Abstract
Neurotransmitter identity is a defining feature of all neurons because it constrains the type of information they convey, but it has become clear that many neurons in fact release multiple transmitters. Although the physiological role for co-release has remained poorly understood, the vesicular uptake of one transmitter can regulate filling with the other by influencing expression of the H+ electrochemical driving force. In addition, the sorting of vesicular neurotransmitter transporters and other synaptic vesicle proteins into different vesicle pools suggests the potential for distinct modes of release. Co-release thus serves multiple roles in synaptic transmission.
Keywords: neurotransmitter co-release, neurotransmitter co-storage, synaptic vesicle pools, vesicular neurotransmitter transporters
INTRODUCTION TO THE NEUROTRANSMITTER CYCLE
Chemical neurotransmission depends on the regulated synthesis and release of a range of soluble mediators. In the case of lipophilic or gaseous molecules such as endocannabinoids and nitric oxide, which readily penetrate biological membranes, release is regulated at the level of synthesis. However, the hydrophilic compounds that mediate most forms of both synaptic transmission and neuromodulation are packaged into vesicles that undergo regulated release by exocytosis. For neural peptides, synthesis and translocation into the secretory pathway occur at the endoplasmic reticulum, with subsequent packaging into large dense core vesicles (LDCVs) at the trans-Golgi network. LDCVs then translocate to release sites in the axon or dendrites and undergo regulated release in response to the appropriate physiological stimulus. However, the time required for passage through the secretory pathway and along neuronal processes limits the capacity for sustained release and hence high frequency transmission. Fast synaptic transmission is thus mediated by classical neurotransmitters which undergo local synthesis and recycling. Indeed, synaptic vesicles recycle locally, at the nerve terminal, through a carefully orchestrated process of exo- and endocytosis known as the synaptic vesicle cycle (1). In addition, release from rapidly recycling synaptic vesicles depends on their capacity to refill with transmitter at the nerve terminal, and presynaptic boutons have developed mechanisms to recapture released transmitter as well as to synthesize it de novo, as part of a parallel, integrated process known as the neurotransmitter cycle. The expression of specialized biosynthetic enzymes and transporters required for the neurotransmitter cycle thus define transmitter phenotype. A recent proteomic analysis indeed shows that glutamatergic and GABAergic synaptic vesicles differ primarily in the expression of vesicular transporters for glutamate and GABA (2).
NEUROTRANSMITTER CO-RELEASE
Although it has generally been assumed that neurons release only one classical neurotransmitter, exceptions continue to accumulate. The first demonstration of co-release involved adenosine triphosphate (ATP) and acetylcholine (ACh) in the electric organ of Torpedo californica (3, 4). Subsequent work showed that ATP is frequently stored and released with other, often cationic classical transmitters in the central and peripheral nervous systems of both invertebrates and vertebrates [for review see (5)]. Since the vesicular GABA transporter (VGAT) also transports glycine (6), it is not surprising that some neurons have been shown to release both inhibitory transmitters (7–9). Similarly, the vesicular monoamine transporter (VMAT2) recognizes serotonin and histamine as well as catecholamines, and is expressed by essentially all monoamine neurons. The biosynthetic enzymes for different monoamines are expressed by specific subpopulations, but the plasma membrane monoamine transporters show only modest substrate selectivity, indicating the potential for uptake, storage and release of one monoamine by a neuron which does not produce that particular transmitter. For example, the anti-depressant drug fluoxetine, which selectively inhibits the plasma membrane serotonin transporter (SERT), redistributes serotonin from serotonergic to dopaminergic terminals, where it also undergoes release, and this redistribution may contribute to its antidepressant action (10). In addition, glutamate-releasing thalamocortical neurons (as well as some retinal ganglion cells) express SERT and VMAT2 transiently during development, conferring the ability to take up and release serotonin during the critical period for maturation of this projection (11). Conversely, many monoamine neurons co-release glutamate when grown in culture (12, 13), and dopaminergic periglomerular cells in the olfactory bulb also co-release GABA (14). Even motor neurons thought to release only ACh may co-release glutamate from collateral synapses within the spinal cord (15).
Although the evidence for the co-release of classical neurotransmitters in vivo is clear, and the occurrence more widespread than originally anticipated, the physiological significance remains largely unknown. In this review, we therefore focus on the consequences of co-release for vesicle filling and for neurotransmission, synaptic plasticity and behavior.
VESICLE FILLING
Proton Electrochemical Driving Force
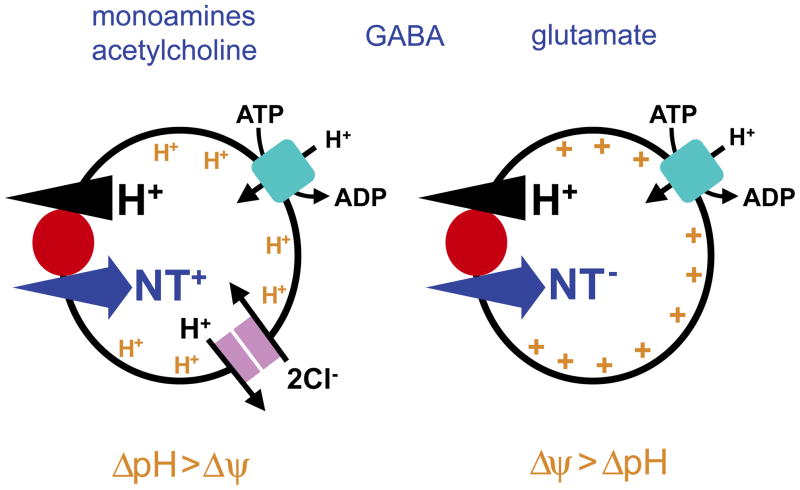
The filling of synaptic vesicles with neurotransmitter depends on the energy stored in a H+ electrochemical gradient (ΔμH+) produced by the vacuolar-type H+-ATPase. The vacuolar H+ pump resembles the F0/F1 ATPase (ATP synthase) of mitochondria in structure and function, but rather than using H+ flux to produce ATP, the vacuolar H+ pump uses ATP hydrolysis to drive H+ transport into membranes of the secretory pathway, including endosomes, lysosomes, synaptic vesicles, and LDCVs (16). ΔμH+ in turn comprises both a chemical gradient (ΔpH) and membrane potential (Δψ), and the transport of all classical transmitters into synaptic vesicles depends on both components (Figure 1). However, classical studies have shown that the different transport activities depend to differing extents on ΔpH and Δψ due to the charge on the substrate and the stoichiometry of coupling to H+.
Figure 1. Vesicular neurotransmitter transporters depend differentially on the chemical and electrical components of the H+ electrochemical gradient.
The vacuolar-type H+-ATPase generates the H+ electrochemical gradient (ΔμH+) required for transport of all classical neurotransmitters into synaptic vesicles. However, different vesicular neurotransmitter transporters rely to differing extents on the two components of ΔμH+, the chemical gradient (ΔpH) and the electrical gradient (Δψ). The vesicular accumulation of monoamines and ACh (left) involves the exchange of protonated cytosolic transmitter for two lumenal H+. The resulting movement of more H+ than charge dictates a greater dependence on ΔpH than Δψ for both VAChT and VMAT. Vesicular glutamate transport (right) may not involve H+ translocation. In the absence of Δψ, however, disruption of ΔpH inhibits uptake, suggesting that the transport of anionic glutamate involves exchange for nH+, resulting in the movement of n + 1 charge and hence greater dependence on Δψ than ΔpH. Transport of the neutral zwitterion GABA (and glycine) involves the movement of an equal number of H+ and charge, consistent with the similar dependence of VGAT on ΔpH and Δψ. These differences suggest that vesicles storing monoamines or ACh may have mechanisms to favor the accumulation of ΔpH at the expense of Δψ, whereas those storing glutamate may promote a larger Δψ. The extent to which vesicles differ in their expression of these two components remains unknown, but intracellular chloride carriers such as the synaptic vesicle-associated ClC-3 promote vesicle acidification by dissipating the positive Δψ developed by the vacuolar H+ pump, thereby disinhibiting the pump to make larger ΔpH. The VGLUTs can also contribute to formation of ΔpH because as an anion, glutamate entry similarly dissipates Δψ to promote ΔpH. Interestingly, a Cl− conductance associated with the VGLUTs may also promote acidification by Cl−.
Vesicular Transporters
The vesicular transporters for monoamines (VMAT) and ACh (VAChT) exchange 2 lumenal H+ for each molecule of cytosolic transmitter (17–19). However, only the charged monoamine is recognized, and ACh is permanently protonated, so each transport cycle results in a net loss from the lumen of 2 H+ but only +1 charge, accounting for the greater dependence of these activities on ΔpH than Δψ. The greater consumption of ΔpH than Δψ in turn requires the replacement of more H+ than charge by the H+ pump. Since the number of charges pumped by the H+-ATPase must equal the number of H+, regeneration of the gradients dissipated by vesicular monoamine and ACh transport thus requires an additional mechanism that can restore the necessary balance.
The vesicular GABA transporter (VGAT, also known as vesicular inhibitory amino acid transporter, or VIAAT) recognizes glycine as well as GABA. GABA and glycine exchange for an unknown number of H+ and as zwitterions, their uptake depends more equally on ΔpH and Δψ (20, 21). Despite the clear role for ΔpH in vesicular GABA transport, recent work using functional reconstitution of purified mammalian VGAT has suggested that the activity requires cotransport of 2 Cl− and hence relies predominantly if not exclusively on Δψ (22). Previous work had not identified a requirement for Cl−, but the apparent affinity of VGAT for Cl− appears high, suggesting that only low concentrations may be required (22). However, it remains possible that the assays used reflect only kinetics, and it will be important to determine the stoichioimetry using thermodynamic measurements at equilibrium.
In contrast to VMAT and VAChT, the vesicular glutamate transporters (VGLUTs) depend primarily on Δψ. The three isoforms (VGLUT1-3) exhibit generally complementary patterns of expression in the brain but very similar transport activity, and this topic has recently been reviewed (23–25). Despite the primary reliance on Δψ, VGLUT activity retains some dependence on ΔpH even after dissipation of Δψ (26, 27), suggesting that the mechanism involves H+ exchange. Independent of H+ coupling, however, glutamate uptake depends more on Δψ because at neutral pH, glutamate is anionic. If exchanged for nH+ (and the stoichiometry of coupling remains unknown), glutamate influx results in the efflux of n + 1 charge. The VGLUTs thus produce an imbalance between charge and H+ similar to but opposite that created by VMAT and VAChT, implicating additional mechanisms to balance the two components of ΔμH+ so that the H+ pump can continue to function.
It is widely assumed that the expression of a vesicular neurotransmitter transporter confers the potential for regulated release of available substrate. Indeed, all of the known transporters contain signals which target them to endocytic vesicles even in non-neural cells (28), and the expression of ΔμH+ by endosomes should in principle drive their activity. Heterologous expression of the VMATs by a range of cell lines indeed confers robust monoamine uptake by endosomes. However, it has been extremely difficult to measure the activity of other vesicular transporters after heterologous expression, perhaps because they have a much lower apparent affinity (low millimolar Km) for substrate than the VMATs (Km <1 μM), but at least in some cases perhaps because the endosomes of non-neural cells lack essential components such as factors that regulate the expression of ΔμH+ as ΔpH or Δψ.
THE REGULATION OF ΔpH BY ANION FLUX
More attention has focused on the factors that promote formation of ΔpH than on those promoting Δψ because organelle ΔpH is easier to measure than Δψ and because it is presumed to have a more important biological role – in ligand dissociation from receptors within the endocytic pathway, in the processing of propeptides within the biosynthetic pathway and in proteolytic degradation within lysosomes, as well as vesicular neurotransmitter transport. Importantly, in vitro studies have repeatedly shown that the simple addition of ATP to activate the H+ pump does not suffice to produce substantial ΔpH. With activation of the H+ pump, Δψ accumulates before the bulk concentration of H+ increases, arresting the activity of the pump before development of ΔpH. Dissipation of Δψ, generally considered to involve the entry of anion, allows the ATPase to continue pumping H+ and produce ΔpH.
Chloride
The principal anion involved in vesicle acidification is presumed to be Cl−. In the absence of Cl−, synaptic vesicles and other isolated organelles show only a small acidification upon addition of ATP. The addition of Cl− then leads to a concentration-dependent increase in ΔpH, presumably by dissipating Δψ (26, 27, 29). Intracellular members of the ClC chloride channel family are considered to mediate the Cl− permeability of acidic vesicles, with ClC-3 the predominant but probably not the only isoform on synaptic vesicles (30). Interestingly, work on the related ClCs 4–7 as well as a bacterial homologue show that these proteins do not function as channels but rather as Cl−/H+ exchangers with a stoichiometry of 2 Cl− : 1 H+ (31–35). In this case, Cl− entry is coupled to H+ efflux, which seems counterproductive since Cl− entry acts primarily to increase ΔpH. In the case of ClCs, however, the loss of 1 H+ is accompanied by the loss of +3 charge, dissipating Δψ more than ΔpH, and thus stimulating the H+-ATPase to replenish these gradients. For an equivalent [Cl−] gradient, 2Cl− : 1H+ exchange would thus produce a larger ΔpH than a simple Cl− channel (29, 36, 37): for 2Cl− : 1H+ exchange, the concentration gradient of Cl− at equilibrium is predicted by the equation
| (1) |
where R is the gas constant, T the absolute temperature, F Faraday’s constant, and the vATPase determines ΔpH and Δψ. Estimating that the proton pump can generate a total ΔμH+ ~3 (i.e., ΔpH ~3 pH units, Δψ ~180 mV or a combination of both) (38, 39):
| (2) |
Replacing Δψ in equation (1) with 2.3 RT/F (3 − log10([H+]i/[H+]o) predicts
| (3) |
On the other hand, if the ClC or another protein present on synaptic vesicles functions as a simple Cl− channel, the concentration gradient of Cl− at equilibrium would be predicted by the Nernst equation:
Replacing Δψ with 2.3 RT/F (3 − log10([H+]i/[H+]o) as above,
| (4) |
For an equivalent concentration gradient of anion, the H+ exchange mechanism thus counter-intuitively produces a substantially larger ΔpH (by 1.5 pH units) than a simple ion channel. Conversion of two ClCs into Cl− channels in knock-in mice indeed impairs the function of the endocytic pathway (36, 37). However, no change in acidification was observed, raising the possibility that the two mechanisms differ primarily in the luminal concentration of Cl−. It is unclear why changes in luminal Cl− would affect the function of the endocytic pathway if not through a change in ΔpH, but it does seem likely that the anion gradients differ between the two mechanisms.
Considering the established role of ClCs in endosome/lysosome acidification, it is surprising to note that recent work has suggested a primary role for the VGLUTs in Cl− flux by synaptic vesicles. Originally, the analysis of ClC-3 knockout (KO) mice had suggested a role for that isoform in the acidification of synaptic vesicles, but the analysis was complicated by severe degeneration of hippocampus and retina (30). Using younger ClC-3 KO mice, the defect appeared much less significant (40, 41). In contrast, synaptic vesicles from VGLUT1 KO mice showed a more profound defect in the acidification due to Cl− (41), suggesting that they mediate Cl− flux by synaptic vesicles. Indeed, the expression of other so-called type I phosphate transporters of the VGLUT family have been shown to confer a Cl− conductance (42), and the VGLUTs also promote acidification of synaptic vesicles by Cl− (41, 43). In addition to their essential role in packaging glutamate, the VGLUTs may thus exhibit Cl− channel activity, and vesicular glutamate transport shows a clear biphasic dependence on Cl− (26, 27). In addition, the Cl− dependence of glutamate transport has been suggested to reflect allosteric activation rather than effects on the driving force (44).
On the other hand, it remains unclear how a Cl− conductance might contribute to the kinetic properties of glutamate transport. Recent work has indeed failed to detect any Cl− flux after functional reconstitution of purified VGLUT2 (45), and the analysis involved direct measurement of flux rather than indirect effects on acidification. It thus remains uncertain whether the VGLUTs and/or ClCs mediate Cl− entry into glutamatergic synaptic vesicles. Taken together, however, the data suggest that synaptic vesicles storing glutamate, which are the most abundant in brain, express more VGLUT than ClC – Cl− entry would indeed dissipate the Δψ required for vesicular glutamate transport, and previous work has suggested that substrates can inhibit the Cl− conductance associated with VGLUTs and related proteins (42, 43).
What then would be the role for a Cl− conductance associated with glutamatergic vesicles? Recent work in reconstituted proteoliposomes has suggested that Cl− efflux can promote glutamate uptake (41). Immediately after endocytosis, synaptic vesicles should contain large amounts of Cl− captured from the extracellular space. Although it was suggested that lumenal Cl− exchange directly for cytosolic glutamate, it seems more likely that Cl− efflux generates the Δψ required for vesicular glutamate transport, and this possibility requires direct testing. It will also be very important to determine whether luminal Cl− influences the filling of native synaptic vesicles, rather than simply artificial membranes whose much larger size may confer new properties. In any case, the acidification of non-glutamatergic synaptic vesicles presumably depends on ClCs, and ClC-3 may be only one of several isoforms involved. Indeed, recent work using ClC-3 KO mice has shown major defects in GABA release, apparently due to the impaired acidification of GABAergic synaptic vesicles (40).
Previous work has also demonstrated the synergistic effect of ATP (also an anion) on serotonin uptake by chromaffin granules (46). Although ATP is present in all cells, this effect presumably requires vesicular nucleotide transport, which may only occur in cells that actually release ATP. Manipulation of the recently described vesicular nucleotide transporter VNUT (47) will therefore be required to assess its physiological role.
Glutamate
Independent of the Cl− flux that may be mediated by VGLUTs, vesicular glutamate transport itself has profound effects on ΔpH (26, 27). As an anion, glutamate will, like Cl−, dissipate Δψ and hence promote ΔpH. Indeed, glutamate alone acidifies synaptic vesicles in the presence of ATP to activate the H+ pump, presumably reflecting the abundance of glutamatergic vesicles in the mammalian brain. We do not know the stoichiometry of ionic coupling by the VGLUTs, but the sensitivity to ΔpH (27, 43, 48) supports a H+ exchange mechanism despite their primary dependence on Δψ. Assuming the exchange of 1 H+ for 1 glutamate and hence the movement of +2 charge,
| (5) |
Again replacing Δψ with 2.3 RT/F (3 − log10([H+]i/[H+]o),
| (6) |
For a given anion gradient, glutamate flux through the VGLUTs (equation 6) is therefore predicted to generate ΔpH 1.5 units greater than Cl− flux through even an intracellular ClC (equation 3) and 3 units greater than Cl− flux through a channel (equation 4).
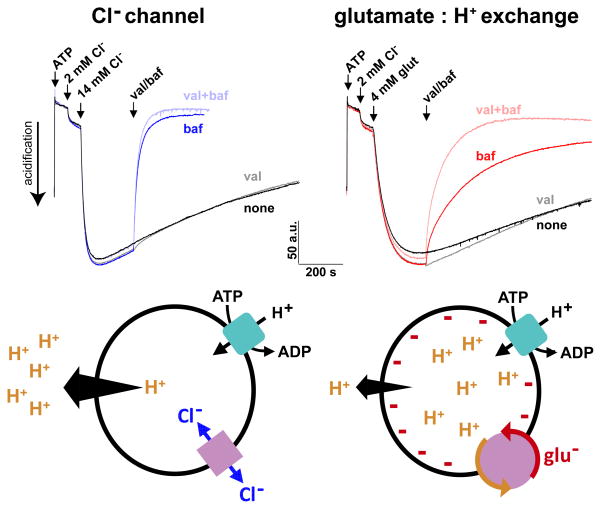
Consistent with these predictions, we have found that different anions have non-redundant effects on vesicle filling with transmitter (29), presumably by producing different ΔpH. Although Cl− suffices to promote ΔpH and stimulate the ΔpH-dependent storage of cationic transmitters (29, 49), we and others have found that glutamate can also increase the packaging of monoamines (29, 50, 51) and ACh (52) into isolated synaptic vesicles. Indeed, a subset of monoamine and cholinergic neurons express VGLUTs: a number of catecholamine populations including midbrain dopamine neurons in the ventral tegmental area (VTA) express VGLUT2 (53, 54) while serotonergic neurons in the dorsal raphe and cholinergic interneurons in the striatum express VGLUT3 (55–57) [reviewed in (25)]. However, it has remained unclear how glutamate promotes vesicle filling in the presence of substantially higher cytosolic Cl− concentrations, and most previous work showing stimulation of vesicle filling by glutamate has relied on very low Cl− (50–52). We have recently found that the effects of glutamate on monoamine filling persist even at physiological Cl− (20 mM) (29), indicating that the two anions do not have redundant roles. Surprisingly, glutamate produces larger synaptic vesicle pH gradients than Cl− at concentrations up to ~12 mM. The acidification by glutamate saturates at concentrations greater than 2–4 mM, consistent with the known VGLUT Km (1–3 mM). In addition, the acidification produced by glutamate is more stable than that produced by Cl−: after inhibition of the H+ pump, ΔpH collapses immediately in vesicles acidified with Cl−, but much more slowly in those acidified with glutamate (29). Although glutamate has a much higher pKa than Cl− and can thus serve as a better buffer, the increased stability of ΔpH is in large part attributable to the mechanism of anion flux. In the absence of an electrical shunt, synaptic vesicle ΔpH is quite stable because the efflux of H+ will create a negative Δψ that opposes further efflux. In the case of vesicles acidified with Cl−, however, H+ can leave the vesicle because Cl− efflux through a channel-like mechanism dissipates Δψ. In the case of vesicles acidified with glutamate, glutamate cannot leave the vesicle because the H+ exchange mechanism opposes coupled H+ influx into acidic vesicles. In contrast to a channel, the H+ exchange mechanism thus serves to “lock” H+ inside synaptic vesicles, stabilizing ΔpH and promoting vesicular uptake of monoamines and ACh (Figure 2) (29).
Figure 2. Glutamate flux produces larger and more stable changes in vesicular ΔpH than chloride.
Changes in ΔpH of isolated synaptic vesicles were monitored using acridine orange (5 μM) in 140 mM choline gluconate, 10 mM K+ gluconate, 10 mM HEPES, pH 7.4. Acidification was triggered by the sequential addition of 1 mM ATP and 2 mM Cl− followed by either 14 mM Cl− (top left) or 4 mM glutamate (top right); more Cl− is required to produce an equivalent initial change in ΔpH. The traces in black indicate vesicles without any further addition. At the arrow, the K+ ionophore valinomycin (50 nM, gray), the proton pump inhibitor bafilomycin (250 nM, dark blue/red) or both (light blue/pink) were added. The rate of alkalinization immediately after bafilomycin addition (dark blue/red) is much faster in the vesicles acidified with Cl−, indicating that vesicles acidified with glutamate maintain a more stable ΔpH. Although increased buffering may contribute to the stabilization of ΔpH by glutamate, valinomycin accelerates the bafilomycin-induced collapse in ΔpH across membranes acidified with glutamate (pink) but not with Cl− (light blue), indicating an important role for negative Δψ in the stability of ΔpH in glutamate-acidified vesicles. We hypothesize that the negative Δψ developing upon H+ efflux impedes further dissipation of ΔpH. In the case of vesicles acidified with Cl−, anion efflux through a channel (bottom left) would shunt the developing negative Δψ, allowing the continued efflux of H+ and rapid collapse of ΔpH. In the case of vesicles acidified with glutamate, a H+ : anion exchange mechanism (bottom right) would impede anion efflux because it would be coupled to the uphill movement of H+ into acidic vesicles. Since glutamate efflux is disfavored, H+ efflux is slow and ΔpH more stable. Thus, the differences in mechanism of anion flux (channel versus H+ exchange) confer differences in the stability of ΔpH. Glutamate thus serves to ‘lock’ H+, and hence cationic transmitters such as ACh and monoamines, inside secretory vesicles.
Using knockout mice, recent work has demonstrated the physiological significance of VGLUT co-expression with VMAT2 or VAChT on synaptic vesicles in vivo. Originally, there was some concern that adult dopamine neurons did not in fact express VGLUT2 (58, 59), and expression does appear to be highest early in development or after injury (60–63). However, mature conditional knockout (cKO) mice lacking VGLUT2 selectively in dopamine neurons clearly show a reduction in both dopamine storage and evoked dopamine release (29) that presumably accounts for their reduced response to psychostimulants (29, 64). The reduction is anatomically restricted to the ventral striatum, consistent with the expression of VGLUT2 by VTA dopamine neurons projecting to the ventral striatum but not their neighbors in the substantia nigra pars compacta (SNc) that innervate the dorsal striatum (53, 54). These data are also consistent with the presence of TH+ asymmetric (presumably excitatory) synapses in ventral but not dorsal striatum (65, 66).
Since VGLUT proteins usually localize exclusively to axon terminals, identification of VGLUT+ cell populations has generally required quantitative PCR, in situ hybridization, or alternatively, immuno- electron or confocal microscopy to examine nerve terminals directly. However, the low levels of VGLUT2 in mature dopamine neurons have sometimes eluded detection with the less sensitive of these methods, leading to conflicting conclusions about the expression of VGLUT2 by midbrain dopamine neurons (53, 54, 58–64, 67, 68). Using VGLUT2-GFP BAC transgenic mice, we have observed clear colocalization of GFP with tyrosine hydroxylase in a medial subset of VTA neurons (29), consistent with a recent comprehensive report using in situ hybridization (54). Since the co-expressing neurons comprise only a fraction of all dopamine neurons in the VTA, the effect of the knockout on dopamine stores in vivo may in fact greatly underestimate the effect on this subset. Thus, midbrain dopamine neurons may differ dramatically in the storage and release of dopamine, due to the heterogeneous expression of VGLUT2.
A knockout of VGLUT3 has also been used to assess the role of glutamate storage and release by cholinergic interneurons of the striatum, which along with serotonin neurons in the raphe, express high levels of VGLUT3. Constitutive disruption of VGLUT3 produces increased locomotor activity that can be reversed by inhibition of acetylcholinesterase, and the animals show a reduction in vesicular ACh (and serotonin) uptake and release (50, 52). In contrast to wild type animals, they also show no stimulation of vesicular ACh or serotonin transport by glutamate. However, the expression of VGLUT3 by a number of neuronal populations and the unconditional inactivation of VGLUT3 in these animals make it difficult to conclude that the behavioral abnormalities reflect a specific alteration in ACh release by striatal interneurons. The biochemical effect of glutamate on ACh and monoamine co-storage thus seems clear, but the conditional inactivation of VGLUT3 or even VAChT in genetically defined cell populations will be required to address the role of this phenomenon in behavior.
Although the dissipation of Δψ required for vesicle acidification has generally been attributed to anion entry, recent observations from non-neural cells suggest a role for cation efflux in lysosome ΔpH (69). Chloride clearly promotes lysosome acidification in vitro, but this report suggests a smaller role in intact (or at least permeabilized) cells, with the efflux of lumenal cation (apparently K+) responsible in vivo. Nonetheless, the considerable data from ClC KO mice documenting effects on acidification within the endosome/lysosome pathway make it very difficult to exclude a role for Cl− and these proteins in the formation of ΔpH.
THE REGULATION OF Δψ BY CATION FLUX
Do endocytic vesicles have a specific mechanism to promote formation of Δψ? Or does Δψ result simply from the absence of a counterion such as Cl− or glutamate? In general, Δψ has received little attention for an independent role in the secretory pathway, but vesicular glutamate transport clearly depends on Δψ. Although recent attention has focused on the expression of VGLUTs as a presynaptic determinant of quantal size (70–72) the number of transporters per vesicle will change primarily the kinetics of transport, not the thermodynamic equilibrium reached at steady-state (1). However, changes in the driving force should have dramatic effects on the extent as well as rate of vesicle filling, so the regulation of Δψ has important implications for transmitter release.
Although very little is known about the factors that promote formation of Δψ, recent work has identified intracellular members of the Na+/H+ exchanger (NHE) family that could serve this function. NHEs catalyze the electroneutral exchange of monovalent cation for H+, and plasma membrane isoforms have an important role in the regulation of cytosolic pH (73). Intracellular isoforms recognize K+ as well as Na+ and several localize to endosomes (74) where they should dissipate ΔpH and thus enable the H+ pump to increase Δψ. Interestingly, recent human genetic studies have implicated intracellular isoform NHE6 in Angelman syndrome (75), and NHE9 in autism (76).
Cation channels may also influence formation of Δψ. In this case, K+ entry would promote formation of Δψ independent of the H+ pump. Interestingly, the TRPM7 channel localizes to synaptic vesicles and influences quantal size, although it also interacts with proteins involved in fusion and affects the frequency of release (77, 78). However, it is important to note that the work on TRPM7 has involved cholinergic neurons, whereas the presence of an active K+ conductance on synaptic vesicles might shunt the Δψ required for vesicular glutamate transport.
INDEPENDENT ROLES FOR CO-RELEASED NEUROTRANSMITTER
In addition to the presynaptic consequences for vesicle filling, co-release has implications for the activation of postsynaptic receptors. The two transmitters may both activate receptors, with the potential for distinct modes of signaling, and recent work has begun to elucidate the physiological role of co-release.
Co-release of GABA and ACh from starburst amacrine cells
Starburst amacrine cells (SACs) contribute to direction-selective motion-sensing by the vertebrate retina. SACs have a radially symmetric dendritic morphology that overlaps with dendrites from neighboring SACs as well as direction-selective ON-OFF ganglion cells (DSGCs) in the inner plexiform layer. Dual recordings show that SACs release more GABA onto DSGCs in response to light moving in the non-preferred than the preferred direction. Indeed, GABA release, presumably from SACs, appears to be essential for direction selectivity (79).
In addition to the inhibitory GABA, SACs release ACh, activating nicotinic (nACh) receptors on DSGCs. However, the activation of nACh receptors is not required for direction selectivity (79). To characterize the release of both transmitters, a recent study using paired recordings demonstrated that, whereas GABA release by SACs is selective for movement in the null direction, the cholinergic response is greater with movement in the preferred direction (80). Both GABA and ACh currents depend on external Ca++, supporting a vesicular release mechanism, but release of ACh shows much less sensitivity to Ca++ than GABA release, requiring stronger stimulation, and providing physiological evidence that different vesicle populations mediate release of the two transmitters. These observations are consistent with a proposed dual role for SACs, encoding direction selectivity through the release of GABA, and motion sensitivity through the release of ACh.
GABA/glutamate co- release from MNTB
Neurons in the lateral superior olive (LSO) function as interaural coincidence detectors essential for sound localization. They accomplish this by integrating tonotopically precise excitatory input from the ipsilateral cochlear nucleus with inhibitory GABAergic and glycinergic inputs from the contralateral medial nucleus of the trapezoid body (MNTB). During development, however, MNTB neurons transiently express VGLUT3 and co-release glutamate between P0 and P12 (81). In VGLUT3 KO mice, MNTB cells still form synapses onto LSO neurons that are indistinguishable from those in control animals at P1-2, however, the strengthening of these inhibitory synapses that normally occurs by P10-12 fails to occur in VGLUT3-null mice (82). Further, tonotopic projections from MNTB that project diffusely within the LSO at P1, fail to sharpen normally in the absence of VGLUT3. But why is glutamate release important when GABA is itself excitatory (due to a shift in ECl) during the same time frame? Presumably, the specific activation of NMDA receptors confers the plasticity required for normal development (81). The results thus support a role for glutamate co-release inrefinement of the synapse that underlies sound localization in the auditory system.
GABA/glutamate co-release from hippocampal mossy fibers
In the hippocampus, mossy fibers derived from granule cells in the dentate gyrus form glutamatergic synapses onto CA3 pyramidal neurons where they also co-release GABA. Early in development, pyramidal neurons express VGAT and glutamic acid decarboxylase, the enzyme responsible for GABA biosynthesis, but these genes subsequently downregulate (83–85). For the first 3 weeks after birth, stimulation of mossy fiber inputs indeed produces GABA-mediated currents in pyramidal neurons (86). However, the significance of this transient GABA co-release remains unknown, and it is important to note that at this time, GABA currents are still excitatory due to the shift in Cl− reversal potential.
Interestingly, epileptic activity rekindles expression of the GABAergic phenotype in the adult granule cells (87–90). At this point, GABA transmission is inhibitory and may thus serve a distinct, possibly homeostatic role to restrain the excitability responsible for epilepsy.
Monoamine/glutamate co-release
The first clear evidence that monoamine neurons co-release glutamate derived from dissociated neurons grown in isolation so that they can only form synapses onto themselves. Stimulation of both serotonin (12) and dopamine (13) neurons produced fast excitatory currents blocked by glutamate receptor antagonists, indicating the potential for glutamate co-release to activate postsynaptic receptors. However, the postnatal decline in VGLUT2 expression by midbrain dopamine neurons (60, 63) raised the possibility that VGLUT2 expression in vitro (67) might simply reflect dedifferentiation. The low level of VGLUT2 expression by midbrain dopamine neurons in the adult raised further questions about the physiological relevance of these in vitro observations. The phenotype of mice lacking VGLUT2 specifically in dopamine neurons and the anatomical evidence for VGLUT2 expression by a medial subset of VTA neurons have provided clear evidence for the effects of glutamate on co-stored dopamine, but not directly addressed the role of glutamate as an independent signal.
In 2004, the laboratory of Steve Rayport published a landmark study that used an acute, horizontal slice preparation to demonstrate the presence of a monosynaptic glutamatergic projection from VTA to nucleus accumbens (NAc) at both P10 and P21 (91). The next year, the laboratory of Jeremy Seamans showed that VTA stimulation in vivo rapidly leads to glutamate release in the prefrontal cortex (PFC) (92). Although both of these studies supported an independent role for the glutamate released by dopamine neurons, questions remained about the specificity of stimulation, particularly after the identification of purely glutamatergic neurons in the ventral midbrain (53, 59) that we now know also project to both ventral striatum and PFC (54, 93).
On the other hand, genetic approaches have recently provided definitive physiological evidence that glutamate released by at least a subset of DA neurons in adult mice activates ionotropic glutamate receptors on postsynaptic medium spiny neurons in the striatum. Using cre recombinase selectively expressed by dopamine neurons to activate a conditional allele of the light-activated cation channel channelrhodopsin-2, we and others have observed glutamate responses evoked by direct illumination of the striatum (94, 95). In addition to the increased specificity, the ability to stimulate glutamate release directly at presynaptic boutons circumvented the unavoidable transection of mesolimbic projections in horizontal slices, resulting in larger postsynaptic responses. Robust glutamate-mediated AMPA receptor currents were observed in the ventral but not dorsal striatum even though light evoked dopamine release at both sites (94), consistent with the restricted expression of VGLUT2 by dopamine neurons in the VTA but not SNc (53, 54). Further, the conditional knockout of VGLUT2 in dopamine neurons completely abolished these responses (94).
What then is the role of this glutamate signal? The most robust phenotype observed in cKO mice that lack glutamate co-release from dopamine neurons is a reduction in psychostimulant-induced locomotion (29, 64). This may be most easily explained by the reduction in dopamine release that we attribute to a reduction in vesicular dopamine storage (29). However, the activation of postsynaptic ionotropic receptors by the glutamate released from dopamine neurons likely encodes distinct information.
One possibility is that the glutamate released by dopamine terminals contributes to the prediction-error signal encoded in the firing rates of dopamine neurons (96, 97). A subset of tonically active midbrain (presumaby dopamine) neurons burst fire in response to unexpected rewards or to rewards better than predicted by a conditioned cue. Conversely, they slow or pause firing in response to rewards worse than predicted (98). Consistent with these changes in firing, extracellular dopamine measured by fast-scan cyclic voltammetry changes as predicted in rodents performing goal-directed tasks (99). However, it is not clear how dopamine signaling by metabotropic G protein-coupled dopamine receptors could maintain the fidelity of synaptic transmission required for learning tasks dependent on subsecond cue discrimination. As a neuromodulator activating G protein-coupled receptors, dopamine presumably acts on slower time scales (i.e., seconds to minutes). In contrast, the glutamate co-released by dopamine neurons produces a rapid, transient postsynaptic response more tightly coupled to dopamine neuron firing and thus well-positioned to convey temporally precise information about reward [for excellent reviews see (100, 101)]. This hypothesis predicts deficits in reward-learning by cKO mice lacking VGLUT2 in dopamine neurons, but initial assessment using conditioned place preference showed no such deficits (29). However, mice can also learn CPP in the absence of dopamine (102, 103), and the cue-reward pairing involved in CPP occurs continuously over the course of 20 minutes and may therefore not depend on transient sub-second bursts in dopamine neuron firing.
The expression of channelrhodopsin in raphe nuclei has also revealed an optically evoked glutamate-mediated response in hippocampus, presumably from the population of serotonergic neurons expressing VGLUT3 (104). However, these experiments did not use genetic manipulation to limit channelrhodopsin expression to serotonergic neurons, so it remains possible that the responses derived from neighboring non-serotonergic neurons in the raphe. Indeed, despite the strong expression of VGLUT3 mRNA in raphe nuclei, it has remained unclear to what extent VGLUT3 and serotoninergic markers are coexpressed or comprise separate neuronal populations, similar to the non-dopaminergic VGLUT2+ population of neurons in the medial midbrain. On the other hand, the anatomical evidence supports expression of VGLUT3 by at least a subset of serotonergic neurons (105–109).
ACh/glutamate co-release
Channelrhodopsin was also used recently to demonstrate that in addition to the role of glutamate co-storage in promoting vesicular ACh filling in striatal interneurons (52), the released glutamate activates ionotropic receptors on medium spiny neurons. Consistent with the expression of VGLUT3 by these cells, the response was abolished in VGLUT3 KO mice (110). Recent work has also identified co-release of ACh and glutamate by neurons of the medial habenula. Expressed in cholinergic neurons, channelrhodopsin confers light-evoked release of glutamate as well as ACh within the interpeduncular nucleus of the midbrain (111). However, brief illumination evokes primarily the glutamate response, with the ACh response requiring more sustained stimulation. Released from the same neuron, the two transmitters may thus subserve distinct roles in signaling, perhaps due to differences in the distance between release site and postsynaptic receptors (i.e., between synaptic and volume transmission), or perhaps as a function of release from different vesicle populations.
DISTINCT AND OVERLAPPING POOLS OF SYNAPTIC VESICLES
The ability of one transmitter to affect the storage of another through changes in the H+ electrochemical driving force requires localization of the two vesicular transporters to the same secretory vesicle, but several recent observations suggest that release can also occur from distinct vesicle populations. In retinal SACs, the release of GABA and ACh respond differently to Ca++ (80), providing unequivocal evidence for release from different vesicles. Immunolabeling for endogenously expressed proteins also suggest that dopaminergic release sites are heterogeneous in their capacity to store glutamate (13, 53, 60–62, 67, 68). In midbrain dopamine neurons, heterologous expression of differentially tagged vesicular glutamate and monoamine transporters shows colocalization at most boutons, but a significant fraction express only one or the other (112), consistent with the original suggestion that catecholamine and glutamate markers might segregate to distinct synapses both in vitro and in vivo (13, 65). However, in contrast to the VGLUTs which generally reside only at presynaptic boutons, VMAT2 localizes to dendrites as well as axons, but the segregation occurs even with the analysis restricted to axonal sites. The segregation of monoamine and glutamate markers to different release sites may indeed contribute to the failure to detect VGLUT expression in tyrosine hydroxylase-positive striatal projections by immuno-electron microscopy (60, 68). Hippocampal neurons show no evidence of such segregation, indicating mechanisms specific to dopamine neurons. In addition, optical imaging with a pHluorin-based reporter shows that field stimulation evokes release of a greater proportion of the VGLUT1 at boutons than VMAT2 (112), suggesting that the two proteins exhibit overlapping but differential localization to synaptic vesicle pools.
Considerable previous work has shown that only a fraction of the synaptic vesicles in a presynaptic bouton are available for evoked release, even after prolonged stimulation (113). This so-called recycling pool can be only a small fraction of all the vesicles present, with the remaining, so-called resting pool of uncertain physiological role. Since the proportion of several synaptic vesicle proteins in this recycling pool is generally the same (~50–60%), it has been assumed that they will all exhibit the same distribution between recycling and resting (unresponsive) pools. However, the relatively small recycling pool size of VMAT2 (20–30%) indicates that in addition to the segregation of dopamine and glutamate vesicles at different boutons, dopamine and glutamate vesicles also segregate to at least some extent within individual boutons where they both reside. Interestingly, the differential exocytosis of VMAT2 and VGLUT occurs in hippocampal as well as midbrain dopamine neurons, indicating the potential for differential co-release of classical transmitters by many if not all neuronal populations.
Recent work has suggested that the VGLUTs may in fact control the probability of transmitter release, perhaps accounting for the differential release of two transmitters by the same neuron. The distribution of VGLUT1 and 2 originally suggested a correlation of VGLUT1 with synapses having a low probability of release (such as hippocampal synapses and parallel fiber synapses in the cerebellum) and VGLUT2 with synapses having a high probability of release (114). Although it has been difficult to understand how the transporter might control fusion, recent work has indeed suggested that the known interaction of VGLUT1 with the endocytic protein endophilin (115) may also influence exocytosis (116). Alternatively, the two transporters may simply recycle through slightly different mechanisms, consistent with the role of endophilin in endocytosis, and these mechanisms may generate vesicles with different release probability. Rather than influencing the release machinery, the transporter may thus simply target to vesicles with different properties. The difference between VMAT2 and the VGLUTs in overall recycling pool size supports this possibility, but it may be more difficult to assess directly the targeting of VGLUT1 and 2 to distinct subsets within the recycling pool. Since synaptic vesicles have generally been considered homogeneous in terms of biochemical composition, considerable basic work will now be required to characterize the properties of these subsets and identify the proteins responsible for these properties, as well as those responsible for sorting them into functionally distinct vesicle pools.
Summary Points.
The filling of synaptic vesicles with different transmitters relies on different components of ΔμH+.
ΔμH+ can be expressed as either ΔpH, Δψ or a combination of both.
The entry of Cl− and other anions promote the formation of ΔpH by dissipating Δψ, thereby disinhibiting the H+ pump.
Cation flux may promote the formation of Δψ.
Many neuronal populations co-release two classical transmitters.
Co-storage with glutamate promotes the vesicular transport of monoamines and ACh.
Co-released neurotransmitters can activate their cognate postsynaptic receptors.
Co-release of two transmitters can also occur from independent vesicle populations.
Acronyms
- ΔμH+
H+ electrochemical gradient
- ΔpH
pH gradient
- Δψ
Organelle membrane potential
- VMAT
Vesicular monoamine transporter
- LDCV
Large dense core vesicles
- SERT
Serotonin transporter
- ACh
Acetylcholine
- VAChT
Vesicular ACh transporter
- VGLUT
Vesicular glutamate transporter
- GABA
γ-aminobutyric acid
- VGAT
Vesicular GABA (and glycine) transporter
- VNUT
Vesicular nucleotide transporter
- ATP
Adenosine triphosphate
- NHE
Na+/H+ exchanger
- VTA
Ventral tegmental area
- SNc
Substantia nigra, pars compacta
- NAc
Nucleus accumbens
- MNTB
Medial nucleus of the trapezoid body
- LSO
Lateral superior olive
- SAC
Starburst amacrine cell
- DSGC
Direction-selective ganglion cell
- nAChR
Nicotinic acetylcholine receptors
- ClC
Chloride channel
- KO
Knockout
Definitions list (20 max)
- Exocytosis
The fusion of a vesicle with the plasma membrane
- Classical neurotransmitters
Small molecules synthesized or recycled locally, transported into vesicles and released to convey an extracellular signal
- Vacuolar type H+-ATPase (or H+ pump)
A complex of V0 and V1 subunits homologous to the F0 and F1 subunits of mitochondrial ATP synthase, that uses ATP hydrolysis to pump H+ into organelles
- Vesicular neurotransmitter transporter
Located on secretory vesicles, these transporters use the electrochemical gradient produced by the vacuolar proton pump to fill vesicles with neurotransmitter
- Plasma membrane neurotransmitter transporter
Active at the plasma membrane, these proteins transport neurotransmitter from the extracellular space back into the presynaptic neuron or glia
- Quantal size
The postsynaptic response to release of a single secretory vesicle
Contributor Information
Thomas S. Hnasko, Email: thomas.hnasko@ucsf.edu.
Robert H. Edwards, Email: robert.edwards@ucsf.edu.
LITERATURE CITED
- 1.Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–58. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H, Jahn R. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci. 2010;30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker VP, Dowdall MJ, Boyne AF. The storage and release of acetylcholine by cholinergic nerve terminals: recent results with non-mammalian preparations. Biochem Soc Symp. 1972:49–68. [PubMed] [Google Scholar]
- 4.Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975;247:145–62. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–87. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Awatramani GB, Turecek R, Trussell LO. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol. 2005;93:819–28. doi: 10.1152/jn.00798.2004. [DOI] [PubMed] [Google Scholar]
- 8.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–24. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 9.Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- 10.Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–35. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12:433–42. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 13.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008;99:1559–64. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- 15.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–9. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–29. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RG, Carty SE, Scarpa A. Proton: substrate stoichiometries during active transport of biogenic amines in chromaffin ghosts. J Biol Chem. 1981;256:5773–80. [PubMed] [Google Scholar]
- 18.Knoth J, Zallakian M, Njus D. Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts. Biochemistry. 1981;20:6625–9. doi: 10.1021/bi00526a016. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen ML, Cox GD, Parsons SM. Kinetic parameters for the vesicular acetylcholine transporter: two protons are exchanged for one acetylcholine. Biochemistry. 1998;37:13400–10. doi: 10.1021/bi9802263. [DOI] [PubMed] [Google Scholar]
- 20.Hell JW, Maycox PR, Jahn R. Energy dependence and functional reconstitution of the gamma-aminobutyric acid carrier from synaptic vesicles. J Biol Chem. 1990;265:2111–7. [PubMed] [Google Scholar]
- 21.Kish PE, Fischer-Bovenkerk C, Ueda T. Active transport of gamma-aminobutyric acid and glycine into synaptic vesicles. Proc Natl Acad Sci U S A. 1989;86:3877–81. doi: 10.1073/pnas.86.10.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juge N, Muroyama A, Hiasa M, Omote H, Moriyama Y. Vesicular inhibitory amino acid transporter is a Cl−/gamma-aminobutyrate Co-transporter. J Biol Chem. 2009;284:35073–8. doi: 10.1074/jbc.M109.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 25.El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–16. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 26.Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–8. [PubMed] [Google Scholar]
- 27.Tabb JS, Kish PE, Van Dyke R, Ueda T. Glutamate transport into synaptic vesicles. Roles of membrane potential, pH gradient, and intravesicular pH. J Biol Chem. 1992;267:15412–8. [PubMed] [Google Scholar]
- 28.Tan PK, Waites C, Liu Y, Krantz DE, Edwards RH. A leucine-based motif mediates the endocytosis of vesicular monoamine and acetylcholine transporters. J Biol Chem. 1998;273:17351–60. doi: 10.1074/jbc.273.28.17351. [DOI] [PubMed] [Google Scholar]
- 29.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–56. doi: 10.1016/j.neuron.2010.02.012. Using conditional knockout mice lacking VGLUT2 specifically in dopamine neurons, this paper demonstrates the co-storage and co-release of glutamate by VTA dopamine neurons. It also elucidates the non-redundant roles of glutamate and Cl− in formation and stabilization of ΔpH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–96. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 31.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–7. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 32.Graves AR, Curran PK, Smith CL, Mindell JA. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–92. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- 33.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–3. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 34.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–7. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 35.Neagoe I, Stauber T, Fidzinski P, Bergsdorf EY, Jentsch TJ. The late endosomal ClC-6 mediates proton/chloride countertransport in heterologous plasma membrane expression. J Biol Chem. 2010;285:21689–97. doi: 10.1074/jbc.M110.125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novarino G, Weinert S, Rickheit G, Jentsch TJ. Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science. 2010;328:1398–401. doi: 10.1126/science.1188070. [DOI] [PubMed] [Google Scholar]
- 37.Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, Rademann J, Stauber T, Kornak U, Jentsch TJ. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl− accumulation. Science. 2010;328:1401–3. doi: 10.1126/science.1188072. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RG, Carty SE, Scarpa A. Coupling of H+ gradients to catecholamine transport in chromaffin granules. Ann N Y Acad Sci. 1985;456:254–67. doi: 10.1111/j.1749-6632.1985.tb14874.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RG, Scarpa A. Protonmotive force and catecholamine transport in isolated chromaffin granules. J Biol Chem. 1979;254:3750–60. [PubMed] [Google Scholar]
- 40.Riazanski V, Deriy LV, Shevchenko PD, Le B, Gomez EA, Nelson DJ. Presynaptic CLC-3 determines quantal size of inhibitory transmission in the hippocampus. Nat Neurosci. 2011;14:487–94. doi: 10.1038/nn.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenck S, Wojcik SM, Brose N, Takamori S. A chloride conductance in VGLUT1 underlies maximal glutamate loading into synaptic vesicles. Nat Neurosci. 2009;12:156–62. doi: 10.1038/nn.2248. This study uses purified, reconstituted VGLUT1 and vesicle acidification to suggest that Cl− efflux promotes vesicular glutamate uptake. [DOI] [PubMed] [Google Scholar]
- 42.Busch AE, Schuster A, Waldegger S, Wagner CA, Zempel G, Broer S, Biber J, Murer H, Lang F. Expression of a renal type I sodium/phosphate transporter (NaPi-1) induces a conductance in Xenopus oocytes permeable for organic and inorganic anions. Proc Natl Acad Sci U S A. 1996;93:5347–51. doi: 10.1073/pnas.93.11.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–60. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 44.Wolosker H, de Souza DO, de Meis L. Regulation of glutamate transport into synaptic vesicles by chloride and proton gradient. J Biol Chem. 1996;271:11726–31. doi: 10.1074/jbc.271.20.11726. [DOI] [PubMed] [Google Scholar]
- 45.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. A long with showing that ketone bodies influence vesicular glutamate transport by competing with Cl− at an allosteric site, this work could not detect a VGLUT-mediated Cl− conductance by directly measureing Cl− flux. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–8. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 47.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–6. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 49.Erickson JD, Masserano JM, Barnes EM, Ruth JA, Weiner N. Chloride ion increases [3H]dopamine accumulation by synaptic vesicles purified from rat striatum: inhibition by thiocyanate ion. Brain Res. 1990;516:155–60. doi: 10.1016/0006-8993(90)90912-u. [DOI] [PubMed] [Google Scholar]
- 50.Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zander JF, Munster-Wandowski A, Brunk I, Pahner I, Gomez-Lira G, Heinemann U, Gutierrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–45. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. Focusing on cholinergic interneurons of the striatum, this paper demonstrates an important role for glutamate entry through VGLUT3 on synaptic vesicle filling with ACh. [DOI] [PubMed] [Google Scholar]
- 53.Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–92. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–90. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–48. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 58.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–18. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–91. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- 61.Dal Bo G, Berube-Carriere N, Mendez JA, Leo D, Riad M, Descarries L, Levesque D, Trudeau LE. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 62.Descarries L, Berube-Carriere N, Riad M, Bo GD, Mendez JA, Trudeau LE. Glutamate in dopamine neurons: synaptic versus diffuse transmission. Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–18. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 2010;107:389–94. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hattori T, Takada M, Moriizumi T, Van der Kooy D. Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J Comp Neurol. 1991;309:391–401. doi: 10.1002/cne.903090308. [DOI] [PubMed] [Google Scholar]
- 66.Meredith GE, Wouterlood FG. Identification of synaptic interactions of intracellularly injected neurons in fixed brain slices by means of dual-label electron microscopy. Microsc Res Tech. 1993;24:31–42. doi: 10.1002/jemt.1070240105. [DOI] [PubMed] [Google Scholar]
- 67.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- 68.Moss J, Ungless MA, Bolam JP. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur J Neurosci. 2011;33:1205–11. doi: 10.1111/j.1460-9568.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- 69.Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, Grinstein S. A cation counterflux supports lysosomal acidification. J Cell Biol. 2010;189:1171–86. doi: 10.1083/jcb.200911083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Gois S, Jeanclos E, Morris M, Grewal S, Varoqui H, Erickson JD. Identification of endophilins 1 and 3 as selective binding partners for VGLUT1 and their co-localization in neocortical glutamatergic synapses: implications for vesicular glutamate transporter trafficking and excitatory vesicle formation. Cell Mol Neurobiol. 2006;26:679–93. doi: 10.1007/s10571-006-9054-8. [DOI] [PubMed] [Google Scholar]
- 73.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–65. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–72. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 75.Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, Sjoholm H, Server A, Samuelsson L, Christianson A, Tarpey P, Whibley A, Stratton MR, Futreal PA, Teague J, Edkins S, Gecz J, Turner G, Raymond FL, Schwartz C, Stevenson RE, Undlien DE, Stromme P. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82:1003–10. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci U S A. 2008;105:8304–8. doi: 10.1073/pnas.0800881105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–96. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 79.Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–86. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–72. doi: 10.1016/j.neuron.2010.11.031. This elegant study shows that starburst amacrine cells of the retina release GABA and ACh from distinct vesicle pools, and the differential release contributes to direction selectivity in retinal ganglion cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–8. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- 82.Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–8. doi: 10.1038/nn.2478. This study demonstrates a developmental role for VGLUT3 in the co-release of glutamate by inhibitory MNTB neurons and its importance for synaptic refinement within the lateral superior olive that contributes to sound localization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–8. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maqueda J, Ramirez M, Lamas M, Gutierrez R. Glutamic acid decarboxylase (GAD)67, but not GAD65, is constitutively expressed during development and transiently overexpressed by activity in the granule cells of the rat. Neurosci Lett. 2003;353:69–71. doi: 10.1016/j.neulet.2003.08.077. [DOI] [PubMed] [Google Scholar]
- 85.Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–15. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 86.Trudeau LE, Gutierrez R. On cotransmission & neurotransmitter phenotype plasticity. Mol Interv. 2007;7:138–46. doi: 10.1124/mi.7.3.5. [DOI] [PubMed] [Google Scholar]
- 87.Gutierrez R. Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol. 2000;84:3088–90. doi: 10.1152/jn.2000.84.6.3088. [DOI] [PubMed] [Google Scholar]
- 88.Lehmann H, Ebert U, Loscher W. Immunocytochemical localization of GABA immunoreactivity in dentate granule cells of normal and kindled rats. Neurosci Lett. 1996;212:41–4. doi: 10.1016/0304-3940(96)12777-4. [DOI] [PubMed] [Google Scholar]
- 89.Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–58. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 90.Romo-Parra H, Vivar C, Maqueda J, Morales MA, Gutierrez R. Activity-dependent induction of multitransmitter signaling onto pyramidal cells and interneurons of hippocampal area CA3. J Neurophysiol. 2003;89:3155–67. doi: 10.1152/jn.00985.2002. [DOI] [PubMed] [Google Scholar]
- 91.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–81. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–23. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The Glutamatergic Component of the Mesocortical Pathway Emanating from Different Subregions of the Ventral Midbrain. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–33. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–10 . doi: 10.1523/JNEUROSCI.0265-10.2010. Along with Stuber et al. 2010 (ref. 94), this work uses optogenetics to show that glutamate released by dopamine neurons activates ionotropic glutamate receptors on post-synaptic medium spiny neurons in the NAc of adult mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 97.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 98.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 99.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–8. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 100.Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–25. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lapish CC, Seamans JK, Judson Chandler L. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–65. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 102.Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–7. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- 103.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–8. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–53. doi: 10.1126/science.1178307. This work uses channelrhodopsin to demonstrate the release of glutamate from hippocampal projections of serotonergic raphe nuclei. [DOI] [PubMed] [Google Scholar]
- 105.Hioki H, Fujiyama F, Nakamura K, Wu SX, Matsuda W, Kaneko T. Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb Cortex. 2004;14:1266–75. doi: 10.1093/cercor/bhh088. [DOI] [PubMed] [Google Scholar]
- 106.Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–86. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- 107.Jackson J, Bland BH, Antle MC. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse. 2009;63:31–41. doi: 10.1002/syn.20581. [DOI] [PubMed] [Google Scholar]
- 108.Mintz EM, Scott TJ. Colocalization of serotonin and vesicular glutamate transporter 3-like immunoreactivity in the midbrain raphe of Syrian hamsters (Mesocricetus auratus) Neurosci Lett. 2006;394:97–100. doi: 10.1016/j.neulet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 109.Shutoh F, Ina A, Yoshida S, Konno J, Hisano S. Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci Lett. 2008;432:132–6. doi: 10.1016/j.neulet.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 110.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–52. doi: 10.1016/j.neuron.2010.12.038. This study uses optogenetics to demonstrate a glutamatergic response in the interpeduncular nucleus elicited by optical stimulation of cholinergic projections from the medial habenula. [DOI] [PubMed] [Google Scholar]
- 112.Onoa B, Li H, Gagnon-Bartsch JA, Elias LA, Edwards RH. Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J Neurosci. 2010;30:7917–27. doi: 10.1523/JNEUROSCI.5298-09.2010. Using heterologous expression in primary dissociated culture, this study shows that VGLUT2 and VMAT2 traffic to overlapping but distinct synaptic boutons, and respond differently to stimulation when expressed in midbrain dopamine neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 114.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 115.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 116.Weston MC, Nehring RB, Wojcik SM, Rosenmund C. Interplay between VGLUT isoforms and endophilin A1 regulates neurotransmitter release and short-term plasticity. Neuron. 2011;69:1147–59. doi: 10.1016/j.neuron.2011.02.002. [DOI] [PubMed] [Google Scholar]