Abstract
Objective
The purpose of this study was to use proton magnetic resonance spectroscopy, at 4.0 T, to explore the glutamine and glutamate levels in the anterior cingulate cortex of children and adolescents with bipolar disorder (BPD; medicated and unmedicated) and healthy comparison subjects (HCSs). We hypothesized that unmedicated children with BPD would have reduced glutamine and glutamate levels compared with HCSs and medicated children with BPD.
Method
Spectra were acquired from the anterior cingulate cortex in 22 children and adolescents with DSM-IV-TR BPD, type 1 (13 female: age 12.6 ± 4.4 years: 7 of the subjects with BPD were unmedicated at the time of the scan) and 10 HCSs (7 female: age 12.3 ± 2.5 years).
Results
Unmedicated subjects with BPD had significantly lower glutamine levels than HCSs or medicated subjects with BPD. There were no differences in glutamate levels between the three groups.
Conclusions
These results are consistent with there being an abnormality in anterior cingulate cortex glia in untreated children and adolescents with BPD. The results of this pilot study may be important in helping us better understand the pathophysiology of child and adolescent BPD. In addition, this observation may help to develop better and more targeted treatments, in particular those affecting the metabolism of glutamine, perhaps by regulation of glutamine synthetase activity.
Keywords: magnetic resonance spectroscopy, glutamate, glutamine, bipolar disorder
The lifetime prevalence of bipolar disorder (BPD) is between 1% and 3%, and BPD can manifest in both children and adolescents (Geller et al., 1994; Lewinsohn et al., 1995). Although the presence and prevalence of prepuberty-onset BPD is controversial, an emerging literature supports its prevalence, validity, and associated morbidity (Biederman, 2003; Biederman et al., 2000; Giedd, 2000; Post et al., 2004; Wozniak, 2003).
A number of studies have implicated the corticolimbic region as an area with significant pathology in BPD (Coyle and Duman, 2003; Leibenluft et al., 2003). Using different imaging modalities, including magnetic resonance imaging (MRI) and functional MRI, abnormalities in anterior cingulate cortex (ACC) volume and function have been found in adults with BPD (Drevets, 2001; Kruger et al., 2003; Lyoo et al., 2004; Sassi et al., 2004; Yurgelun-Todd et al., 2000). These alterations in the ACC in adult BPD also appear to be present in children and adolescents with the illness (Frazier et al., 2005). Using MRI, Kaur et al. (2005) measured reduced ACC volume in children and adolescents with BPD and Chang et al. have used functional MRI to demonstrate altered corticolimbic activity, including abnormalities in the ACC, in children and adolescents with familial BPD (Chang et al., 2004). In addition, many magnetic resonance spectroscopy (MRS) studies have shown altered metabolite levels in the ACC of both adults and children with BPD (Cecil et al., 2002, 2003; Davanzo et al., 2001, 2003; DelBello et al., 2006; Moore et al., 2000; Moore and Galloway, 2002).
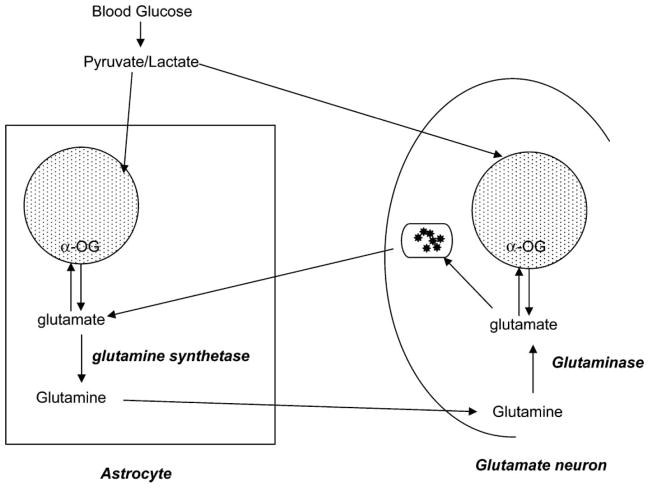
Postmortem studies in adults with BPD have demonstrated reductions in the total number of glia and increases in glial cell size in the prefrontal cortex and ACC in adults with BPD (Ongur et al., 1998; Rajkowska, 2002). Glia provide a pathway for neuronal glutamate synthesis and reuptake though the glutamate/glutamine cycle (Fig. 1; Erecinska and Silver, 1986). Glutamate is the most abundant excitatory neurotransmitter in the brain (Cooper et al., 2003). Brain glutamate concentrations are on the order of 8 to 13 mmol/g, and the glutamate-to-glutamine ratio in vivo has been shown to range from ~2.4 to 3.8 (Gruetter et al., 2003). Brain tissue glutamine content reflects primarily glial glutamine concentrations. The enzyme glutamine synthetase, which catalyzes glutamate to glutamine, is found exclusively in glia (Martinez-Hernandez et al., 1977; Tansey et al., 1991). Glia take up glutamate from the synaptic cleft of glutamatergic neurons; glutamate is then metabolized to glutamine (by glutamine synthetase). Some glutamine is then transported back to the glutamatergic neuron where it is metabolized back to glutamate (by glutaminase) (Cooper et al., 2003).
Fig. 1.
Cycling of glutamate and glutamine in glia and glutamatergic neurons. Reductions in glial cell density could result in decreased glutamine and glutamate, each of which may be measured using proton magnetic resonance spectroscopy (MRS). This figure does not include all metabolic pathways that exist between neurons and glia. α-OG = α-oxoglutarate. = Krebs
 cycle.
cycle.
A recent postmortem study by Choudary et al. (2005) demonstrated reduced glutamine synthetase in the ACC of depressed subjects, 40% of whom had BPD. Abnormalities in glia may lead to reductions in glutamine and/or glutamate levels. Frye et al. (2007) reported reduced CSF levels of glutamate in unmedicated adults with refractory affective illness, two thirds of whom had BPD. The CSF glutamate levels in these subjects increased following treatment with the anticonvulsant mood stabilizer lamotrigine; a number of medications that are effective in treating BPD appear to alter glutamatergic function (Heresco-Levy, 2003; Ketter and Wang, 2003; Ketter et al., 2003; Krystal et al., 2002).
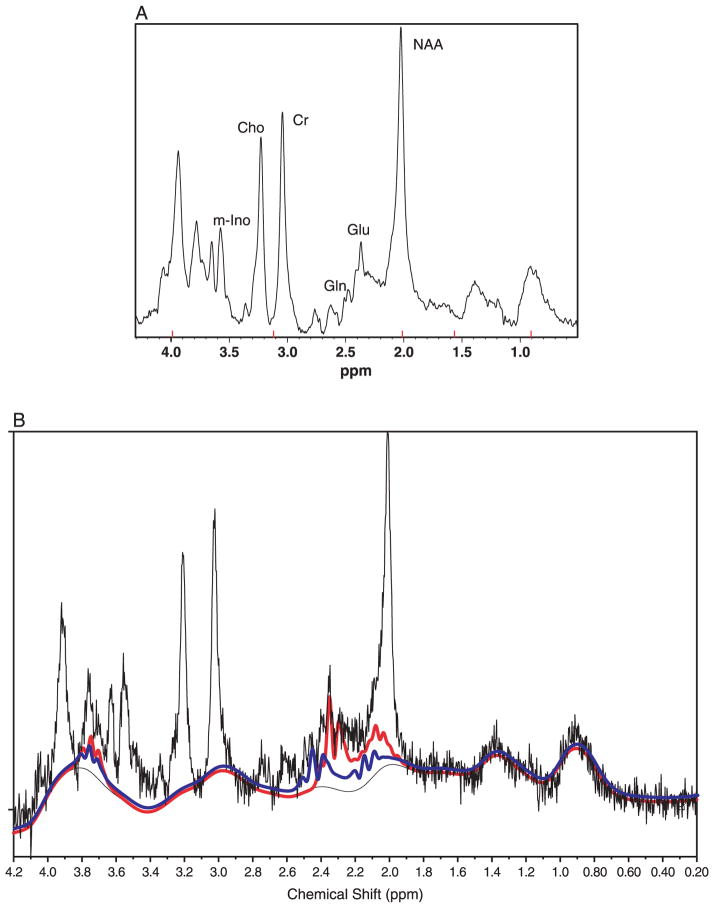
Using proton MRS (1H-MRS), it is possible to measure a number of cerebral metabolites. The most prominent resonances in proton spectra are N-acetyl aspartate (NAA), creatine plus phosphocreatine (Cr), choline-containing compounds (Cho), and myo-inositol–containing compounds (m-Ino; Fig. 2A). Because of the substantial overlap of glutamate, glutamine, and γ-aminobutyric acid (composite referred to as Glx) in 1H-MRS, the individual components of Glx may be difficult to distinguish at the relatively low magnetic field strengths used in human MRS studies (Stork and Renshaw, 2005). Alterations in Glx have been suggested to reflect primarily altered glutamate levels (Dager et al., 2004). Since the U.S. Food and Drug Administration (2003) released its “Criteria for Significant Risk: Investigations of Magnetic Resonance Diagnostic Devices,” the use of a 4.0-T scanner for MRS examinations is allowed in research. Using higher magnetic strengths allows for the evaluation of the independent contributions of glutamine and glutamate. Glutamate resonates at 2.11, 2.35, and 3.75 ppm; this overlaps with glutamine at 2.11 and 3.75 ppm, which also resonates at 2.45 ppm (Fig. 2B; Cady, 1990). At 4.0 T, it is possible to distinguish the 2.35-ppm resonance of glutamate and the 2.45-ppm resonance of glutamine using conventional spectroscopy techniques (Moore et al., 2006; Theberge et al., 2002). γ-Aminobutyric acid resonates at 1.9, 2.27, and 3.02 ppm and without the application of special spectroscopic techniques, even at 4.0 T, is difficult to measure (Hetherington et al., 1997, 1998).
Fig. 2.
A: A proton magnetic resonance spectrum acquired from the anterior cingulate cortex of a 17-y-old boy with untreated BPD. This spectrum was acquired at 4.0 T using point-resolved spectroscopy (PRESS) pulse sequence (echo time = 30 msec, repetition time = 2 sec and averages = 128). A 2-Hz exponential filter has been applied to this spectrum. Glutamine is visible at 2.45 ppm and glutamate at 2.35 ppm. Glu = glutamate; Gln = glutamine; NAA = N-acetyl aspartate; Cr = creatine plus phosphocreatine; Cho = choline-containing compounds; M-Ino = myo-inositol–containing compounds. B: LCModel fit for glutamine (blue line) and glutamate (red line) for the anterior cingulate cortex spectrum shown.
The purpose of this study was to measure glutamine and glutamate, using 1H-MRS at 4.0 T, in the ACC of children and adolescents with BPD given that (1) ACC abnormalities have been shown in children, adolescents, and adults with BPD using MRI, MRS, and functional MRI techniques and (2) ACC glial cell abnormalities (which would affect glutamate and glutamine levels) have been shown in postmortem studies of adults with BPD. We hypothesized that unmedicated children with BPD would have reduced glutamine and glutamate levels, reflecting abnormal glia, compared with HCSs, and medicated children with BPD would have higher glutamine and glutamate levels compared with unmedicated children with BPD because many medications that are effective in the treatment of BPD may do so through altering glutamatergic activity.
METHOD
The institutional reviews boards at both McLean Hospital and Cambridge Health Alliance approved this study. All of the subjects were recruited through McLean Hospital and the Cambridge Health Alliance (outpatient, partial, and inpatient programs). Male and females subjects of multiple ethnicities were recruited.
All of the children, including the healthy controls, underwent diagnostic semistructured (Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic version (K-SADS-E) and clinical interviews. Psychiatric comorbidity and the diagnosis of BPD in all of the children was assessed by the K-SADS-E and the Structured Clinical Interview for DSM-IV TR in adults ≥18 years of age (note that one subject was 19 at the time of this study; First et al., 1997). Parents also were administered a K-SADS-E regarding their children by trained raters. The diagnosis of BPD in the children, adolescents, and young adults, based on DSM-IV-TR criteria, was made following the clinical interview by board-certified child and adolescent psychiatrists.
All of the subjects with BPD in this study meet criteria for BPD I, which is the narrow phenotype (Leibenluft et al., 2003). For a diagnosis of BPD I, manic episode, subjects must meet the DSM-IV-TR Criterion A of extreme and persistently elevated, expansive, or irritable mood lasting at least 1 week (or any duration if hospitalization is necessary). In addition, subjects must manifest three (four if the mood is irritable only) of seven Criterion B symptoms during the period of mood disturbance. The seven Criteria B symptoms are grandiosity, decreased need for sleep, talkativeness, and/or pressured speech, flights of ideas and/or racing thoughts, distractibility, increase in goal-directed activity and/or psychomotor agitation, and “excessive involvement in pleasurable activities that have a high potential for painful consequences.”
Current mood state that is mixed, manic, depressed, or euthymic was determined based on the K-SADS-E. Measures of current mood symptoms and impairment ratings on all patients were obtained by child and adolescent psychiatrists based on their interviews of both the parent and child using the Young Mania Rating Scale (YMRS; Young et al., 1978) and the Children’s Depression Rating Scale-Revised (Poznanski, 1996; Poznanski et al., 1979). Exclusion criteria included a history of a uncontrolled general medical disorder; a history of neurological illness (including head trauma with loss of consciousness, seizure disorder, multiple sclerosis, cerebral ischemia or infarction, neoplasia), major sensorimotor handicaps, mental retardation (Full Scale IQ <70), learning disability, autism, schizophrenia, alcohol or drug dependence/abuse (during 2 months before scan or total history of ≥12 months), electroconvulsive therapy, a contraindication to MR scan including metal fragments or implants, claustrophobia, lactation, pregnancy (all females of child-bearing age were using an effective contraceptive method and passed a negative pregnancy urine test before scanning). Unique exclusion criteria for the HCSs included an Axis I diagnosis and a family history of a mood disorder in a first-degree relative. Family history of DSM-IV psychiatric diagnoses was obtained during the telephone screening and during the clinical assessment and interview about the child with the parents. Parents were asked to report on the psychiatric history of their child’s first-degree relatives (parents and siblings).
After the study was described, all of the parents signed a written informed consent form and all of the children signed a written informed assent form.
MRI and MRS
MRS studies were performed on a 4.0-T Varian Unity/Inova whole-body MR scanner (Varian NMR Instruments, Palo Alto, CA) equipped with a proton head coil (MR Instruments, Minneapolis, MN). A three-plane set of fast localizers was acquired followed by an axial fast spin echo series. (Subjects also had images acquired that were read by a radiologist for the purposes of ruling out any clinical abnormalities.) These images were used for localization of the ACC and for tissue segmentation purposes. Spectra were then acquired using the PRESS (Bottomley, 1987) technique following local shimming and pulse optimization.
The proton PRESS spectrum was acquired from a 2 × 2 × 2-cm voxel localized on the ACC. (The voxels were consistently placed and all 4.0-T data acquired and analyzed by the same person.) The voxel was placed over the anterior cingulate gyrus, in a predominantly gray matter area, superior to the orbits and inferior to the genu of the corpus callosum (Fig. 3). PRESS parameters included a repetition time (TR) = 2 seconds, echo time (TE) = 30 milliseconds and averages = 128. The total PRESS acquisition time was less than 5 minutes. Following data acquisition, the spectra were fit using LCModel (Version 6.1-0; Provencher, 2001) and a simulated basis set. The basis set used for this study included alanine, aspartate, creatine, phosphocreatine, GABA, glucose, glutamate, glutamine, glycerophosphocholine, phosphocholine, inositol, lactate, NAA, N-acetyl aspartyl glutamate, scyllo-inositol, and taurine. Version 6.1-0 of LCModel also fits the macromolecule resonances present at 2.4 and 3.2 ppm. The Cramer-Rao spectral inclusion criteria for the spectra were NAA, Cho, Cr, and m-Ino SD<15% and glutamine and glutamate SD <25%.
Fig. 3.
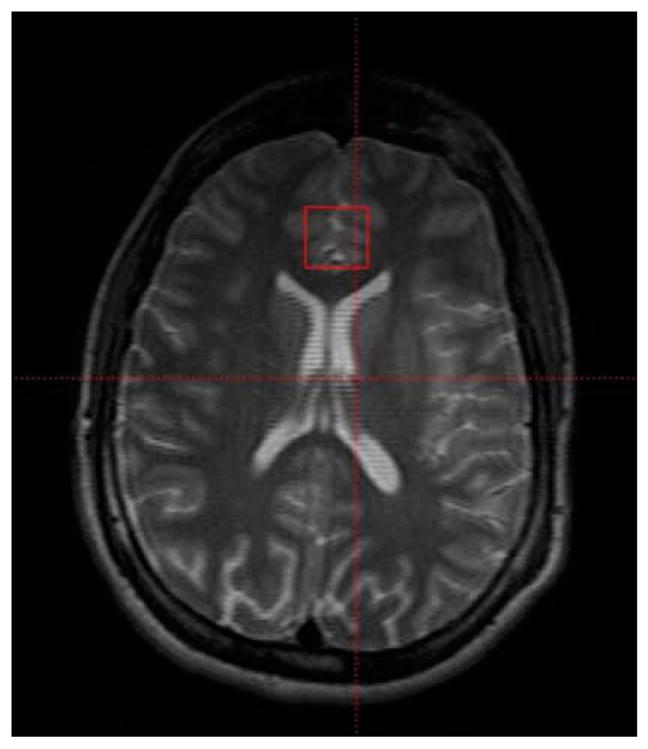
Localization of a 2 × 2 × 2 cm voxel in the anterior cingulate cortex.
Structural 4.0-T MRI scans were segmented using open source software, “NVM” (freely available from Neuromorphometrics, Inc. at http://neuromorphometrics.org:8080/nvm), to determine gray matter, white matter, and CSF contributions to the voxel of interest.
Statistical Analysis
Linear regression modeling was chosen as the primary analysis method. Analysis of variance (ANOVA), multiple analysis of variance (MANOVA) and nonparametric tests (Kruskal-Wallis) were conducted for some secondary analyses. For the linear regression model, backward analysis was used. For hypothesis-driven tests, statistical significance was defined at an α level of .05 using two-tailed tests. Some exploratory analysis was also performed; for these tests; statistical significance was defined at an α level of .01 using two-tailed tests to correct for multiple comparisons. SPSS 11.0 for Macintosh OS X was used for all computations.
RESULTS
A total of 32 subjects were examined: 22 subjects with BPD (13 female: age 12.5 ± 4.2 years) and 10 healthy comparison subjects (7 female: age 12.3 ± 2.5 years; Table 1). Seven of the subjects with BPD were unmedicated at the time of the scan (three female: age 12.86 ± 3.8 years). Three of these subjects were medication naïve. The remaining four subjects were medication free for 10, 55, 85, and 188 days each before participating in this study (Table 2). The mean age, height, weight, and IQ for the three groups studied are shown in Table 3. There were no significant differences in age or sex between the three groups (unmedicated and medicated subjects with BPD and HCSs) examined (ANOVA for age: df = 2, 32; F = 0.05; p = .95; Kruskal-Wallis for sex: , p = .90), nor was there a significant difference in height (MANOVA df = 2, 31; F = 0.58; p = .56), weight (MANOVA df = 2, 31; F = 0.83; p = .45), or IQ (df = 2; 29, F = 0.076; p = .927) between the three groups examined.
TABLE 1.
Diagnosis (BPD or HCS), Medication Status (Yes or No), Age (in Years), Sex, Height (in Centimeters), Weight (in Kilograms), IQ, and CDRS and YMRS Scores for the Children and Adolescents Who Participated in This Study
| ID | Diagnosis | Medication | Age | Sex | Height | Weight | IQ | CDRS | YMRS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | HCS | HCS | 11 | F | 151.77 | 61.23 | 102 | na | 0 |
| 2 | HCS | HCS | 12 | F | 157.48 | 62.60 | 120 | na | na |
| 3 | HCS | HCS | 15 | M | 157.48 | 44.45 | 103 | 21 | 0 |
| 4 | HCS | HCS | 13 | F | 168.91 | 51.71 | 103 | 17 | 0 |
| 5 | HCS | HCS | 15 | F | 140.34 | 58.51 | 104 | 17 | 1 |
| 6 | HCS | HCS | 15 | F | 162.56 | 62.14 | 100 | na | 1 |
| 7 | HCS | HCS | 12 | F | 158.75 | 62.60 | 83 | 17 | 0 |
| 8 | HCS | HCS | 13 | M | 173.36 | 84.82 | 106 | 19 | 1 |
| 9 | HCS | HCS | 8 | M | 121.92 | 22.68 | 123 | 17 | 1 |
| 10 | HCS | HCS | 9 | F | 124.46 | 23.13 | 125 | 18 | 1 |
| 11 | BPD | No | 11 | M | 149.86 | 41.41 | 75 | 37 | 19 |
| 12 | BPD | No | 12 | F | 153.67 | 41.73 | 135 | 64 | na |
| 13 | BPD | No | 8 | M | na | na | na | 36 | na |
| 14 | BPD | No | 9 | M | 127.00 | 28.12 | 106 | 44 | 27 |
| 15 | BPD | No | 16 | F | 154.94 | 54.43 | na | 62 | 21 |
| 16 | BPD | No | 17 | F | 165.10 | 68.04 | 101 | 48 | 42 |
| 17 | BPD | No | 17 | M | 162.56 | 49.90 | 114 | 71 | 24 |
| 18 | BPD | Yes | 11 | F | 160.02 | 53.52 | 106 | 42 | 26 |
| 19 | BPD | Yes | 15 | M | 170.18 | 68.04 | 102 | 43 | 16 |
| 20 | BPD | Yes | 19 | F | 154.94 | 52.16 | 113 | 17 | 0 |
| 21 | BPD | Yes | 9 | F | 121.92 | 22.68 | 97 | 23 | 12 |
| 22 | BPD | Yes | 15 | F | 165.10 | 58.97 | 102 | 50 | na |
| 23 | BPD | Yes | 15 | M | na | na | na | 26 | 5 |
| 24 | BPD | Yes | 15 | F | 170.18 | 61.23 | 116 | 20 | 26 |
| 25 | BPD | Yes | 14 | M | 167.01 | 70.31 | 80 | na | na |
| 26 | BPD | Yes | 8 | M | 116.84 | 27.22 | 113 | 50 | 31 |
| 27 | BPD | Yes | 5 | F | 106.68 | 22.68 | 98 | 28 | 31 |
| 28 | BPD | Yes | 5 | F | 110.49 | 22.68 | 119 | 44 | 26 |
| 29 | BPD | Yes | 16 | F | 163.83 | 58.06 | 112 | 35 | 13 |
| 30 | BPD | Yes | 16 | F | 163.83 | 77.11 | 110 | 47 | 28 |
| 31 | BPD | Yes | 16 | F | 167.64 | 62.60 | 100 | 34 | 40 |
| 32 | BPD | Yes | 7 | M | 135.89 | 36.74 | 99 | na | na |
Note: BPD = bipolar disorder; HCSs = healthy comparison subjects; CDRS = Children’s Depression Rating Scale; YMRS = Young Mania Rating Scale.
TABLE 2.
Mood State, Comorbid Diagnoses of, and Medications Subjects With Bipolar Disorder Were Receiving at the Time of Their Spectroscopy Examination
| ID | Mood | Comorbid Diagnosis | Medicated Subjects With Bipolar Disorder
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mood Stabilizers | Atypical Antipsychotics | Antidepressants | Others | |||||
| 18 | Manic | None | Oxcarbazepine | Risperidone, aripiprazole | ||||
| 19 | Manic | ODD | Oxcarbazepine | Lithium | Risperidone | |||
| 20 | Manic | None | Gabapentin | Quetiapine | Nefazadone | Sertraline | ||
| 21 | Euthymic | ADHD | Risperidone | Methylphenidate | ||||
| 22 | Manic | ODD | Quetiapine | |||||
| 23 | Mixed | ADHD | Valproate | Aripiprazole | ||||
| 24 | Manic | ADHD | Clozapine | Trazodone | Sertraline | Atomoxetine | ||
| 25 | Mixed | PTSD | Lithium | Olanzapine | ||||
| 26 | Mixed | ADHD | Aripiprazole | Clonidine | ||||
| 27 | Manic | ADD | Quetiapine | |||||
| 28 | Manic | None | Oxcarbazepine | Clonidine | ||||
| 29 | Mixed | ADHD | Oxcarbazepine | Trazodone | Sertraline | |||
| 30 | Mixed | PTSD | Lamotrigine | Quetiapine | Lorazepam | |||
| 31 | Euthymic | None | Quetiapine | |||||
| 32 | Mixed | ADHD | Oxcarbazepine | Clonidine | ||||
| Unmedicated Subjects With Bipolar Disorder
|
||||||||
| Days Unmedicated | Previous Medications | |||||||
|
|
||||||||
| 11 | Mixed | None | 10 | Risperidone | ||||
| 12 | Manic | None | 55 | Escitalopram | ||||
| 13 | Depressed | None | Drug naïve | None | ||||
| 14 | Manic | ADHD | Drug naïve | None | ||||
| 15 | Mixed | None | Drug naïve | None | ||||
| 16 | Manic | ADHD/ODD | 188 | Methylphenidate, topiramate, trazadone | ||||
| 17 | Mixed | None | 85 | Oxacarbazepine, bupropion | ||||
Note: ODD = oppositional defiant disorder; ADHD = attention-deficit/hyperactivity disorder; PTSD = posttraumatic stress disorder.
TABLE 3.
Mean ± SD of Age, Weight, Height, IQ, and CDRS and YMRS Scores for the Children and Adolescents Who Participated in This Study
| HCSs (10) | BPD (22) | BPD-no med (7) | BPD-med (15) | |
|---|---|---|---|---|
| Age, y | 12.30 ± 2.50 | 12.50 ± 4.20 | 12.86 ± 3.80 | 12.40 ± 4.50 |
| Weight, kg | 53.39 ± 19.04 | 48.88 ± 17.46 | 42.27 ± 13.57 (n = 6) | 49.57 ± 19.32 (n = 14) |
| Height, cm | 151.70 ± 17.50 | 149.38 ± 21.30 | 152.19 ± 13.59 (n = 6) | 148.18 ± 24.22 (n = 14) |
| IQ | 106.90 ± 12.65 | 105.16 ± 13.41 (n = 19) | 106.20 ± 21.74 (n = 5) | 104.79 ± 10.14 (n = 14) |
| CDRS | 18.00 ± 1.53 (n = 7) | 41.05 ± 14.51 (n = 20) | 51.71 ± 13.94 (n = 7) | 35.31 ± 11.69 (n = 13) |
| YMRS | 0.56 ± 0.53 (n = 9) | 22.76 ± 1.15 (n = 17) | 26.60 ± 9.13 (n = 5) | 21.17 ± 11.87 (n = 12) |
Note: CDRS = Children’s Depression Rating Scale; YMRS = Young Mania Rating Scale; HCSs = healthy comparison subjects; Med = medication.
Nine subjects were medicated with a mood stabilizer (eight with an anticonvulsant and two with lithium; Table 2). Twelve subjects were medicated with an atypical antipsychotic. Three subjects were receiving antidepressant medication, three were receiving clonidine, one was receiving atomoxetine, and one was receiving methylphenidate. Two subjects were receiving quetiapine monotherapy, five subjects were taking two medications concomitantly, four were taking three medications concomitantly, and two were taking four medications concomitantly. Nine of the subjects with BPD had comorbid attention-deficit/hyperactivity disorder, three had oppositional defiant disorder, two had posttraumatic stress disorder, and one had anxiety in addition to BPD.
The YMRS scores (Table 3) differed significantly between unmedicated subjects with BPD, medicated subjects with BPD, and HCSs (ANOVA; df = 2, 25; F = 18.323; p = .000); this was driven by both unmedicated subjects with BPD and medicated subjects with BPD being significantly more manic than the HCSs (p = .000 and p = .000, respectively). There were no significant differences in the YMRS scores between the unmedicated subjects with BPD and medicated subjects with BPD (p = .271).
The CDRS scores differed significantly between unmedicated subjects with BPD, medicated subjects with BPD, and HCSs (ANOVA: df = 2, 26; F = 17.116; p = .000). Unmedicated subjects with BPD were significantly more depressed than both HCSs (p = .000) and medicated subjects with BPD (p = .003). Additionally, the medicated subjects with BPD were significantly more depressed than the HCSs (p = .002).
The mood state of the subjects with BPD was determined using the K-SADS-E. Two subjects were described as euthymic, 1 subject was depressed, 10 subjects were manic, and 9 subjects were described as in a mixed mood state. Of the unmedicated subjects with BPD, one was depressed, three were manic, and three were mixed.
All of the spectra in this study were fit using LCModel and a simulated basis-set (Fig. 1B). The mean metabolite levels (institutional units) and SDs for all of the measured metabolites are shown in Table 4.
TABLE 4.
Metabolite Levels in Institutional Units (Mean ± SD) for the Spectra Included in This Study
| Glna | Glu | NAA | Cho | Cr | m-Ino | |
|---|---|---|---|---|---|---|
| HCSs (10) | 9.53 ± 4.09 | 11.91 ± 2.96 | 11.47 ± 2.66 | 2.68 ± 0.90 | 10.17 ± 2.75 | 7.11 ± 2.19 |
| BPD (21) | 8.56 ± 3.20 | 12.59 ± 6.61 | 11.78 ± 5.87 | 2.92 ± 1.18 | 11.17 ± 4.09 | 7.80 ± 3.49 |
| BPD no med (6) | 5.77 ± 1.36 | 9.60 ± 5.49 | 10.14 ± 6.54 | 2.28 ± 1.28 | 9.05 ± 4.38 | 6.59 ± 3.99 |
| BPD med (15) | 9.68 ± 3.04 | 13.79 ± 6.80 | 12.44 ± 5.68 | 3.18 ± 1.08 | 12.02 ± 3.80 | 8.29 ± 3.30 |
Note: Glu = glutamate; Gln = glutamine; NAA = N-acetyl aspartate; Cr = creatine plus phosphocreatine; Cho = choline-containing compounds; m-Ino = myo-inositol–containing compounds; BPD = bipolar disorder; HCSs = healthy comparison subjects; med = medication.
Unmedicated children with BPD had lower glutamine levels than HCSs (df = 1, 16; F = 6.035; p = .027), and medicated subjects with BPD (df = 1, 21; F = 11.549; p = .003).
Structural 4.0-T MRI scans were segmented using open source software to determine gray matter, white matter, and CSF contributions to the voxel of interest. One HCS and four subjects with BPD (one unmedicated) did not have structural scans that allowed us to calculate their white matter, gray matter, and CSF contributions. Using MANOVA, there were no significant differences between voxel tissue content in unmedicated children with BPD (gray matter: 75.24 ± 9.95%, white matter: 20.32 ± 10.31%, and CSF: 4.44 ± 3.88%), medicated children with BPD (gray matter: 73.48 ± 9.47%, white matter: 19.62 ± 7.51%, and CSF: 6.90 ± 7.88%), and HCSs (gray matter: 71.48 ± 8.87%, white matter: 20.82 ± 9.25%, and CSF: 7.70 ± 6.99%; gray [df = 2, 27; F = 0.30; p = .74]; white [df = 2, 27; F = 0.05; p = .90]; CSF [df = 2, 29; F = 0.42; p = .66].
Diagnosis and Medication
Linear regression analysis was conducted to look at the effects of diagnosis, medication, age, and sex on glutamate and glutamine.
Glutamine
Unmedicated children with BPD had lower glutamine levels than HCSs (df = 1, 16; F = 6.035; p = .027), and medicated subjects with BPD (df = 1, 21; F = 11.549; p = .003). There were no significant differences between medicated children with BPD and HCSs or between subjects with BPD (as a whole) and HCSs.
All but three of the subjects in this study met criteria for having a mixed- and/or manic mood state. One unmedicated subject was depressed, and two medicated subjects were euythmic. Excluding these three subjects a linear regression analysis looking at the effects of diagnosis, age, and sex on glutamine showed unmedicated children with BPD had lower glutamine levels than HCSs (df = 1, 15; F = 4.939; p = .043) and medicated subjects with BPD (df = 1, 18; F = 8.917; p = .008).
Glutamate
There were no significant effects for unmedicated children with BPD versus HCSs, unmedicated children with BPD versus medicated children with BPD, medicated children with BPD versus HCSs, or children with BPD versus HCSs. This was the case including and excluding the one depressed and the two euythmic subjects.
Exploratory linear regression analysis was conducted to look at group differences in Cho, m-Ino, NAA, and Cr between unmedicated children with BPD and HCSs, unmedicated children with BPD and medicated children with BPD, medicated children with BPD and HCSs, and children with BPD and HCSs. No significant effects were found. This was the case including and excluding the one depressed and the two euythmic subjects.
Effects of Mood
Pearson correlations were performed to look at the interaction between YMRS and CDRS scores and metabolites glutamine, glutamate, NAA, Cho, Cr, and m-Ino. There were no significant effects of the metabolites on YMRS or CDRS scores. However, for all of the subjects, there was a trend for the CRDS score to correlate negatively with the ACC glutamine levels (n = 27, r = −0.348, p = .075); for just the BPD subjects, this trend was stronger (n = 20, r = −0.348, p = .064). There were no trends for the other metabolites and YMRS or CDRS scores.
IQ
There was no significant correlation between IQ and any of the measured metabolites for the study group as a whole or for the subgroups of HCSs, subjects with BPD, unmedicated subjects with BPD, and medicated subjects with BPD.
DISCUSSION
Unmedicated children with BPD had significantly lower glutamine levels than HCSs or medicated subjects with BPD. There were no differences in glutamate levels between the three groups. Reduced glutamine in unmedicated subjects with BPD compared with HCSs is consistent, with there being an abnormality in glial cells in the ACC in BPD (Ongur et al., 1998; Rajkowska et al., 2001). Brain tissue glutamine content reflects primarily glial glutamine concentrations (Gruetter, 2002; Hyder et al., 2006; Martinez-Hernandez et al., 1977). Glutamine in glia is a source for glutamate synthesis (Cooper et al., 2003; Hyder et al., 2006).
Medication in children with BPD appears to increase ACC Gln levels. Because the subjects with BPD in this study were taking various medications that have diverse modes of action, it is difficult to interpret what these medication effects on glutamine may mean. Glutamine is synthesized from glutamate by the enzyme glutamine synthetase; therefore, one could speculate that by increasing the efficiency of glutamine synthetase, one could increase the low glia glutamine levels, which in turn would increase the neuronal glutamate levels. Many anticonvulsant medications do not appear to upregulate glutamine synthetase activity (Fraser et al., 1999; Pavone and Cardile, 2003). For example, Fraser et al. (1999) report that in mouse cortical neurons, sodium valproate, lamotrigine, gabapentin, and levetiracetam have no effect on glutamine synthetase activity, and carbamazepine may reduce glutamine synthetase activity. Conversely, Collins et al. (1994) demonstrated that sodium valproate increased glutamate and glutamine but decreased glutamine synthetase activity in rat astrocytes. Pavone and Cardile (2003) demonstrated that in rat astrocytes low doses of lamotrigine, gabapentin, levetiracetam, and topiramate have little effect on glutamine synthetase, but at higher doses downregulate the enzyme’s activity, and carbamazepine and oxacarbazepine downregulate the enzyme’s activity even at low doses. Chlorpromazine has been shown to increase glutamine synthetase acutely in all brain regions in the rat and acutely and chronically in rat cerebral cortex (Chandrakala et al., 1987). Perhaps other antipsychotics have a similar effect. Twelve of the fifteen medicated subjects in this study were given an atypical antipsychotic. Marcus et al. (1986) have shown lithium increases glutamine and glutamate acutely and chronically in rat brain. However, lithium acutely upregulated glutamate synthesis in the cerebellum and brainstem, but not the cerebral cortex. Decreased glutamine synthetase activity has also been measured in the prefrontal cortex of subjects with schizophrenia (Burbaeva et al., 2003). Perhaps atypical antipsychotics play a role in upregulating glutamine synthetase activity. More work needs to be done to fully understand how psychotropic medications may be affecting glutamine synthetase activity.
Alternatively, the reduced levels of glutamine in the unmedicated subjects with BPD, compared with medicated subjects with BPD and HCSs, may be a result of the unmedicated subjects with BPD being significantly more depressed than either the medicated subjects with BPD or HCSs. There was a trend for CRDS score to correlate negatively with the ACC glutamine levels. In a recent postmortem study, Choudary et al. (2005) demonstrated reduced glutamine synthetase in the ACC of depressed subjects, 40% of whom had BPD. A reduction in glutamine synthetase activity could lead to reduced glutamine levels. What effect this would have on glutamate is not clear (Choudary et al., 2005).
We found no significant differences in glutamate between the three groups, although glutamate was lower in unmedicated subjects with BPD compared with HCSs or medicated subjects with BPD. Indeed, the highest glutamate levels were noted in the medicated subjects with BPD. The reasons for a lack of a significant change in glutamate may be multiple. While brain glutamate levels are high, the percentage of glutamate in glia (~10%) is small (Hyder et al., 2006). Therefore, any perturbation in the glial glutamate pool would be small to detect. Our lack of a finding for glutamate is consistent with a recent study at this center in which the anticonvulsant topiramate increased ACC glutamine levels in healthy male adults (Moore et al., 2006), but did not increase glutamate levels.
Limitations
This study has some limitations: the lack of a pre-and postmedication design, the diversity of medications that the medicated children were receiving, the small sample size, and the different mood states between the groups examined. These are issues that need to be addressed in future studies using larger samples and a pre- and posttreatment study design. Nevertheless, we believe that this study provides important new preliminary information that may help in understanding the pathophysiology of BPD, particularly early onset.
Clinical Implications
Unmedicated children with BPD have lower glutamine than HCSs and medicated children with BPD. These results are consistent with there being an abnormality in ACC glia in untreated child and adolescent BPD. These results are important in helping us better understand the pathophysiology of child and adolescent BPD and helping to develop better and more targeted treatments, in particular, those treatments affecting the metabolism of glutamine by the regulation of glutamine synthetase activity.
Acknowledgments
This study was supported by National Institute of Mental Health grant no. MH01978.
Footnotes
Disclosure: Dr. Frazier has received research support from, acted as a consultant to, and/or served on the speakers’ bureaus of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, Johnson & Johnson, Otsuka, and Pfizer. Dr. Renshaw is a consultant to Novartis, GlaxoSmithKline, and Kyowa Hakko and has received research support from Eli Lilly. Dr. Glod receives research funding from the Apollo Light Company. The other authors have no financial relationships to disclose.
References
- Biederman J. Pediatric bipolar disorder coming of age. Biol Psychiatry. 2003;53:931–934. doi: 10.1016/s0006-3223(03)00297-x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J. Pediatric mania: a developmental subtype of bipolar disorder? Biol Psychiatry. 2000;48:458–466. doi: 10.1016/s0006-3223(00)00911-2. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:675–680. doi: 10.1016/s0278-5846(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Cady EB. Clinical Magnetic Resonance Spectroscopy. New York: Plenum; 1990. [Google Scholar]
- Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- Cecil KM, DelBello MP, Sellars MC, Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- Chandrakala MV, Marcus SR, Nadiger HA, Sadasivudu B. Acute and long-term effects of chlorpromazine on glutamine synthetase and glutaminase in rat brain. J Neurochem. 1987;49:32–34. doi: 10.1111/j.1471-4159.1987.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RM, Jr, Zielke HR, Woody RC. Valproate increases glutaminase and decreases glutamine synthetase activities in primary cultures of rat brain astrocytes. J Neurochem. 1994;62:1137–1143. doi: 10.1046/j.1471-4159.1994.62031137.x. [DOI] [PubMed] [Google Scholar]
- Cooper J, Bloom F, Roth R. The Biochemical Basis of Neuropharmacology. 8. New York: Oxford University Press; 2003. Amino acid transmitters; pp. 105–150. [Google Scholar]
- Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Parow A, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- Davanzo P, Yue K, Thomas MA, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Cecil KM, Adler CM, Daniels JP, Strakowski SM. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuro-psychopharmacology. 2006;31:1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. The role of glial cells in regulation of neurotransmitter amino acids in the external environment. I. Trans-membrane electrical and ion gradients and energy parameters in cultured glial-derived cell lines. Brain Res. 1986;369:193–202. doi: 10.1016/0006-8993(86)90528-7. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorder-Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fraser CM, Sills GJ, Forrest G, Thompson GG, Brodie MJ. Effects of anti-epileptic drugs on glutamine synthetase activity in mouse brain. Br J Pharmacol. 1999;126:1634–1638. doi: 10.1038/sj.bjp.0702472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic resonance imaging studies in early-onset bipolar disorder: a critical review. Harv Rev Psychiatry. 2005;13:125–140. doi: 10.1080/10673220591003597. [DOI] [PubMed] [Google Scholar]
- Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6-to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994;33:461–468. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Bipolar disorder and attention-deficit/hyperactivity disorder in children and adolescents. J Clin Psychiatry. 2000;9:31–34. [PubMed] [Google Scholar]
- Gruetter R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int. 2002;41:143–154. doi: 10.1016/s0197-0186(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U. Glutamatergic neurotransmission modulation and the mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1113–1123. doi: 10.1016/j.pnpbp.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Newcomer BR, Pan JW. Measurements of human cerebral GABA at 4.1 T using numerically optimized editing pulses. Magn Reson Med. 1998;39:6–10. doi: 10.1002/mrm.1910390103. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Chu WJ, Mason GF, Newcomer BR. Biological and clinical MRS at ultra-high field. NMR Biomed. 1997;10:360–371. doi: 10.1002/(sici)1099-1492(199712)10:8<360::aid-nbm477>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassi RB, Axelson D, et al. Cingulate anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW. The emerging differential roles of GABAergic and antiglutamatergic agents in bipolar disorders. J Clin Psychiatry. 2003;64:15–20. [PubMed] [Google Scholar]
- Ketter TA, Wang PW, Becker OV, Nowakowska C, Yang YS. The diverse roles of anticonvulsants in bipolar disorders. Ann Clin Psychiatry. 2003;15:95–108. doi: 10.1023/a:1024636309185. [DOI] [PubMed] [Google Scholar]
- Kruger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Marcus SR, Nadiger HA, Chandrakala MV, Rao TI, Sadasivudu B. Acute and short-term effects of lithium on glutamate metabolism in rat brain. Biochem Pharmacol. 1986;35:365–369. doi: 10.1016/0006-2952(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Moore CM, Breeze JL, Gruber SA, et al. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord. 2000;2:207–216. doi: 10.1034/j.1399-5618.2000.20302.x. [DOI] [PubMed] [Google Scholar]
- Moore CM, Wardrop M, Frederick BD, Renshaw PF. Topiramate raises anterior cingulate cortex glutamine levels in healthy male adults; A 4.0 T magnetic resonance spectroscopy study. Psychopharmacology. 2006;188:236–243. doi: 10.1007/s00213-006-0451-y. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Galloway MP. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone A, Cardile V. An in vitro study of new antiepileptic drugs and astrocytes. Epilepsia. 2003;44:34–39. doi: 10.1046/j.1528-1157.44.s10.5.x. [DOI] [PubMed] [Google Scholar]
- Post RM, Chang KD, Findling RL, et al. Prepubertal bipolar I disorder and bipolar disorder NOS are separable from ADHD. J Clin Psychiatry. 2004;65:898–902. doi: 10.4088/jcp.v65n0703. [DOI] [PubMed] [Google Scholar]
- Poznanski E. Children’s Depression Rating Scale, Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Tansey FA, Farooq M, Cammer W. Glutamine synthetase in oligodendrocytes and astrocytes: new biochemical and immunocyto-chemical evidence. J Neurochem. 1991;56:266–272. doi: 10.1111/j.1471-4159.1991.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Theberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. [Accessed November 7 2006];Guidance for Industry and FDA Staff: Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices. 2003 Available at: http://www.fda.gov/cdrh/ode/guidance/793.html.
- Wozniak J. Pediatric bipolar disorder: the new perspective on severe mood dysfunction in children. J Child Adolesc Psychopharmacol. 2003;13:449–451. doi: 10.1089/104454603322724832. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]