Abstract
Objective
To measure and analyze motor unit number estimation (MUNE) values longitudinally in spinal muscular atrophy (SMA).
Methods
Sixty-two children with SMA types 2 and 3 were observed prospectively for up to 42 months. Longitudinal electrophysiological data were collected, including compound motor action potential (CMAP), single motor unit action potential (SMUP), and MUNE.
Results
Significant motor neuron loss and compensatory collateral reinnervation were noted at baseline. Over time, there was a significant mean increase in MUNE (4.92 units/year, P = 0.009), a mean decrease in SMUP amplitude (−6.32 μV/year, P = 0.10) and stable CMAP amplitude.
Discussion
The unexpected longitudinal results differ from findings in amyotrophic lateral sclerosis, perhaps indicating that compensatory processes in SMA involve new motor unit development. A better understanding of the mechanisms of motor unit decline and compensation in SMA is important for assessing novel therapeutic strategies and for providing key insights into disease pathophysiology.
Keywords: Spinal muscular atrophy, Motor neuron disease, Motor unit number estimation, Compound motor action potential, Electrophysiology
Introduction
Research advances have increased dramatically our understanding of spinal muscular atrophy (SMA) over the past 2 decades1,2 and have fostered a growing number of clinical trials that have raised hope for the discovery of effective treatments in the foreseeable future3,4. Reliable and informative outcome measures are critical for the design and success of any clinical trial; however, standardized outcome measures have only been developed recently or have been adapted for use in SMA, as its era of clinical therapeutics has only begun in earnest recently5. Clinical outcome measures in SMA have included traditional6, modified7, and expanded8 versions of the Hammersmith Functional Motor Scale, the Gross Motor Function Measure9, and the PedsQL10.
Electrophysiological outcome measures provide unique information regarding the primary cause of clinical decline in SMA, the loss of functional motor neurons. They also provide unique insights into compensatory motor unit responses to disease. Motor unit number estimation (MUNE) and the compound motor action potential (CMAP)11 have been used extensively to track disease progression in ALS and have begun to be employed in SMA in recent years12. MUNE provides a quantitative estimate not only of the number of functional motor units, but also of their sizes in the form of the single motor unit potential (SMUP) amplitude, providing a measurement of the extent of collateral reinnervation. CMAP amplitude, as the product of both motor unit size and motor unit number, provides less detailed information as a solitary measure than a full set of MUNE studies, which detail the full the contribution of both motor unit size and number to the CMAP. However, the CMAP is easy and quick to perform and is obtained during routine diagnostic motor nerve conduction studies.
In this study, the Pediatric Neuromuscular Clinical Research (PNCR) Network for Spinal Muscular Atrophy collected MUNE, SMUP, and CMAP data on subjects with SMA types 2 and 3 to determine how well each of these measures correlates with standard clinical outcome measures and to analyze their changes over time. More detailed clinical data and some limited electrophysiological data from this study are reported separately13.
Methods
The PNCR Network enrolled 85 children with SMA types 2 and 3 and confirmed homozygous deletions in exon 7 of the SMN1 gene in a natural history study at 3 sites (Boston Children’s Hospital, Columbia University, and Children’s Hospital of Philadelphia). The study was approved by the institutional review boards at each participating institution, and written informed consent was obtained in all cases, either from the parents/guardians or the participants. Patients who had severe respiratory or other medical conditions that precluded safe participation or who did not live within a reasonable driving distance from a participating site were excluded. Data were excluded on 6 subjects who had inadequate evaluations13 and 17 others who did not have electrophysiological testing; thus, this investigation focuses on the 62 participants in whom electrophysiological testing was performed. Detailed methods regarding recruitment, follow-up, and evaluation of study participants, as well as quality control, have been described previously.13, 14
Participants were evaluated at baseline and at months 2, 4, 6, 9, and 12, and every 6 months thereafter for up to 42 months. Because electrophysiological testing was not performed at entry into the larger clinical study in all subjects, the first visit at which such testing was performed was used to summarize the “baseline” data and to perform cross-sectional analyses (see below). Forty-eight of the 62 participants (77%) had at least 2 electrophysiological evaluations and were included in longitudinal analyses.
Traditional criteria were used for subtype classification based on maximum gross motor function achieved at some point in the course: type 2 participants were able to sit independently and consistently when placed in that position (n = 30), and type 3 participants were able to walk consistently for at least 25 steps (n = 32)15. Type 3 participants were subdivided further into those who were non-ambulatory (n = 12) or ambulatory (n = 20) at their initial electrophysiological evaluation.
All electrophysiological studies were performed or supervised by a practicing electromyographer on site with certification in either Electrodiagnostic Medicine by the American Board of Electrodiagnostic Medicine or Clinical Neurophysiology by the American Board of Psychiatry and Neurology (PBK, CLG, and RLF). Prior to the start of the study, a standardized and technically detailed MUNE protocol was developed based upon best available evidence by the head of the central EMG laboratory (CLG), who has extensive experience in a variety of MUNE techniques and particular experience in the application of MUNE to motor neuron disease, in concert with supervising electrophysiologists at each site. A training session conducted by the head of the central EMG laboratory was attended by the electromyographers who were responsible for the other 2 sites (PBK and RLF). An online system was developed to enable rapid transmission of both numerical and waveform data to the central EMG laboratory from each site. All datasets, including waveforms, from every subject at every session were reviewed personally by the head of the central EMG laboratory for technical errors prior to submission to a centralized database for storage and future analysis. Where applicable, each technologist at each site passed a detailed certification protocol prior to the start of data collection. This consisted of multiple rounds of practice studies on normal subjects and SMA patients, using the above system, for review by the head of the central EMG laboratory for technical acceptability and reproducibility. A digital EMG machine capable of recording motor amplitudes in microvolts was used at all 3 sites. Whenever possible, medial wrist skin temperatures of 32-34°C were recorded at the beginning of each study, and the extremity was heated if necessary. Standard motor nerve conduction studies were performed with stimulation of the ulnar nerve at the wrist, as well as below and above the elbow. The recording site was the right abductor digiti minimi (ADM) muscle (also known as the abductor digiti quinti), with the active recording electrode placed over the midpoint of the lateral hypothenar eminence and the reference electrode placed over the distal interphalangeal joint of the fifth digit. The ground electrode was placed over the lateral aspect of the palm or dorsum of the hand. When an ulnar neuropathy at the elbow was identified, the left side was screened and, if no ulnar neuropathy was found, this side was used instead; otherwise, the evaluation was not performed. The distal CMAP amplitude was measured and recorded. Multiple point stimulation MUNE was performed in the ulnar nerve with recording over the abductor digiti minimi muscle using a technique described previously16. Stimulus intensities of 0-10 mA were generally used. Stimulations were first attempted at or near the wrist crease, and a SMUP was confirmed if: (a) an “all or nothing” response was observed via slight adjustments in stimulus intensities and (b) it could be reproduced and recorded at least 10 times. Once a set of these potentials was recorded, the stimulation site was moved, usually by a distance of less than a centimeter, in an attempt to find a different single motor unit potential. SMUPs were determined to be distinct based on morphologies and average amplitudes. This process was repeated until: (a) 10-20 distinct sets of SMUPs were recorded, (b) all reasonable stimulation sites along the ulnar nerve between the wrist crease and the ulnar groove at the elbow were exhausted, or (c) the limit of patient tolerance was reached. Motor unit number estimates were calculated from the CMAP and the mean SMUP.
Clinical outcome measures included the Hammersmith Functional Motor Scale (HFMS)6, the Expanded Hammersmith Functional Motor Scale (HFMSE)8, the Gross Motor Function Measure (GMFM)9, forced vital capacity (FVC, percent of predicted for age and height)13, and strength of elbow flexion, knee flexion, and knee extension measured using a hand-held dynamometer13. Other demographic and clinical data such as age, gender, and SMN2 copy number were recorded.
Spearman rank correlation coefficients were used to describe the associations between the electrophysiological measures (MUNE, CMAP amplitude, and SMUP amplitude) and measures of motor function (GMFM, HFMSE), pulmonary function (FVC), and strength, overall and within SMA subtypes. To assess the associations between a number of variables and the extent of collateral reinnervation, participants with severe denervation (MUNE < 50) were divided into those with SMUP amplitude ≤ 100 μV (less reinnervation) versus those with SMUP amplitude > 100 μV (more reinnervation). These 2 groups were compared with respect to age, gender, SMA type, walking status, SMN2 copy number, motor function (GMFM, HFMSE), and elbow flexion strength using Wilcoxon rank sum tests or chi-square tests, as appropriate.
Mixed effects linear regression models were used to estimate the rates of change of the electrophysiological measures over time. These models included SMA type as a covariate and year of follow-up (continuous) as the independent variable of interest. These models allowed for subject-specific intercepts and slopes, with the average slope being of primary interest. Potential baseline correlates of change over time were examined by adding appropriate main effect and interaction terms to the mixed effects linear regression models. Variables considered were SMA type (2, 3), SMN2 copy number (3, 4), gender (male, female), and age group (< 5 years, 5-10 years, 10-15 years, ≥ 15 years).
Results
At baseline, consistent clinical and electrophysiological patterns were seen across SMA subtypes (Table 1). Mean MUNE and mean CMAP amplitude were substantially lower in the type 2 patients, intermediate in non-ambulatory type 3 patients, and highest in ambulatory type 3 patients. Also, mean SMUP amplitude was higher in the non-ambulatory patients (type 2 and type 3) and lower in the ambulatory (type 3) patients. Measures of motor function (GMFM, HFMSE), pulmonary function (FVC), and strength all demonstrated the expected patterns with SMA subtype (Table 1). There were no significant differences in demographic, phenotypic, or genetic variables between the group that underwent electrophysiological studies (n = 62) and those who did not (n = 17), though the latter were slightly younger and were more likely to be type 2 (Supplemental Table 1). Among the 62 participants with electrophysiological data, those who had only 1 MUNE evaluation (n = 14) were much more likely to be Type 2, have 3 SMN2 copies, display lower MUNE and CMAP (and higher SMUP) values, and poorer muscle function and strength than those who had longitudinal MUNE data (n = 48) (Supplemental Table 2).
Table 1.
Participant characteristics at the initial electrophysiological evaluation, overall and by SMA subtype
| Variable | Type 2 (n = 30) |
Type 3 Non-Ambulatory (n = 12) |
Type 3 Ambulatory (n = 20) |
All (n = 62) |
|---|---|---|---|---|
|
| ||||
| Age (years) | 9.6 (7.2) 0.9-32.5 |
16.8 (9.5) 3.9-39.6 |
13.1 (11.6) 2.5-45.1 |
12.1 (9.5) 0.9-45.1 |
|
| ||||
| Age group (%) | ||||
| < 5 years | 30.0 | 16.7 | 20.0 | 24.2 |
| 5-10 years | 33.3 | 0.0 | 40.0 | 29.0 |
| 10-15 years | 23.3 | 33.3 | 15.0 | 22.6 |
| ≥ 15 years | 13.3 | 50.0 | 25.0 | 24.2 |
|
| ||||
| Female gender (%) | 63.3 | 33.3 | 55.0 | 54.8 |
|
| ||||
|
SMN2 copy number (%) |
||||
| 3 | 100.0 | 75.0 | 45.0 | 77.4 |
| 4 | 0.0 | 25.0 | 55.0 | 22.6 |
|
| ||||
| MUNE | 15.4 (15.6) 1.6-79.9 |
62.5 (56.9) 7.7-197.4 |
110.2 (72.2) 9.4-281.0 |
55.1 (64.1) 1.6-281.0 |
|
| ||||
| CMAP (mV) | 1.4 (1.2) 0.3-5.5 |
4.3 (2.0) 1.1-7.7 |
7.1 (3.0) 1.0-12.2 |
3.8 (3.3) 0.3-12.2 |
|
| ||||
| SMUP (μV) | 109.1 (64.7) 48.1-292.8 |
106.5 (72.4) 39.0-281.8 |
83.9 (47.2) 39.8-244.3 |
100.5 (61.4) 39.0-292.8 |
|
| ||||
| GMFM | 15.3 (8.4) 2.0-30.0 |
32.2 (9.3) 17.6-45.7 |
82.8 (15.2) 44.5-99.7 |
42.4 (33.0) 2.0-99.7 |
|
| ||||
| HFMSE | 8.9 (7.4) 0-22 |
24.6 (5.7) 16-31 |
53.7 (7.8) 34-66 |
27.0 (21.5) 0-66 |
|
| ||||
| FVC | 49.5 (22.3) 11-86 |
83.3 (12.4) 60-101 |
98.9 (15.9) 72-131 |
72.6 (28.9) 11-131 |
|
| ||||
| Strength (kg) | ||||
| Elbow flexion | 2.2 (1.1) 0.0-5.3 |
9.3 (9.8) 1.7-35.8 |
14.9 (10.1) 2.8-41.6 |
7.5 (8.9) 0.0-41.6 |
| Knee flexion | 2.3 (1.0) 0.3-4.7 |
5.6 (3.7) 1.2-13.7 |
10.5 (7.4) 0.7-30.8 |
5.5 (5.8) 0.3-30.8 |
| Knee extension | 0.9 (1.0) 0.0-4.2 |
1.6 (1.2) 0.0-4.1 |
4.7 (3.3) 0.8-14.2 |
2.3 (2.6) 0.0-14.2 |
Values are mean (standard deviation) and range unless otherwise indicated
MUNE, motor unit number estimate; CMAP, compound motor action potential; SMUP, single motor unit potential; GMFM, Gross Motor Function Measure; HFMSE, Expanded Hammersmith Functional Motor Scale; FVC, forced vital capacity (percent predicted)
The correlations between the electrophysiological measures and measures of motor function, pulmonary function, and strength at initial evaluation were variable (Table 2). In type 2 SMA, MUNE was correlated moderately with the GMFM (r = 0.45), HFMSE (r = 0.49), FVC (r = 0.44), elbow flexion strength (r = 0.43), and knee extension strength (r = 0.52). The associations of SMUP and CMAP amplitudes with function and strength measures were weaker, apart from a moderate negative correlation between SMUP amplitude and the GMFM (r = −0.51) (Table 2). In non-ambulatory type 3 participants, MUNE showed moderate correlations with the GMFM (r = 0.58) and the HFMSE (r = 0.57) and a strong correlation with elbow flexion strength (r = 0.83). CMAP amplitude was also associated with the GMFM (r = 0.53) and the HFMSE (r = 0.63) as well as elbow flexion strength (r = 0.49) (Table 2), although these associations did not reach statistical significance, possibly due to the very small sample size in this subgroup (n = 12). In type 3 ambulatory patients, MUNE was associated weakly with the function and strength measures, but CMAP amplitude demonstrated moderate correlations with the GMFM (r = 0.45), HFMSE (r = 0.55), elbow flexion strength (r = 0.70), and knee flexion strength (r = 0.79) (Table 2). The associations of MUNE and CMAP amplitude with measures of function and strength were quite strong in the sample as a whole (Table 2), likely reflecting the major differences between the SMA subtypes with respect to each of these variables.
Table 2.
Spearman rank correlations between electrophysiological measures and clinical measures at the initial electrophysiological evaluation, overall and by SMA subtype
| SMA Type | Variable | MUNE | CMAP | SMUP |
|---|---|---|---|---|
| Type 2 | GMFM | 0.45 | 0.04 | −0.51 |
| (n = 30) | HFMSE | 0.49 | 0.17 | −0.37 |
| FVC | 0.44 | 0.27 | −0.34 | |
| Elbow Flexion | 0.43 | 0.25 | −0.14 | |
| Knee Flexion | 0.05 | 0.19 | 0.06 | |
| Knee Extension | 0.52 | 0.06 | −0.43 | |
| Type 3 | GMFM | 0.58 | 0.53 | −0.28 |
| Non- Ambulatory |
HFMSE | 0.57 | 0.63 | −0.35 |
| (n = 12) | FVC | 0.04 | −0.47 | −0.24 |
| Elbow Flexion | 0.83 | 0.49 | −0.45 | |
| Knee Flexion | 0.39 | 0.21 | −0.21 | |
| Knee Extension | −0.17 | 0.10 | 0.61 | |
| Type 3 | GMFM | 0.12 | 0.45 | 0.27 |
| Ambulatory | HFMSE | 0.24 | 0.55 | 0.18 |
| (n = 20) | FVC | 0.25 | 0.20 | −0.20 |
| Elbow Flexion | 0.38 | 0.70 | −0.06 | |
| Knee Flexion | 0.43 | 0.79 | −0.01 | |
| Knee Extension | 0.02 | 0.30 | 0.27 | |
| Overall | GMFM | 0.77 | 0.77 | −0.30 |
| (n = 62) | HFMSE | 0.79 | 0.80 | −0.25 |
| FVC | 0.70 | 0.67 | −0.28 | |
| Elbow Flexion | 0.82 | 0.79 | −0.22 | |
| Knee Flexion | 0.65 | 0.69 | −0.12 | |
| Knee Extension | 0.58 | 0.56 | −0.12 |
P < 0.01 for values in bold
MUNE, motor unit number estimate; CMAP, compound motor action potential; SMUP, single motor unit potential; GMFM, Gross Motor Function Measure; HFMSE, Expanded Hammersmith Functional Motor Scale; FVC, forced vital capacity (percent predicted)
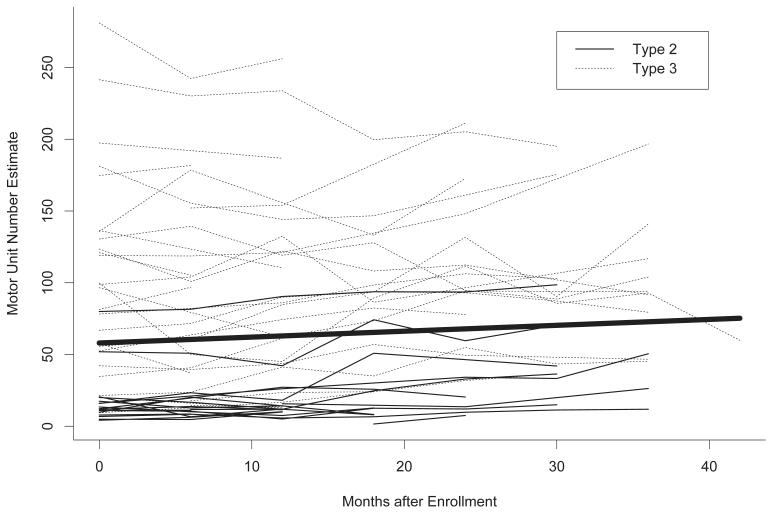
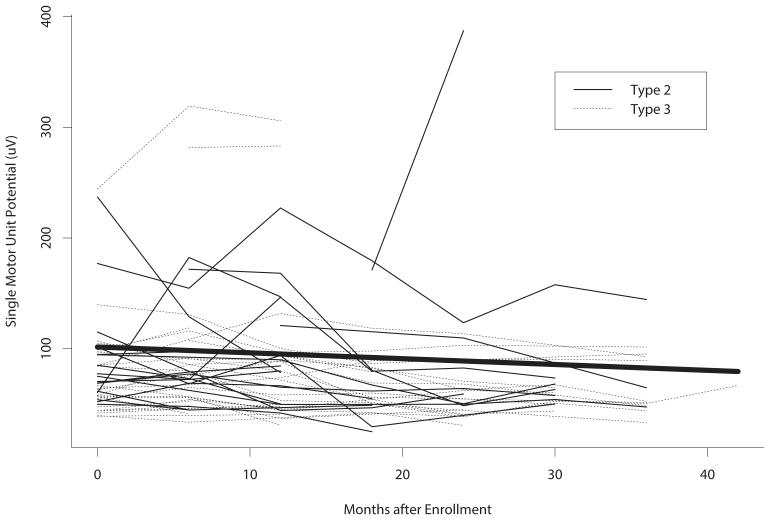
For the overall sample, there was a significant mean increase in MUNE value over time (4.92 units/year, 95% confidence interval [CI] 1.26-8.57, P = 0.009), corresponding to a mean decrease in SMUP amplitude (−6.32 μV/year, 95% CI −13.94-1.31, P = 0.10) and stable CMAP amplitude (−0.03 mV/year, 95% CI −0.18-0.11, P = 0.65) (Table 3). Figures 1 and 2 demonstrate the changes over time in MUNE and SMUP amplitude in individual participants. The findings concerning MUNE were remarkably consistent across subgroups defined by age, gender, SMA subtype, and SMN2 copy number (Supplemental Table 3). While mean increases in MUNE and mean decreases in SMUP amplitude were numerically largest in the 5-10 year-old children, significant associations with age were not detected (Supplemental Tables 3 and 4). A pattern of a mean increase in CMAP amplitude in younger children and a mean decrease in older participants was evident (P = 0.008, Supplemental Table 5). Male participants appeared to have a greater mean decline in SMUP amplitude than female participants, but this difference was not statistically significant (P = 0.12, Supplemental Table 4).
Table 3.
Mean annual rates of change in electrophysiological measures
| Variable | Slope | 95% CI | P-value |
|---|---|---|---|
| MUNE | 4.92 | (1.26, 8.57) | 0.009 |
| CMAP (mV) | −0.03 | (−0.18, 0.11) | 0.65 |
| SMUP (μV) | −6.32 | (−13.94, 1.31) | 0.10 |
Mean annual rates of change (slopes), confidence intervals, and p-values were obtained from a mixed effects linear regression model that included SMA type as a covariate and year of follow-up (continuous) as the independent variable of interest; see text for details.
CI, confidence interval; MUNE, motor unit number estimate; CMAP, compound motor action potential; SMUP, single motor unit potential
Figure 1.
Plot of individual subject trajectories in motor unit number estimate (MUNE) over time. The line in bold is the average trajectory estimated using a mixed-effects linear regression model. On average, MUNE values increased by 4.92 units/year.
Figure 2.
Plot of individual subject trajectories in single motor unit potential (SMUP) amplitude (μV) over time. The line in bold is the average trajectory estimated using a mixed-effects linear regression model. On average, SMUP amplitudes decreased by 6.32 μV/year.
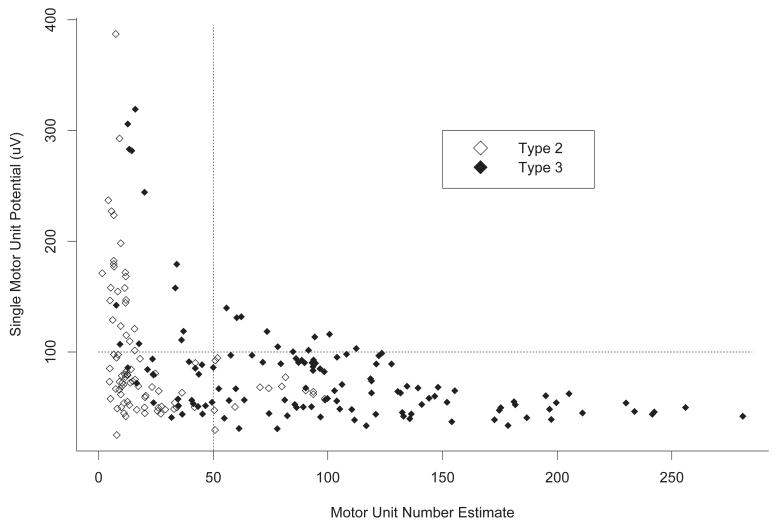
The relationship between MUNE and SMUP amplitude is depicted for all participant evaluations in Figure 3. In participants who had a MUNE > 100, SMUP amplitudes remained in the 39-100 μV range. In a subgroup of participants with MUNE ≤ 100 (consistent with greater denervation), SMUP amplitudes increased well above 100 μV (ranging up to 400 μV). This pattern was even more pronounced in participants with MUNE < 50.
Figure 3.
Plot of average single motor unit potential (SMUP) amplitude (μV) vs. motor unit number estimate (MUNE) using data from all participant visits. Plot symbols indicate SMA type. When MUNE > 100, SMUP amplitudes remain in the 39-100 μV range. When MUNE ≤ 100, however, SMUP amplitudes increase well above 100 μV in a subgroup of participants, consistent with ongoing collateral reinnervation in this more denervated group. This is particularly evident in those with MUNE < 50.
To identify characteristics that distinguish participants with significant denervation (MUNE < 50) who exhibit a large (> 100 μV) SMUP amplitude (i.e., robust collateral reinnervation) from those who exhibit a smaller (≤ 100 μV) SMUP amplitude despite a low MUNE, we compared these subgroups with respect to age, gender, SMA type, SMN2 copy number, motor function, and strength (Supplemental Table 6). The subgroups were comparable with respect to motor function, elbow flexion strength, gender distribution, and SMN2 copy number, but those with average SMUP amplitude > 100 μV tended to be older (mean 12.6 years vs. 8.3 years, P = 0.03) and to more frequently have type 3 SMA (42.1% vs. 15.0%, P = 0.06) than those with average SMUP amplitude ≤ 100 μV.
Discussion
A typical pattern of denervating disease emerged in the baseline cross-sectional analysis, with lower mean MUNE values and CMAP amplitudes observed in children with SMA type 2 compared to those with SMA type 3. Overall, SMUP amplitudes were largest in SMA type 2, consistent with ongoing collateral reinnervation in that group, though high SMUP amplitudes were also observed in some participants with SMA type 3 who also had significant loss of motor neurons by MUNE analysis. Clinical function scales and strength measures also showed predictable associations with SMA subtype. The clinical and electrophysiological differences between the cohort that yielded longitudinal data versus the one that did not indicate that the results of the longitudinal analysis may be most applicable to patients with milder phenotypes, although the changes in electrophysiological measures over time did not appear to depend on the severity of the phenotype. Also, there are important implications for the overall pathophysiology of this disease.
MUNE correlated moderately with functional measures in SMA type 2 and in non-ambulatory SMA type 3, but less well with these outcomes in ambulatory SMA type 3 participants. Up to 50% of motor units may be lost before clinical weakness appears in a given muscle group17. Consequently, if MUNE is measured longitudinally in a muscle with normal baseline strength in a patient with a global denervating disease, there will be a pre-symptomatic period of “silent” denervation, during which a declining MUNE will correlate poorly with both strength and function, which remain normal. It is only after weakness appears that the strong correlations between MUNE and strength/function appear16. In the least affected ambulatory Type 3 SMA patients in this study, this confounding feature would likely weaken the associations between MUNE and functional measures. A very strong correlation was seen between MUNE and elbow flexion strength in type 3 non-ambulatory subjects. This association was also seen, though to a lesser degree, in the other SMA types. CMAP amplitude showed moderate to high correlations with elbow flexion strength in type 3 participants as a whole, but not in type 2 participants, possibly because of the severe and paralytic weakness in elbow flexion of type 2 participants compared to the other groups. Just as correlations between MUNE and strength may be weaker in the pre-symptomatic phase of denervation when muscle strength is still normal, MUNE will still be detectable at very low levels and will decrease for a period of time in a near- to completely-paralyzed muscle before reaching zero. This will result in weaker correlations with low or absent strength.
Increased SMUP size and decreased MUNE were seen in the more severe SMA type 2 group and to a lesser extent in SMA type 3 participants. Figure 3 demonstrates progressive increases in SMUP size as MUNE values fall below 50. These findings indicate that collateral reinnervation is occurring in SMA type 2 participants and in those with type 3 who are experiencing more severe denervation. However, longitudinal data demonstrated an unexpected mean increase in MUNE over time with a corresponding mean decrease in SMUP amplitude for the group as a whole. The mean increase in MUNE and mean decline in SMUP amplitude appeared to be most marked in the 5-10 year-old age group, although the associations with age were not significant statistically. We considered potential confounding factors such as the increase in hand size that occurs during normal growth and development, but such variables are not likely to affect the recordings in question, as motor unit growth is not known to be related directly to somatic growth of the extremities. We also considered potential technical factors, but the statistically significant finding of an increase in MUNE over time emerged in a reasonably large cohort of SMA type 2 and type 3 subjects and was consistent among various subgroups (Supplemental Table 3). Also, the testing was performed by trained electromyographers at 3 tertiary care academic medical centers. Moreover, 38 of the 48 participants (79%) who contributed longitudinal electrophysiological data had 3 or more evaluations, reducing the likelihood that technical errors from isolated recording sessions would lead to spurious results. For these reasons, we believe that our findings are important to confirm.
Our results appear to differ from those from a previous study12, which found that both MUNE and CMAP declined with age in a cohort that included all 3 major SMA subtypes. That study analyzed the longitudinal measurements according to age rather than according to time starting from a common baseline (as in the setting of a clinical trial), which may account for at least some of the differences in the observations between the 2 studies. The prior study included patients with SMA type 1 (we did not) and relatively few SMA type 3 patients; this may have potentially contributed to the divergent findings, particularly since the authors used age as the time indicator in the analysis12.
Collateral reinnervation is a pattern seen in adult motor neuron diseases such as amyotrophic lateral sclerosis, in which motor unit loss (reflected by decreases in MUNE) is offset by increases in the size of surviving motor units due to collateral reinnervation (reflected by increases in SMUP amplitude). However, the longitudinal increase in MUNE and concurrent decrease in SMUP size in this study is unexpected and could indicate that compensation for denervation in young patients with SMA (especially prepubertal children) includes an element of new motor unit development, perhaps as an outgrowth of the normal motor neuron maturation process in children. It is widely believed that motor neuron regeneration does not occur in mammals18; however, it has become apparent in recent years that zebrafish have a robust capacity for regeneration in a number of cell types, including motor neurons in the spine19-21. This raises the question of whether a subtle or latent regenerative capacity in mammalian motor neurons may become apparent upon further investigation. An alternative explanation for our longitudinal findings is that existing motor neurons in the spinal cord may exhibit axonal growth across spinal levels and into nerves to which they ordinarily do not contribute, resulting in an increasing number of small motor units that innervate a given muscle over time. This phenomenon has been documented in a rat model of brachial plexus injury22. A third potential explanation is that some affected motor neurons may develop presynaptic abnormalities, leading to loss of synapse occupation by their axons, as has been demonstrated in mouse23 and zebrafish24,25 models. If a child with SMA regenerates a limited number of such axons, apparent new motor unit potentials may appear on electrophysiological testing.
Experimental evidence suggests that the need for SMN in developing motor neurons varies over time26,27 with the highest requirement early in life. In a setting of SMN deficiency, early on, axons of surviving motor neurons may fail to establish functional neuromuscular synapses with muscle fibers or may even retract. As the SMN protein requirements diminish over time, it is possible that axons may grow and create new neuromuscular synapses and, hence, nascent motor units. As the new motor unit potentials might be smaller than average, and as the proportion of developing motor units might increase gradually, a higher proportion of small motor unit potentials might accrue, resulting in a lower mean SMUP amplitude over time. The prominence of this pattern in the 5 to 10 year age group is consistent with this hypothesis, though its absence in older age groups suggests that this process is temporally limited and may have a modest or imperceptible clinical impact without interventions to augment the response. Prior studies have also found that SMA subjects have a higher than expected proportion of small motor unit potentials28, especially when compared to patients with ALS29,30. Though some other studies suggest that ALS patients may have a higher number of smaller motor unit potentials than normal controls when the full spectrum of potentials is analyzed31, all ALS patients experience progressive loss of motor unit numbers over time. No ALS study has ever documented increases in MUNE or decreases in SMUP amplitude over time.
Further study is needed to confirm these observations, and the normal process of motor unit maturation during healthy growth and development needs to be explored. However, the possibility that some young SMA subjects may still be in a phase of childhood development when motor units continue to develop. During this phase that developmental phenomenon could conceivably be harnessed to provide partial compensation against ongoing denervation; this is a novel concept and suggests a new avenue for possible therapeutic investigation. There are currently over a dozen different mouse models that mimic the genetic lesion and phenotype of SMA32, and histological studies that quantify motor neuron populations in the spinal cords of some of these mice over time might be a way to confirm these observations.
Electrophysiological measures, particularly MUNE and SMUP and CMAP amplitudes, continue to provide novel insights into motor unit decline and compensation in denervating diseases such as SMA. The results of this study illustrate the value of measuring MUNE, SMUP, and CMAP values to track disease progression and potential responses to therapeutic interventions, as these measurements complement each other. MUNE and SMUP provide not only a quantitative estimate of surviving motor units, but also a concurrent measure of average single motor unit size. This key information enables assessment of whether expansion of single motor unit size during collateral reinnervation might be masking the loss of motor units. These studies provide important and unique data that are critical to understanding the full milieu of SMA in children, as well as the role of development and compensation in functional clinical outcomes. Such information is vital to developing and measuring the effects of new therapeutic interventions.
Supplementary Material
Acknowledgements
The authors thank all the study participants and their families for their valuable contributions to this study. The Pediatric Neuromuscular Research Network for SMA (PNCR) includes Columbia University Medical Center (Clinical Coordination and Molecular Genetics), The Children’s Hospital of Philadelphia, and Boston Children’s Hospital. Data management was performed at the Muscle Study Group (MSG) Coordination and Biostatistics Centers at the University of Rochester (NY). The authors thank the following members of the PNCR for their assistance with study coordination, data collection, and data management: Maryam Oskoui, MD; Andrei Constantinescu, MD, PhD; A. Reghan Foley, MD; Rabi Tawil, MD; William B. Martens, BA; Jacqueline Montes, PT, MA; Vanessa Battista, CPNP; Jessica O’Hagen, DPT; Sally Dunaway, PT, DPT; Jean Flickinger, PT, PCS; Janet Quigley, PT, PCS; Susan Riley, PT, MS, DPT, PCS; Erica Sanborn, MS; Allan M. Glanzman, PT, DPT, PCS, ATP; Maryjane Benton, RN; Patricia Ryan, MA, OT; Mark Punyanitya, MD; Megan J. Montgomery, MPH; Jonathan Marra, MA; Benjamin Koo, BS. This study was funded by the Spinal Muscular Atrophy Foundation. Additional clinical research support was provided to Columbia University through CTSA grant No. UL1 RR024156 from NCATS NCRR/NIH and the NSADA K12 program (NINDS Training Grant); to The Children’s Hospital of Philadelphia through CTSA Award 1 NIH UL1-RR-024134 (NCRR/NIH); and to Harvard University through the UL1 RR025755–01 Harvard Catalyst Clinical & Translational Science Center (NCRR/NIH). The content of this report is solely the responsibility of the authors and should not be considered as the opinion or position of the NIH or its affiliates.
Abbreviations
- CMAP
compound motor action potential
- FVC
forced vital capacity
- GMFM
Gross Motor Function Measure
- HFMS
Hammersmith Functional Motor Scale
- HFMSE
Expanded Hammersmith Functional Motor Scale
- MUNE
motor unit number estimation
- PNCR
Pediatric Neuromuscular Clinical Research Network
- SMUP
single motor unit potential
- SMA
spinal muscular atrophy
References
- 1.Darras BT, Kang PB. Clinical trials in spinal muscular atrophy. Curr Opin Pediatr. 2007;19(6):675–679. doi: 10.1097/MOP.0b013e3282f1884c. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz JA, Singh P, Darras BT. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46(1):1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Chen TH, Chang JG, Yang YH, Mai HH, Liang WC, Wu YC, Wang HY, Huang YB, Wu SM, Chen YC, Yang SN, Jong YJ. Randomized, double-blind, placebo-controlled trial of hydroxyurea in spinal muscular atrophy. Neurology. 2010;75(24):2190–2197. doi: 10.1212/WNL.0b013e3182020332. [DOI] [PubMed] [Google Scholar]
- 4.Mercuri E, Bertini E, Messina S, Solari A, D’Amico A, Angelozzi C, Battini R, Berardinelli A, Boffi P, Bruno C, Cini C, Colitto F, Kinali M, Minetti C, Mongini T, Morandi L, Neri G, Orcesi S, Pane M, Pelliccioni M, Pini A, Tiziano FD, Villanova M, Vita G, Brahe C. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68(1):51–55. doi: 10.1212/01.wnl.0000249142.82285.d6. [DOI] [PubMed] [Google Scholar]
- 5.Montes J, Gordon AM, Pandya S, De Vivo DC, Kaufmann P. Clinical outcome measures in spinal muscular atrophy. J Child Neurol. 2009;24(8):968–978. doi: 10.1177/0883073809332702. [DOI] [PubMed] [Google Scholar]
- 6.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith functional motor scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol. 2003;7(4):155–159. doi: 10.1016/s1090-3798(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 7.Krosschell KJ, Scott CB, Maczulski JA, Lewelt AJ, Reyna SP, Swoboda KJ. Reliability of the Modified Hammersmith Functional Motor Scale in young children with spinal muscular atrophy. Muscle Nerve. 2011;44(2):246–251. doi: 10.1002/mus.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glanzman AM, O’Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, Quigley J, Montes J, Dunaway S, Deng L, Chung WK, Tawil R, Darras BT, De Vivo DC, Kaufmann P, Finkel RS. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26(12):1499–1507. doi: 10.1177/0883073811420294. [DOI] [PubMed] [Google Scholar]
- 9.Nelson L, Owens H, Hynan LS, Iannaccone ST. The gross motor function measure is a valid and sensitive outcome measure for spinal muscular atrophy. Neuromuscul Disord. 2006;16(6):374–380. doi: 10.1016/j.nmd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Iannaccone ST, Hynan LS, Morton A, Buchanan R, Limbers CA, Varni JW. The PedsQL in pediatric patients with Spinal Muscular Atrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Neuromuscular Module. Neuromuscul Disord. 2009;19(12):805–812. doi: 10.1016/j.nmd.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewelt A, Krosschell KJ, Scott C, Sakonju A, Kissel JT, Crawford TO, Acsadi G, D’Anjou G, Elsheikh B, Reyna SP, Schroth MK, Maczulski JA, Stoddard GJ, Elovic E, Swoboda KJ. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve. 2010;42(5):703–708. doi: 10.1002/mus.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann P, McDermott MP, Darras BT, Finkel RS, Sproule DM, Kang PB, Oskoui M, Constantinescu A, Gooch CL, Foley AR, Yang ML, Tawil R, Chung WK, Martens WB, Montes J, Battista V, O’Hagen J, Dunaway S, Flickinger J, Quigley J, Riley S, Glanzman AM, Benton M, Ryan PA, Punyanitya M, Montgomery MJ, Marra J, Koo B, De Vivo DC. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79(18):1889–1897. doi: 10.1212/WNL.0b013e318271f7e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann P, McDermott MP, Darras BT, Finkel R, Kang P, Oskoui M, Constantinescu A, Sproule DM, Foley AR, Yang M, Tawil R, Chung W, Martens B, Montes J, O’Hagen J, Dunaway S, Flickinger JM, Quigley J, Riley S, Glanzman AM, Benton M, Ryan PA, Irvine C, Annis CL, Butler H, Caracciolo J, Montgomery M, Marra J, Koo B, De Vivo DC. Observational study of spinal muscular atrophy type 2 and 3: functional outcomes over 1 year. Arch Neurol. 2011;68(6):779–786. doi: 10.1001/archneurol.2010.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol. 2007;22(8):946–951. doi: 10.1177/0883073807305673. [DOI] [PubMed] [Google Scholar]
- 16.Gooch CL, Kaufmann P. Multiple point stimulation motor unit number estimation with single motor unit tracking in a therapeutic ALS trial. In: Bromberg MB, editor. Supplements to Clinical Neurophysiology. Volume 55. Elsevier; 2003. pp. 284–285. [Google Scholar]
- 17.Daube JR. Electrophysiologic studies in the diagnosis and prognosis of motor neuron diseases. Neurol Clin. 1985;3(3):473–493. [PubMed] [Google Scholar]
- 18.Bareyre FM. Neuronal repair and replacement in spinal cord injury. J Neurol Sci. 2008;265(1-2):63–72. doi: 10.1016/j.jns.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Reimer MM, Kuscha V, Wyatt C, Sorensen I, Frank RE, Knuwer M, Becker T, Becker CG. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J Neurosci. 2009;29(48):15073–15082. doi: 10.1523/JNEUROSCI.4748-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimer MM, Sorensen I, Kuscha V, Frank RE, Liu C, Becker CG, Becker T. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28(34):8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias TB, Yang YJ, Ogai K, Becker T, Becker CG. Notch signaling controls generation of motor neurons in the lesioned spinal cord of adult zebrafish. J Neurosci. 2012;32(9):3245–3252. doi: 10.1523/JNEUROSCI.6398-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korak KJ, Tam SL, Gordon T, Frey M, Aszmann OC. Changes in spinal cord architecture after brachial plexus injury in the newborn. Brain. 2004;127(Pt 7):1488–1495. doi: 10.1093/brain/awh155. [DOI] [PubMed] [Google Scholar]
- 23.McGovern VL, Gavrilina TO, Beattie CE, Burghes AH. Embryonic motor axon development in the severe SMA mouse. Hum Mol Genet. 2008;17(18):2900–2909. doi: 10.1093/hmg/ddn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWhorter ML, Monani UR, Burghes AH, Beattie CE. Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J Cell Biol. 2003;162(5):919–931. doi: 10.1083/jcb.200303168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boon KL, Xiao S, McWhorter ML, Donn T, Wolf-Saxon E, Bohnsack MT, Moens CB, Beattie CE. Zebrafish survival motor neuron mutants exhibit presynaptic neuromuscular junction defects. Hum Mol Genet. 2009;18(19):3615–3625. doi: 10.1093/hmg/ddp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darras BT. More can be less: SMN1 gene duplications are associated with sporadic ALS. Neurology. 2012;78(11):770–771. doi: 10.1212/WNL.0b013e318249f754. [DOI] [PubMed] [Google Scholar]
- 27.Le TT, McGovern VL, Alwine IE, Wang X, Massoni-Laporte A, Rich MM, Burghes AH. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet. 2011;20(18):3578–3591. doi: 10.1093/hmg/ddr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galea V, Fehlings D, Kirsch S, McComas A. Depletion and sizes of motor units in spinal muscular atrophy. Muscle Nerve. 2001;24(9):1168–1172. doi: 10.1002/mus.1128. [DOI] [PubMed] [Google Scholar]
- 29.Buchthal F, Olsen PZ. Electromyography and muscle biopsy in infantile spinal muscular atrophy. Brain. 1970;93(1):15–30. doi: 10.1093/brain/93.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Erminio F, Buchthal F, Rosenfalck P. Motor unit territory and muscle fiber concentration in paresis due to peripheral nerve injury and anterior horn cell involvement. Neurology. 1959;9:657–671. doi: 10.1212/wnl.9.10.657. [DOI] [PubMed] [Google Scholar]
- 31.Vogt T, Nix WA. Functional properties of motor units in motor neuron diseases and neuropathies. Electroencephalogr Clin Neurophysiol. 1997;105(4):328–332. doi: 10.1016/s0924-980x(97)00028-3. [DOI] [PubMed] [Google Scholar]
- 32.Bebee TW, Dominguez CE, Chandler DS. Mouse models of SMA: tools for disease characterization and therapeutic development. Hum Genet. 2012;131(8):1277–1293. doi: 10.1007/s00439-012-1171-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.