Abstract
We previously showed that NF-κB inactivation in astrocytes leads to improved functional recovery following spinal cord injury (SCI). This correlated with reduced expression of pro-inflammatory mediators and chondroitin sulphate proteoglycans, and increased white matter preservation. Hence we hypothesized that inactivation of astrocytic NF-κB would create a more permissive environment for axonal sprouting and regeneration. We induced both contusive and complete transection SCI in GFAP-IκBα-dn and WT mice and performed retrograde (fluorogold) and anterograde (biotinylated dextran amine) tracing eight weeks after injury. Following contusive SCI, more fluorogold-labeled cells were found in motor cortex, reticular formation, and raphe nuclei of transgenic mice. Spared and sprouting biotinylated dextran amine-positive corticospinal axons were found caudal to the lesion in GFAP-IκBα-dn mice. Higher numbers of fluorogold-labeled neurons were detected immediately rostral to the lesion in GFAP-IκBα-dn mice, accompanied by increased expression of synaptic and axonal growth-associated molecules. After transection, however, no fluorogold-labeled neurons or biotinylated dextran amine-filled axons were found rostral and caudal to the lesion, respectively, in either genotype. These data demonstrated that inhibiting astroglial NF-κB resulted in a growth-supporting terrain promoting sparing and sprouting, rather than regeneration, of supraspinal and propriospinal circuitries essential for locomotion, hence contributing to the improved functional recovery observed after SCI in GFAP-IκBα-dn mice.
Keywords: transgenic mice, astrocytes, retrograde tracing, anterograde tracing, GAP-43, neuroprotection
INTRODUCTION
After SCI, the first traumatic phase of mechanical damage to the spinal cord is immediately followed by a phase of secondary injury due to local disturbance of the blood supply and inflammation, which lead to permanent impairment of motor, sensory and autonomic functions (Bethea et al. 1999; Schwab 2002; Hagg and Oudega 2006; Maier and Schwab 2006). Such an outcome can be ultimately attributed to the failure of damaged axons to regenerate and the lack of self-renewal properties of the spinal cord nervous tissue, which prevents the formation of axonal circuits involved in locomotor function.
The clinical outcome of SCI can be improved by limiting the extent of secondary tissue damage, which is largely dependent on the aggressive inflammatory response mounted simultaneously by peripheral leukocytes infiltrating the CNS and resident astrocyte and microglia activation (Bethea 2000; Fleming et al. 2006; Trivedi et al. 2006; Donnelly and Popovich 2008). We previously demonstrated that activated astrocytes play a critical role in this process as they synthesize and release pro-inflammatory mediators (e.g. cytokines, chemokines, adhesion molecules) through the sustained activation of the transcription factor NF-κB (Bethea et al. 1998; Brambilla et al. 2005; Brambilla et al. 2009). Indeed, by inhibiting the activation of NF-κB specifically in astrocytes (GFAP-IκBα-dn transgenic mice) the inflammatory response is reduced, leading to significant improvement in locomotor recovery (Brambilla et al. 2005). Furthermore, in these transgenic mice white matter preservation after SCI is increased compared to WT mice (Brambilla et al. 2005), indicating that the improved functional outcome might be correlated with a higher number of spared axons extending through the intact white matter and, possibly, with a degree of regeneration or regenerative sprouting.
Regenerative sprouting is a spontaneous growth response of CNS neurons in reaction to injury and is characterized by the up-regulation of immediate early genes, such as c-Jun, c-Fos (Jenkins et al. 1993; Vaudano et al. 1998; Hayashi et al. 2000), and the growth-associated protein GAP-43 (Tetzlaff et al. 1991; Li et al. 1996; Di Giovanni et al. 2005), which are typically expressed in developing neurons. Re-expression of these molecules following trauma enhances the growth potential of damaged neurons. However, the concomitant activation of astrocytes secreting scar-forming and growth-inhibitory molecules, such as chondroitin sulphate proteoglycans (CSPGs), counteracts the potential effects of the increased expression of growth-associated molecules, thereby limiting the capacity of axonal re-growth and sprouting (Busch and Silver 2007). As proof of this concept, treatments aimed at preventing the synthesis (deoxyribozyme against XT-1) or cleaving (chondroitinase) the glycosaminoglycan chains from the CSPG protein core have been shown to facilitate axonal growth and regeneration in various experimental SCI models (Bradbury et al. 2002; Fouad et al. 2005; Barritt et al. 2006; Houle et al. 2006; Massey et al. 2006; Crespo et al. 2007; Hurtado et al. 2008).
We previously demonstrated that inhibition of NF-κB activation in astrocytes, in addition to reducing inflammation, highly diminishes the presence of CSPGs following injury (Brambilla et al. 2005), therefore we now hypothesize that constitutive inactivation of the NF-κB pathway attenuates the non-permissive properties of reactive astrocytes, leading to an increased ability of neurons to sustain injury and regenerate their axons. In order to directly address this point, we performed anterograde and retrograde tracing experiments on WT and GFAP-IκBα-dn transgenic mice 8 weeks after contusion or complete transection of the spinal cord. Our findings indicate that, compared to WT mice, GFAP-IκBα-dn transgenic mice exhibit increased sparing of corticospinal, reticulospinal and raphespinal axons, in addition to sprouting of corticospinal and, possibly, raphespinal axons. Furthermore, GFAP-IκBα-dn mice show sparing of propriospinal axons from spinal cord segments immediately rostral to the lesion, as well as increased gene expression, in the same area, of synaptic and axonal growth-associated molecules (SNAP25, synaptotagmin and GAP-43), indicating sparing as well as sprouting of propriospinal projections.
MATERIALS AND METHODS
Animals
GFAP-IκBα-dn mice were generated at the Transgenic Core Facility of the University of Miami, as previously published (Brambilla et al. 2005). All mice used in our experiments (total of 15 WT and 15 GFAP-IκBα-dn female mice for spinal cord contusion, and 6 WT and 6 GFAP-IκBα-dn female mice for spinal cord transection) were 3–4 months old, obtained by breeding heterozygous GFAP-IκBα-dn males with WT females. WT littermates from the same breeding were used as controls. All animals were housed in a 12 hr light/dark cycle in a virus/antigen free facility with controlled temperature and humidity and provided with water and food ad libitum.
Spinal cord contusion and transection
Contusion injuries and complete transection injuries were performed in the Animal and Surgical Core Facility of the Miami Project to Cure Paralysis according to protocols approved by the Institutional Animal Care and Use Committee of the University of Miami. Contusion injury was induced with the Electromagnetic Spinal Cord Injury Device (ESCID) adapted to the mouse (Jakeman et al. 2000). Briefly, in adult female mice (3–4 months old, 20–24 g weight) a laminectomy was performed at the T9 vertebral level to expose the underlying spinal cord. The tip of the contusion device was then lowered onto the spinal cord until a force of 2 kDyn was recorded on the force transducer. Approximately 2 seconds after this pre-load force was reached, the mice were injured by a rapid displacement of the impounder (0.5 mm, moderate injury), the muscles and skin were sutured, and the mice returned to their individual cages. In the transection injury, the exposed spinal cord was completely transected at the T9 level with surgical scissors and a suture thread was passed through to ensure the full severance of the spinal cord tissue. Mice were prepared and cared for after the procedure as for the contusion injury paradigm. After surgery, the mice were treated with topical antibiotics and subcutaneous Ringer’s solution to replace lost fluids. Manual bladder expression was performed twice a day. Prophylactic antibiotic (Gentocin; 50–80 mg/day) was administered daily for one week post-injury to prevent urinary tract infections.
Retrograde neuronal tracing
At 8 weeks after contusion or transection, the mice were injected with 0.2 μl 3% aqueous fluorogold (FG; Fluorochrome LLC, Denver, CO) about 5 mm caudal to the contusion or transaction epicenter (in between vertebra T11 and T12). Injections of 0.1 μl FG were made at 0.5 mm depth and bilaterally to the midline to avoid injecting FG into the central canal. A pulled glass needle fixed to a 1 μl Hamilton syringe attached to a micromanipulator was used. All tracer injections were performed by the same individual. The muscles and skin were closed and the mice returned to their cages for an additional 2 weeks. Nine mice/group were used in the contusion study, and 6 mice/group in the transection study.
Anterograde tracing of corticospinal tract axons
Mice were injected with 10% biotinylated dextran amine (BDA; Molecular Probes) in both cerebral cortices at 8 weeks after contusion or transection. A total of 0.2 μl was injected into each cortex using a 1 μl Hamilton syringe with a pulled glass needle (tip diameter = 100 μm) attached according to a protocol described previously (Oudega et al. 1999). All tracer injections were performed by the same individual. The skin was closed and the mice returned to their cages for an additional 2 weeks. The anterograde tracing was performed simultaneously on the same animals (9 mice/group in the contusion study; 6 mice/group in the transection study) used for retrograde tracing with FG.
Histological procedures and immunohistochemistry
Animals were fixed by transcardial perfusion with normal saline followed by ice-cold 4% paraformaldehyde in 0.1 M PBS. After perfusion, spinal cords and brains were dissected and post-fixed overnight in the same fixative. The next day, the tissues were cryoprotected in 0.1 M PBS + 30% sucrose. Tissue blocks, both brains and spinal cords, were embedded in gelatin and cut longitudinally along the horizontal plane (40 μm thickness) into 6 series using a freezing microtome, as described previously (Oudega and Hagg 1996). Series were kept in phosphate buffer containing 0.1% sodium azide at 4°C until further processing. Two series of sections were set aside for FG counting (see below). Two series were used for BDA-labeling and quantification (see below). The remaining series were mounted on glass slides, incubated overnight at 4°C with antibodies against GFAP (1:1,000; Dako), CD11b (1:500; Serotec), GAP-43 (1:1,000; GeneTex) and 5-HT (1:5,000; Immunostar), followed by secondary species-specific fluorescent antibodies (Alexa Fluor 488 and 594, 1:750; Invitrogen) for 1h at room temperature. Slides were then covered with glass slips using Vectashield mounting medium with DAPI (Vector, Burlingame) and micrographs were taken with a Zeiss Axiovert 200M fluorescence microscope. Nine mice/group were used in the contusion study, and 6 mice/group in the transection study.
Quantitative assessment of anterogradely labeled CST axons and axonal dieback
Estimated total numbers of BDA-labeled CST axons were calculated as follows. Serial longitudinal sections of the spinal cord were incubated overnight with streptavidin-Alexa Fluor 488 (1:500, Invitrogen) for detection of BDA-labeled axons, and with anti-GAP43 followed by species-specific AlexaFluor 594 secondary antibody. Sections were then visualized with a 63X oil immersion objective and the perimeter of the lesion area outlined (Stereoinvestigator, MicroBrightField). At the caudal margin of the lesion area a fractionator grid of 250 μm2 intervals was placed. All BDA-labeled fibers crossing the grid at a distance of 0, 250, 500, 750, 1000, 1500, 2000 and 2500 μm from the caudal margin of the lesion were separately counted by placing a 5-μm2 dissector probe at the level of the 0, 250, 500, 750, 1000, 1500, 2000 and 2500 μm vertical lines of the grid (optical fractionator method, (West and Collins 1989). Additionally, BDA-positive fibers double-labeled with GAP-43 were separately counted using a different probe marker. On the same images, the maximum axonal dieback was assessed by measuring of the distance of the rostral most endbulb from the rostral margin of the lesion area using a ruler tool.
Fluorogold counting
Counting of FG-labeled neurons was performed according to a method previously described (Lo et al. 2009). Briefly, two series of sections were separately mounted on glass slides and coverslipped with Vectashield mounting medium with DAPI. In the spinal cord, each FG-positive cell up to 10 mm rostral to the injury site was counted in every third section and the total number was calculated by multiplying the cell count by 3 to account for all the series. In the brain, every FG-positive cell was counted and classified into specific areas or nuclei of the brain, following anatomic guidelines set by an atlas of the C57BL/6 mouse brain. The sum of the counts obtained from all those areas was defined as “whole brain” FG counts. Results from the two series were averaged and then multiplied by 6 to account for the six series obtained from each tissue sample. The Abercrombie formula (Abercrombie and Johnson 1946) was applied to correct for overcounting: section thickness/(section thickness + mean diameter of the counted object). For this formula, the average diameter of 20 FG-positive neurons in each neuroanatomical region was recorded. Throughout the whole experiment, the FG-labeled cells were counted by the same investigator blinded to the animal genotype. Nine animals per experimental group were used for this experiment.
Total RNA isolation and real time RT-PCR
Total RNA was extracted with TRIZOL® (Invitrogen). Reverse transcription was performed with Superscript II (Invitrogen), according to manufacturer’s instructions. cDNA equal to 10–50 ng of total RNA was used as template in each PCR reaction. Real time PCR was performed in the Rotor-Gene 3000 Real Time Cycler (Corbett Research) with TAQurate GREEN Real-Time PCR MasterMix (Epicentre Biotechnologies). Relative expression was calculated by comparison with a standard curve after normalization to β-actin. The primers for gene amplification are as follows: SNAP25 Forward 5′ gcaataatcaggatggagtagtgg 3′, SNAP25 Reverse: 5′ cattttcccgggcatcgtttgtta 3′; Synaptotagmin XI Forward 5′ gggcctccgtgctggtggtgt 3′, Synaptotagmin XI Reverse 5′ tggcggggtcttgtgcttcttctc 3′; GAP-43 Forward 5′ taccactgataactccccgtcctc 3′, GAP-43 Reverse 5′ ccttggtggcagcatcctct 3′. Six animals per experimental group were used for this experiment.
Statistical analysis
Real time PCR and cell number data were analyzed with one-way ANOVA followed by Tukey test for multiple comparisons. For single comparisons, Student’s T test was applied. With a P value equal to or less than 0.05 results were considered significantly different.
RESULTS
Reduced astrocytic activation in GFAP-IκBα-dn transgenic mice 10 weeks after contusive SCI
Ten weeks after the induction of moderate contusive SCI, histological analysis was conducted on longitudinal sections of the spinal cord in both WT and transgenic mice. Astroglial and microglial activation was evaluated by double immunolabeling with the astrocyte-specific marker GFAP in combination with the microglia/macrophage-specific marker CD11b. In order to assure the accuracy of the analysis, we immunolabeled the same series for each animal (specifically, series #2 out of the 6 series collected) and took photomicrographs of matching sections, located at the same depth into the spinal cord. Additionally, we measured the distance from the bottom of the lesion area and compared images taken 1) at the interface between the lesion and the tissue below (Fig. 1A and D), and 2) at the distance of 3 mm caudally to the bottom margin of the lesion. In the immediate proximity of the lesion site both WT and transgenic mice displayed high levels of astroglial and microglial activation as indicated by strong GFAP and CD11b immunoreactivity (Fig. 1A and D). The injury was clearly defined by a surrounding glial scar in both genotypes and was filled with large CD11b-positive macrophages. Caudally from the injury site, the immunoreactivity for GFAP and CD11b decreased, with a more robust reduction, particularly for GFAP, in the transgenic mice compared to WT (Fig. 1B, C, E and F). This finding is relevant, since a reduced reactive gliosis may create an environment more favorable to axonal survival and regeneration, which could explain the improved functional outcome observed in transgenic mice compared to WT (Brambilla et al., 2005).
Figure 1. Immunostaining for GFAP and CD11b in spinal cords of WT and GFAP-IκBα-dn mice 10 weeks after contusive SCI.
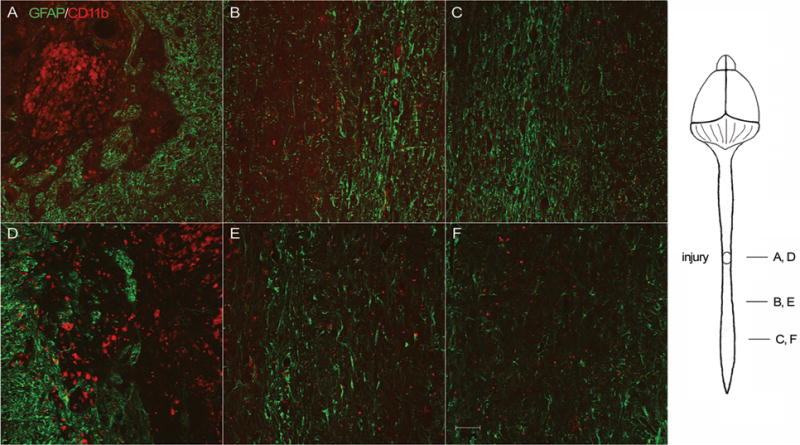
Longitudinal spinal cord sections of WT (A, B, C) and GFAP-IκBα-dn (D, E, F) mice, cut along the horizontal plane, were double labeled for GFAP (green) and CD11b (red). Micrographs were taken at the lesion site (A, D), immediately below (B, E), and caudally to the lesion site (C, F). Scale bar: 50 μm.
GFAP-IκBα-dn mice have higher numbers of FG-labeled neuronal cell bodies in the spinal cord and brain 10 weeks after contusive SCI
In order to evaluate whether transgenic mice demonstrated increased axonal sparing or sprouting compared to WT, we performed retrograde tract tracing via bilateral injection of FG caudally to the injury site 8 weeks after spinal cord contusion (Fig. 2A), and let the animals survive for an additional 2 weeks. Caution was used to avoid the injection of FG into the central canal. Indeed, the needle was small enough to allow for easy manipulation and insertion into the spinal cord away from the midline. This was confirmed using the surgery microscope during the injection procedure. Furthermore, absence of FG leakage was assessed in the mounted cryostat sections of the spinal cord and brain prior to counting. Representative images of spinal cord and brain FG-positive neurons are displayed in Fig. 2A. At week 10, 2 weeks after injection of the tracer, the number of FG-labeled neurons was counted in the spinal cord segments immediately rostral to the lesion and in the brain. FG is retrogradely transported along the axon to the cell body, thus only neurons with a viable axon that runs uninterrupted through the injury site will be backfilled with the tracer. Consequently, the counting of FG-labeled neurons provides a quantitative measure of spared and/or regenerated axons. In addition, counting the FG-labeled cells in individual nuclei in the brainstem allows the identification of the responding descending axonal tracts. In both spinal cord (Fig. 2B) and whole brain (Fig. 2C) the number of FG-positive cell bodies was significantly higher in GFAP-IκBα-dn mice compared to WT (spinal cord: WT = 768±262, GFAP-IκBα-dn = 1792±391; brain: WT = 4302±872, GFAP-IκBα-dn = 7950±1345; P<0.05, Student’s T test). Furthermore, when counted separately, the areas of the brain where transgenic mice showed a significantly higher number of FG-positive cells were the motor cortex, the reticular formation, and the raphe nucleus (Fig. 2D, E and F), where descending tracts important for locomotion have their origin, namely the corticospinal tract (CST), reticulospinal tract and raphespinal tract. The presence of a higher number of FG-positive cells in the spinal cord demonstrates that, in addition to sparing of supraspinal projections, GFAP-IκBα-dn mice exhibit significant sparing of propriospinal axons, which are known to play an important role in reflex control and coordination during locomotion. These data demonstrate that the inhibition of NF-κB in astroglial cells establishes more favorable conditions for axonal sparing, and possibly sprouting.
Figure 2. Determination of FG-labeled neurons following retrograde tracing with FG in WT and GFAP-IκBα-dn mice 10 weeks after contusive SCI.
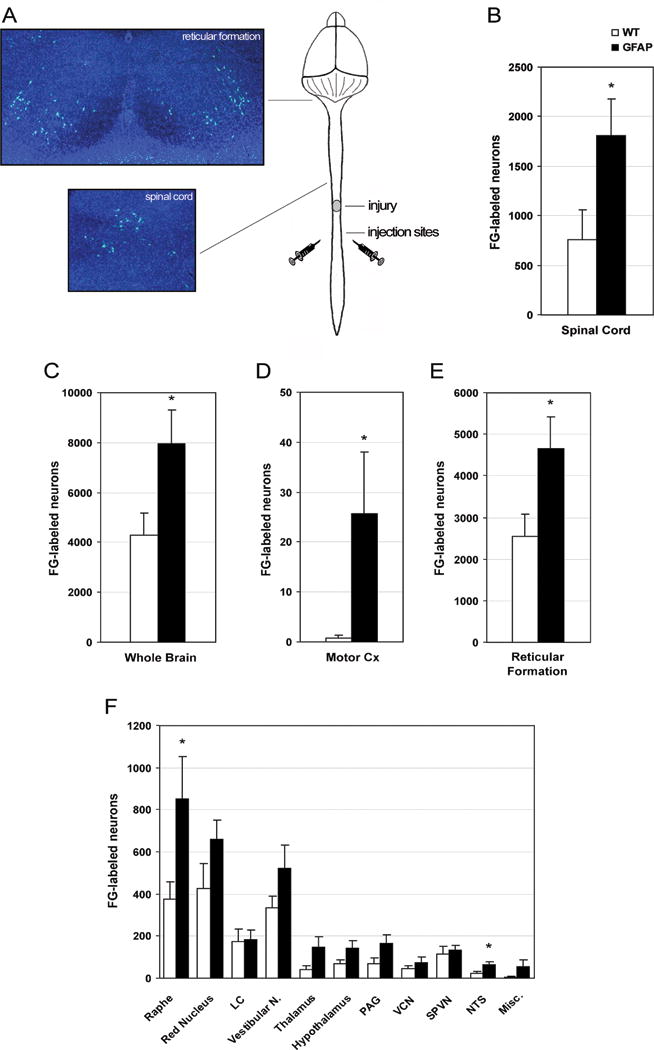
(A) Schematic representation of the FG injection sites located bilaterally to the midline 3 mm below the injury site. Micrographs represent FG-positive cells in the spinal cord and reticular formation of the brain stem. Panels B and C summarize the total number of FG-positive cell bodies counted in spinal cord (B) and whole brain (C). A brakedown of FG-positive counts in the various nuclei and regions of the brain is provided in panels D, E and F. *P<0.05, Student’s T test. N= 9 per group. LC: locus ceruleus; PAG: periaqueductal gray matter; VCN: vestibulocerebellar nucleus; SPVN: spinal vestibular nucleus; NTS: nucleus of the solitary tract; Misc: miscellaneous.
GFAP-IκBα-dn mice show sparing and sprouting of CST axons 10 weeks after contusive SCI
In order to evaluate sparing and potential sprouting/regeneration of CST axons, anterograde tracing with bilateral injection of BDA in the motor cortex was performed. BDA is incorporated by the neurons in the motor cortex and anterogradely transported along their axons, which travel without interruptions to the spinal cord. The numbers of BDA-labeled fibers present caudally to the lesion area, as well as the numbers of BDA-labeled fibers double positive for GAP-43, were quantified. In order to assure that the efficiency of BDA labeling was equal between WT and transgenic mice, the total number of BDA-labeled axons was quantified in each animal in cross sections of the spinal cord at the cervical level, well above the distance of maximum CST axon die back. Since no difference in the numbers of BDA-labeled axons was found between the two genotypes (WT= 240.12 ± 32.7, GFAP-IκBα-dn = 266.10 ± 23.2, Student’s T test), we proceeded to the quantification of BDA-labeled fibers below the injury, confident that this measurement would not be affected by experimental artifacts. Following contusion injury, an absence of BDA-labeled fibers was observed in WT mice below the lesion site (Fig. 3A, Table 1), as virtually all axons died back from the lesion. Significant die back was observed in both genotypes, going as far as 3.2 mm rostral to the injury (WT5, Table 1). However, unlike in WT mice, spared CST axons, capable of traveling through the lesion and remaining viable past the lesion site, were found in GFAP-IκBα-dn mice (Fig. 3B–E).
Figure 3. Fluorescent labeling of CST axons following anterograde tracing with BDA of WT and GFAP-IκBα-dn spinal cords 10 weeks after contusive SCI.
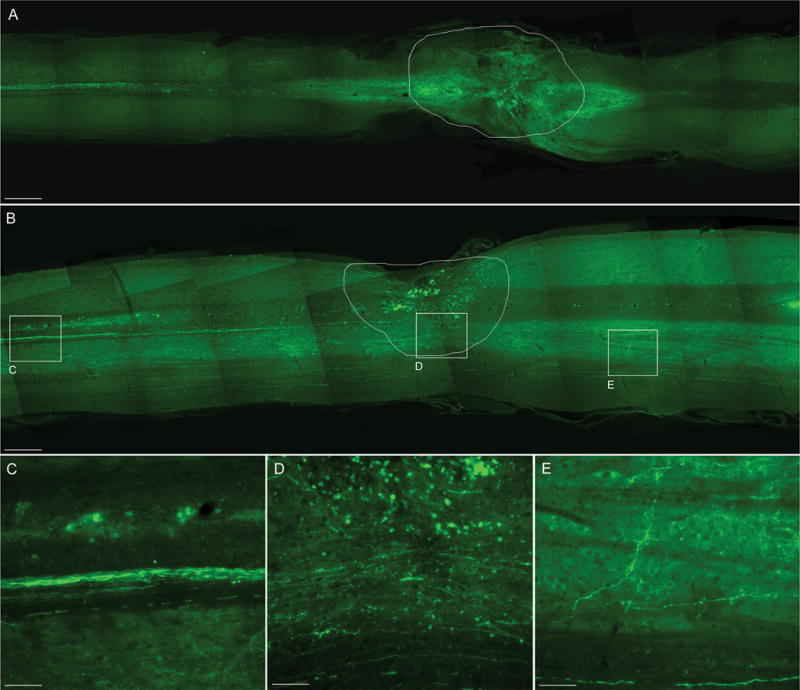
CST axons which anterogradely transported BDA were identified by fluorescent labeling with streptavidin conjugated with Alexa-488 in longitudinal sections of the spinal cord cut along the horizontal plane. WT mice (A) did not show any labeled axon below the injury site, contrary to GFAP-IκBα-dn mice (B), that showed a significant presence of labeled CST fibers through and below the lesion site (C, D, E). The contour in A and B delineates the lesion area. Scale bars: A, B = 500 μm. C, D, E = 80 μm
Table 1. Quantification of BDA-labeled and GAP-43-double-labeled fibers 10 weeks after contusion injury in WT and GFAP-IκBα-dn mice.
In each single animal (n=6 per experimental group), the numbers of BDA-labeled and GAP-43-double-labeled (in parenthesis) fibers were quantified at increasing distances from the caudal end of the lesion site. The maximum die back distance from the rostral margin of the lesion site was also measured. The data are compared to the corresponding numbers of FG-labeled neurons quantified in the motor cortex of each single animal. TG: transgenic animals.
| Animal # | FG neurons in cx | BDA-labeled fibers (in parenthesis GAP43 double-labeled fibers) Distance from injury (μm) | Max die back (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 250 | 500 | 750 | 1000 | 1500 | 2000 | 2500 | |||
| WT 1 | 0 | 0 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 3.1 |
| WT 2 | 5.4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| WT 3 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.6 |
| WT 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| WT 5 | 1.5 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2.6 |
| WT 6 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 3.2 |
| TG 1 | 34.6 | 12 | 14 (2) | 36 (5) | 15 | 25 (3) | 7 (2) | 7 (2) | 16 (3) | 2.1 |
| TG 2 | 11.5 | 6 | 12 | 18 | 3 | 9 (3) | 5 (1) | 3 (1) | 1 (1) | 2.3 |
| TG 3 | 6.1 | 1 | 3 | 15 | 6 | 5 | 8 | 0 | 0 | 2.5 |
| TG 4 | 3.1 | 0 | 0 | 6 | 7 | 7 (2) | 7 (2) | 7 (2) | 3 | 2.6 |
| TG 5 | 1.5 | 0 | 1 | 9 | 5 | 2 (1) | 2 (1) | 0 | 0 | 2.5 |
| TG 6 | 0 | 0 | 0 | 4 | 2 | 5 (1) | 2 | 0 | 0 | 3.0 |
When we counted the numbers of BDA-labeled fibers at various distances below the lesion, we found that in most transgenic animals (Table 1, TG 2 through TG 6) their numbers far exceeded the total number of FG-labeled neurons measured in the motor cortex of the same animal, particularly at a distance of 500 μm from the caudal extent of the lesion (Table 1). Some BDA-labeled fibers were found as far as 2.5 mm caudal to the lesion. This suggests that, in addition to sparing, a certain degree of sprouting is occurring in the GFAP-IκBα-dn mice, a phenomenon that was not observed in WT mice (Table 1). To further support this conclusion, a subset of such BDA-labeled fibers caudal to the lesion was also found to be positive for GAP-43, a protein that in the adult nervous system is generally expressed in axons undergoing sprouting and/or regeneration (Campbell et al. 1991; Benowitz and Routtenberg 1997; Gupta et al. 2006) (Fig. 4, Table 1). These results are particularly relevant since CST fibers, which are highly susceptible to degeneration following injury, innervate the motor neurons located in the ventral horns of the spinal cord and are therefore involved in proper hindlimb locomotor function. This might explain, at least in part, the significant functional improvement observed in transgenic mice compared to WT.
Figure 4. Co-labeling of BDA-positive CST axons with GAP-43 in GFAP-IκBα-dn spinal cords 10 weeks after contusive SCI.
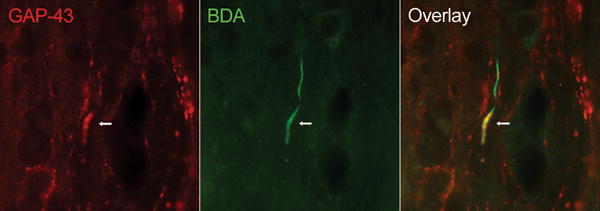
The high power micrograph shows the presence of CST fibers (green; BDA/Streptavidin-Alexa 488), which co-express the growth-associated molecule GAP-43 (red) far below the lesion site in GFAP-IκBα-dn mice. No double-labeled fibers were found in WT mice. Scale bar: 20 μm.
GFAP-IκBα-dn mice show sparing and possible sprouting of serotonergic raphespinal axons 10 weeks after contusive SCI
The presence of serotonergic raphespinal axons in longitudinal spinal cord sections was evaluated using 5-HT immunostaining. In both WT and transgenic animals 5-HT-positive axons were clearly identified in the ventrolateral funiculi above the lesion 10 weeks after SCI (Fig. 5A, B, E and F). In WT mice, the majority of those axons were found rostral to the lesion, and only a few caudal (Fig. 5D). Conversely, in GFAP-IκBα-dn mice numerous 5-HT-positive axons were found at (Fig. 5G, H) and far below the lesion (Fig. 5I). At this level 5-HT-positive axons appeared more spread and less organized, suggesting that sprouting might have occurred, in addition to sparing.
Figure 5. Immuno-fluorescent labeling of serotonergic raphespinal projections in WT and GFAP-IκBα-dn spinal cords 10 weeks after contusive SCI.
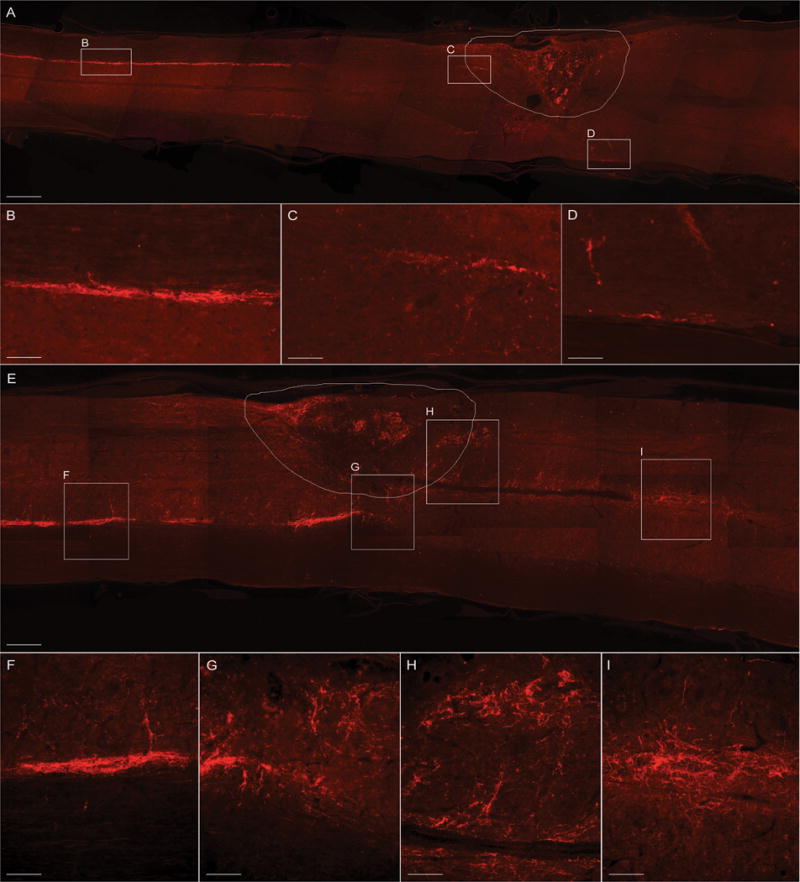
Raphespinal axons were identified by immunolabeling with an anti-5-HT antibody in longitudinal sections of the spinal cord cut along the horizontal plane. WT mice (A–D) showed minimal 5-HT labeling below the injury site, contrary to GFAP-IκBα-dn mice (E–H), who showed a significant presence of 5-HT-labeled raphespinal projections fibers throughout and below the lesion site (H, I). Convoluted and arborized fibers in GFAP-IκBα-dn mice are an anatomical indication of collateral regenerative sprouting (I). The contour in A and E delineates the lesion area. Scale bars: A, E = 500 μm; B, C, D = 50 μm; F, G, H, I: 80 μm.
Increased expression of neuronal-specific genes in GFAP-IκBα-dn mice 10 weeks after contusive SCI
Since we demonstrated a significant sparing and sprouting of propriospinal and supraspinal projections in GFAP-IκBα-dn mice compared to WT 10 weeks after SCI, we proceeded to evaluate the expression of neuronal-specific genes associated with synaptic function and regeneration in the areas of the CNS where those projections originate. Indeed, by real-time RT-PCR we measured gene expression in the spinal cord (segments immediately rostral to the injury site), frontal motor cortex and brainstem (Fig. 6). Although no difference between the two genotypes was detected in motor cortex and brainstem where most of the descending axonal tracts originate, a significantly higher expression of SNAP25, Synaptotagmin and GAP-43 was measured in the spinal cord of GFAP-IκBα-dn mice compared to WT rostral to the lesion site, which paralleled the significant increase in FG-positive neurons found in the same area. Since the majority of the neuronal mRNA is located in soma and dendrites, these data indicate a greater survival of those neurons which have the cell body within the gray matter of the spinal cord, such as motor neurons as well as interneurons and propriospinal neurons.
Figure 6. Differential expression of neuronal-specific molecules in WT and GFAP-IκBα-dn mice 10 weeks mice following contusive SCI.
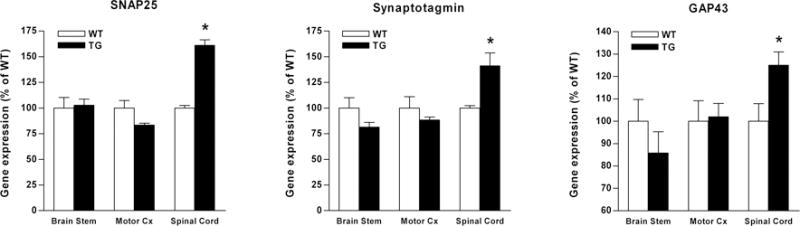
SNAP-25, Synaptotagmin and GAP-43 gene expression was assessed in the brainstem, motor cortex and spinal cord of WT and transgenic animals 8 weeks after SCI. For each gene, results are expressed as % of corresponding WT ± SEM after normalization to β-actin. Six animals per group were analyzed. *P<0.05 with respect to corresponding WT, one-way ANOVA, Tukey test.
Absence of axonal regeneration in WT and GFAP-IκBα-dn mice following complete transection of the spinal cord
Since using a contusion model of spinal cord injury does not allow to unequivocally discriminate between axonal sparing/sprouting and actual regeneration, for which a transection model is better suited, we subjected two groups of WT and GFAP-IκBα-dn mice to complete transection of the spinal cord followed, eight weeks later, by retrograde and anterograde tracing with FG and BDA respectively. Two weeks after injection of the tracers, we set out to count FG-positive neuronal cell bodies above the transection site and BDA-labeled axons below the lesion. In both genotypes we failed to find any FG-labeled neurons in the spinal cord and brain above the lesion, and similarly, we did not find any evidence of BDA labeling of CST axons blow the injury (Figure 7). All BDA-labeled CST fibers degenerated as approaching the transection site (Figure 7C and F), which was surrounded by intense GFAP immunoreactivity indicating the formation of a glial scar (Figure 7G and H). These results show that actual regeneration is highly unlikely to occur not only in WT but also in the transgenic mice. Therefore, the BDA-labeled axonal fibers found below the injury following contusive SCI in the GFAP-IκBα-dn mice are to be attributed to sparing or sprouting rather than regeneration.
Figure 7. Fluorescent labeling of CST axons following anterograde tracing with BDA of WT and GFAP-IκBα-dn spinal cords 10 weeks after spinal cord transcection.
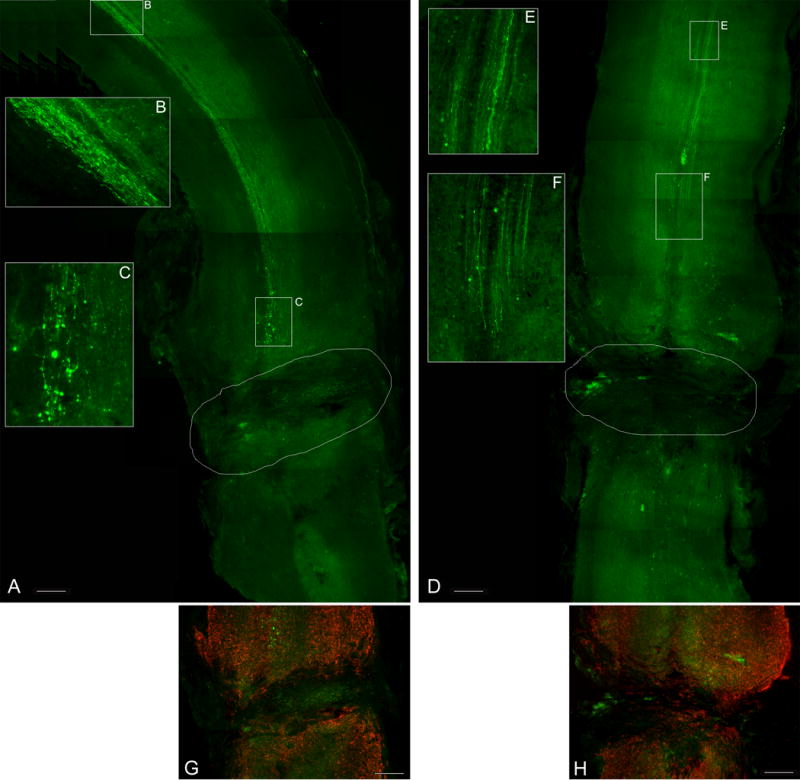
CST axons which anterogradely transported BDA were identified by fluorescent labeling with streptavidin conjugated with Alexa-488 (green) in longitudinal sections of the spinal cord cut along the horizontal plane. Both WT (A) and GFAP-IκBα-dn mice (D) did not show any labeled axon below the injury site. BDA labeling was observed only above the lesion site (B, C, E, F). Panels G (WT) and H (GFAP-IκBα-dn) show the complete transection of the spinal cord surrounded by intense GFAP immunofluorescence (red). The contour in A and D delineates the lesion area. Scale bars: 500 μm.
DISCUSSION
In the present work we show evidence that specific inhibition of the NF-κB pathway in astrocytes results in enhanced sparing and sprouting of axonal tracts critical for locomotion, thereby providing a physiological correlate to the improved functional recovery previously demonstrated in GFAP-IκBα-dn mice following contusive SCI as compared to WT mice (Brambilla et al. 2005).
We first evaluated the profile of glial activation 10 weeks after spinal cord contusion and found that both WT and transgenic mice showed astroglial and microglial activation, especially surrounding the lesion area. However, CD11b and, even more pronouncedly, GFAP immunoreactivity were strongly diminished in GFAP-IκBα-dn mice, particularly when we analyzed areas of the spinal cord caudal to the injury core. These findings demonstrated that the transgenic GFAP-IκBα-dn mice have a reduced chronic gliotic response after contusive SCI. This could be dependent on the fact that by inhibiting NF-κB in astrocytes the production of pro-inflammatory mediators, especially cytokines which are known to be potent glial activators, is reduced (Brambilla et al. 2005; Brambilla et al. 2009), thus limiting the reactivity of astrocytes themselves establishing a negative feedback mechanism. An excessive gliotic response hampers the regenerative capabilities of damaged neurons, as shown in multiple models of trauma and neurodegenerative diseases (Yiu and He 2006; Mi et al. 2007). Indeed, experimental strategies resulting in reduced gliosis proved to be effective in promoting axonal survival and regeneration following SCI (Goldshmit et al. 2004; Silver and Miller 2004). Mice with genetic ablation of GFAP and vimentin showed reduced astroglial reactivity paralleled by increased sprouting of supraspinal axons and restoration of circuits essential for locomotor recovery (Menet et al. 2003). In line with those reports, the reduced reactive astrogliosis found in our GFAP-IκBα-dn transgenic model following SCI indicates the existence of a necessary pre-requisite for the creation of a favorable environment for axonal survival and regeneration.
Following contusive SCI, retrograde tracing with FG demonstrated a significantly increased sparing and/or regeneration of axons in transgenic mice compared to WT. In particular, three descending tracts important for fine motor movements and locomotion were selectively spared, the CST, the reticulospinal and the raphespinal tracts. As a consequence of a lesion, the CST, more than any other spinal tract, has the tendency to retract and rapidly degenerate forming swollen end bulbs that abruptly terminate at the lesion margin (Fishman and Kelly 1984; Pallini et al. 1988; Fabes et al. 2007; Seif et al. 2007). Although by anterograde BDA tracing we detected numerous of these formations in both WT and transgenic mice, we also observed a remarkable sprouting of CST fibers caudal to the injury site selectively in the GFAP-IκBα-dn mice, which could be recognized by the extensive branching, convoluted trajectory (Steward et al. 2003), and positive staining for GAP-43. The high susceptibility of the CST to damage following a weight-drop contusion injury is greatly dependent on the fact that the epicenter of the primary mechanical injury is centered upon the dorsal columns (Young 2002), an area in which the majority (about 95%) of the CST is located, thus subjecting this descending tract to significant, if not almost complete injury, according to the severity of the contusive SCI. However, the fragility of the CST is also partly dependent on the demand of trophic support required for normal function. Several groups have reported on the role of neurotrophins in promoting survival of CST neurons, both under physiological and pathological conditions (Giehl 2001). BDNF, insulin-like growth factor 1 (IGF-1), and more recently, insulin-like growth factor 2 (IGF-2), pleiotrophin, CXCL12, have all been found to be important survival factors for early postnatal, as well as injured, CST motor neurons (Schnell et al. 1994; Giehl et al. 1998; Ozdinler and Macklis 2006; Vavrek et al. 2006; Dugas et al. 2008). Therefore, we cannot exclude that, in the GFAP-IκBα-dn mice, the ability of damaged CST axons to sprout beyond the inhibitory terrain at the lesion site, could be associated with an increased expression of neurotrophic factors, a hypothesis that we are currently investigating.
Rather than the CST, automated locomotor behavior such as walking is mostly dependent upon the proper functioning of descending tracts originating from nuclei or areas of the brainstem, namely the reticluo-, vestibulo-, rubro- and raphespinal tracts (Raineteau and Schwab 2001). The raphe nuclei represent the origin of the serotonergic fibers in the CNS, and the descending raphespinal projections play a key role in activating and facilitating the function of spinal motor circuits (Schmidt and Jordan 2000). After SCI, when all or most serotonergic afferents below the lesion are obliterated, infusion of serotonergic agonists or transplantation of serotonin-secreting cells can improve motor functions (Majczynski et al. 2005; Gerasimenko et al. 2007), demonstrating that serotonergic reinnervation is crucial for locomotor recovery.
The reticulospinal tact is a heterogeneous tract originating from dispersed nuclei in the reticular formation of the brainstem with a crucial role in coordinating rhythmic stepping movements (Shefchyk et al. 1984). In fact, various studies have shown that even limited sparing of the ventrolateral funiculi where the reticulospinal fibers are located is sufficient for spontaneous locomotor recovery (Brustein and Rossignol 1998; Schucht et al. 2002; Ballermann and Fouad 2006). Interestingly, our retrograde tracing experiments indicate that the raphespinal and reticulospinal tracts are significantly spared in the GFAP-IκBα-dn mice compared to WT. In addition, immunostaining for 5-HT revealed the possible occurrence of sprouting in GFAP-IκBα-dn mice, as demonstrated by the presence of fine tortuous and disorganized 5-HT-labeled fibers far below the injury site. These data provide the physiologic correlate to the ability of the transgenic mice to perform partially coordinated movements and plantar stepping two months after injury, improvements not achieved by the WT mice (Brambilla et al. 2005).
Counting of FG-positive neurons in the spinal cord rostrally to the lesion site revealed a significantly higher number of labeled cells in the cord of GFAP-IκBα-dn mice compared to WT. This indicates that, beside the sparing of supraspinal tracts, a greater sparing of propriospinal connections is also occurring in the transgenic mice. These tracts, which are completely confined within the boundaries of the spinal cord, represent a large percentage of the fibers traveling through it (Chung and Coggeshall 1983). They are believed to be important in reflex control and coordination of hind- and forelimbs during locomotion (Jankowska et al. 1973; Schomburg et al. 1978; Meinck and Piesiur-Strehlow 1981; Jordan and Schmidt 2002). In a recent paper Courtine and colleagues (Courtine et al. 2008) elegantly demonstrated the importance of the propriospinal circuitry in mediating spontaneous functional recovery and supraspinal control of stepping following SCI. In fact, they showed that pronounced locomotor recovery can occur even without the maintenance or regeneration of direct supraspinal projections, but simply through the reorganization of descending and propriospinal connections (Courtine et al. 2008). In line with these observations, the significantly greater sparing of propriospinal tracts in the GFAP-IκBα-dn mice compared to WT provides an additional explanation for the improved functional recovery observed following contusive SCI (Brambilla et al. 2005). This could be due to the fact that, in the GFAP-IκBα-dn mice, the fibers that constitute the local propriospinal circuitry encounter an environment far less hostile to their survival, meaning a reduced expression of pro-inflammatory cytokines and chemokines, as well as a reduced expression of CSPGs (Brambilla et al. 2005) which can constitute a physical barrier to sprouting and regeneration.
The increased sparing and sprouting of axonal tracts is paralleled by the finding that several neuronal-specific genes are expressed at significantly higher levels in transgenic mice versus WT 10 weeks after contusion injury. Increased expression of the synaptic molecules SNAP25 and synaptotagmin may indicate both the sparing of axons, and therefore of existing synapses, or the presence of newly generated axons accompanied by the formation of new synapses. On the other hand, the expression of the growth-associated molecule GAP-43, a well-known marker of axonal growth (Campbell et al. 1991; Bisby et al. 1996; Benowitz and Routtenberg 1997; Gupta et al. 2006), represents direct evidence that regenerative sprouting is taking place in the GFAP-IκBα-dn mice. When it occurs, GAP-43 up-regulation has been reported to be transient in injured CNS neurons (Doster et al. 1991; Dijk et al. 2007), suggesting that this may represent a biological basis as to why, differently from PNS axons, CNS axons fail to regenerate following injury (Dusart et al. 2005). It is important to point out that in the GFAP-IκBα-dn injured mice GAP-43 expression is chronically sustained in the spinal cord, since we found expression 10 weeks after SCI, both by real-time PCR and immunohistochemistry, suggesting that the activation of a gene expression program adequate to support long term sprouting and/or regeneration of propriospinal circuits is taking place.
The question of whether the GFAP-IκBα-dn mice are capable of true regeneration as opposed to simply increased sprouting and sparing of axons could not be fully addressed using a contusion model of SCI. Therefore we subjected both WT and transgenic mice to a complete transection of the spinal cord and evaluated, using the same approaches as for the contusive SCI, FG and BDA labeling above and below the lesion site, respectively. In neither case we found evidence of labeling, suggesting the absence of true regenerating axons. It should be noted however, that the complete transection model we used normally results in a very fibrotic lesion, due to the massive infiltration of fibroblasts from the periphery. This is accompanied by the productions of high levels of axon growth inhibitory molecules (e.g., semaphorins), which is not deminished in our GFAP-IκBα-dn mice since the genetic manipulation does not target this cell type. Therefore, not achieving a result in the complete transection model does not fully rule out the possibility that regeneration may occur in our transgenic mice after contusion.
In conclusion, our results provide evidence that by inhibiting NF-κB-dependent cascades specifically in astrocytes we can contain the development of damage following SCI, improve the sparing of axons, and promote regenerative sprouting of spinal tracts ultimately leading to significant functional improvement. The challenge is now to translate this cell-targeted approach into a clinical strategy that could provide an additional tool, perhaps in combination with other emerging treatments (e.g. Nogo-A antibodies, chondroitinase ABC, Rho inhibitors, autologous macrophages, neural stem-based remyelination strategies; for review see (Rossignol et al. 2007) to address a pathology as complex and multifaceted as SCI.
Acknowledgments
This work was supported by the NIH grant NS051709 to JRB and by The Miami Project to Cure Paralysis.
Footnotes
The authors have no conflicting financial interests.
References
- Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration: I. Nuclear population in rabbit sciatic nerve. J Anat. 1946;80:37–50. [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res. 2000;128:33–42. doi: 10.1016/S0079-6123(00)28005-9. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Tetzlaff W, Brown MC. GAP-43 mRNA in mouse motoneurons undergoing axonal sprouting in response to muscle paralysis of partial denervation. Eur J Neurosci. 1996;8:1240–1248. doi: 10.1111/j.1460-9568.1996.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappaB improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J Neurophysiol. 1998;80:1245–1267. doi: 10.1152/jn.1998.80.3.1245. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Campbell G, Anderson PN, Turmaine M, Lieberman AR. GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts. Exp Brain Res. 1991;87:67–74. doi: 10.1007/BF00228507. [DOI] [PubMed] [Google Scholar]
- Chung K, Coggeshall RE. Propriospinal fibers in the rat. J Comp Neurol. 1983;217:47–53. doi: 10.1002/cne.902170105. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007;206:159–171. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Faden AI, Yakovlev A, Duke-Cohan JS, Finn T, Thouin M, Knoblach S, De Biase A, Bregman BS, Hoffman EP. Neuronal plasticity after spinal cord injury: identification of a gene cluster driving neurite outgrowth. Faseb J. 2005;19:153–154. doi: 10.1096/fj.04-2694fje. [DOI] [PubMed] [Google Scholar]
- Dijk F, Bergen AA, Kamphuis W. GAP-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp Eye Res. 2007;84:858–867. doi: 10.1016/j.exer.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster SK, Lozano AM, Aguayo AJ, Willard MB. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6:635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusart I, Ghoumari A, Wehrle R, Morel MP, Bouslama-Oueghlani L, Camand E, Sotelo C. Cell death and axon regeneration of Purkinje cells after axotomy: challenges of classical hypotheses of axon regeneration. Brain Res Brain Res Rev. 2005;49:300–316. doi: 10.1016/j.brainresrev.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- Fishman PS, Kelly JP. Identified central axons differ in their response to spinal cord transection. Brain Res. 1984;305:152–156. doi: 10.1016/0006-8993(84)91131-4. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–2536. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Giehl KM. Trophic dependencies of rodent corticospinal neurons. Rev Neurosci. 2001;12:79–94. doi: 10.1515/revneuro.2001.12.1.79. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Schutte A, Mestres P, Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J Neurosci. 1998;18:7351–7360. doi: 10.1523/JNEUROSCI.18-18-07351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Rummler LS, Palispis W, Truong L, Chao T, Rowshan K, Mozaffar T, Steward O. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp Neurol. 2006;200:418–429. doi: 10.1016/j.expneurol.2006.02.134. [DOI] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17:203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado A, Podinin H, Oudega M, Grimpe B. Deoxyribozyme-mediated knockdown of xylosyltransferase-1 mRNA promotes axon growth in the adult rat spinal cord. Brain. 2008 doi: 10.1093/brain/awn206. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Stuart D. Propriospinal control of last order interneurones of spinal reflex pathways in the cat. Brain Res. 1973;53:227–231. doi: 10.1016/0006-8993(73)90786-5. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Tetzlaff W, Hunt SP. Differential expression of immediate early genes in rubrospinal neurons following axotomy in rat. Eur J Neurosci. 1993;5:203–209. doi: 10.1111/j.1460-9568.1993.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Li GL, Farooque M, Holtz A, Olsson Y. Increased expression of growth-associated protein 43 immunoreactivity in axons following compression trauma to rat spinal cord. Acta Neuropathol. 1996;92:19–26. doi: 10.1007/s004010050484. [DOI] [PubMed] [Google Scholar]
- Lo TP, Jr, Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, Stagg M, Abril RM, Patel S, Dietrich WD, Pearse DD. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol. 2009;514:433–448. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majczynski H, Maleszak K, Cabaj A, Slawinska U. Serotonin-related enhancement of recovery of hind limb motor functions in spinal rats after grafting of embryonic raphe nuclei. J Neurotrauma. 2005;22:590–604. doi: 10.1089/neu.2005.22.590. [DOI] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck HM, Piesiur-Strehlow B. Reflexes evoked in leg muscles from arm afferents: a propriospinal pathway in man? Exp Brain Res. 1981;43:78–86. doi: 10.1007/BF00238812. [DOI] [PubMed] [Google Scholar]
- Menet V, Prieto M, Privat A, Gimenez Y, Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci U S A. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES, Yuan Q, Wong WM, Wang L, Su H, Chu TH, Guo J, Zhang W, So KF, Pepinsky B, Shao Z, Graff C, Garber E, Jung V, Wu EX, Wu W. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–1233. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Oudega M, Vargas CG, Weber AB, Kleitman N, Bunge MB. Long-term effects of methylprednisolone following transection of adult rat spinal cord. Eur J Neurosci. 1999;11:2453–2464. doi: 10.1046/j.1460-9568.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Pallini R, Fernandez E, Sbriccoli A. Retrograde degeneration of corticospinal axons following transection of the spinal cord in rats. A quantitative study with anterogradely transported horseradish peroxidase. J Neurosurg. 1988;68:124–128. doi: 10.3171/jns.1988.68.1.0124. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Meinck HM, Haustein J, Roesler J. Functional organization of the spinal reflex pathways from forelimb afferents to hindlimb motoneurones in the cat. Brain Res. 1978;139:21–33. doi: 10.1016/0006-8993(78)90057-4. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- Seif GI, Nomura H, Tator CH. Retrograde axonal degeneration “dieback” in the corticospinal tract after transection injury of the rat spinal cord: a confocal microscopy study. J Neurotrauma. 2007;24:1513–1528. doi: 10.1089/neu.2007.0323. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jell RM, Jordan LM. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56:257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clin Neurosci Res. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudano E, Campbell G, Hunt SP, Lieberman AR. Axonal injury and peripheral nerve grafting in the thalamus and cerebellum of the adult rat: upregulation of c-jun and correlation with regenerative potential. Eur J Neurosci. 1998;10:2644–2656. doi: 10.1046/j.1460-9568.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- West NR, Collins GH. Cellular changes during repair of a cryogenic spinal cord injury in the rat: an electron microscopic study. J Neuropathol Exp Neurol. 1989;48:94–108. doi: 10.1097/00005072-198901000-00008. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]


