Abstract
Objectives
Oncologic outcomes of robotic cystectomy have focused on pathology and not on survival endpoints. We compared pathology, recurrence, and survival in a single surgeon series of open and robotic cystectomy since the introduction of robotic cystectomy.
Methods
We identified all patients treated by a single surgeon with radical cystectomy for urothelial cancer from June 2007 – June 2010. Clinical, demographic, and pathologic data was abstracted from chart review. Mortality was obtained from institutional cancer registry and chart review. Patients were excluded from analysis for a relative contraindication to robotic surgery. The remaining cohort of patients undergoing robotic (n=36) versus open (n=29) cystectomy with median follow-up 12.2 months were evaluated.
Results
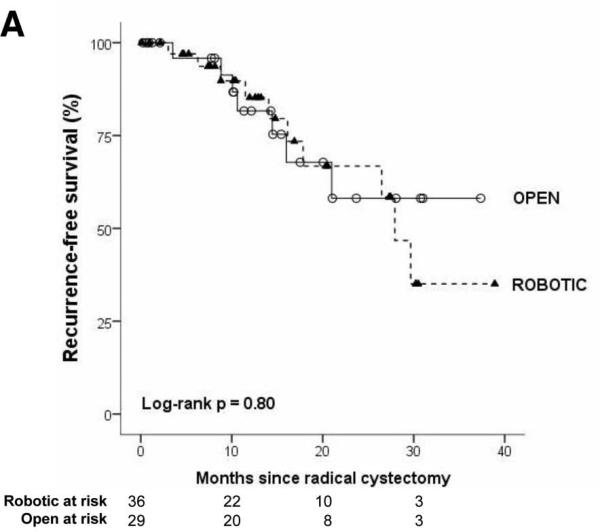
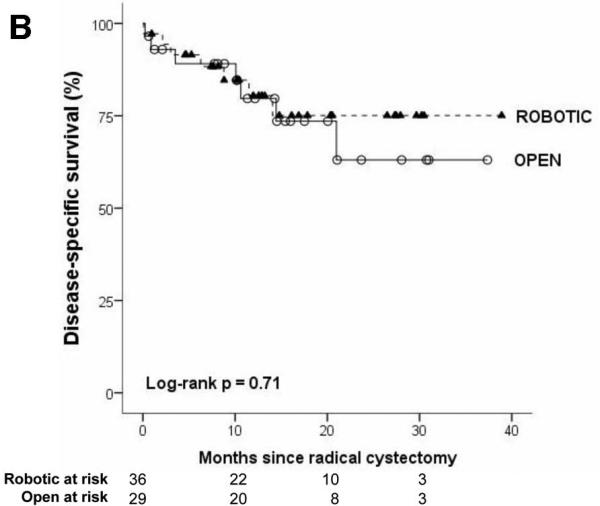
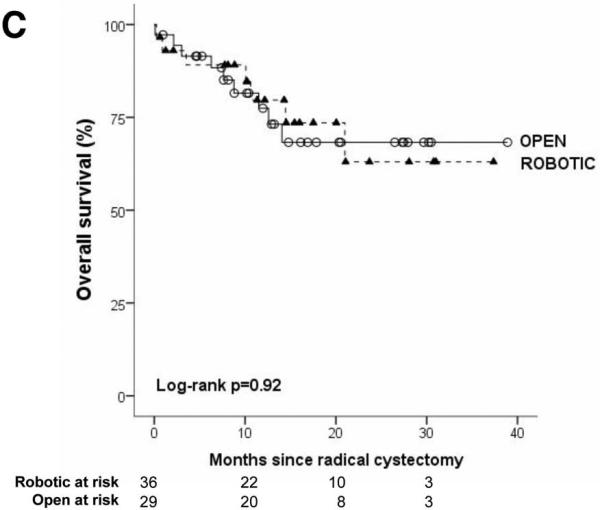
The robotic cohort was more likely to be older and male (p<0.05). Obesity, comorbidity, preoperative pathology, and receipt of neoadjuvant chemotherapy were not different between groups. Three patients had conversion from robotic to open cystectomy because of difficult dissection. Mean surgical time was longer in robotic cystectomy (410 vs. 345 minutes, p<0.01). Cystectomy pathology was not different for robotic versus open surgery for stage, margin status, or mean node count (robotic: 17.0, open: 15.5). On survival analysis which accounts for differences in follow-up time (Figure 1), robotic and open cystectomy outcomes were similar with respect to recurrence-free, disease-specific, and overall survival (all log-rank p values >0.05). The Kaplan-Meier estimate for 2-year outcome for recurrence-free, disease-specific, and overall survival was 67% (95% CI: 41-83), 75% (95% CI: 53-88), 68% (95% CI: 47-82) for robotic cystectomy and 58% (95% CI: 29-79), 63% (95% CI: 34-82), 63% (95% CI: 34-82) for open cystectomy
Conclusions
Short-term oncologic outcomes were similar for open and robotic cystectomy. Increased sample size and further follow-up are necessary before claiming equivalent long-term survival.
Keywords: carcinoma, transitional cell; urinary bladder neoplasms; cystectomy; robotics; comparative effectiveness research
Introduction
Robotic surgery has been increasingly utilized in urologic oncology.[1] The first experience with robotic cystectomy was reported in 2003 by Menon et al.[2] Since that time large multi-institutional studies have demonstrated acceptable immediate pathologic outcomes with respect to margin rate[3] and lymph node counts.[4] One limitation of these large collaborative series is the lack of a comparison with open cystectomy, the current gold standard for the surgical treatment of muscle invasive bladder cancer.[5]
The Institute of Medicine, based on a government mandate, recently established 100 research topics with the highest priority for comparative effectiveness research which included “compare the effectiveness of robotic assistance surgery and conventional surgery” in the second quartile.[6] The optimal comparison of open and robotic cystectomy would be a randomized trial. The only published randomized comparison of open and robotic cystectomy by Nix et al reported equivalent pathologic outcomes in a total of 41 patients, but did not report survival data.[7] Therefore, at the current time we are limited to analysis of observational data and there are no published reports of early oncologic outcomes comparing open to robotic approaches.
At our institution, robotic cystectomy was implemented in June 2007; however, open cystectomy has continued to be performed. In patients without a contraindication to robotic surgery, the choice of surgical approach was based primarily on robotic equipment availability which created two similarly sized cohorts. We thus evaluated a consecutive single surgeon contemporary series of open and robotic cystectomy in a cohort of patients who were all candidates for a robotic approach and compared pathology, recurrence, and survival.
Material and Methods
After obtaining institutional review board approval, we used the Barnes-Jewish Hospital Oncology Data Service cancer registry and billing codes to identify all patients with localized urothelial bladder cancer (n=74) treated by a single surgeon (ASK) with radical cystectomy between June 2007 and July 2010, a time period beginning with the introduction of robotic cystectomy at our institution. We selected all patients who were offered surgery by a robotic or open approach. Patients were excluded from analysis for a relative contraindication to robotic surgery (5 with prior radiation, 3 for prior pelvic surgery, 1 with BMI>50). The remaining cohort of patients had robotic (n=36) or open (n=29) surgical approach. All patients were offered both robotic and open approaches. The primary decision to proceed with robotic or open surgery was based on robot availability; however, patient preference was also a factor for some patients.
Preoperative demographic, clinical, and pathologic data was compared between groups to evaluate for selection bias. Medical comorbidity was assessed by Adult Comorbidity Evaluation Index-27 (ACE-27), a validated comprehensive comorbidity index developed and validated by Piccirillo et al[8], which assigns a comorbidity severity of none, mild, moderate, or severe based on the presence of 27 comorbid ailments.[9]
For the robotic approach, cystectomy and standard pelvic lymph node dissection were performed robotically and all urinary diversions were performed extracorporeally. Patients with concomitant procedure (robotic: 1 urethrectomy, 1 nephro-ureterectomy for carcinoma in situ; open: 1 nephro-ureterectomy for T1 urothelial cancer, 1 nephro-urethrectomy for nonfunctioning kidney) were not excluded.
Oncologic and survival data was evaluated and compared based on an intention to treat analysis with conversions from robotic to open included in the robotic group. There were 3 conversions to open because of difficult dissection. Postoperative follow-up and cause of death were obtained from the institutional cancer registry which examines social security death records and chart review. Patients were censored for recurrence type (local versus distant) at the time of first recurrence.
Patients undergoing robotic and open cystectomy were compared using Student's t-test for continuous data and chi square test for categorical variables with Fisher's exact test used for categorical data with sparse data. Oncologic outcomes assessed were cystectomy and lymph node dissection pathology, bladder cancer recurrence (local, distant), and disease-specific mortality. Recurrence and survival data was compared with Kaplan Meier survival analysis with log-rank test. P value of <0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.2 (Cary, NC) and SPSS 19 (Chicago, IL).
Results
Preoperative characteristics are compared in Table 1 for patients who underwent robotic (n=36) and open (n=29) cystectomy. The robotic group was more likely to be older and male. Obesity, medical comorbidity, preoperative clinical stage, and receipt of neoadjuvant chemotherapy were not different between groups.
Table 1.
Preoperative characteristics of patients undergoing radical cystectomy
| Robotic (n=36) | Open (n=29) | p value | |
|---|---|---|---|
| Gender (%) | <0.01 | ||
| Female | 5 (14) | 13 (45) | |
| Male | 31 (86) | 16 (55) | |
| Median age, years (IQR) | 72 (67-77) | 67 (57-79) | 0.04 |
| Median body mass index (IQR) | 27.7 (24.1-31.4) | 26.2 (22.6-29.0) | 0.07 |
| Comorbidity Index (%) | 0.18 | ||
| None | 9 (25) | 4 (14) | |
| Mild | 10 (28) | 14 (48) | |
| Moderate | 8 (22) | 8 (28) | |
| Severe | 9 (25) | 3 (10) | |
| Clinical stage (%) | 0.60 | ||
| CIS, TaHG | 5 (14) | 5 (17) | |
| T1 high grade | 6 (17) | 4 (14) | |
| T2 | 23 (66) | 20 (69) | |
| T4a | 2 (6) | 0 (0) | |
| Neoadjuvant chemotherapy (%) | 0.39 | ||
| Yes | 2 (6) | 4 (14) | |
| No | 34 (94) | 25 (86) |
IQR=Interquartile range
Ileal conduit was more common in the robotic group than in the open group (29/36 v. 18/29, p=0.10) and orthotopic neobladder was more commonly performed in the open group than in the robotic group (11/29 v. 6/36, p=0.052). One patient in the robotic group had a continent pouch urinary diversion. Mean surgical time (from incision to closure) was 410 minutes for robotic approach compared to 345 minutes for open (p=0.0004). When excluding cases with urethrectomy (n=1) or nephro-ureterectomy (n=3) mean surgical time was 60 minutes longer in robotic cystectomy than open (398 vs. 338 minutes, p=0.0002).
Blood loss was significantly less with the robotic approach (mean: 675cc vs. 1497cc, p=0.0002). The proportion receiving intraoperative blood transfusion was 39% (14/36) in robotic and 83% (24/29) in open (p=0.0004). In patients who received a blood transfusion, the mean number of units transfused was 2.1 units in robotic and 3.0 units in open (p=0.11). The mean length of hospital stay was shorter in the robotic approach but it did not reach statistical significance (7.9 days in the robotic group vs. 9.6 days in the open group, p=0.16).
Surgical pathology from cystectomy and receipt of adjuvant chemotherapy were not different for robotic versus open surgery (Table 2). The positive surgical margin rate for pathologic T2 or lower disease was 0% for both robotic and open cystectomy. Lymph node counts were similar for each approach. If the robotic conversions to open (n=3) are excluded because the node dissection was performed open, lymph node dissection performed robotically were similar to open (17.3 vs. 15.5, p=0.36). Two of the three conversions from robotic to open had extravesical and positive nodal disease on final pathology. The other conversion had a prior history of bladder perforation during transurethral resection at an outside institution and cystectomy pathology was T1 high grade with negative surgical margins and lymph nodes.
Table 2.
Oncologic outcomes of patients undergoing radical cystectomy
| Robotic (n=36) | Open (n=29) | p value | |
|---|---|---|---|
| Median lymph nodes examined (IQR) | 17 (12-20) | 14 (10-20) | 0.43 |
| Lymph node involvement (%) | 0.86 | ||
| Positive | 8 (22) | 7 (24) | |
| Negative | 28 (78) | 22 (76) | |
| Lymphovascular invasion (%) | 0.52 | ||
| Yes | 19 (53) | 13 (45) | |
| No | 17 (47) | 16 (55) | |
| Cystectomy pathology (%) | 0.46 | ||
| pT0 | 5 (14) | 1 (3) | |
| pCIS/Ta/T1 | 10 (28) | 12 (41) | |
| pT2 | 4 (11) | 4 (14) | |
| pT3 | 11 (31) | 6 (21) | |
| pT4a | 6 (17) | 6(21) | |
| Cystectomy margin (%) | 1.00 | ||
| Positive | 2 (6) | 2 (7) | |
| Negative | 34 (94) | 27 (93) | |
| Ureteral margin (%) | 1.00 | ||
| Positive | 3 (8) | 2 (7) | |
| Negative | 33 (92) | 27 (93) | |
| Adjuvant chemotherapy (%) | 0.38 | ||
| Yes | 8 (22) | 4 (14) | |
| No | 28 (78) | 25 (86) | |
| Median follow-up, months (IQR) | 12.3 (7.5-20.4) | 12.2 (8.1-20.0) | 0.85 |
| 2-year recurrence-free survival (95% CI) | 67% (41-83) | 58% (29-79) | 0.80 |
The median follow-up was 12 months in both treatment groups (mean: 14 months, IQR: 8-20 months). Recurrence in robotic and open approaches was 28% (10/36) and 24% (7/29), respectively. Local recurrence occurred in 11% (4/36) of robotic and 3% (1/29) of open. Distant recurrence was observed in 22% (8/36) of robotic and 21% (6/29) of open cystectomy patients. Two patients who underwent robotic approach recurred with concurrent local and metastatic disease. On survival analysis which accounts for differences in follow-up time (Figure 1), robotic and open cystectomy outcomes were similar with respect to recurrence-free, disease-specific, and overall survival (all log-rank p values >0.05). The Kaplan-Meier estimate for 2-year outcome for recurrence-free, disease-specific, and overall survival was 67% (95% CI: 41-83), 75% (95% CI: 53-88), 68% (95% CI: 47-82) for robotic cystectomy and 58% (95% CI: 29-79), 63% (95% CI: 34-82), 63% (95% CI: 34-82) for open cystectomy.
Figure.
Kaplan-Meier survival analysis of recurrence-free (1A), disease-specific (1B), and overall survival (1C) after robotic and open radical cystectomy.
Comment
The primary outcome of importance in extirpative surgery for bladder cancer is cancer control and subsequent survival. The relationship between high quality surgery and improved outcome in radical cystectomy has been an area of focus.[10] In an effort to establish standards for surgical quality, Herr and the Bladder Cancer Collaborative Group proposed standards for radical cystectomy and pelvic lymph node dissection of positive margin rate <10% and a median of 10 to 14 lymph nodes retrieved[11] One of the concerns with implementation of a new technology like robotic surgery is that patients may be harmed during the learning curve. However, at the start of robotic cystectomy at our institution, both robotic and open cystectomy met the pathology-based benchmarks for margin status and node dissection.
With respect to oncologic outcome, short-term pathologic outcomes have been the focus of most published robotic series, which have reported positive surgical margin rates of 0-8.6% from single institution data[2,12-16] and 6.8% in the multi-institutional IRCC[3]. Substantial concerns have existed over the ability to perform an adequate lymph node dissection robotically. However, reported series found median lymph node yield of 16-19 in single institution series[12-14,16] and 17 in the IRCC.[4] The possibility exists of publication bias against robotic series with less robust pathologic outcomes, but the available data thus far during early adoption of robotic cystectomy strongly supports adequate margin rate and node dissection with robotic cystectomy.
Recently, robotic cystectomy series have matured to allow reporting on the outcomes of recurrence and survival. Dasgupta et al reported 10% bladder cancer mortality in a series of 20 patients with a median follow-up of 23 months.[16] Pruthi et al reported on 100 consecutive robotic cystectomy cases. At a median follow-up of 18 months the cancer recurrence rate was 15% and 6% had died.[13] Hayn et al reported on their 164 robotic cystectomy cases, and at a median follow-up of 8 months death occurred in 27%.[15] In the robotic group in our series (median follow-up of 12.3 months) recurrence occurred in 28%, bladder cancer mortality was 19%, and all-cause mortality was 25%. Longer oncologic follow-up to five years and beyond is not yet available for robotic cystectomy, however, shorter term outcomes have been reported to be potential intermediate surrogates for 5-year survival.[17]
The performance of comparative effectiveness research, which has received increased attention by the Institute of Medicine, requires the presence of a comparison group. Our series evaluated pathologic outcomes, recurrence, disease-specific survival, and overall survival in a direct comparison to open cystectomy. We found the available oncologic outcomes were similar for robotic cystectomy and open surgery. One concern in the evaluation of a robotic cystectomy series is selection bias as surgeons whom institute the robotic approach may be more apt to choose younger and healthier candidates. Our series compared the oncologic outcomes of patients undergoing robotic and open cystectomy in groups with similar characteristics as the practice during this time was to offer robotic or open approach to patients without prior radiation or pelvic surgery. Even though this was not a prospective randomized comparison, the two groups in our study appeared to have similar preoperative characteristics with a trend toward less favorable characteristics in the robotic group.
To our knowledge, thus far other direct comparison studies of robotic and open cystectomy are limited to pathologic outcomes without subsequent follow-up data. Institutional non-randomized reports have included Wang et al[18] series (n=54; 33 robotic) and Richards et al[12] series (n=70; 35 robotic) which both reported similar pathologic outcomes. One series has performed prospective randomized comparison of robotic (n=21) versus open (n=20) cystectomy and this study reported similar pathologic outcomes without follow-up data reported.[7]
Our data showed no effect on oncologic outcomes of the initial portion of the learning curve during implementation of robotic cystectomy. Notably, the operating surgeon was an experienced urologic oncologist with experience in open cystectomy and robotic prostatectomy. Hayn et al evaluated a group of 21 surgeons performing robotic cystectomy and found that experienced robotic surgeons had higher lymph node yields while surgical margin status was not affected.[19]
Our experience thus far has shown similar oncologic outcomes of robotic and open cystectomy. The optimal choice of surgical approach remains unsettled. In our series, robotic surgery was associated with lower blood loss and transfusion but longer operative times and no statistically significant change in hospital stay. Other outcomes including cost, complication rate, postoperative recovery, functional outcomes, ureteral stricture rates, and patient satisfaction must also be considered when comparing techniques.
Limitations are inherent to this retrospective study. With respect to study design, we selected patients who were candidates for either approach to avoid bias against the open approach. Our robotic and open cystectomy groups appeared similar but a randomized trial of open versus robotic cystectomy would be the ideal study design to compare surgical techniques. Additionally, our study may be underpowered to detect small differences in outcomes, as evidenced by the wide confidence intervals on survival analysis. Larger sample sizes from multi-institutional cohorts are needed. Our goal was to evaluate all available comparable patients during a contemporary cohort to see if the early oncology outcomes indicated an inadequacy of the robotic approach, but we were unable to identify any differences in our cohort. Multivariate analysis would not be informative due to the lack of differences in our robotic and open cystectomy group.
Our patients had a median follow-up of 12 months due to the recent introduction robotic cystectomy. While this is limited follow-up, to our knowledge, ours is the first series to report survival outcomes in a series with open and robotic cystectomy performed by the same surgeon. Even in the case of robotic prostatectomy, which was adopted several years before robotic cystectomy, recent reports[20] of oncologic outcomes may still have median follow-up of less than a year. Although Sonpavde et al[17] reported that shorter term oncologic follow-up is an acceptable surrogate for five-year data, a longer follow-up period with a larger sample size preferably in the context of a randomized trial will be needed to establish equivalence.
Conclusions
Early oncologic outcomes were similar for robotic and open cystectomy. Increased sample size and further follow-up are necessary before claiming equivalent long-term survival.
Acknowledgement
Dr. Nepple is supported by a Washington University Comparative Effectiveness Research Mentored Career Development Award KM1 Grant (NIH Grant Number 1KM1CA156708-01)
Abbreviations
- IRCC
International Robotic Cystectomy Consortium
- IQR
Interquartile range
- CI
Confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med. 2010 Aug 19;363(8):701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 2.Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003 Aug;92(3):232–236. doi: 10.1046/j.1464-410x.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 3.Hellenthal NJ, Hussain A, Andrews PE, et al. Surgical margin status after robot assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2010 Jul;184(1):87–91. doi: 10.1016/j.juro.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Hellenthal NJ, Hussain A, Andrews PE, et al. Lymphadenectomy at the time of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. BJU Int. 2010 Jun 18; doi: 10.1111/j.1464-410X.2010.09473.x. [DOI] [PubMed] [Google Scholar]
- 5.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001 Feb 1;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 6.InstituteOfMedicine Initial national priorities for comparative effectiveness research. 2009 www.iom.edu/cerpriorities.
- 7.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010 Feb;57(2):196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004 May 26;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo JF. Web-based comorbidity calculator. http://oto2.wustl.edu/clinepi/comorbid.html.
- 10.Cooperberg MR, Birkmeyer JD, Litwin MS. Defining high quality health care. Urol Oncol. 2009 Jul-Aug;27(4):411–416. doi: 10.1016/j.urolonc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Herr H, Lee C, Chang S, Lerner S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004 May;171(5):1823–1828. doi: 10.1097/01.ju.0000120289.78049.0e. discussion 1827-1828. [DOI] [PubMed] [Google Scholar]
- 12.Richards KA, Hemal AK, Kader AK, Pettus JA. Robot assisted laparoscopic pelvic lymphadenectomy at the time of radical cystectomy rivals that of open surgery: single institution report. Urology. 2010 Dec;76(6):1400–1404. doi: 10.1016/j.urology.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM. Robotic radical cystectomy for bladder cancer: surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010 Feb;183(2):510–514. doi: 10.1016/j.juro.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DG, Challacombe BJ, Elhage O, et al. Robotic-assisted laparoscopic radical cystectomy with extracorporeal urinary diversion: initial experience. Eur Urol. 2008 Sep;54(3):570–580. doi: 10.1016/j.eururo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Hayn MH, Hellenthal NJ, Seixas-Mikelus SA, et al. Is patient outcome compromised during the initial experience with robot-assisted radical cystectomy? Results of 164 consecutive cases. BJU Int. 2010 Dec 16; doi: 10.1111/j.1464-410X.2010.09904.x. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta P, Rimington P, Murphy D, et al. Robotic assisted radical cystectomy: short to medium-term oncologic and functional outcomes. Int J Clin Pract. 2008 Nov;62(11):1709–1714. doi: 10.1111/j.1742-1241.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 17.Sonpavde G, Khan MM, Lerner SP, et al. Disease-Free Survival at 2 or 3 Years Correlates With 5-Year Overall Survival of Patients Undergoing Radical Cystectomy for Muscle Invasive Bladder Cancer. J Urol. 2010 Dec 16; doi: 10.1016/j.juro.2010.09.110. [DOI] [PubMed] [Google Scholar]
- 18.Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008 Jan;101(1):89–93. doi: 10.1111/j.1464-410X.2007.07212.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayn MH, Hellenthal NJ, Hussain A, et al. Does previous robot-assisted radical prostatectomy experience affect outcomes at robot-assisted radical cystectomy? Results from the International Robotic Cystectomy Consortium. Urology. 2010 Nov;76(5):1111–1116. doi: 10.1016/j.urology.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Barocas DA, Salem S, Kordan Y, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol. 2010 Mar;183(3):990–996. doi: 10.1016/j.juro.2009.11.017. [DOI] [PubMed] [Google Scholar]