sedation group (19.3%) and those in the deep sedation group (29.8%; HR, 0.61; 95% confidence interval [CI], 0.28–1.33; P=0.21. Figure 1A and Table 2). However, among the subset of patients with Charlson score >4, 1-year mortality was reduced in the light sedation group (22.2%) compared to deep sedation group (43.6%; HR, 0.43; 95% CI, 0.19–0.97; P=0.04. Figure 1C and Table 2) during spinal anesthesia. Time to death was also 4.47 times longer in the light sedation group than in the deep sedation group (relative time [RT], 4.47; 95% CI, 1.16–17.31; P=0.03). Similarly, among the subset of patients with Nottingham score >4, 1-year mortality was reduced in the light compared to deep sedation group (HR, 0.44; 95% CI, 0.21–0.96; P=0.04), and time to death was longer after light compared to deep sedation (RT, 4.37; 95% CI, 1.16–16.41; P=0.03) during spinal anesthesia. As shown in Table 2, the beneficial effect of light sedation on mortality was magnified in patients with higher comorbidity scores (Charlson or Nottingham score >6), as reflected by even greater decrease in mortality and longer time to death after light compared to deep sedation during spinal anesthesia.
Figure 1.
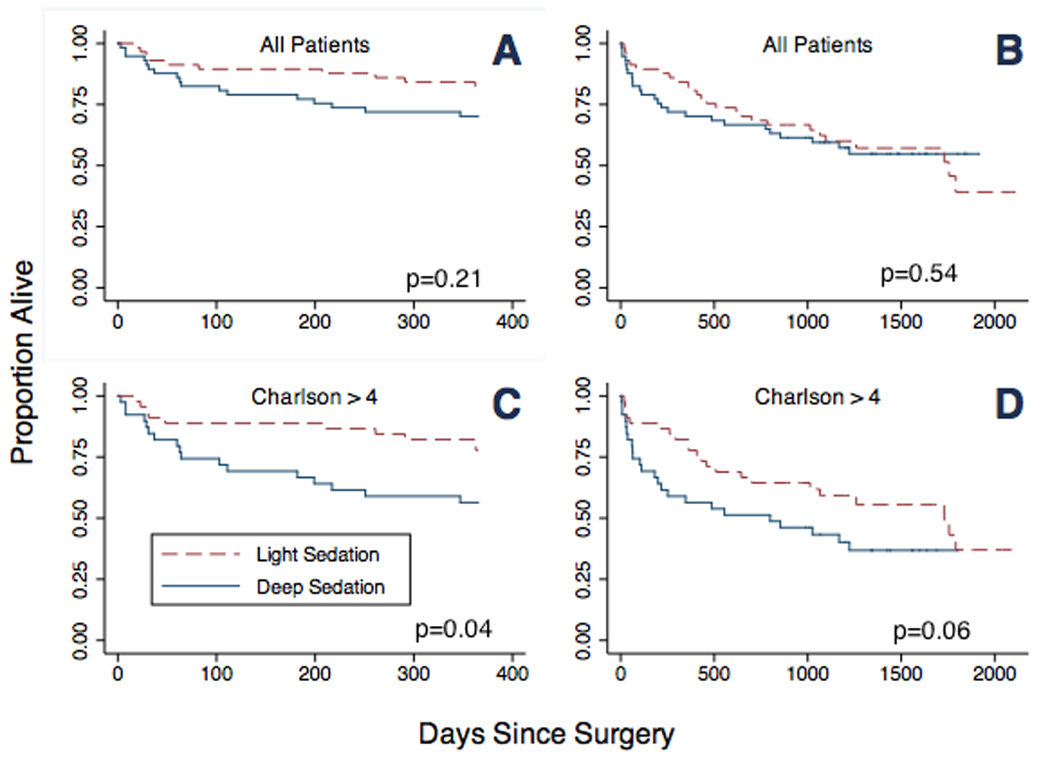
Kaplan-Meier survival curves (1-year and overall) for all patients and for patients with Charlson score >4. Survival of all patients (A) and patients with Charlson score >4 (C) at 1 year. Survival of all patients (B) and patients with Charlson score >4 (D) in overall follow-up. (P values for 1-year mortality are given by adjusted Cox proportional hazards models, and P values for overall mortality are given by adjusted parametric models)
Focusing on survival beyond 1 year, assumptions of proportionality in the Cox model were not fully supported for all groups of patients, so parametric models were used. In overall survival in all patients, no difference in time to death was observed between the light and deep sedation groups (Figure 1B). As shown in Table 2 and Figure 1D, there was a trend towards increased time to death in the light sedation group compared to the deep sedation group for patients with Charlson score >4 (RT, 2.97; 95% CI, 0.94–9.35; P= 0.06) or Nottingham score >4 (RT, 2.69; 95%CI, 0.93–7.77; P=0.07). Among patients with increased comorbidity (Charlson score >6 or Nottingham score >6), the beneficial effect of light sedation on survival during spinal anesthesia was more pronounced, and time to death was significantly increased in the light compared to deep sedation group (Table 2).
To assess whether postoperative delirium was a mediator between depth of sedation and mortality, we adjusted for delirium and found that the estimated hazard ratio of 1-year mortality for patients with light compared to deep sedation did not change, indicating that delirium was unlikely to be a mediator. In addition, we found no significant interaction between depth of sedation and delirium in determining mortality at 1 year (P=0.34) or in overall follow-up (P=0.37).
Discussion
We present survival data of patients enrolled in a previously reported trial. We demonstrate that among patients with high comorbidity scores, patients originally randomized to light sedation during spinal anesthesia had reduced 1-year mortality compared to those patients randomized to deep sedation. Although light sedation was previously shown to reduce postoperative delirium in these patients, our results do not demonstrate a clear role for delirium as a mediator between depth of sedation and mortality. Previous studies examining the association between low intraoperative BIS values and mortality have been criticized for being observational,1,2 so that low BIS values might have simply identified vulnerable patients. In this study, randomization to distinct BIS targets addressed this limitation, and our results support the hypothesis that light sedation may reduce mortality. In contrast to previous studies which included multiple noncardiac1,2,5 and cardiac operations,3,4 this study population is unique because only hip fracture surgeries planned for spinal anesthesia were included. Mortality at 1-year substantially exceeded that reported in previous BIS-related trials,1–3 so these results may not be generalizable to less severely ill patients. Our study was limited because of small sample size, lack of knowledge of cause of death, post hoc analysis, and limitation to a specific surgery. In conclusion, light sedation during repair of hip fracture under spinal anesthesia may reduce mortality in patients with high comorbidity scores.
Acknowledgments
Funding: 1 R01 AG033615-01A1 NIH KL-2 Clinical Research Scholars Program RO3 AG042331 Jahnigen Career Development Award
Footnotes
The authors declare no conflicts of interest.
This report was previously presented, in part, at the Translational Science Meeting
DISCLOSURES:
Name: Charles H. Brown IV, MD MHS
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Charles H. Brown IV has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Andrew S. Azman, MS
Contribution: This author helped analyze the data and write the manuscript
Attestation: Andrew S. Azman has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Allan Gottschalk, MD, PhD
Contribution: This author helped analyze the data and write the manuscript
Attestation: Allan Gottschalk has seen the original study data and approved the final manuscript
Name: Simon C Mears, MD, PhD
Contribution: This author helped write the manuscript
Attestation: Simon C Mears approved the final manuscript
Name: Frederick E Sieber, MD
Contribution: This author helped conduct the study and write the manuscript
Attestation: Frederick E Sieber approved the final manuscript
This manuscript was handled by: Sorin J. Brull, MD, FCARCSI (Hon)
Contributor Information
Charles H. Brown, IV, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Andrew S. Azman, Bloomberg School of Public Health, Johns Hopkins, Baltimore, Maryland.
Allan Gottschalk, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Simon C Mears, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Frederick E Sieber, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
References
- 1.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic Management and One-Year Mortality After Noncardiac Surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm ML, Träff S, Granath F, Greenwald SD, Ekbom A, Lennmarken C, Sandin RH. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009;108:508–512. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 3.Leslie K, Myles PS, Forbes A, Chan MTV. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010;110:816–822. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 4.Kertai MD, Pal N, Palanca BJA, Lin N, Searleman SA, Zhang L, Burnside BA, Finkel KJ, Avidan MS B-Unaware Study Group. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010;112:1116–1127. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 5.Kertai MD, Palanca BJA, Pal N, Burnside BA, Zhang L, Sadiq F, Finkel KJ, Avidan MS B-Unaware Study Group. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011;114:545–556. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 6.Sieber F, Zakriya K, Gottschalk A, Blute M-R, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85(1):18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Neuhaus V, King J, Hageman MG, Ring DC. Charlson Comorbidity Indices and in-hospital deaths in patients with hip fractures. Clin Orthop Relat Res. 2013;471:1712–1719. doi: 10.1007/s11999-012-2705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vestergaard P, Rejnmark L, Mosekilde L. Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoperosis. 2007;18:1583–1593. doi: 10.1007/s00198-007-0403-3. [DOI] [PubMed] [Google Scholar]
- 10.Swain DG, O Brien AG, Nightingale PG. Cognitive assessment in elderly patients admitted to hospital: the relationship between the Abbreviated Mental Test and the Mini-Mental State Examination. Clin Rehabil. 13:503–508. doi: 10.1191/026921599670895633. 199. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell MJ, Moran CG, Moppett IK. Development and validation of a preoperative scoring system to predict 30-day mortality in patients undergoing hip fracture surgery. Br J Anaesth. 2008;101:511–517. doi: 10.1093/bja/aen236. [DOI] [PubMed] [Google Scholar]
- 12.Wiles MD, Moran CG, Sahota O, Moppett IK. Nottingham hip fracture score as a predictor of 1-year mortality in patients undergoing surgical repair of fractured neck of femur. Brit J Anaesth. 2011;106:501–504. doi: 10.1093/bja/aeq405. [DOI] [PubMed] [Google Scholar]
- 13.Kirkland LL, Kashiwagi DT, Burton MC, Cha S, Varkey P. The Charlson Comorbidity Index Score as a predictor of 30-day mortality after hip fracture surgery. Am J Med Qual. 2011;26:461–467. doi: 10.1177/1062860611402188. [DOI] [PubMed] [Google Scholar]
- 14.Cox C, Chu H, Schneider MF, Muñoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Statist Med. 2007;26:4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]


