Abstract
Various types of nanoparticles efficiently heat in radiofrequency fields, which can potentially be used to produce cancer cell cytotoxicity within minutes. Multifunctional and targeted nanoparticles have demonstrated effective cancer control in vivo without significant toxicity associated with radiofrequency field exposure. Importantly, animals treated systemically with targeted nanoparticles smaller than 50 nm demonstrate tumor necrosis after radiofrequency field exposure without acute or chronic toxicity to normal tissues. Likewise, the future holds great promise for multifunctional imaging as well as multimodality therapy with chemotherapeutic molecules and ionizing radiation sensitizing agents attached to nanoparticle constructs. However, the appropriate balance of safety and efficacy for diagnosis, therapy, and therapeutic monitoring with these nanoparticles remains to be fully elucidated.
With few notable exceptions, treatment for solid-organ malignancies consists of surgical resection, radiotherapy and chemotherapy. For some cancers, such as hepatocellular carcinoma, hyperthermic therapy is an option when resection is not feasible [1]. Thermal ablation is produced with probes that release non-ionizing radiation such as microwaves or radiofrequency (RF) fields. Unlike cryoablation where freeze–thaw cycles produce cancer cell death, hyperthermic therapy raises the temperature to well above 55°C, often 100°C, which induces not only protein denaturation but also vaporization at higher temperatures [2]. Obviously, these probes produce extracellular heat and destroy any tissue, malignant or normal, within a specified volume that is usually 1–3 cm in diameter. Of course, for oncologically sound operations, it is beneficial to have cancer-free margins. However, the destruction of normal tissue, creation of scar tissue and eventual fibrosis may result in adverse effects that negatively effect patient care.
In general, thermal ablation of malignancies is an adjunct treatment when resection is not possible. For example, long-term survival of colorectal hepatic metastases is highest after resection and less so after thermal ablation [3]. Furthermore, RF ablation has been utilized in the multi disciplinary treatment of renal-cell carcinoma and lung cancers [4,5]. The success of RF ablation in nonhepatic organ sites (lung, kidneys and breast) has not been nearly as successful as that seen for unresectable hepatic lesions. Part of this is due to the success and safety of resection at these organ sites (i.e, breast lesions) or the complications associated with thermal injury (i.e., pancreatic duct injury during pancreatic cancer ablation). Finally, while the focus of this review is on cancer therapy, it should be noted that in the future it may be possible to utilize these techniques for the treatment of infections and benign growths, two topics that will not be discussed herein.
Unlike invasive clinical RF ablation, RF field therapy is a non-invasive method to expose cancers to nonionizing radiation that is relatively nontoxic in and of itself. Patients receiving standard of care RF ablation undergo therapy with invasive probes that create a hyperthermic region of 2–4 cm around the probe's tip. However, nanoparticle-mediated RF field hyperthermia induces heating on the scale of approximately 100 μm. Fortunately, non-invasive RF fields easily penetrate human tissues and pass through the entire body with minimal perturbations until the RF fields interact with metal [6,7]. The size of the field can be scaled from small, animal-sized devices (Figure 1), up to very large volumes that could theoretically treat small (local tumor) or large regions of the human body. Samples (cells, animals or, theoretically, patients) are placed in an RF field created by a parallel plate capacitor (Figure 1). This establishes a directional electromagnetic field that passes through tissues and organs without significant absorbance. Metal, however, absorbs RF energy and quickly releases heat to the surrounding region. Nanoparticles in general, and metal nanoparticles specifically, are utilized because of this general principle, as well as their unique qualities that absorb even more energy (and release even more heat), due to their very small size and quantum characteristics.
Figure 1. A radiofrequency field is created between the source plate (top) and the ground plate (bottom) when connected to an RF generator (not imaged).
The sample holder can hold nanoparticle solutions or cells by changing out the Teflon holder. Likewise, animals are placed directly on the ground plate for in vivo radiofrequency field exposure.
RF: Radiofreqeuncy.
Recent advancements in nanotechnology have resulted in multiple types of nanoparticles that can be targeted with antibodies, peptides/proteins and sugar residues to cancer cells [8,9]. Thermal therapy is induced with either focused laser irradiation, manipulation of magnetic fields or RF field exposure [8,10,11]. While these nanoparticles may be more selective than specific, animal studies have demonstrated promising results without major toxicity [10,12,13]. Unlike the previously described forms of ablation, nanoparticles induce intracellular hyper-thermia. Unique physicochemical properties of metallic and non-metallic nanoparticles result in different heating rates for various types of non-ionizing radiation exposure.
The safety of nanoparticles has been a controversial topic in recent years [14]. We have found that solid gold nanoparticles less than 50 nm in diameter are safely taken up by macrophages in the liver and spleen without major toxicity [10,13]. Since gold is a fairly inert material, gold nanoparticles do not interact chemically with most biologic substances. Furthermore, the very small sizes may prevent cellular injury or inflammatory response as gold nanoparticles may be excreted unmodified. However, large nanoparticles with a large aspect ratio (i.e., rods or tubes) have been associated with fibrosis and cellular injury [14]. Short single-walled carbon nanotubes (SWNTs) with long axes <200 nm are likely to pass through pulmonary and renal filtering systems without injury, but this remains to be fully evaluated. Finally, it remains unclear which nanoparticles, if any, will pass the blood–brain barrier and what the consequences will be if so.
This review will primarily focus on RF field heating of gold nanoparticles as these particles appear to be the safest and are similarly effective compared with other nanoparticles. Likewise, SWNTs and quantum dots will also be discussed as these nanoparticles have interesting physical properties that may make them more effective theranostic nanoparticles in the future. The focus will be on those applications that are most likely to have favorable risk:benefit ratios and those that are most likely to result in improved patient care.
Nanoparticles heat in radiofrequency fields
Nanoparticle heating in RF fields is a very complex phenomenon. The end result is that RF fields induce nanoparticle heating rates of 1–3°C/s in various solutions and at various power levels [10,15]. Most RF field devices are based on shortwave RF fields (13.56 MHz) as licensing agencies permit this frequency for ‘medical use’ (Figure 1). The power of these devices is typically from 100–800 W. The energy transfer efficiency from the field generator to actual field strength varies amongst the devices; determining the exact field strength is problematic [10,12,16]. The RF electrical field strength in general, however, is approximately 5–15 kV/m [15].
In a nanoparticle concentration and field strength-dependent manner, nanoparticles in aqueous solutions can reach boiling temperatures in 2–3 min. Interestingly, deionized water negligibly heats in RF fields, while antibody solutions (e.g., with ions or proteins) typically heat around five- to ten-times less than nanoparticle solutions [17]. In the RF field, SWNTs heat in the range of 2–6°C/s, slighter faster than gold nanoparticles [18]. Quantum dots heat in a similar manner to gold nanoparticles, but they do not demonstrate significant differences when antibodies or proteins are bound to the quantum dots [17].
There remains some controversy regarding the mechanism in which nanoparticles heat in an RF field. Our group has demonstrated that gold nanoparticles heat primarily via Joule heating [15,17]. Temperatures were measured with an infrared camera in such a way as to not interfere with the RF field. This work has demonstrated that gold nanoparticles behave as ‘mini-resistors’, where free electrons on the surface have limitations to their movement. In this way, friction is created at the individual nanoparticle level, which release heat into the surrounding aqueous solution. Furthermore, based on these findings, one would predict less heating for larger nanoparticles as well as much less for aggregates where there are far fewer limitations placed on the movement of electrons. However, a single manuscript has shown that RF induced heating of gold nanoparticles is less than as previously described [19]. Issues with their research methodology include temperature measurements with a device inside the RF field itself (and, hence, may change the local field) and their use of aggregated gold nanoparticles. As described previously, aggregated gold nanoparticles are not expected to heat well in RF fields due to changes in electron mean free path on the surface of the aggregates compared with the surface of an individual gold nanoparticle. Furthermore, it is well known that aggregates of gold nanoparticles have different physio-optic properties than individual gold nanoparticles [20].
Animal cancer models utilizing RF-induced, nanoparticle-mediated hyperthermic therapy
Unlike external hyperthermia utilized clinically in humans, nanoparticle-mediated ablation of cancer cells offers numerous benefits. First, destruction is limited to regions around the nanoparticle (i.e., the cell). Second, RF fields are safely used clinically in MRI and for long-range communication such as in cellular phones and by the aviation community. Third, there are no clinical contra-indications to RF field exposure with the exception of metal implants in patients (e.g., joint replacements and pacemakers).
Selective delivery of nanoparticles is important to prevent off-target RF-induced injury. Thus far, small animal studies have not demonstrated off-target clinical toxicity. This is due to [21]:
■ Minimal absorption in nontargeted tissues [23];
■ Rapid excretion of nanoparticles not taken up by cells [13,24];
■ Theoretically, organ macrophage repopulation from activated circulating macrophages if Kupffer cells are destroyed after RF exposure.
An intact blood–brain barrier may protect the brain from nanoparticle exposure to neuronal cells while high blood flow organs such as lungs and the heart dissipate heat quickly. The exact protective mechanism is unknown since no injury has been seen in these organs.
Targeting with antibodies or proteins usually can result in selective delivery and hence, selective hyperthermia. That is, not only are nanoparticles delivered to the tumor, but also to scavenger cells in the liver, spleen, kidneys and lungs. RF-induced heating would then occur in all of those sites. Fortunately, small gold nanoparticles less than 50 nm in diameter have negligible delivery to the brain, heart, kidneys or lungs [10,13]. Furthermore, in the liver and spleen, the gold nanoparticles are endo cytosed into macrophage-family cells (i.e., Kupffer cells in the liver) that are efficiently replaced by circulating macrophages without clinical sequela or acute toxicity in animals [13]. Large nanoparticle constructs (>500 nm) are not expected to behave in a completely benign fashion. Specifically, these much larger nano particles and microparticles may interact in the human pulmonary and renal capillary vasculature in a different, and perhaps more inflammatory fashion, than the small gold nanoparticles described herein.
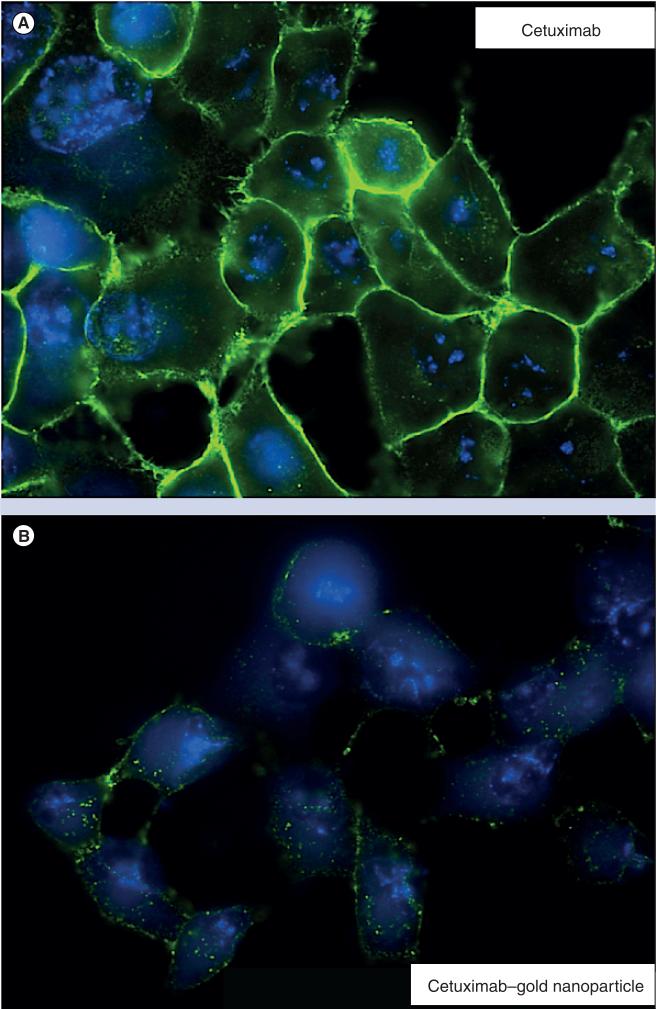
Gold nanoparticles have been used to ablate pancreatic xenografts non-invasively in a murine model [17]. Interestingly, the pattern of internalization is quite different when gold nanoparticles are conjugated to antibodies compared with free antibodies in vitro (Figure 2). In the in vivo study, mildly sedated mice tolerated 10 min weekly of whole-body RF field exposure without any evidence of complications over a 6-week time period. Importantly, weekly whole-body RF treatments resulted in significant pancreatic cancer xenograft control (p < 0.05) without any evidence of chronic injury to the nontargeted gold nanoparticle containing organs (liver and spleen) in murine models [10]. Gold was injected intraperitoneally 36 h prior to application of an RF field at a dose of 10 mg/kg.
Figure 2.
After 1 h of treatment, (A) Panc-1 cells in vitro have the majority of free cetuximab located on the cellular membrane; while (B), the same molar concentration of cetuximab–gold nanoparticles is internalized with a distinct punctate morphology.
Carbon SWNTs have also been demonstrated effective in inducing acute tumor necrosis after RF field exposure in vivo as well [18]. The model utilized was Vx2 tumors implanted in rabbit livers. Intratumoral injection of SWNTs induced necrosis of all tumors within 5 min of RF field exposure. Regions of necrosis were identified with 2–5 mm of margins. Importantly, SWNTs alone or RF field exposure alone did not induce any measurable tumor necrosis or liver injury. While this model did not utilize long-term treatment or measured survival, it did demonstrate proof-of-principle that RF-induced necrosis can be mediated by direct nanoparticle injection.
Multifunctional nanoparticles & theranostic approaches
Multifunctional nanoparticles offer the potential to provide diagnosis, therapy and monitoring in a compact and measurable construct. Gold nanoparticles, for example, can be functionalized with MRI contrast agents while other iron-based nanoparticles are MRI contrast agents by definition [25,26]. Depending on the size of the nanoparticle, conjugation with multiple targeting antibodies and chemotherapeutic agents can be done with relative ease. The challenge remains doing so in a safe, efficient and effective manner.
Quantum dots are quickly becoming useful research options for theranostics approaches to cancer therapy with the potential clinical applications. Quantum dots are small (<7 nm) semiconducting nanoparticles that are ‘tunable’ to fluoresce at very specific wavelengths [27]. In addition, quantum dots heat nearly as well as gold in RF fields suggesting that they may be used in the future for optical identification of cancer and monitoring of RF-induced thermal therapy [17]. Other groups have demonstrated that quantum dots conjugated to trastuzumab, the HER-2 antibody used for certain breast cancers, can be used for diagnosis and localization in vivo [28]. While there was some toxicity seen with free quantum dots injected intravenously, the toxicity was significantly less when conjugated to the antibody. While the free quantum dot did not localize to HER-2 positive cancer cells, the antibody-conjugated quantum dots did so quite well. This is indicative of a synergistic risk:benefit ratio where targeting decreased toxicity, increased diagnostic yield and potentially served a therapeutic benefit by having any toxicity due to quantum dots focused primarily on the cancer cells. Finally, multifunctional nanoparticles have been used to overcome cellular defenses to chemotherapy and increase ionizing radiation sensitivity [29,30].
Future perspective
Multifunctional nanoparticles clearly offer many potential benefits in the early diagnosis of cancer and monitoring of therapeutic effects. By utilizing RF-sensitive nanoparticles (i.e., gold) as the basis for the multifunctional platforms, a non-invasive and thus far safer therapy can be added to a diagnostic nanoparticle construct. Likewise, targeted chemotherapeutic agents offer the potential for simultaneous multimodality treatment at the individual cell level. Multifunctional nanoparticles in early preclinical models have demonstrated the potential selective delivery of chemotherapeutics that previously have only been given nonselectively via intravenous infusions [29].
Obviously, this would be the best-case scenario over the next 5–10 years. A major advantage of multifunctional platforms is to provide synergistic and simultaneous cytotoxic insults such as intracellular thermal ablation combined with chemotherapeutics that prevent hyperthermic cellular repair mechanisms. The major disadvantage of ‘multifunctional nanoparticles’ is the toxicity associated with the diagnostic portions, as well as the therapeutic adverse effects. Furthermore, identifying the appropriate balance of imaging agents, therapeutic agents and overall nanoparticle size remains a logistical, functional and chemical problem. However, we believe the relative safety of RF fields coupled with the biosafety of gold nanoparticles minimizes toxicity and enhances therapy compared with other combinations of ionizing radiation and heavy metal-based nanoparticles. Selective and specific multi-functional cancer treatments primarily work by minimizing normal cell toxicity, not necessarily enhancing cancer cell death. Minimizing risks and enhancing benefits applies to nanoparticle-based translational research, just as it does in clinical care.
Key Terms.
Nanoparticle: Homogenous particle with width less than 1 μm, usually less than 500 nm in diameter for a sphere.
Gold nanoparticles: Solid spheres of gold with diameters of 2–100 nm.
Carbon nanotubes: Hollow tubes of carbon with a length much longer than its width.
Quantum dot: Fluorescent nanoparticle (semiconducting nanoparticle) with a shell composed of various metals.
Executive summary.
■ Gold nanoparticles, quantum dots and carbon nanotubes quickly heat in otherwise nontoxic radiofrequency fields.
■ Small gold nanoparticles (<50 nm) delivered systemically control human pancreatic cancer xenografts in murine models without injury to other organs such as the liver, kidney or spleen after weekly radiofrequency field exposure.
■ Quantum dots, carbon nanotubes and multifunctional nanoparticles have the potential to allow pretherapeutic MRI imaging coupled with radiofrequency hyperthermic treatment monitoring.
■ By conjugating active medications to gold nanoparticles, we predict that in the future multimodality therapy will take place at cellular level with chemotherapy, ionizing radiation sensitization and radiofrequency-induced hyperthermia all occurring nearly simultaneously.
Acknowledgements
The authors would like to acknowledge Kristine Ash for assistance in preparation of this manuscript.
Evan S Glazer is funded by the Medical Sciences Graduate Program, Department of Surgery, The University of Arizona. Steven A Curley is funded from National Cancer Institute (NIH U54 CA143837), The University of Texas M.D. Anderson Cancer Center and The V Foundation.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Curley SA. Radiofrequency ablation leads to excellent local tumor control and durable longterm survival in specific subsets of early stage HCC patients confirming to the Milan criteria. Ann. Surg. 2010;252(6):913–914. doi: 10.1097/SLA.0b013e3182034862. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur. J.Radiol. 2011;79(1):124–130. doi: 10.1016/j.ejrad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Curley SA. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann. Surg. Oncol. 2008;15(1):11–13. doi: 10.1245/s10434-007-9668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J. Thorac. Cardiovasc. Surg. 2005;129(3):639–644. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Abdellaoui A, Watkinson AF. Radiofrequency ablation of renal tumors. Future Oncol. 2008;4(1):103–111. doi: 10.2217/14796694.4.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Roschmann P. Radiofrequency penetration and absorption in the human body: limitations to high-field whole-body nuclear magnetic resonance imaging. Med. Phys. 1987;14(6):922–931. doi: 10.1118/1.595995. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AY, Neyzari A. Deep local hyperthermia for cancer therapy: external electromagnetic and ultrasound techniques. Cancer Res. 1984;44(Suppl. 10):4736s–4744s. [PubMed] [Google Scholar]
- 8.Letfullin RR, Iversen CB, George TF. Modeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomedicine. 2011;7(2):137–145. doi: 10.1016/j.nano.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010;62(3):339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin. Cancer Res. 2010;16(23):5712–5721. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi WI, Kim JY, Kang C, Byeon CC, Kim YH, Tae G. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers. ACS Nano. 2011;5(3):1995–2003. doi: 10.1021/nn103047r. [DOI] [PubMed] [Google Scholar]
- 12.Cardinal J, Klune JR, Chory E, et al. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery. 2008;144(2):125–132. doi: 10.1016/j.surg.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazer ES, Zhu C, Hamir AN, Borne A, Thompson CS, Curley SA. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2010 doi: 10.3109/17435390.2010.516026. DOI: 10.3109/17435390.2010.516026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine. 2011;6(1):143–156. doi: 10.2217/nnm.10.139. [DOI] [PubMed] [Google Scholar]
- 15.Moran CH, Wainerdi SM, Cherukuri TK, et al. Size-dependent joule heating of gold nanoparticles using capacitively coupled radiofrequency fields. Nano Res. 2009;2(5):400–405. [Google Scholar]
- 16.Curley SA, Cherukuri P, Briggs K, et al. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J. Exp. Ther. Oncol. 2008;7(4):313–326. [PubMed] [Google Scholar]
- 17.Glazer ES, Curley SA. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer. 2010;116(13):3285–3293. doi: 10.1002/cncr.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gannon CJ, Cherukuri P, Yakobson BI, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110(12):2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Jung YS, Tan S, Kim HK, Chory E, Geller DA. Negligible absorption of radiofrequency radiation by colloidal gold nanoparticles. J. Colloid. Interface Sci. 2011;358(1):47–53. doi: 10.1016/j.jcis.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 20.Nergiz SZ, Singamaneni S. Reversible tuning of plasmon coupling in gold nanoparticle chains using ultrathin responsive polymer film. ACS Appl. Mater. Interfaces. 2011;3(4):945–951. doi: 10.1021/am200109r. [DOI] [PubMed] [Google Scholar]
- 21.Wardle EN. Kupffer cells and their function. Liver. 1987;7(2):63–75. doi: 10.1111/j.1600-0676.1987.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 22.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin. Cancer Res. 2010;16(23):5712–5721. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan JA, Kudgus RA, Szabolcs A, et al. Designing nanoconjugates to effectively target pancreatic cancer cells in vitro and in vivo. PLoS One. 2011;6(6):e20347. doi: 10.1371/journal.pone.0020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida JP, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine. 2011;6(5):815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 25.Ali MM, Yoo B, Pagel MD. Tracking the relative in vivo pharmacokinetics of nanoparticles with PARACEST MRI. Mol. Pharm. 2009;6(5):1409–1416. doi: 10.1021/mp900040u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warsi MF, Chechik V. Strategies for increasing relaxivity of gold nanoparticle based MRI contrast agents. Phys. Chem. Chem. Phys. 2011;13(20):9812–9817. doi: 10.1039/c0cp02508a. [DOI] [PubMed] [Google Scholar]
- 27.Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 2006;384(3):620–630. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari DK, Jin T, Behari J. Bio-distribution and toxicity assessment of intravenously injected anti-HER2 antibody conjugated CdSe/ZnS quantum dots in Wistar rats. Int. J. Nanomedicine. 2011;6:463–475. doi: 10.2147/IJN.S15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo SM, Oh IJ. Release of adriamycin from poly(lactide-co-glycolide)-polyethylene glycol nanoparticles. J. Nanosci. Nanotechnol. 2011;11(2):1795–1798. doi: 10.1166/jnn.2011.3406. [DOI] [PubMed] [Google Scholar]
- 30.Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology. 2010;21(29):295101. doi: 10.1088/0957-4484/21/29/295101. [DOI] [PMC free article] [PubMed] [Google Scholar]