Abstract
Rationale
Prepulse inhibition (PPI), a preattentional information-filtering mechanism, is disrupted by serotonin (5-HT) or norepinephrine (NE) agonists to model deficits seen in schizophrenia, but whether this effect occurs through interactions between these systems is not known.
Objectives
These studies investigated whether PPI/activity changes induced by agonists of one system were dependent on neurotransmission within the other.
Methods
Male Sprague-Dawley rats received the 5-HT2 receptor agonist DOI (1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane) (0, 0.3 mg/kg), with or without antagonists for α1 (prazosin:0, 0.3, or 1 mg/kg) or β (timolol:0, 3, or 10 mg/kg) receptors or their combination (0 or 0.3 mg/kg prazosin + 3 mg/kg timolol), or the 5-HT2 antagonist ritanserin (0, 2 mg/kg). Separately, the α1-adrenergic receptor agonist cirazoline (0, 0.68 mg/kg) was given with and without ritanserin (0, 0.5, or 2 mg/kg) or the NE antagonists (0 or 0.3 mg/kg prazosin + 3 mg/kg timolol). Finally, combinations of subthreshold doses of DOI (0, 0.01, 0.025 mg/kg) and cirazoline (0, 0.1, 0.25 mg/kg) were tested for their ability to disrupt PPI, and concomitant administration of all three antagonists (0 vs. 0.3 mg/kg prazosin + 3 mg/kg timolol + 2 mg/kg ritanserin) was assessed for its ability to modify PPI. Locomotion was assessed in an additional set of experiments.
Results
Doses/combinations of prazosin and timolol that reversed cirazoline-induced effects did not alter DOI-induced effects, and ritanserin did not affect cirazoline at doses that blocked DOI-mediated effects. Concomitant antagonism of α1+β+5-HT2 receptors did not modify PPI, nor did combinations of subthreshold doses of cirazoline and DOI.
Conclusions
5-HT2 receptors and α1 and β NE receptors may act through independent mechanisms to modulate sensorimotor gating and locomotor activity.
Keywords: Startle, Schizophrenia, Noradrenergic, Serotonergic, Locomotion, Stereotypy
Introduction
Sensorimotor gating is an involuntary mechanism by which organisms filter sensory stimuli before they reach motor response networks, and is hypothesized as a gauge of information-filtering processes that may confer some protection from potential sensory inundation and cognitive deficits (Geyer et al. 1990). Prepulse inhibition (PPI) is a phenomenon found in many species, including humans and rats, and can serve as a translational paradigm that provides a functional measure of sensorimotor gating (Braff et al. 2001; Geyer et al. 2001; Swerdlow et al. 2001). PPI occurs when a brief, nonstartling stimulus (prepulse) decreases the startle reflex to a subsequent, more intense stimulus (pulse) (Hoffman and Ison 1980; Ison and Hoffman 1983). While some shifts in an individual's level of PPI can be considered a component of healthy information processing to flexibly adapt to changing environmental demands, consistently and significantly reduced levels of PPI have been observed in patients with schizophrenia, obsessive-compulsive disorder, and Tourette syndrome, compared to healthy control subjects. Deficient PPI, accordingly, has been proposed as an endophenotype with which to study the etiology of such illnesses (Braff et al. 2008). Indeed, animal models of deficient PPI have been shown for the past several decades to possess face, construct, and predictive validity for gating deficits in schizophrenia, and have been a valuable tool in identifying antipsychotic treatments (Braff 2010; Castagne et al. 2009; Ellenbroek 2004; Geyer 2008; Swerdlow et al. 2008; van den Buuse 2010; Weiss and Feldon 2001). Thus, understanding the neural substrates behind PPI could be a promising step towards understanding these disorders.
Multiple neurotransmitters regulate PPI, including the norepinephrine (NE) and serotonin (5-HT) systems (Geyer et al. 2001). The NE system has three main receptor types: α1, α2, and β. Within these receptor types are nine known subtypes (α1A, α1B, α1D,α2A, α2b, α2c,β1, β2, and β3,). The predominant ‘heteroreceptors’ (i.e., postsynaptic receptors localized on the dendrites or terminal of a target neuron) are α1 and β receptors, whereas α2 receptors are both presynaptic autoreceptors and postsynaptic heteroreceptors depending on region and their effects on PPI are thus complex (Bylund et al. 2009; Pupo and Minneman 2001; Sallinen et al. 2007; Starke 2001). In accord with the notion that increasing central NE transmission can reduce PPI, it has been found consistently and repeatedly that stimulation of α1 and β NE receptors results in PPI disruption, and that PPI deficits can be reversed by α1 and β NE receptor antagonists (Alsene et al. 2006; Alsene et al. 2010; Alsene et al. 2011; Carasso et al. 1998; Shilling et al. 2004; Swerdlow et al. 2006a; Varty et al. 1999). Thus, overactivation of NE transmission results in stimulation of postsynaptic α1 and β NE receptors, and models in animals the deficits in PPI that have been reported clinically.
Among the seven known 5-HT receptor classes (5-HT1− 5-HT7) (Andrade et al. 2010; Hoyer and Martin 1996), both 5-HT1 (which includes an autoreceptor) and 5-HT2 receptor families have been linked to PPI, with 5-HT2 heteroreceptors being perhaps the predominant postsynaptic receptors studied in this regard. Stimulation of 5-HT2 receptors, which are thought to be especially important in schizophrenia (Maier et al. 2008), causes PPI deficits that can be reversed by 5-HT2 receptor antagonists (Brea et al. 2006; Briody et al. 2010; Farid et al. 2000; Feifel et al. 2003; Kohnomi et al. 2008; Shilling and Feifel 2002; Shilling et al. 2004; Sipes and Geyer 1994; Sipes and Geyer 1995; 1997; Swerdlow et al. 2006b; Varty and Higgins 1995; Wadenberg et al. 2000). Thus, increased 5-HT transmission also reduces PPI, and postsynaptic 5-HT2 receptors play a prominent role in this effect.
NE and 5-HT systems can influence each other through a complex set of interactions at multiple levels. The locus coeruleus (LC) and the dorsal raphe (DR), the primary brainstem sources of NE and 5-HT, respectively, project to each other (Imai et al. 1986; Kim et al. 2004; Peyron et al. 1996; Segal 1979; Sim and Joseph 1993), and enhanced NE signaling can increase DR firing, while decreased NE transmission has the opposite effect (Baraban and A ghaja-nian 1980; Vandermaelen and Aghajanian 1983). 5-HT and agonists inhibit tonic LC cell firing but facilitate phasic LC cell firing (Aghajanian 1980; Haddjeri et al. 1997; Rasmussen and Aghajanian 1986). Indeed, extensive electrophysiological studies by Blier et al. have characterized multiple mechanisms by which 5-HT and NE transmission are interrelated, with stimulation of 5-HT1A and 5-HT2A receptors inhibiting tonic LC firing, and antagonism of α2 receptors on LC NE cells facilitating 5-HT cell firing (Blier 2001; Szabo and Blier 2001). NE and 5-HT also regulate each other's release in forebrain terminal regions with, for example, NE-increasing drugs enhancing 5-HT transmission at postsynaptic 5-HT receptors and α1 NE agonists elevating 5-HT in prefrontal cortex (Aloyo and Walker 1988; Amargos-Bosch et al. 2003; Done and Sharp 1992; Haddjeri et al. 1995). Importantly, functional effects caused by increased 5-HT signaling can be blocked by reducing NE transmission, and vice versa. For example, depletion of NE prevents 5-HT agonist-induced release of adrenocorticotropic hormone and corticosterone, and conversely, 5-HT depletion prevents α1 agonist-induced release of these hormones (Weidenfeld et al. 2002). Consistent with the idea that elevations in the transmission of one system could contribute to effects induced by the other are the findings that NE depletion attenuates behavioral effects of certain serotonin-selective reuptake inhibitors, and that neurokinin 1 receptor antagonists, which have putative antidepressant properties, cannot increase 5-HT cell firing without NE (Cryan et al. 2004; Gobbi et al. 2007; O'Leary et al. 2007a; O'Leary et al. 2007b). Hence, there is strong evidence that the NE and 5-HT systems interact in the treatment of depression (Blier and Briley 2011). NE and 5-HT also interact in mediating sensitization of psychostimulant-induced hyperactivity (Salomon et al. 2006). Therefore, there is significant potential for these two systems to interact in the regulation of behavioral processes.
Although there is evidence that both 5-HT and NE modulate PPI, and that these two systems interact at multiple levels, it is unclear whether or not they interact to regulate PPI. Determining if 5-HT and NE systems modulate PPI through independent mechanisms or through mutual interactions could aid in understanding the basis of sensorimotor gating impairments in a number of psychiatric disorders. The present studies thus tested the hypothesis that PPI deficits caused by one system were dependent on the other; this was done by examining whether or not the PPI-disruptive effects of the 5-HT2 receptor agonist DOI (1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane) could be reversed by α1 (prazosin) or β (timolol) NE receptor antagonists, and if PPI deficits induced by the α1 NE receptor agonist cirazoline could be normalized by the 5-HT2 receptor antagonist ritanserin. These receptors were studied because these are the primary postsynaptic receptors within their respective transmitter systems that are well-characterized to regulate PPI (Alsene et al. 2006; Briody et al. 2010; Carasso et al. 1998; Farid et al. 2000; Shilling and Feifel 2002; Sipes and Geyer 1997).
Materials and methods
Subjects
One hundred seventy-three experimentally naïve adult male Sprague-Dawley rats weighing between 300 and 400 g (Harlan Laboratories, Madison, WI, USA) were pairhoused in clear polycarbonate cages in a temperature- and light-controlled vivarium with food and water available ad libitum. Lights were on from 0700 h until 1900 h, with experiments conducted between 0900 h and 1700 h. Rats were handled daily for 1 week after arrival before testing began. Facilities and procedures complied with animal use and care guidelines from the National Institutes of Health of the USA, and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Startle and PPI testing
Startle chambers (San Diego Instruments, San Diego, CA, USA) consisted of Plexiglas cylinders, 8″ in length and 4″ in diameter, resting inside of a ventilated and illuminated chamber. A high frequency loudspeaker inside each chamber produced both a continuous background noise of 65 dB and various other acoustic stimuli. Whole-body startle responses of the rats caused vibrations in the cylinder, which were transduced into analogue signals by the platform’s piezoelectric unit. These signals were then digitized and stored by a microcomputer. Monthly calibrations were performed to ensure accuracy (using the dB(A) scale). The PPI test session consisted of a background noise (65 dB) that was presented alone for 5 min and remained on for the length of the session, followed by presentation (in a pseudorandom order) of Pulse-Alone trials (40-ms, 120-dB broadband bursts), Prepulse + Pulse trials (20-ms noises that were 3, 9, or 15 dB above the background noise and were presented 100 ms before the onset of the 120-dB pulse), and No Stimulus trials (only the background noise). The session contained 80 trials (16 each of the 3-, 9-, and 15-dB Prepulse + Pulse trials, 16 Pulse-Alone trials and 16 No Stimulus trials). Four Pulse-Alone trials were presented at the beginning and the end of the session to ensure that startle magnitude was stable during the portion of the session when PPI was measured, as the most rapid habituation of the startle response occurs within the first several presentations (Geyer et al. 1990); these Pulse-Alone trials were excluded from the calculations of startle and %PPI. During the week before the drug testing began, all rats were exposed to the startle test session once per day on three separate days to familiarize rats with the testing procedures prior to the commencement of drug testing. The final baseline test occurred 5–7 days before the experiment, and PPI and startle magnitude values from this final baseline day were used to create equally matched treatment groups for subsequent drug testing.
Activity cages
Locomotor activity was measured using a Photobeam Activity System (San Diego Instruments, San Diego, CA, USA) consisting of wire floor clear plastic cages (8×8×18″) surrounded by a grid of photobeams. Interruptions in the horizontal beams provided a measure of cage crossings (locomotion), breaks in the vertical beams measured rearing, and repeated breaks of the same beam provided a measure of fine motor movements (an assay of stereotypy). No food or water was provided during locomotor activity testing. Rats for each locomotor activity experiment were habituated for 2 h to the photocell cages on two separate days, occurring several days before the drug test.
Drugs
Prazosin hydrochloride, timolol maleate, DOI (1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane), ritanserin, and cirazoline were obtained from Sigma (St Louis, MO, USA). DOI, timolol, and cirazoline were dissolved in sterile isotonic saline. Prazosin was dissolved with sonication in a vehicle solution containing 95% distilled water and 5% DMSO (Sigma). This solution was used as the vehicle injection for all prazosin experiments. Ritanserin was dissolved with sonication in a vehicle solution containing 95% isotonic saline, 2.5% pure ethanol, and 2.5% cremaphor (Fluka BioChemiKa, Buchs, Switzerland). The ritanserin suspension was injected at a volume of 2 mL/kg body weight. All other drugs were injected at 1 mL/kg body weight.
Experimental design
Each experiment tested startle/PPI and locomotor activity using separate sets of experimentally naïve rats. For experiments 1a- and 2a–c, ppl was tested first, and then after a 10-day drug washout period, locomotor activity was measured in the same subjects. The drug treatments from PPI testing were equally represented in each new treatment group for locomotor testing (therefore, each treatment group in the locomotor test had an equal number of rats from the vehicle, low dose antagonist, or high dose antagonist groups of the PPI test). In addition, the total locomotion (cage crossings) recorded from the final photocell acclimation day was used to balance these groups for the subsequent drug test day so that the different groups had equivalent baseline activity levels prior to drug testing. For experiments 1d–h, experimentally naïve rats were used for PPI testing, according to the designs described in detail below. For all experiments, doses/injection parameters were based on studies in which these drugs were found to affect PPI or antagonize behavioral effects induced by agonists for their respective receptors (Alsene et al. 2006; Alsene et al. 2010; Carasso et al. 1998; Sipes and Geyer 1994; Sipes and Geyer 1997; Wing et al. 1990).
Experiment 1: PPI and startle
Experiment 1a: prazosin/DOI/PPI
Rats received one dose of prazosin (N=8/dose; 0, 0.3, or 1 mg/kg, intraperitoneally (IP)) and 20 min later, half of the rats in each dose condition received DOI (0.3 mg/kg, subcutaneously (SC)); the remaining rats in each prazosin dose condition got vehicle injections. Ten minutes after the DOI/vehicle, all rats were tested in startle chambers. One week later, this protocol was repeated except that rats previously receiving DOI now got vehicle and vice versa. Thus, prazosin dose was a between-subjects factor, and DOI treatment was within-subjects.
Experiment 1b: timolol/DOI/PPI
Using the same design as above, experimentally naïve rats received timolol (0, 3, or 10 mg/kg, IP, 15 min before testing, N=8/dose) and DOI (0, 0.3 mg/kg, SC, 10 min before testing).
Experiment 1c: ritanserin/cirazoline/PPI
Using the same design, new rats received ritanserin (0, 0.5, 2 mg/kg, SC, 30 min before PPI testing, N=8/dose) and cirazoline (0, 0.68 mg/kg, IP, 5 min before PPI testing).
Experiment 1d: prazosin+timolol/DOI/PPI
Rats (N=12) received both prazosin (0.3 mg/kg, IP, 30 min before PPI testing) and 15 min later, timolol (3 mg/kg, IP); 12 other rats received vehicle injections at those same timepoints. Ten minutes before testing, half the rats in each group received DOI (0.5 mg/kg, SC), and the other half received SC vehicle.
Experiment 1e: prazosin+timolol/cirazoline/PPI
Using the same doses and injection parameters as the previous experiment, rats received either vehicle or the prazosin/ timolol combination prior to cirazoline (0.68 mg/kg, IP, N=8) or vehicle (N=8) and were tested in startle chambers 5 min later. One week later, this protocol was repeated but rats that previously got prazosin+timolol now received vehicle injections and vice versa (cirazoline status remained the same as before).
Experiment 1f: ritanserin/DOI/PPI
Rats received either vehicle (N=10) or DOI (0.5 mg/kg, SC, 10 min before testing, N=8) 20 min after half of the rats in each group had received vehicle and the other half had received ritanserin (2 mg/kg, SC); 1 week later, the protocol was repeated with previous ritanserin rats getting vehicle prior to their second injection, and vice versa.
Experiment 1g: cirazoline/DOI/PPI
Rats received a dose of DOI (0, 0.01, 0.025 mg/kg, SC), and 5 min later received either IP vehicle (N=9), 0.1 mg/kg cirazoline (N=7), or 0.25 mg/kg cirazoline (N=7); testing took place 5 min after the second injection. This protocol was repeated twice more, with cirazoline condition staying constant (i.e., between-subjects factor) and DOI as a within-subjects factor so rats in each cirazoline condition received all three doses of DOI in a counterbalanced order over the three test days. Prior to this experiment, we first conducted extensive pilot dose-response studies to determine doses of cirazoline and DOI that were below the threshold for disrupting PPI using our test parameters.
Experiment 1h: ritanserin+prazosin+timolol/PPI
Eight rats received injections of 2 mg/kg of ritanserin (SC) and 0.3 mg/kg prazosin (IP), and then after 15 min received timolol (3 mg/kg, IP). A separate group of eight rats received three vehicle injections according to the same routes and timeline. Fifteen minutes after the final injection, all rats were tested for PPI.
Experiment 2: locomotor activity
Experiment 2a: prazosin/DOI/locomotor activity
Rats (N=6/group, with pretreatment and treatment as between-subjects factors) were placed in photocell cages for 30 min, then given prazosin (0 or 1 mg/kg, IP), placed back in the photocell cages, and given DOI (0 or 0.3 mg/kg, SC) 20 min later. Motor activity was measured for 60 min after this final injection.
Experiment 2b: timolol/DOI/locomotor activity
Using the same design as above, rats (N=7/group) were treated with timolol (0 or 10 mg/kg, IP) and 5 min later given DOI (0 or 0.3 mg/kg, SC).
Experiment 2c: ritanserin/cirazoline/locomotor activity
Using the same design, rats (N=6/group) received ritanserin (0 or 2 mg/kg, SC) followed 25 min later by cirazoline (0 or 0.68 mg/kg, IP).
Data analysis
The startle response to the onset of the 120-dB burst was recorded for 100 ms for each Pulse-Alone and Prepulse +Pulse trial. Two measurements were calculated for each rat: startle magnitude was the average of the startle responses to the Pulse-Alone trials, PPI was a percent score for each Prepulse + Pulse trial type: %PPI=100-{[(startle response for Prepulse+Pulse trial)/(startle response for Pulse-Alone trial)] × 100}. For every experiment, a significant main effect of prepulse intensity was seen upon analyzing %PPI data; this is a standard parametric feature of PPI in which increasing prepulse intensities elicit higher levels of PPI (Braff et al. 2001). For brevity, this main effect is not repeated throughout the text. Because no significant interactions were seen with prepulse intensity and any other factor in these experiments, a single composite %PPI score (average PPI collapsed across all three prepulse intensities) was calculated for each subject, and this is what is shown in the figures. Startle and PPI data were analyzed with separate 2-way analyses of variance (ANOVA) using pretreatment as a between-subjects factor and treatment as a within-subjects factor.
The total frequency of locomotion (cage crossings), rears, and fine motor movements were calculated in 10-min intervals (see Fig. 4–Fig. 6). The first 30 min were the ‘habituation’ phase prior to drug treatments and were followed by injections; the second injection (the treatment factor) was followed immediately by a 60-min ‘testing’ phase. Values from this 60-min period were used for separate 3-factor ANOVAs with time (10-min intervals) as the repeated measure and with pretreatment and treatment as between-subjects variables. Unpaired t-tests using Bonferroni-adjusted α values were used for post hoc analyses.
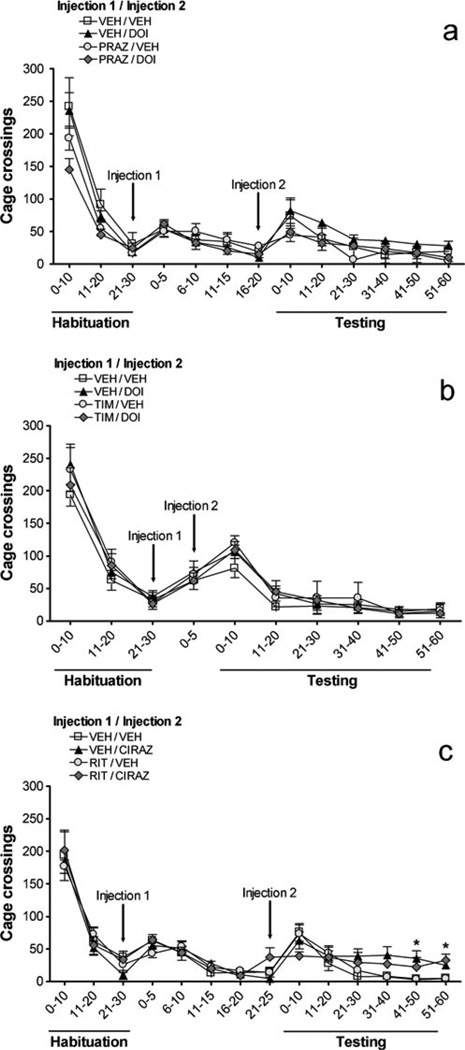
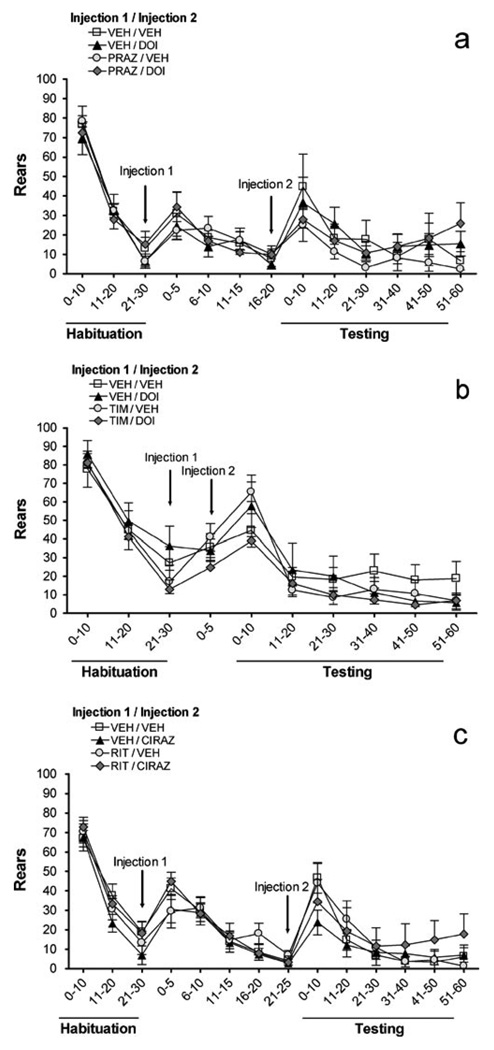
Fig. 4.
Effects on locomotion (number of cage crossings) of a prazosin (PRAZ, 1 mg/kg) vs. DOI (0.3 mg/kg), b timolol (TIM, 10 mg/kg) vs. DOI (0.3 mg/kg), c ritanserin (RIT, 2 mg/kg) vs. cirazoline (0.68 mg/kg). VEH = vehicle, x-axis is in minutes, and all values represent means ± SEM. ‘Habituation’ is the 30 min following entry of rats into photocell cages; injections (indicated by arrows) took place after this habituation; ‘Testing’ is the 60 min immediately after Injection 2. *P<0.05, compared to VEH/VEH
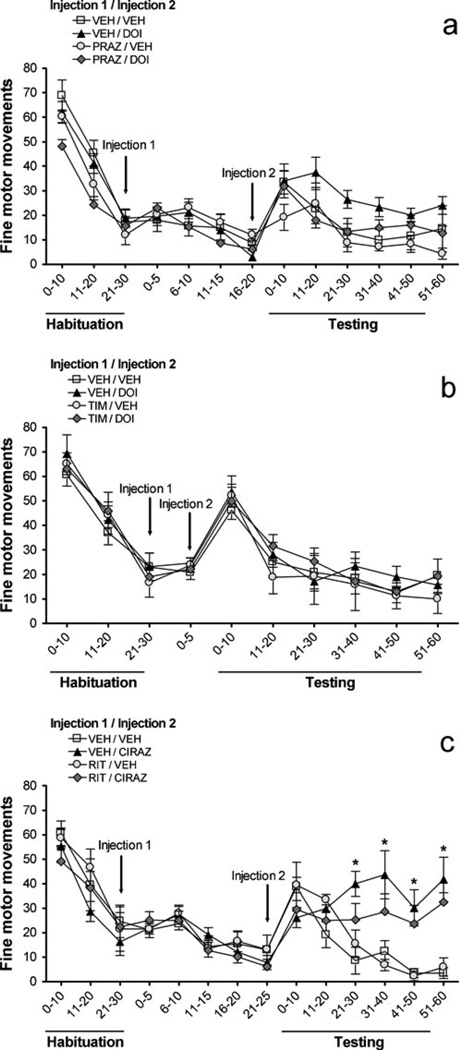
Fig. 6.
Effects on number of fine motor movements of a prazosin (PRAZ, 1 mg/kg) vs. DOI (0.3 mg/kg), b timolol (TIM, 10 mg/kg) vs. DOI (0.3 mg/kg), c ritanserin (RIT, 2 mg/kg) vs. cirazoline (0.68 mg/ kg). VEH = vehicle, χ-axis is in minutes, and all values represent means ± SEM. ‘Habituation’ is the 30 min following entry of rats into photocell cages; injections (indicated by arrows) took place after this habituation; ‘Testing’ is the 60 min immediately after Injection 2. a Main effects of both pretreatment and treatment, but no interactions. c *P<0.05, relative to groups receiving vehicle (VEH)
Results
Prepulse inhibition
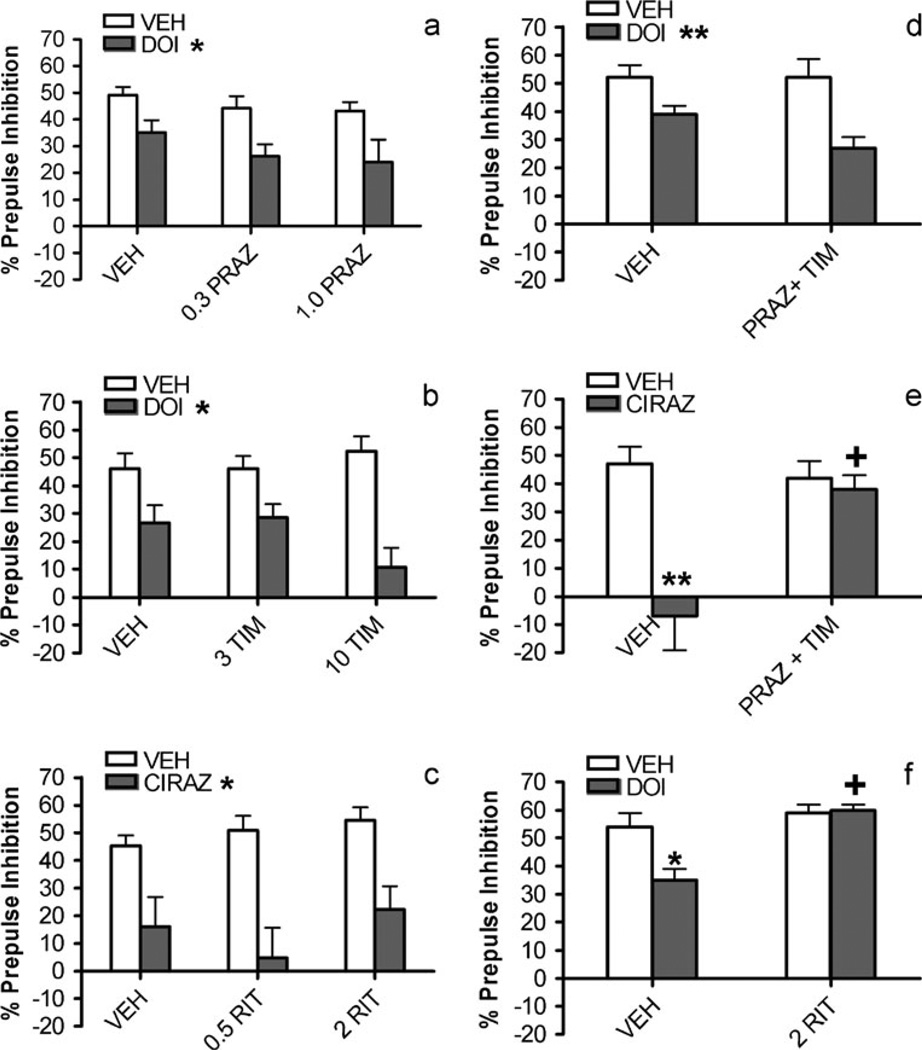
Figure 1a shows the effects of prazosin and DOI on PPI. There was a main effect of DOI [F(1,18)=14.0, P<0.002]; PPI values in all DOI groups were lower than those for vehicle treatment. Thus, DOI significantly decreased PPI. There was no main effect of prazosin [F(2,18)=2.2, not significant (NS)], nor was there a prazosin × DOI interaction [F(2,18)=0.2, NS], indicating that prazosin had no effect on its own or on DOI-induced PPI deficits. Thus, prazosin pretreatment did not reverse the DOI-induced disruption of PPI. Figure 1b illustrates the timolol/DOI results. Again, there was a main effect of DOI [F(1,20)= 32.4, P<0.001], with DOI reducing PPI. There was no main effect of timolol [F(2,20)=0.6, NS], nor was there a timolol × DOI interaction [F(2,20)=2.9, NS], indicating that timolol had no effect on its own or on DOI-induced PPI deficits. Thus, timolol, like prazosin, was unable to reverse DOI-induced PPI disruption. Similarly, even when both NE receptor antagonists were administered together, they did not affect DOI-induced PPI deficits (Fig. 1d). In this experiment, there was a main effect of DOI treatment [F(1,20)=15.8, P<0.001], but no pretreatment effect [F(1,20)=1.8, NS] or pretreatment × treatment interaction [F(1,20)=1.7, NS]. Figure 1c illustrates the effects of ritanserin and cirazoline on PPI. There was a main effect of cirazoline [F(1,19)=35.5, P<0.001], with cirazoline impairing PPI. Ritanserin, however, had no effect [F(2,19)= 0.9, NS], and the ritanserin × cirazoline interaction was not significant [F(2,19)=0.7, NS], demonstrating that ritanserin had no effect on its own or on cirazoline-induced PPI deficits. Thus, DOI and cirazoline disrupted PPI, but these effects were not reversed by antagonists of the other transmitter system.
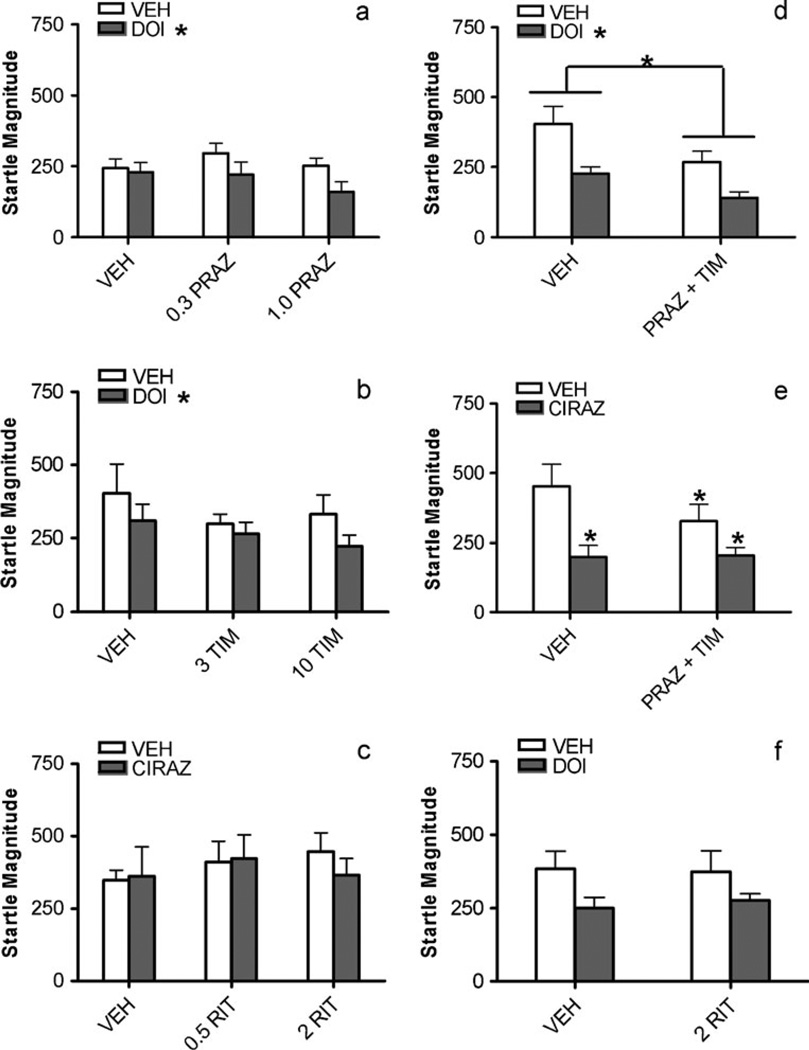
Fig. 1.
Effects on % prepulse inhibition of a prazosin (PRAZ) vs. DOI, b timolol (TIM) vs. DOI, c ritanserin (RIT) vs. cirazoline (CIRAZ), d prazosin (0.3 mg/kg)+timolol (3 mg/kg) vs. DOI, e prazosin (0.3 mg/kg)+ timolol (3 mg/kg) vs. cirazoline, f ritanserin vs. DOI. Values represent means ± SEM and doses are in mg/kg. VEH = vehicle; cirazoline dose is 0.68 mg/kg and DOI dose is 0.5 mg/kg. a–d *P<0.05, **P<0.01, main effect of DOI or cirazoline treatment. e–f *P<0.05, **P<0.01, compared to VEH/VEH; +P<0.05, compared to VEH/CIRAZ or VEH/DOI
Nevertheless, the present doses of prazosin, timolol, and ritanserin were sufficient to completely block PPI deficits that were elicited by agonists of the same neurotransmitter system, as depicted in Fig. 1e – f. There were significant main effects of the prazosin (0.3 mg/kg)+timolol (3 mg/kg) combination pretreatment [F(1,14)=5.9, P<0.03] and the cirazoline treatment [F(1,14)=14.8, P<0.002], and a significant interaction between the two [F(1,14)=8.9, P<0.01]. Post hoc analyses revealed that in vehicle-pretreated rats, cirazoline profoundly reduced PPI compared to the vehicle-vehicle group (P<0.01), but that this cirazoline-induced PPI reduction was not seen in rats that were pretreated with prazosin+timolol; PPI values for this groups were significantly higher than those for the vehicle- cirazoline group (P<0.05). In the ritanserin/DOI experiment, there was similarly a significant interaction between pretreatment and treatment [F(1,16)=5, P<0.04], which was due to a marked reduction in PPI in the vehicle/DOI condition compared to the vehicle-alone condition (P<0.05). The ritanserin/DOI condition was significantly higher than vehicle/DOI (P<0.01), and was in fact indistinguishable from vehicle/vehicle. Therefore, the prasozin+timolol combination that failed to reverse DOI-induced PPI deficits did block cirazoline-induced effects, and the same dose of ritanserin that did not block cirazoline-induced deficits did reverse DOI-induced effects.
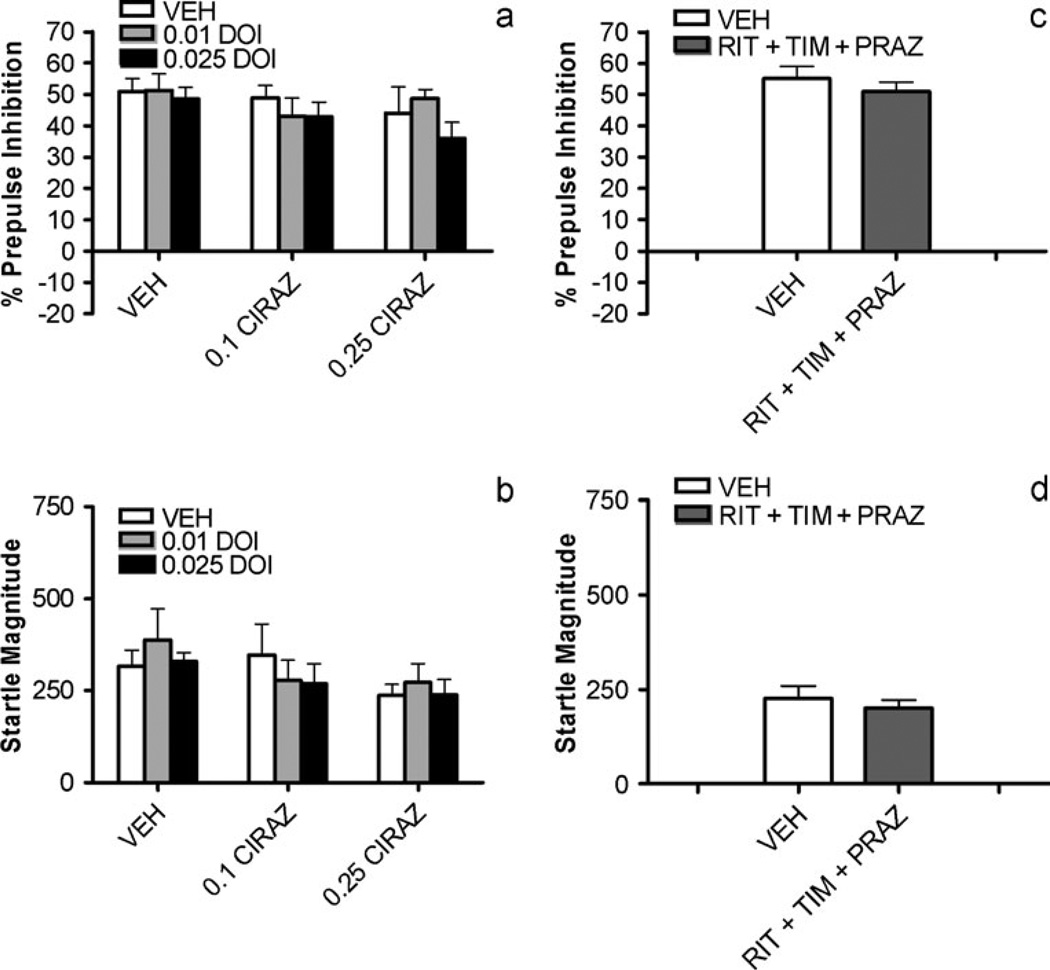
To test in other ways for possible interactions between these receptors in the regulation of PPI, the ability of subthreshold doses of cirazoline and DOI to synergistically disrupt PPI when given together was examined. No main effects of cirazoline dose [F(2,20)=1.3, NS], DOI dose [F(2,20)=1.3, NS], nor any interaction between the two factors [F(4,40)=0.6, NS] was seen (Fig. 3a). Thus, this experiment also failed to reveal interactions between these receptor systems in PPI. Similarly, to determine if simultaneous antagonism of α1, β, and 5-HT2 receptors would be synergistic and alter PPI (since the above studies showed that individual blockade of each receptor was insufficient to do so), rats treated with prazosin+timolol+ritanserin were compared to vehicle-treated animals. Again, as depicted in Fig. 3c, no effect on PPI was seen [F(1,14)=1.1, NS]. Thus, neither simultaneous stimulation nor simultaneous blockade of NE and 5-HT2 receptors indicated interactions between these systems in the modulation of PPI.
Fig. 3.
Effects of subthreshold doses of DOI combined with subthreshold doses of cirazoline (CIRAZ) on a % prepulse inhibition and b startle magnitude; doses are in mg/kg. Effects of combined injections of ritanserin (RIT, 2 mg/kg)+timolol (TIM, 3 mg/kg) + prazosin (PRAZ, 0.3 mg/kg) vs. vehicle (VEH) on c % prepulse inhibition and d startle magnitude. All values represent means ± SEM
Startle magnitude
Figure 2a shows the effects of prazosin and DOI on baseline startle magnitude. DOI had a significant main effect [F(1,18)=9.8, P<0.006], with DOI treatment producing lower startle values than vehicle. On closer examination, this effect seemed mainly driven by rats receiving DOI in combination with prazosin, although prazosin had no main effect [F(2,18)=0.7, NS], and there was no prazosin×DOI interaction [F(2,18)=1.5, NS]. Figure 2b illustrates the effects of timolol and DOI on startle magnitude. Again, there was a main effect of DOI treatment on startle magnitude [F(1,20)=5.9, P<0.025] that, as with the prazosin experiment, seemed to be due primarily to the DOI and timolol combination. Timolol pretreatment had no main effect [F(2,20)=0.7, NS], and there was no timolol×DOI interaction [F(2,20)=0.5, NS]. When the combination of prazosin (0.3 mg/kg) and timolol (3 mg/ kg) was administered prior to DOI, there was a main effect of pretreatment [F(1,20)=7.7, P<0.02] and a main effect of DOI treatment [F(1,20)=14.8, P<0.001], but no interaction between factors [F(1,20)=0.5, NS], as depicted in Fig. 2d. Figure 2c shows the effects of ritanserin and cirazoline on startle magnitude. Ritanserin [F(2,19)=0.3, NS] and cirazoline [F(1,19)=0.2, NS] had no main effects, and there was no interaction [F(2,19)=0.6, NS]. Thus, neither ritanserin nor cirazoline significantly affected startle magnitude.
Fig. 2.
Effects on baseline startle magnitude of a prazosin (PRAZ) vs. DOI, b timolol (TIM) vs. DOI, c ritanserin (RIT) vs. cirazoline (CIRAZ), d prazosin (0.3 mg/kg) + timolol (3 mg/kg) vs. DOI, e prazosin (0.3 mg/kg)+timolol (3 mg/kg) vs. cirazoline, f ritanserin vs. DOI. Values represent means ± SEM and doses are in mg/kg. VEH = vehicle; cirazoline dose is 0.68 mg/kg and DOI dose is 0.5 mg/kg. a–d *P<0.05, main effects of pretreatment (drawn with lines in d) or treatment (DOI or cirazoline). e *P<0.05, compared to VEH/VEH
In Fig. 2e, one sees that there were significant main effects of the prazosin (0.3 mg/kg)+timolol (3 mg/kg) combination pretreatment [F(1,14)=4.7, P<0.05] and the cirazoline treatment [F(1,14)=6.3, P<0.03], and a significant interaction between the two [F(1,14)=5.7, P<0.03]. Post hoc analyses revealed that all three drug conditions caused significantly lower baseline startle magnitude compared to the vehicle-alone condition (P<0.05). No statistically significant effects on startle magnitude were seen in the ritanserin/DOI experiment ANOVA (Fig. 2f). Similarly, there were no significant effects on baseline startle with either factor nor an interaction between the two in the subthreshold cirazoline+DOI study (Fig. 3b) and also in the prazosin+timolol+ritanserin experiment (Fig. 3d) (all F values≤1.1).
Locomotor activity
In every experiment, there was a significant main effect of time on each measure of locomotor activity (all F values were greater than or equal to 6.2, and all P values were less than 0.001). For brevity, the description of this main effect is not repeated throughout this section.
Locomotion (cage crossings)
The effects of prazosin and DOI on locomotion (cage crossings) are shown in Fig. 4a. There was no main effect of DOI [F(1,20)=1.1, NS] or prazosin [F(1,20)=2.6, NS] on locomotion. The prazosin × DOI interaction was not significant [F(1,20)=0.3, NS], and there were no time×prazosin [F(5, 100)=1.3, NS], time × DOI [F(5,100)=0.2, NS], or 3-way [F(5,100)=0.8, NS] interactions. To summarize, neither prazosin nor DOI affected locomotion. Similarly, there were no significant effects in the timolol/DOI experiment (Fig. 4b): no main effect of DOI [F(1, 24)=0.1, NS] or timolol [F(1,24)=0.5, NS]; no timolol × DOI interaction [F(1,24)= 0.8, NS]; no time × timolol [F(5,120)=1.0, NS], time×DOI [F(5,120)=0.6, NS], or time×DOI×timolol [F(5,120)= 0.5, NS] interactions. Figure 4c illustrates the effects of ritanserin and cirazoline on locomotion. There was no main effect of cirazoline [F(1,20)=3.9, NS] or ritanserin [F(1,20)=0.2, NS]. No ritanserin × cirazoline [F(1,20)= 0.9, NS], time × ritanserin [F(5,100)=0.8, NS], or 3-way [F(5,100)=0.4, NS] interactions were seen. The time × cirazoline interaction was significant [F(5,100)=5.3, P< 0.001]. Further analyses revealed that cirazoline treatment produced a small increase in locomotion for the last 20 min of the test session (P<0.05), and ritanserin did not reverse this effect.
Rearing
Figure 5a shows how prazosin and DOI affected rearing. Similar to the profile observed with locomotion, there was no main effect of DOI [F(1,20)=0.8, NS], nor prazosin [F(1,20)=0.8, NS], no significant prazosin × DOI [F(1,20)= 0.7, NS], time × prazosin [F(5,100)=0.9, NS], time × DOI [F(5, 100)=1.1, NS], or time × prazosin × DOI interactions [F(5,100)=0.4, NS]. Thus, neither prazosin nor DOI affected rearing. Similarly, in the timolol/DOI experiment (Fig. 5b), there were no main effects of DOI [F(1,24)=0.7, NS] or timolol [F(1,24)=1.2, NS], and no timolol × DOI [F(1,24)=0.1, NS], time × DOI [F(5,120)=0.8, NS] or time × timolol [F(=5,120)=0.4, NS] interactions. There was a small 3-way interaction [F(5,120)=2.5, P<0.038] that did not reveal any significant timolol/DOI effects or interactions at any of the individual timepoints with subsequent analyses. Thus, rearing was not significantly altered by DOI or timolol. Figure 5c illustrates how ritanserin and cirazoline affected rearing. There were no main effects of cirazoline [F(1,20)=0.0, NS] or ritanserin [F(1,20)=0.5, NS], nor any ritanserin × cirazoline [F(1,20)=0.2, NS], time × ritanserin [F(5,100)=0.3, NS], or time × ritanserin × cirazoline [F(5,100)=0.9, NS] interactions. Despite a significant time × cirazoline interaction [F(5,100)=5.3, P<0.001], no significant simple main effects of cirazoline were found at any time bin; this 2-way interaction in the omnibus ANOVA may have been driven primarily by a nonsignificant trend for a simple main effect of cirazoline in the first 10 min after injection.
Fig. 5.
Effects on number of rears of a prazosin (PRAZ, 1 mg/kg) vs. DOI (0.3 mg/kg), b timolol (TIM, 10 mg/kg) vs. DOI (0.3 mg/kg), c ritanserin (RIT, 2 mg/kg) vs. cirazoline (0.68 mg/kg). VEH = vehicle, x-axis is in minutes, and all values represent means ± SEM. ‘Habituation’ is the 30 min following entry of rats into photocell cages; injections (indicated by arrows) took place after this habitua-tion; ‘Testing’ is the 60 min immediately after Injection 2
Fine motor movements
Figure 6a shows how prazosin and DOI affected fine motor movements. DOI [F(1,20)=5.2, P<0.034] and prazosin [F(1,20)=4.8, P<0.042] each produced a significant main effect, with DOI increasing and prazosin decreasing this measure. There were no prazosin × DOI [F(1,20)=0.4, NS], time × prazosin [F(5,100)=0.4, NS], time × DOI [F(5,100)=0.3, NS], or 3-way interactions [F(5,100)=1.8, NS]. Therefore, DOI increased fine motor movements and prazosin decreased fine motor movements, but these were separate independent effects, with no interaction between the drugs. Figure 6b shows the effects of the timolol/DOI experiment. In this experiment, DOI did not have a main effect [F(1,24)=0.7, NS]; timolol also had no main effect [F(1,24)=0.1, NS], and there were no significant timolol × DOI [F(1,24)=0.1, NS], time × timolol [F(5,120)=0.4, NS], time × DOI [F(5,120)=0.3, NS], or time × timolol × DOI [F(5,120)=1.2, NS] interactions. In summary, neither timolol nor DOI affected fine motor movements in this experiment. In contrast, cirazoline treatment (Fig. 6c) had a main effect on fine motor movements [F(1,20)=24.5, P<0.001], with cirazoline robustly increasing this measure. There was no ritanserin main effect [F(1,20)=0.6, NS], and no time × ritanserin [F(5,100)= 1.1, NS], or 3-way interactions [F(5,100)=0.8, NS]. The time × cirazoline treatment interaction was significant [F(5,100)=11.7, P<0.001], and subsequent post hoc tests showed that cirazoline-treated rats had more fine motor movements 21– 60 minutes after injection (P<0.05). In conclusion, cirazoline significantly increased the amount of fine motor movements, and ritanserin may have slightly reduced this effect; however, this interaction was not significant. Although it appeared that ritanserin/cirazoline rats may have had slightly lower values than vehicle/cirazoline rats, the ritanserin × cirazoline interaction did not reach statistical significance [F(1,20)=2.9, NS].
Discussion
In this study, we tested the hypothesis that 5-HT2 and α1/β NE receptors interact reciprocally to regulate PPI. Several important results were obtained. First, confirming multiple previous reports, both the 5-HT2 receptor agonist DOI and the α1 NE receptor agonist cirazoline markedly reduced PPI after systemic injection (Alsene et al. 2006; Alsene et al. 2010; Brea et al. 2006; Briody et al. 2010; Carasso et al. 1998; Farid et al. 2000; Feifel et al. 2003; Kohnomi et al. 2008; Shilling and Feifel 2002; Shilling et al. 2004; Sipes and Geyer 1994; Sipes and Geyer 1995; 1997; Swerdlow et al. 2006b; Varty et al. 1999; Wadenberg et al. 2000). Thus, individual direct stimulation of either 5-HT2 or α1 NE disrupts PPI. Neither the α1 receptor antagonist prazosin nor the β receptor antagonist timolol reversed the DOI-induced PPI deficits (individually or when administered in combination), indicating that this effect was independent of NE transmission. Conversely, the 5-HT2 receptor antagonist ritanserin did not reverse the PPI deficits caused by cirazoline, suggesting that likewise, α1 NE receptor-mediated PPI deficits are not dependent on 5-HT transmission at the 5-HT2 receptor. Locomotor activity measures followed a similar profile, with either DOI- or cirazoline-induced effects not being reversed by prazosin/timolol or ritanserin, respectively. Thus, two of the cardinal effects of 5-HT2 and α1 NE receptor stimulation (PPI deficits and increased stereotyped motor responses) seem to be mediated independently of the other transmitter system. As is true for any study in which one is demonstrating a negative result, it is possible that doses other than those used presently might indicate an interaction between the 5-HT2 and α1/β NE systems in PPI. Nevertheless, the antagonist doses that were used completely reversed PPI deficits caused by agonism of the same receptor, indicating that they were sufficiently high to enact functional antagonism in this paradigm. Similarly, multiple combinations of subthreshold doses of NE α1 and 5-HT2 agonists did not affect PPI, and conversely, concomitant administration of α1, β, and 5-HT2 receptor antagonists together also failed to alter PPI. Therefore, to a reasonable extent, our studies provide evidence that PPI deficits induced by either agonist do not seem to require stimulation of the ‘opposite’ system and that these receptors may act independently of each other in the regulation of PPI.
To the best of our knowledge, this is the first study to systematically determine if α1, β, and 5-HT2 receptors interact to modulate several behavioral processes; the finding that α1 NE receptor antagonism does not block DOI-induced effects has not been reported previously. Similarly, the β receptor antagonist timolol also failed to reverse DOI-induced effects, which is consistent with a previous study using the nonselective β receptor antagonist propranolol (Sipes and Geyer 1994). The finding that ritanserin did not reverse cirazoline-induced PPI deficits further supports the notion that these NE and 5-HT systems may be mutually independent in the regulation of PPI, and agrees with the previous result that M100907, a 5-HT2A subtype-selective receptor antagonist, also did not reverse the effects of cirazoline on PPI (Varty et al. 1999). As shown in the present studies, these same doses of prazosin, timolol, and ritanserin do reverse behavioral effects induced by agonists of their respective systems, indicating that these doses were sufficiently high to reverse effects that were putatively caused by indirect increases in NE and 5-HT transmission (Alsene et al. 2006; Alsene et al. 2010; Carasso et al. 1998; Wing et al. 1990). The failure of the antagonists to reverse PPI deficits produced by the ‘opposite’ system in the present study indicates that DOI does not depend on α1/β NE receptors and that cirazoline does not depend on 5-HT2 receptors to disrupt PPI. Therefore, 5-HT2 receptors as well as α1 and β NE receptors regulate PPI, but these systems may act in parallel instead of through mutual interactions with each other for these effects.
Although a significant main effect of DOI treatment on startle was seen in some experiments, we do not believe that the DOI-induced deficits are simply due to changes in baseline startle. If one closely examines Fig. 2a–b, startle values for the vehicle-vehicle condition are nearly identical to those of the vehicle-DOI condition, suggesting that this main effect was due mostly to the DOI and antagonist combinations. Furthermore, there was no statistically significant reduction in startle by DOI in the DOI/ritanserin experiment, even though there was a robust DOI-induced PPI deficit. There is ample evidence that the present dose range of DOI disrupts PPI without affecting startle (Briody et al. 2010; Farid et al. 2000; Feifel et al. 2003; Shilling et al. 2004; Sipes and Geyer 1994; Sipes and Geyer 1995; Swerdlow et al. 2006b; Wadenberg et al. 2000). Similarly, cirazoline effects on PPI and startle were also dissociable in the present studies, which corroborates many previous reports (Alsene et al. 2006; Carasso et al. 1998; Gresack and Risbrough 2010; Shilling et al. 2004), although in one study a higher dose did increase baseline startle (Varty et al. 1999). It is also noteworthy that 1/β and 5-HT2 antagonists reversed PPI deficits induced by the agonist for the same receptor without altering agonist-induced effects on baseline startle. These findings suggest that the PPI deficits seen presently were not simply artifacts of altered baseline startle reactivity, and that on the independent measure of startle reactivity, the NE and 5-HT receptor antagonists did not alter the profile of DOI or cirazoline, again indicating a lack of interaction between these systems for these behaviors.
The results of the locomotor activity experiments also support the conclusion that to produce their behavioral effects, DOI does not require NE release, and cirazoline does not require 5-HT release. DOI on its own increased fine motor movements (repeated consecutive breaks of the same photobeam in a localized area of the cage that provides a rough measure of motor stereotypies) but had little to no effect on cage crossings or rearing. Although traditionally 5-HT was thought to reduce locomotor activity, the present profile is consistent with more refined characterizations of motor movement patterns that indicate that DOI increases the tendency for predictable and stereotyped responses (Geyer 1996; Hillegaart et al. 1996; Krebs-Thomson et al. 1998; Wing et al. 1990), and with previous reports that stimulation of 5-HT2 receptors promotes head shakes and wet dog shakes (Berendsen et al. 1996; Chaouloff et al. 1994; Kohnomi et al. 2008; Kouhata et al. 2001; Kugaya et al. 1996; Marek 2009; Millan et al. 1998; Pranzatelli 1990; Schreiber et al. 1995). Similarly, with cirazoline, little to no effect was seen on locomotion and rearing, but a large and prolonged increase in fine motor movements occurred, consistent with previous reports that α1 NE receptors contribute to motor activity induced by a variety of drugs (Fone et al. 1987; Fowler et al. 2007; Harkin et al. 2001; Stone et al. 2004). As reviewed by Stone et al. (2011), α1 receptors in the locus coeruleus are thought to play a critical role in behavioral activation; indeed, infusion of the nonselective α receptor agonist 6FNE in the vicinity of the locus coeruleus produces extreme hyperactivity that is likely mediated by α1 receptors (Stone et al. 2009). This could provide a potential mechanism through which cirazoline stimulates fine motor activity. Again, despite the fact that these doses of prazosin and timolol do reverse NE-mediated PPI deficits and hyperactivity (Alsene et al. 2006; Alsene et al. 2010; Vanderschuren et al. 2003), and the present dose range of ritanserin does reverse the locomotor effects of DOI and other 5-HT2 agonists (Hillegaart et al. 1996; Mittman and Geyer 1991; Wing et al. 1990), the increase in fine motor movements induced by DOI was not reversed by prazosin or timolol, and the cirazoline-induced elevation of fine motor movements was not antagonized by ritanserin. Thus, it appears that the locomotor activity effects of DOI and cirazoline are also independent of the other transmitter system. Higher doses of prazosin have been suggested to reverse 5-HT2 receptor-mediated head shakes in mice, although given the significant reduction of movements with the prazosin alone even at the lower doses of our present study, one wonders if these were independent opposing actions rather than a true interaction-based reversal (Dursun and Handley 1996; Heal et al. 1986). Consistent with the present results, β receptor antagonists do not reverse head twitches induced by 5-HT2 receptor agonists (Darmani et al. 1991). Propranolol has been shown previously to partially reverse the locomotor effects of d-lysergic acid diethylamide, a nonspecific 5-HT agonist, but this effect was likely due to the well-known 5-HT1A receptor-blocking actions of propranolol (Mittman and Geyer 1991). To the best of our knowledge, this is the first study to investigate the effects of 5-HT2 receptor blockade on α1-mediated locomotor changes.
Our data demonstrated that within the dose ranges we tested, the α1 and β NE receptor antagonists prazosin and timolol did not reverse the PPI deficits or the increase in fine motor movements (stereotypies) induced by the 5-HT2 receptor agonist DOI, and that the 5-HT2 receptor antagonist ritanserin did not block effects induced by cirazoline, an α1 receptor agonist. Thus, stimulation of postsynaptic NE receptors is not necessary for 5-HT2 receptor-mediated effects, and conversely, 5-HT2 receptor stimulation is not required for α1-mediated effects. The neural substrates of these potentially independent effects on PPI are not clear right now. Forebrain regions including the prefrontal cortex, ventral striatum, amygdala, and hippocampus ultimately regulate PPI via descending projections to the pedunculopontine tegmentum, which converges directly onto the primary acoustic startle circuit (Davis et al. 1982; Fendt et al. 2001; Swerdlow et al. 2001). Thus, there are multiple sites within which NE and 5-HT receptors could influence PPI, and it may be that the mutual independence of DOI-and cirazoline-mediated effects seen presently could reflect differential distributions of α1, β, and 5-HT2 receptors across these PPI modulatory regions such that certain sites may subserve primarily NE-based PPI effects, and others 5-HT-based effects. Alternatively, separate PPI systems could exist despite the coexistence of 5-HT2 receptors and α1/β receptors in brain regions important for PPI. For example, within the nucleus accumbens, which contains both NE receptors and 5-HT2 receptors, stimulation of α1 plus β NE receptors disrupts PPI, but a 5-HT2 agonist does not (Alsene et al. 2011; Ma et al. 2006; Sipes and Geyer 1997). Therefore, despite the presence of both 5-HT2 receptors and α1/β receptors, there may be regions critical for PPI that are sensitive to manipulations of only one of these systems.
Taken together, these studies suggest that 5-HT2 receptors and α1 and β NE receptors may act independently of each other in the regulation of PPI. One might speculate that it is evolutionarily advantageous to have separate and perhaps redundant systems for PPI, since such redundancy would confer ‘protection’ from a loss of this crucial information processing mechanism if a problem occurred in one PPI regulatory system (other intact PPI systems might compensate and salvage PPI). Such a compensatory change has been reported for locomotor activity, with α1 NE receptor mediated effects ‘shifting’ to being transduced through 5-HT2 receptors when the α1 receptor gene is deleted (Auclair et al. 2004). In disorders with severe PPI disruptions, perhaps there is an abnormal function in multiple PPI-regulating systems. Indeed, second generation antipsychotics used to treat schizophrenia act as antagonists of multiple systems including dopamine, 5-HT, and NE, and they have superior efficacy in improving deficient PPI than the single target first generation antipsychotics like haloperidol (Kumari et al. 2007; Kumari et al. 1999; 2002; Leumann et al. 2002). Conversely, given the heterogeneity in the number and types of disorders with manifested PPI deficits (Braff et al. 2001; Geyer et al. 2001; Swerdlow et al. 2001), it may be that 5-HT2 receptor dysfunction contributes to some subset of these whereas NE receptor dysfunction contributes more to others. Regardless, the present findings could provide important insights into the etiology of information processing deficits in multiple disorders.
Acknowledgments
This work was supported by R01-MH075980 (VPB) and T32-GM007507 (SKB and AKR). The authors would like to thank Akaila Cabell for her technical assistance and Brian Baldo for his comments on the manuscript. Facilities and procedures complied with animal use and care guidelines from the National Institutes of Health of the USA, and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Footnotes
Conflict of interest The authors have full control of all primary data and agree to allow the journal to review their data if requested. None of the authors has any conflict of interest or financial arrangements pertaining to this work.
Contributor Information
Sarah K. Baisley, Department of Psychiatry, UW-Madison, Madison, WI, USA Neuroscience Training Program, UW-Madison, Madison, WI, USA.
Katherine L. Fallace, Department of Psychiatry, UW-Madison, Madison, WI, USA
Abha K. Rajbhandari, Department of Psychiatry, UW-Madison, Madison, WI, USA Neuroscience Training Program, UW-Madison, Madison, WI, USA.
Vaishali P. Bakshi, Email: vbakshi@wisc.edu, Department of Psychiatry, UW-Madison, Madison, WI, USA; Neuroscience Training Program, UW-Madison, Madison, WI, USA; Department of Psychiatry, University of Wisconsin School of Medicine and Public Health, 6001 Research Park Blvd, Madison, WI 53719, USA.
References
- Aghajanian GK. Mescaline and LSD facilitate the activation of locus coeruleus neurons by peripheral stimuli. Brain Res. 1980;186:492–498. doi: 10.1016/0006-8993(80)90997-x. [DOI] [PubMed] [Google Scholar]
- Aloyo VJ, Walker RF. Alpha-adrenergic control of serotonin release from rat pineal glands. Neuroendocrinology. 1988;48:61–66. doi: 10.1159/000124990. [DOI] [PubMed] [Google Scholar]
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacol-ogy. 2006;31:2150–2161. doi: 10.1038/sj.npp.1300989. [DOI] [PubMed] [Google Scholar]
- Alsene KM, Fallace K, Bakshi VP. Ventral striatal noradren-ergic mechanisms contribute to sensorimotor gating deficits induced by amphetamine. Neuropsychopharmacology. 2010;35:2346–2356. doi: 10.1038/npp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsene KM, Rajbhandari AK, Ramaker MJ, Bakshi VP. Discrete forebrain neuronal networks supporting noradrenergic regulation of sensorimotor gating. Neuropsychopharmacology. 2011;36:1003–1014. doi: 10.1038/npp.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Adell A, Bortolozzi A, Artigas F. Stimulation of alpha1-adrenoceptors in the rat medial prefrontal cortex increases the local in vivo 5-hydroxytryptamine release: reversal by antipsychotic drugs. J Neurochem. 2003;87:831–842. doi: 10.1046/j.1471-4159.2003.02044.x. [DOI] [PubMed] [Google Scholar]
- Andrade R, Barnes NM, Baxter G, Bockaert J, Branchek T, Cohen ML, Dumuis A, Eglen RM, Gothert M, Hamblin M, Hamon M, Hartig PR, Hen R, Herrick-Davis K, Hills R, Hoyer D, Humphrey PPA, Latté KP, Maroteaux L, Martin GR, Middlemiss DN, Mylecharane E, Peroutka SJ, Saxena PR, Sleight A, Villalon CM, Yocca F. 5-Hydroxytryptamine receptors, introductory chapter. IUPHAR database (IUPHAR-DB) 2010 [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b–adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Kester RC, Peeters BW, Broekkamp CL. Modulation of 5-HT receptor subtype-mediated behaviours by corticosterone. Eur J Pharmacol. 1996;308:103–111. doi: 10.1016/0014-2999(96)00286-5. [DOI] [PubMed] [Google Scholar]
- Blier P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci. 2001;26(Suppl):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- Blier P, Briley M. The noradrenergic symptom cluster: clinical expression and neuropharmacology. Neuropsychiatr Dis Treat. 2011;7:15–20. doi: 10.2147/NDT.S19613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci. 2010;4:349–371. doi: 10.1007/7854_2010_61. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea J, Castro M, Loza MI, Masaguer CF, Ravina E, Dezi C, Pastor M, Sanz F, Cabrero-Castel A, Galan-Rodriguez B, Fernandez-Espejo E, Maldonado R, Robledo P. QF2004B, a potential antipsychotic butyrophenone derivative with similar pharmacological properties to clozapine. Neuropharmacology. 2006;51:251–262. doi: 10.1016/j.neuropharm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Briody S, Boules M, Oliveros A, Fauq I, Richelson E. Chronic NT69L potently prevents drug-induced disruption of prepulse inhibition without causing tolerance. Behav Brain Res. 2010;207:118–124. doi: 10.1016/j.bbr.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Bond RA, Eikenburg DC, Hieble JP, Hills R, Minneman KP, Parra S. Adrenoceptors introductory chapter. 2009 [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuro-pharmacology. 1998;37:401–404. doi: 10.1016/s0028-3908(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Castagne V, Moser PC, Porsolt RD. Preclinical behavioral models for predicting antipsychotic activity. Adv Pharmacol. 2009;57:381–418. doi: 10.1016/S1054-3589(08)57010-4. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Baudrie V, Coupry I. Effects of chlorisondamine and restraint on cortical [3H]ketanserin binding, 5-HT2A receptor-mediated head shakes, and behaviours in models of anxiety. Neuropharmacology. 1994;33:449–456. doi: 10.1016/0028-3908(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Inhibition of 5-HT2 receptor-mediated head-twitch response by cocaine via indirect stimulation of adrenergic alpha 2 and serotonergic 5-HT1A receptors. Pharmacol Biochem Behav. 1991;38:353–357. doi: 10.1016/0091-3057(91)90290-i. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done CJ, Sharp T. Evidence that 5-HT2 receptor activation decreases noradrenaline release in rat hippocampus in vivo. Br J Pharmacol. 1992;107:240–245. doi: 10.1111/j.1476-5381.1992.tb14493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychophar-macology (Berl) 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA. Pre-attentive processing and schizophrenia: animal studies. Psychopharmacology (Berl) 2004;174:65–74. doi: 10.1007/s00213-003-1684-7. [DOI] [PubMed] [Google Scholar]
- Farid M, Martinez ZA, Geyer MA, Swerdlow NR. Regulation of sensorimotor gating of the startle reflex by serotonin 2A receptors. Ontogeny and strain differences. Neuropsychopharma-cology. 2000;23:623–632. doi: 10.1016/S0893-133X(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28:651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fone KC, Bennett GW, Marsden CA. Involvement of catecholaminergic neurones and alpha-adrenoceptors in the wet-dog shake and forepaw licking behaviour produced by the intrathecal injection of an analogue of thyrotrophin-releasing hormone (CG 3509) Neuropharmacology. 1987;26:1147–1155. doi: 10.1016/0028-3908(87)90261-9. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Pinkston JW, Vorontsova E. Clozapine and prazosin slow the rhythm of head movements during focused stereotypy induced by d-amphetamine in rats. Psychopharmacology (Berl) 2007;192:219–230. doi: 10.1007/s00213-007-0705-3. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox Res. 2008;14:71–78. doi: 10.1007/BF03033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, Hen R, Blier P. Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neuropsycho-pharmacol. 2007;17:328–338. doi: 10.1016/j.euroneuro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB. Corticotropin-releasing factor and noradrenergic signalling exert reciprocal control over startle reactivity. Int J Neuropsychopharmacol. 2010:1–16. doi: 10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Noradrenergic modulation of central serotonergic neurotransmission: acute and long-term actions of mirtazapine. Int Clin Psychopharmacol. 1995;10(Suppl 4):11–17. doi: 10.1097/00004850-199512004-00003. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, de Montigny C, Blier P. Modulation of the firing activity of noradrenergic neurones in the rat locus coeruleus by the 5-hydroxtryptamine system. Br J Pharmacol. 1997;120:865–875. doi: 10.1038/sj.bjp.0700968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin A, Morris K, Kelly JP, O’Donnell JM, Leonard BE. Modulation of MK-801-induced behaviour by noradrenergic agents in mice. Psychopharmacology (Berl) 2001;154:177–188. doi: 10.1007/s002130000630. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Philpot J, O’Shaughnessy KM, Davies CL. The influence of central noradrenergic function on 5-HT2-mediated head-twitch responses in mice: possible implications for the actions of antidepressant drugs. Psychopharmacology (Berl) 1986;89:414–420. doi: 10.1007/BF02412113. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Estival A, Ahlenius S. Evidence for specific involvement of 5-HT1A and 5-HT2A/C receptors in the expression of patterns of spontaneous motor activity of the rat. Eur J Pharmacol. 1996;295:155–161. doi: 10.1016/0014-2999(95)00666-4. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoyer D, Martin GR. Classification and nomenclature of 5-HT receptors: a comment on current issues. Behav Brain Res. 1996;73:263–268. doi: 10.1016/0166-4328(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychol Bull. 1983;94:3–17. [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kohnomi S, Suemaru K, Kawasaki H, Araki H. Effect of aripiprazole on 5-HT2 receptor-mediated wet-dog shake responses and disruption of prepulse inhibition in rats. J Pharmacol Sci. 2008;106:645–650. doi: 10.1254/jphs.fp0071924. [DOI] [PubMed] [Google Scholar]
- Kouhata S, Kagaya A, Nakae S, Nakata Y, Yamawaki S. Effect of acute lipopolysaccharide administration on (+/−)-1-(2,5-dime-thoxy-4-iodophenyl)-2 aminopropane-induced wet dog shake behavior in rats: comparison with body weight change and locomotor activity. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:395–407. doi: 10.1016/s0278-5846(00)00172-x. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuro-psychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Kagaya A, Uchitomi Y, Yokota N, Yamawaki S. Effect of interferon-alpha on DOI-induced wet-dog shakes in rats. J Neural Transm. 1996;103:947–955. doi: 10.1007/BF01291785. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Prepulse inhibition of the startle response in risperidone-treated patients: comparison with typical antipsychotics. Schizophr Res. 2002;55:139–146. doi: 10.1016/s0920-9964(01)00276-6. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsy-chotics. Int J Neuropsychopharmacol. 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol Psychiatry. 2002;52:729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- Ma J, Ye N, Cohen BM. Expression of noradrenergic alpha1, serotoninergic 5HT2a and dopaminergic D2 receptors on neurons activated by typical and atypical antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:647–657. doi: 10.1016/j.pnpbp.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Maier W, Mossner R, Quednow BB, Wagner M, Hurlemann R. From genes to psychoses and back: the role of the 5HT2alpha-receptor and prepulse inhibition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 5):40–43. doi: 10.1007/s00406-008-5011-5. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Activation of adenosine(1) (A(1)) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56:1082–1087. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Schreiber R, Dekeyne A, Rivet JM, Bervoets K, Mavridis M, Sebban C, Maurel-Remy S, Newman-Tancredi A, Spedding M, Muller O, Lavielle G, Brocco M. S 16924 ((R)-2-[1-[2-(2,3-dihydro-benzo[1,4] dioxin-5-yloxy)-ethyl]-pyrrolidin-3yl]-1-(4-fluoro-phenyl)-ethanone), a novel, potential antipsychotic with marked serotonin (5-HT)1A agonist properties: II. Functional profile in comparison to clozapine and haloperidol. J Pharmacol Exp Ther. 1998;286:1356–1373. [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology (Berl) 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007a;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. Eur Neuropsychopharmacol. 2007b;17:215–226. doi: 10.1016/j.euroneuro.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2 (DOI) Neurosci Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- Pupo AS, Minneman KP. Adrenergic pharmacology: focus on the central nervous system. CNS Spectr. 2001;6:656–662. doi: 10.1017/s1092852900001346. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res. 1986;385:395–400. doi: 10.1016/0006-8993(86)91090-5. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Höglund I, Engström M, Lehtimäki J, Virtanen R, Sirviö J, Wurster S, Savola JM, Haapalinna A. Pharmacological characterization and CNS effects of a novel highly selective alpha2C–adrenoceptor antagonist JP-1302. Br J Pharmacol. 2007;150:391–402. doi: 10.1038/sj.bjp.0707005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci USA. 2006;103:7476–7481. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Segal M. Serotonergic innervation of the locus coeruleus from the dorsal raphe and its action on responses to noxious stimuli. J Physiol. 1979;286:401–415. doi: 10.1113/jphysiol.1979.sp012628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. SR146131, a cholecystokinin-A receptor agonist, antagonizes prepulse inhibition deficits produced by dizocilpine and DOI. Psychopharmacology (Berl) 2002;164:285–293. doi: 10.1007/s00213-002-1214-z. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Melendez G, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT2A and an alpha1 agonist. Psychopharma-cology (Berl) 2004;175:353–359. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Dorsal raphe nucleus efferents: termination in peptidergic fields. Peptides. 1993;14:75–83. doi: 10.1016/0196-9781(93)90013-7. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J Neurochem. 2001;78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Ahsan R, Quartermain D. Gross mapping of alpha1-adrenoceptors that regulate behavioral activation in the mouse brain. Behav Brain Res. 2004;152:167–175. doi: 10.1016/j.bbr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. Marked behavioral activation from inhibitory stimulation of locus coeruleus alpha1-adrenoceptors by a full agonist. Brain Res. 2009;1291:21–31. doi: 10.1016/j.brainres.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67:193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bongiovanni MJ, Tochen L, Shoemaker JM. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette Syndrome. Psychopharmacology (Berl) 2006a;186:246–254. doi: 10.1007/s00213-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Shoemaker JM, Light GA, Braff DL, Stevens KE, Sharp R, Breier M, Neary A, Auerbach PP. Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and N40 suppression in rats. Neuropsychopharmacology. 2006b;31:506–515. doi: 10.1038/sj.npp.1300841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Beemster P, Schoffelmeer AN. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl) 2003;169:176–185. doi: 10.1007/s00213-003-1509-8. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology (Berl) 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Varty GB, Bakshi VP, Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague- Dawley and Wistar rats. Neuropsychopharmacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Wadenberg MG, Sills TL, Fletcher PJ, Kapur S. Antipsychotic-like effects of amoxapine, without catalepsy, using the prepulse inhibition of the acoustic startle reflex test in rats. Biol Psychiatry. 2000;47:670–676. doi: 10.1016/s0006-3223(99)00267-x. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Feldman S, Itzik A, Van de Kar LD, Newman ME. Evidence for a mutual interaction between noradrenergic and serotonergic agonists in stimulation of ACTH and cortico-sterone secretion in the rat. Brain Res. 2002;941:113–117. doi: 10.1016/s0006-8993(02)02641-0. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology (Berl) 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]