Abstract
Clinical criteria, primarily young age of cancer onset and family history of signature cancers, have been developed to identify individuals at elevated risk for Lynch Syndrome with the goals of early identification and cancer prevention. In 2007, the Society of Gynecologic Oncology (SGO) codified criteria for women presenting with gynecologic cancers. These criteria have not been validated in a population-based setting. For 412 unselected endometrial cancers, immunohistochemical expression of DNA mismatch repair proteins and MLH1 methylation were assessed to classify tumors as sporadic or probable Lynch Syndrome. In this cohort, 10.5% of patients were designated as probable Lynch Syndrome based on tumor testing. The sensitivity and specificity of SGO criteria to identify these same cases were 32.6% (95% CI 19.2–48.5) and 77% (95% CI 72.7–81.8), respectively. With the exception of tumor location in the lower uterine segment, multivariate analysis of clinical features, family history, and pathologic variables failed to identify significant differences between the sporadic and probable Lynch Syndrome groups. A simplified cost-effectiveness analysis demonstrated that SGO clinical criteria and universal tissue testing strategies had comparable costs per probable Lynch Syndrome patient identified. In conclusion, SGO criteria successfully identify probable Lynch Syndrome cases among women with endometrial cancer who are young or have significant family history of signature tumors. However, a larger proportion of probable Lynch Syndrome patients who are older and have less significant family history are not detected by this screening strategy. Universal tissue testing may be necessary to capture more individuals at risk for having Lynch Syndrome.
Keywords: Lynch Syndrome, Endometrial Cancer, Screening, Cost analysis
INTRODUCTION
Lynch Syndrome is an inherited cancer syndrome due to a germline mutation in a DNA mismatch repair (MMR) gene, primarily MLH1, MSH2, MSH6 or PMS2. Lynch Syndrome is thought to account for 1–3% of all endometrial cancers (1). For women with Lynch Syndrome, a gynecologic cancer is the sentinel cancer diagnosis in 50% of instances (2). The lifetime risks of developing endometrial cancer and colorectal cancer for these women are 39.4% and 42.7%, respectively, and the lifetime risk of developing either one of these carcinomas is 73.4% (3). After sentinel cancer diagnosis, individuals with Lynch Syndrome have a cumulative risk of developing a secondary Lynch Syndrome associated tumor of 1.5–3% per year (4). A diagnosis of Lynch Syndrome provides the patient with the opportunity to undergo surveillance for these cancers, facilitating their prevention and/or early detection, and allows for the opportunity for cancer prevention in first-degree relatives (FDRs).
Computer-based clinical prediction models, PREMM1,2,6, MMRPredict and MMRPro, have emerged to help identify an individual’s risk for having a Lynch Syndrome mutation (5–7). These models were validated in the colorectal cancer population for the detection of MLH1, MSH2, and MSH6 mutations with favorable results. When applied to the endometrial cancer patient population, however, these models fail to perform at a level that would support their use as a clinical screening tool (8).
In 2007, the Society of Gynecologic Oncology (SGO) published a statement with clinical criteria for which gynecologic cancer patients would benefit from further evaluation for Lynch Syndrome. Based on a constellation of criteria dominated by features such as young age of cancer diagnosis and family history of Lynch Syndrome associated tumors, SGO established two groups of patients – those with a 5–10% probability of having a germline mutation in a DNA mismatch repair gene and those with a 20–25% probability. The expert panel stated that genetic risk assessment (genetic counseling with genetic testing if appropriate) for individuals with a 5–10% likelihood of having a germline mutation is reasonable and asserted that individuals with a 20–25% possibility of a germline mutation should undergo risk assessment (9).
Ryan et al. investigated the utility of these criteria in a cohort of 76 endometrial cancer patients with a known germline mutation and found that SGO 5–10% criteria performed the best by correctly identifying 93% of known mutation carriers, whereas SGO 20–25% criteria identified 71%. It should be noted that these patients had a mean age of 47.3 years and were identified through databases from the British Columbia Familial Cancer Registry and Mount Sinai Hospital Familial Gastrontestinal Cancer Registry. In addition, 68/76 of these patients had MLH1 or MSH2 mutations; only 8/76 had an MSH6 mutation, and there were no PMS2 mutation carriers (10). The SGO criteria have not yet been validated in a population-based setting.
In addition to clinical screening criteria, molecular diagnostic techniques such as the PCR-based microsatellite instability analysis (MSI) and immunohistochemistry evaluating expression of the DNA MMR proteins can be used to screen for Lynch Syndrome-associated cancers. For tumors with immunohistochemical loss of MLH1, the PCR-based MLH1 promoter methylation assay assists in delineating sporadic (methylated) from suspicious for Lynch Syndrome (unmethylated) tumors. The overall sensitivity of MSI and immunohistochemistry for identifying Lynch Syndrome germline mutations in colorectal cancers is similar, with rates of 83% and 94%, respectively (11). Current recommendations in the colorectal cancer literature are to perform tissue testing on all newly diagnosed colorectal cancer patients tumors regardless of personal or family history (12).
The “gold standard” for diagnosing Lynch Syndrome is to detect a germline mutation in one of the DNA MMR genes. Sequencing has excellent sensitivity for detecting point mutations and minor insertions and deletions, but large deletions/insertions or gene rearrangements pose limitations of conventional sequencing techniques (13). It is unclear whether individuals with tissue testing results suggestive of Lynch Syndrome with negative germline testing are truly negative or if their mutations have genetic features that make accurate identification of the germline aberration by conventional techniques more difficult.
In many published studies, the data regarding Lynch Syndrome-associated endometrial cancer is derived from hereditary colorectal cancer registries. These patient populations are biased towards young age at cancer diagnosis and having larger families with many family members affected by Lynch Syndrome-associated cancers. The purpose of this study was to evaluate the performance of SGO 5–10% criteria (summarized in supplemental Table 1) in identifying endometrial cancers with tissue testing results consistent with a diagnosis of Lynch Syndrome in a large cohort of unselected endometrial cancer patients.
MATERIALS AND METHODS
Patient Population and Study Design
After obtaining Institutional Review Board approval, cases of endometrial cancer involving women who underwent hysterectomy at MD Anderson Cancer Center through 2011 were identified. Beginning with the most recent cases, endometrial cancers were included if the patient was 18 years of age or greater and sufficient tissue from the surgery was available for molecular analysis. Endometrioid and non-endometrioid histologies of endometrial carcinoma were included. Relevant clinical data were extracted from physician and genetic counselor notes and patient intake forms, all available in the electronic medical record. All hysterectomies were pathologically reviewed by a gynecologic pathologist (RRB). Pathologic data were derived from the pathology report for each hysterectomy specimen. Surgical stage was derived from the pathology report and the surgeon operative note. These data are summarized in Table 1.
Table 1.
Clinicopathologic features of sporadic and probable Lynch Syndrome-associated endometrial cancers.
| Clinical Features | Sporadic EC1 N (%) |
PLS EC2 N (%) |
p -value |
|---|---|---|---|
|
| |||
| Median Age at Diagnosis (yrs) Range |
61 (18–92) | 61 (42–87) | |
|
| |||
| Age (yrs) | |||
| < 50 | 52 (14.2) | 10 (23.3) | 0.12 |
| ≥ 50 | 313 (85.8) | 33 (76.7) | |
|
| |||
| BMI (kg/m2) | |||
| < 30 | 121 (33.2) | 17 (39.5) | 0.41 |
| ≥ 30 | 243 (66.8) | 26 (60.5) | |
|
| |||
| History of Diabetes | |||
| Y | 89 (24.4) | 9 (20.9) | 0.62 |
| N | 276 (75.6) | 34 (79.1) | |
|
| |||
| History of Hypertension | |||
| Y | 201 (55.1) | 18 (41.9) | 0.10 |
| N | 164 (44.9) | 25 (58.1) | |
|
| |||
| 3 Family History of EC | |||
| Y | 35 (9.8) | 4 (9.8) | > 0.99 |
| N | 323 (90.2) | 37 (90.2) | |
|
| |||
| 3 Family History of CRC4 | |||
| Y | 58 (16.2) | 8 (19.0) | 0.63 |
| N | 301 (83.8) | 34 (81.) | |
|
| |||
| Family History of CRC or EC | |||
| Y | 87 (24.2) | 12 (28.6) | 0.54 |
| N | 272 (75.8) | 30 (71.4) | |
| Pathologic Features | Sporadic EC N (%) |
PLS EC N (%) |
p -value |
|---|---|---|---|
|
| |||
| Histology | |||
| Endometrioid | 299 (81.9) | 37 (86.0) | 0.67 |
| Non-endometrioid | 66 (18.1) | 6 (14.0) | |
|
| |||
| FIGO5 Stage | |||
| I & II | 289 (79.2) | 37 (86.0) | 0.42 |
| III & IV | 76 (20.8) | 6 (14.0) | |
|
| |||
| FIGO Grade | |||
| 1 & 2 | 267 (73.2) | 32 (74.4) | 0.86 |
| 3 | 98 (26.8) | 11 (25.6) | |
|
| |||
| Depth of myometrial invasion | |||
| < 50% | 257 (70.4) | 32 (74.4) | 0.58 |
| ≥ 50% | 108 (29.6) | 11 (25.6) | |
|
| |||
| Tumor Location | |||
| Corpus | 357 (97.8) | 38 (88.4) | 0.007 |
| Lower uterine segment | 8 (2.2) | 5 (11.6) | |
|
| |||
| Largest gross tumor dimension (cm) | |||
| Mean ± Standard Deviation | 4.3 ± 3.2 | 3.6 ± 2.9 | 0.13 |
| Median (range)6 | 4 (0–25) | 3.5 (0–13) | |
EC; Endometrial Cancer
PLS EC; Probable Lynch Syndrome-associated endometrial cancer
Family history of EC or CRC; patient has a first- or second-degree relative with a diagnosis of endometrial cancer or colorectal cancer
CRC; Colorectal cancer
FIGO; International Federation of Gynecology and Obstetrics
Some tumors were not apparent grossly, but were identified by microscopic examination of the entire endometrium. These tumors were designated with a largest gross tumor dimension of 0.
The endometrial cancer cohort from this study is derived from a large NCI-designated cancer center. Comparison to published data from a national epidemiologic analysis of 161,513 endometrial cancer patients shows that the MD Anderson cohort is comparable in terms of age at diagnosis, proportion of endometrioid vs. non-endometrioid histologies, and stage distribution. The only notable difference between the two patient populations is that MD Anderson has a higher proportion of women with grade 2 and 3 endometrioid carcinomas (14).
For this work, sporadic and probable Lynch Syndrome patients were defined based on the results of tissue testing studies. Thus, an endometrial cancer patient with intact immunohistochemical expression of MLH1, MSH2, MSH6, and PMS2 would be considered as having a sporadic endometrial carcinoma. Similarly, a patient with immunohistochemical loss of MLH1, but presence of MLH1 methylation, was also considered sporadic. Patients with tumors with immunohistochemical loss of MSH2, MSH6, and PMS2 were considered as probable Lynch Syndrome. Those with tumors with immunohistochemical loss of MLH1 and absence of MLH1 methylation were also considered probable Lynch Syndrome. When reviewing medical records of these patients, a family history of a specific cancer was defined as having a first- or second-degree relative with a diagnosis of that specific cancer.
Molecular Analyses
All tissue-based analyses were performed using formalin-fixed, paraffin-embedded endometrial carcinoma sections derived from the hysterectomies in Clinical Laboratory Improvement Amendments (CLIA) – and College of American Pathology (CAP) – approved clinical pathology laboratories. Immunohistochemistry was performed using standard techniques for MLH1 (G168-15, 1:25; BD Biosciences Pharmingen), MSH2 (FE11, 1:100; Calbiochem), MSH6 (44, 1:300; BD Biosciences Pharmingen), and PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen) (15). MLH1, MSH2, MSH6, and PMS2 immunohistochemistry was scored as protein intact or deficient using light microscopic examination. Complete absence of mismatch repair protein expression was required in order for a case to be designated as mismatch repair deficient. Stromal cells served as an internal positive control. Tumors with immunohistochemical loss of MSH2, MSH6, or PMS2 were designated as probable Lynch Syndrome. For tumors with loss of MLH1 protein expression, PCR-based MLH1 promoter methylation analysis was performed. DNA was isolated from mapped formalin-fixed, paraffin-embedded tissue sections that were dissected with a scalpel blade to provide relatively pure tumor samples for analysis. Isolated DNA was treated with bisulfite to convert unmethylated cytosine nucleotides to uracil using the Zymo EZ DNA Methylation-Gold Kit according to the manufacturer’s instructions (Zymo Research, Orange, CA). Methylation of MLH1 was assessed using a modified version of methylation-specific PCR followed by capillary electrophoresis using FAM labeled reverse primer and unlabeled forward primers (Integrated DNA Technology). The primer sequences were the following: methylated forward, 5′-GAT AGC GAT TTT TAA CGC-3′, unmethylated forward, 5′-AGA GTG GAT AGT GAT TTT TAA TGT-3′ and labeled reverse primer, 5′-FAM-TCT ATA AAT TAC TAA ATC TCT TC-3′. The forward primers were designed to distinguish the methylated amplicon from the unmethylated by difference in size. The bisulfite treated DNA was then amplified by PCR using primers specific for methylated and unmethylated DNA. The methylated PCR product of 85 bp was separated from unmethylated PCR product of 91 bp by capillary electrophoresis using an ABI Prism 3130 Genetic Analyzer. Chromatograms for tumor were compared to those generated for the RKO colon carcinoma cell line (positive control known to have loss of MLH1 protein due to MLH1 promoter methylation) and the leukemia cell line K562 (negative control with no MLH1 methylation). Tumors with MLH1 immunohistochemical loss and presence of MLH1 methylation were designated as sporadic, while tumors with MLH1 loss and absence of MLH1 methylation were designated as probable Lynch Syndrome (16).
Cost Analysis
A simplified cost-effectiveness analysis was performed to compare the direct costs of utilizing SGO 5–10% clinical criteria to universal tissue testing (immunohistochemistry for all and MLH1 methylation analysis when indicated) for the 412 cases included in this study. The analysis was conducted from a third-party payer perspective. Two approaches were used to assess cost-effectiveness. The first method assessed the direct costs associated with identifying patients with probable Lynch Syndrome (PLS) among women diagnosed with endometrial cancer for SGO 5–10% clinical criteria and universal tissue testing. The second method evaluated direct costs associated with identifying cases of PLS among women with endometrial cancer as well as their potentially affected first-degree relatives (FDRs). Since actual germline mutation results were unknown and tissue testing results do not always correlate with identification of a germline mutation, the percentage of tumors identified as PLS that would have corresponding germline mutations was varied between 25–75% to represent the range of germline mutations found in previously published studies (17, 18). Costs included were hospital and professional costs associated with identifying PLS cases. Costs were estimated for initial genetic counseling and follow-up visits, immunohistochemistry for MLH1, MSH2, MSH6 and PMS2, MLH1 promoter methylation assay for tumors with loss of MLH1, and single gene germline mutation testing. Cost estimates were based on using CPT codes and Medicare reimbursement fees which were obtained from the Physician Fee Schedule and Laboratory Fee Schedule (Table 2) (19). All cost amounts were calculated in 2012 U.S. dollars. The following clinical assumptions were made for the cost-effectiveness analysis: 1) 25–75% of endometrial cancer patients with immunohistochemical loss of expression of a mismatch repair protein would have a germline mutation detected; 2) all women with probable Lynch Syndrome identified by tissue testing would undergo genetic counseling and recommended germline testing; 3) all FDRs would undergo recommended genetic counseling and germline testing; 4) for the purposes of costs associated with testing FDRs, it was assumed that 50% of individuals with immunohistochemical loss of a DNA MMR protein would have an identifiable germline mutation.
Table 2.
Technical and professional costs associated with tumor testing, genetic counseling, and germline testing of endometrial carcinomas.
| Medicare reimbursement amounts | Cost with SGO Screening N = 97 |
Cost for Universal Screening N = 412 |
|
|---|---|---|---|
| Initial genetic counseling consultation (1 hr)a | $192.67 | $18,688.99 | $8,862.82 |
| Follow-up genetic counseling visits (30 min)a | $105.20 | $11,151.20 | $4,628.80 |
| IHC for MLH1, MLH2, MSH6, and PMS2b | $422.08 | $40,941.76 | $173,896.96 |
| MLH1 promoter methylation assay for tumor with IHC loss of MHL1c | $90.25 | $1,173.25 | $8122.50 |
| Single gene germ-line testingd | $1300 | $19,500 | $57,200 |
| Single site testinge,f | $475 | N/A | N/A |
Medicare does not reimburse for genetic counseling. Costs shown are estimates for genetic counseling before genetic testing.
CPT code 88342 used for each individual IHC DNA MMR protein
CPT stack codes 83900, 83909, 83912 used in 2012 (no longer effective 01/2013).
Cost of germline testing was obtained from Myriad ABN worksheet
Cost of single site testing was obtained from Myriad ABN worksheet
Single site testing intended for first degree relatives of patients with endometrial cancer who were identified as having Lynch Syndrome based upon positive germline test results
Statistical Analysis
Clinical and pathological criteria were compared across a variety of groups. STATA v 12 was used to perform statistical analyses. Fisher’s, chi-squared, Mann-Whitney, or t-test were conducted to test association across groups depending on the distribution of the data. Clinical and pathological criteria were compared between the sporadic and probable Lynch Syndrome groups. CART analysis was performed to identify a set of variables that would possibly predict Lynch Syndrome in the absence of tissue testing. Sensitivity and specificity were calculated along with their 95% binomial exact confidence intervals for SGO 5–10% clinical criteria in its ability to predict probable Lynch Syndrome tumors.
RESULTS
Comparison of sporadic and probable Lynch Syndrome endometrial cancers
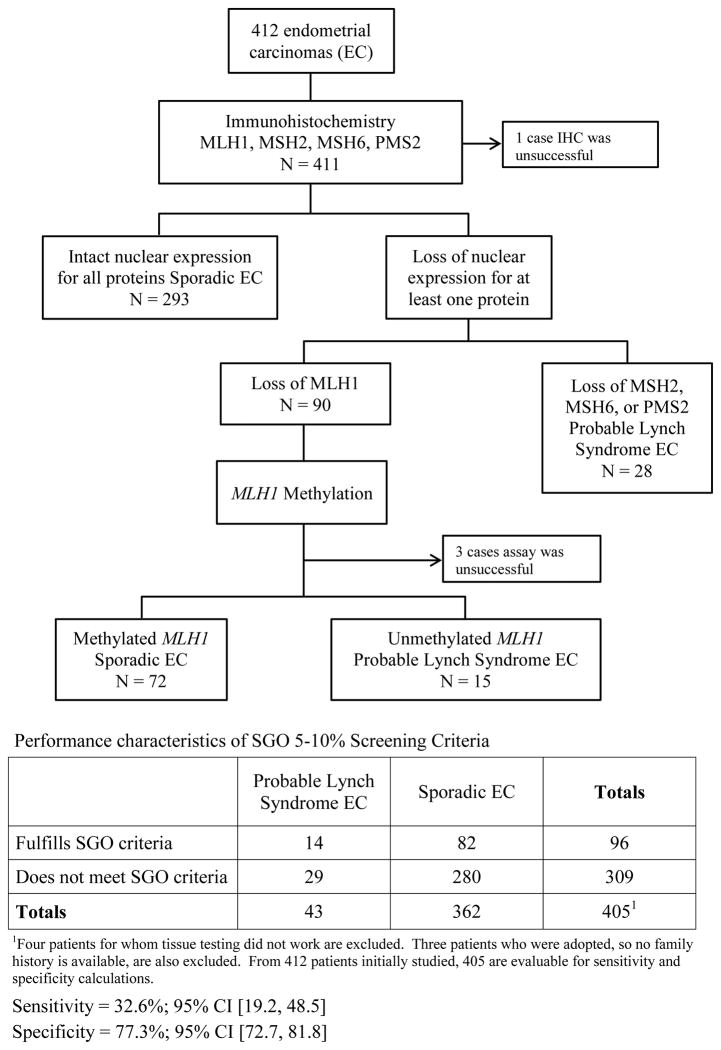
Four-hundred-twelve patients with endometrial carcinoma met inclusion criteria, and their tumors were subjected to tissue testing. Immunohistochemistry was uninterpretable in one case (lack of positive staining in the internal positive control), and the MLH1 methylation testing failed in 3 cases because of insufficient DNA amplification. Of the 411 cases with complete immunohistochemistry results, 293 showed intact nuclear staining for all four DNA mismatch repair proteins, and 118 had loss of at least one mismatch protein (90 MLH1 + PMS2 loss; 12 MSH2 + MSH6 loss; 9 MSH6 loss; 7 PMS2 loss). Of those with loss of MLH1 + PMS2, 72/90 (80%) had associated methylation of MLH1 and were thus considered sporadic (Figure 1). The total number of probable Lynch Syndrome endometrial carcinomas in this series was 43 (10.5%). These tissue testing data were used to stratify the endometrial cancer patients into either the sporadic category or the probable Lynch Syndrome category in Table 1.
Figure 1.
Demographic and pathologic information for the 408 EC cases with complete tissue testing results are summarized in Table 1. The median age at diagnosis in this cohort was 60.5 years with a range of 18–92. The majority of women were older than age 50, obese (BMI ≥ 30 kg/m2), and had unremarkable family histories for endometrial or colorectal cancer. The median largest tumor dimension was 4.3 cm. Most tumors were endometrioid histology, grade 1 or 2, early stage, and located in the uterine corpus.
A comparison between patients with sporadic endometrial cancer and probable Lynch Syndrome-associated endometrial cancer was made using a variety of clinical and pathologic criteria (Table 1). With the exception of tumors arising from the lower uterine segment, there were no pathologic or clinical variables that could distinguish the two groups. Endometrial carcinomas arising in the lower uterine segment were previously identified to be associated with Lynch Syndrome. However, lower uterine segment localization was found to occur in only 3.5% of cases in a large study of 1,009 patients (20). Due to its rarity, lower uterine segment localization cannot solely be used to clinically distinguish sporadic endometrial carcinoma from Lynch Syndrome associated endometrial carcinoma.
Evaluation of SGO 5–10% Screening Criteria
The SGO 5–10% screening criteria were next applied to the endometrial cancer patients who had both informative tissue testing results and evaluable family history information (Figure 1). Using clinical history exclusively irrespective of tumor testing results, 96 patients meet SGO 5–10% criteria. Fourteen of these patients had tissue testing results that would be consistent with Lynch Syndrome, resulting in 82 women that would potentially undergo unnecessary genetic counseling evaluation and genetic testing. Of these 14 patients, 11/14 were diagnosed with endometrial cancer at age less than 50 years and 6/11 fulfilled SGO criteria by age of diagnosis only (no family history of Lynch Syndrome associated cancer). Only 7/14 women had family histories that fulfilled SGO criteria. The specificity of SGO 5–10% criteria was quite favorable (77.3%; 95% CI 72.7–81.8). Sensitivity, however, was poor (32.6%; 95% CI 19.2–48.5).
From the data above, we concluded that the established SGO 5–10% screening criteria were not optimal for detecting probable Lynch Syndrome patients in an unselected endometrial cancer patient population. To determine if the SGO criteria could be adjusted to be more effective, the age cutoff in these guidelines was incrementally adjusted and a receiver-operator characteristic curve (ROC) generated. An area under the ROC curve of 1 indicates perfect predictive ability, and an area under the curve of 0.5 indicates no predictive ability. In this analysis, the area under the curve was 0.5 (data not shown). From the clinical and pathological data summarized in Table 1, a Classification and Regression Tree (CART) analysis was performed to determine if alternative screening criteria to detect Lynch Syndrome could be generated, but this analysis yielded no superior alternative criteria (data not shown).
SGO 5–10% criteria were then evaluated in the specific probable Lynch Syndrome groups according to type of mismatch repair protein loss (Table 3). Approximately 40% of the patients with MLH1+PMS2 loss (and absence of MLH1 methylation) or MSH2+MSH6 loss fulfilled SGO 5–10% screening criteria. Substantially fewer patients with tumors with MSH6 loss (22.2%) or PMS2 loss (14.3%) fulfilled these same clinical screening criteria. Lower BMI, which has previously been associated with Lynch Syndrome associated endometrial cancers (21–23), was more common in the MSH2+MSH6 and MSH6 groups, but this was not significantly different from the other MMR loss groups (Table 3). Obesity’s increasing prevalence in the U.S. may be obscuring a previously significant clinical differentiator between patients with sporadic and Lynch Syndrome associated endometrial carcinomas. Of the 43 probable Lynch Syndrome patients, only 5 had lower uterine segment tumors, with 3/5 of these in the PMS2 loss group (Table 3). Only 9.8% of the probable Lynch Syndrome patients had a family history of endometrial cancer, and only 19.0% of these women had a family history of colorectal cancer. Family history statistics were not significantly different among the MMR protein loss groups (Table 3). From Table 3, it was concluded that SGO 5–10% criteria are most predictive for patients with endometrial carcinomas with MLH1+PMS2 loss or MSH2+MSH6 loss, but more than half of the women in these MMR groups would not be accurately identified on the basis of clinical criteria alone.
Table 3.
Comparison of select historical factors for Lynch Syndrome among endometrial cancers by type of immunohistochemical protein loss.
| MLH1/PMS2 No MLH1 methylation N = 15 |
MSH2/MSH6 N = 12 |
MSH6 N = 9 |
PMS2 N = 7 |
p-value | |
|---|---|---|---|---|---|
|
Median age at diagnosis (yrs) (Range) |
62 (43–79) |
56 (42–71) |
62 (50–76) |
56 (45–87) |
0.50 --- |
| BMI < 30 kg/m2 | 4 (26.7%) | 5 (41.6%) | 6 (66.6%) | 2 (28.6%) | 0.29 |
| Family History EC1 | 3 (21.4%) | 0 | 1 (12.5%) | 0 | 0.31 |
| Family History CRC2 | 3 (20%) | 3 (25%) | 1 (12.5%) | 1 (14.3%) | 0.95 |
| LUS3 Tumor | 1 (6.7%) | 1 (8.3%) | 0 | 3 (42.9%) | 0.05 |
| Endometrioid Histology | 13 (86.7%) | 9 (75%) | 8 (88.9%) | 7 (100%) | 0.62 |
| Stage I or II | 13 (86.7%) | 8 (66.7%) | 9 (100%) | 7 (100%) | 0.14 |
| Meets SGO4 5–10% Criteria | 6 (40.0%) | 5 (41.7%) | 2 (22.2%) | 1 (14.3%) | 0.62 |
EC; patient has a first- or second-degree relative with a diagnosis of endometrial cancer
CRC; patient has a first- or second-degree relative with a diagnosis of colorectal cancer
LUS; Tumor arising from lower uterine segment
SGO; Society of Gynecologic Oncology
Cost Analysis
SGO criteria do not demonstrate a high enough sensitivity to warrant widespread use in the general population of women diagnosed with endometrial cancer. While universal tissue testing identifies a larger number of PLS endometrial cancer patients, its costs may be prohibitive. To examine the costs and outcomes associated with identifying PLS cases for each screening strategy, a simplified cost-effectiveness analysis was performed using the entire cohort of 412 unselected endometrial cancer cases.
SGO 5–10% criteria were applied to the cohort, identifying 97 women who would undergo further evaluation through tissue testing and genetic counseling, resulting in a total cost of $91,455 (Table 4). Of the 97 cases identified by the SGO model, only 15 were found by tissue testing to be PLS. The total cost per PLS case detected in the SGO model was $6,097. It is known that positive tissue testing results are not always associated with the identification of a deleterious germline mutation in a mismatch repair gene. To account for the range of germline mutations published in the literature, the proportion of positive tissue tests associated with germline mutations was estimated at 25%, 50% and 75% in tumors with immunohistochemical loss of a DNA MMR protein resulting in 4,8, and 11 identifiable germline mutations, respectively. To account for the number of potentially affected first-degree relatives, data from the electronic medical records were used to calculate an average of 5.3 first-degree relatives for patients who met SGO Criteria. Based on this number and the range of estimated germline mutation rates among probable Lynch Syndrome endometrial cancer patients, 21, 42, and 48 first- degree relatives would be eligible for single site gene mutation analysis and enhanced Lynch Syndrome screening. The estimated cost for screening both the probable Lynch Syndrome patients and their first degree relatives in this strategy is $3,006–$6,329 per PLS case identified based on germline detection rates of 25–75% (Table 4).
Table 4.
Comparison of direct Medicare costs associated with SGO Criteria and universal tumor testing models.
| Screening Strategy | SGO | Universal |
|---|---|---|
|
Endometrial Cancer Patients (N = 412)
| ||
| # who undergo IHC testing | 97 | 412 |
| # who have loss of expression of IHC | 21 | 118 |
| # who undergo MLH1 methylation testing | 13 | 90 |
| # seen by genetic counselor | 97 | 46 |
| # probable Lynch Syndrome identified by strategy | 15 | 43 |
| # probable Lynch Syndrome with positive germline test (Detection rates of 25%, 50%, and 75% germline detection1) | 4, 8, 11 | 11, 22, 32 |
|
| ||
|
Estimated Costs for Screening Strategies
| ||
| Cost to screen 412 patients | $91,455 | $252,711 |
| Average cost per probable Lynch Syndrome case detected | $6,097 | $5,877 |
|
| ||
|
First degree relatives (FDRs)
| ||
| # FDRs eligible for germline testing if 25%, 50%, or 75% of patients have an identifiable germline mutation | 21, 42, 58 | 60, 121, 176 |
| Assuming 50% of probable Lynch Syndrome cases have an identifiable germline mutation: | ||
| # of FDRs who will be germline positive for LS if 25%, 50% or 75% inherit the same mutation2 | 11, 21, 32 | 30, 61, 91 |
|
| ||
| Estimated Costs For Screening Including both PLS and FDRs (Assuming 50% of probable Lynch Syndrome patients have germline deleterious mutation) | ||
|
| ||
| Cost per LS case identified if 25% of FDRs have positive germline mutation: | $6,329 | $6,455 |
| Cost per LS case identified if 50% of FDRs have a positive germline mutation: | $4,146 | $4,044 |
| Cost per LS case identified if 75% of FDRs have a positive germline mutation: | $3,006 | $2,970 |
Estimate of 25–75% germline mutation detection rate based upon published data from Hampel et al. and Senter et al. (17, 18)
For SGO and Universal screening methods, we made the assumption that 50% of the probable Lynch Syndrome group identified in each screening method would have a germ line mutation identified. This number was then multiplied by the mean number of first degree relatives per cohort (SGO – 5.3; Universal – 5.5) and then multiplied by 25%, 50%, and 75% to estimate the number of potentially impacted first degree relatives.
The universal tumor testing model identified 43 endometrial cancer patients who warranted further work-up through genetic counseling and germline testing. The total cost of this screening strategy was $252,711, with a cost per probable Lynch Syndrome case identified of $5,877. Identifiable germline mutation detection rates of 25%, 50%, and 75% based on positive tumor test resulted in 11,22, and 32 patients who would have a positive germline mutation, respectively (Table 4). The average number of first-degree relatives for those included in the universal tumor-testing model was 5.5. Based on this and the range of estimated germline mutation rates among probable Lynch Syndrome endometrial cancer patients, 60, 121, and 176 first-degree relatives would be eligible for single-site gene mutation analysis and enhanced Lynch Syndrome screening. The estimated cost for screening both probable Lynch Syndrome patients and their first degree relatives in this strategy ranges between $2,970–$6,455 per case (Table 4). To summarize, while universal tumor testing with immunohistochemistry and MLH1 methylation analysis when the tumor has loss of MLH1 protein involves greater total dollar costs, it also identifies a greater number of Lynch Syndrome patients and ultimately costs less per identified patient compared to the SGO clinical screening strategy.
DISCUSSION
A major conclusion from this work is that the existing SGO 5–10% clinical criteria for identifying endometrial cancer patients with Lynch Syndrome misses most patients who could be captured by universal tissue testing (immunohistochemistry for DNA mismatch repair proteins and PCR-based MLH1 methylation analysis for tumors with loss of MLH1). The predominance of MLH1 and MSH2 mutation carriers within Lynch Syndrome registries, including the two registries in which mutation carriers from the Ryan et al. study were derived, results in validation of existing criteria for these individuals with MLH1 and MSH2 germline mutations (10). The relative paucity of MSH6 and PMS2 mutation carriers in Lynch Syndrome registries suggests that these mutations are rare or that they are underestimated and missed by current screening strategies that rely on young age of cancer onset and family history of signature cancers. Consistent with the idea that these mutations are missed using existing clinical screening strategies, in the current study 16/43 (37.2%) women had endometrial cancers with MSH6 or PMS2 protein loss. Senter et al. investigated 99 individuals with immunohistochemical loss of PMS2 in Lynch Syndrome associated carcinomas (91 colorectal, 5 endometrial, 1 gastric, 1 small bowel, and 1 transitional cell of renal pelvis) (18). They found that 62% of patients with immunohistochemical loss of PMS2 in the tumor had a detectable PMS2 germline mutation; 25.5% of these patients did not meet any published Lynch Syndrome clinical screening criteria. Hendriks et al. investigated individuals with MSH6 germline mutations who met Amsterdam II Criteria and found that MSH6 mutations carriers typically do not meet standard clinical criteria for identifying Lynch Syndrome patients (24). Additional studies have reported similar findings for MSH6 mutation carriers (25, 26).
A strong family history of certain cancers played a pivotal role in the initial identification and characterization of Lynch Syndrome and continues to be a principal component in widely accepted clinical screening algorithms. In 2009, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group de-emphasized the role of family history when evaluating colorectal cancer patients for risk of Lynch Syndrome and recommended a universal tumor testing approach. This recommendation was due in part to the poor overall sensitivity and specificity profiles of clinic-based screening criteria such as Amsterdam II or Revised Bethesda guidelines as well as suboptimal patient history recording by clinicians (27). Consistent with the EGAPP recommendations, there were no statistically significant differences in family history of endometrial cancer or colorectal cancer between probable Lynch Syndrome and sporadic cases in the current study. Further, the majority of all patients in the current cohort did not have a family history of either tumor. Average family size in the 21st century is not the same as it was when Lynch Syndrome was first characterized in the early 1900’s. In Dr. Warthin’s original Family G, there was a male proband with 10 children (28). In the cohort examined for this study, the average number of siblings among the endometrial cancer patients was 3.4, and the average number of children was only 2.1. It is not that family history lacks relevance in detecting hereditary cancers; rather, our data support the idea that as family sizes decrease, the probability of detecting a family history of cancers also decreases. The clinical utility of family history in identifying an individual patient’s risk for hereditary cancer syndromes may be decreasing in the current generations.
The median age of sentinel cancer diagnosis for MSH6 and PMS2 mutations carriers has been shown to be older than that of MLH1 or MSH2 mutation carriers in several studies (18, 25, 29). The underlying cause for an older median age at diagnosis for MSH6 or PMS2 mutations has not been elucidated. Chen et al. examined single nucleotide polymorphisms (SNPs) of genes playing key roles in the cell cycle in a population of individuals with identifiable germline Lynch Syndrome mutations identified through a colorectal cancer registry (30). CART analysis showed that certain SNPs were associated with either earlier median onset of colorectal cancer diagnosis or a later age of onset. It is possible that genetic variants may also play a role in endometrial cancers in individuals harboring MSH6 and PMS2 mutations that might explain the older median age at diagnosis in this subgroup.
Several studies have shown that the prevalence of Lynch Syndrome is increased in women diagnosed with endometrial cancer at age younger than 50 years. Lu et al. found that 11% (11/100) of women presenting with endometrial cancer at age younger than 50 years had tumor testing results suggestive of Lynch Syndrome, and 9% had an identifiable germline mutation (21). In a similar study performed by Walsh et al., 18% (26/146) of women with endometrial cancer diagnosed at less than age 50 years had tissue testing results (immunohistochemistry, MSI, and MLH1 methylation) consistent with Lynch Syndrome (31). Their study also found an increased rate of probable Lynch Syndrome EC tumors of 16.1% in the age under 50 population. In the current study, the majority of probable Lynch Syndrome endometrial cancer cases occur at ages greater than 50, and there were no statistically significant different clinical or pathological variables between those diagnosed before or after age 50.
The endometrial cancer patient population examined for this study had comparable demographics to those previously compiled for the general U.S. population (14). While the percentage of probable Lynch Syndrome detected by tissue testing in this study (10.5%) may seem high, it is actually comparable to that of two other studies that also examined unselected endometrial cancer patients (32, 33). A comparison of the three studies is provided in Table 5. All 3 studies employed immunohistochemistry for the four DNA mismatch repair proteins. The Ohio State group did not perform MLH1 methylation analysis, so percent sporadic with MLH1 methylation was estimated based on the data of the two other studies. Despite examining three different endometrial cancer populations, the percent probable Lynch Syndrome based on tissue testing is remarkably similar among the three groups. Note that the percentage probable Lynch Syndrome based on tissue testing from Table 5 is higher than the 1–3% that is typically attributed to Lynch Syndrome associated colorectal and endometrial cancers (34). The 1–3% value represents germline mutations. It is well-known that current sequencing strategies do not detect germline mutations for all patients with tumor tissue testing studies suggestive of Lynch Syndrome. Rodriguez-Soler et al. evaluated 1,705 consecutive colorectal cancer patients for Lynch Syndrome by performing MSI and immunohistochemistry (35). They found that the familial incidence of colorectal cancer was greatest in germline mutation carriers, next highest in patients with positive tumor testing positive but no germline mutation, and lowest in individuals with a sporadic colorectal cancer. Risk of endometrial cancer and other Lynch Syndrome-associated tumors was not included in their study. More investigation is needed to determine the optimal approach to managing patients with positive tumor testing studies, but no identifiable germline mutation. This is especially important since published guidelines support universal tumor testing of all colorectal cancers, and the incidence of tumor test positive/germline mutation negative will increase as these standards become more widely adopted.
Table 5.
Comparison of MD Anderson results to similarly designed, population-based endometrial cancer national and international studies.
| MDACC1 N = 408 |
Ohio State2 N = 140 |
Netherlands3 N = 179 |
|
|---|---|---|---|
|
| |||
| % IHC Loss | 28.9 | 21.4 | 23.5 |
| % MLH1 Loss | 22.0 | 17.1 | 17.9 |
| % MLH1 methylated | 82.7 | Not performed | 96.9 |
| % Probable Lynch Syndrome | 10.5 | 6.7–10.14 | 6 (3–11) |
MDACC, MD Anderson Cancer Center (4 of the initial 412 patients studied excluded because immunohistochemistry and/or MLH1 methylation did not work)
Backes et al. investigation of 140 endometrial carcinomas with immunohistochemistry for expression of DNR mismatch repair proteins (32).
Leenen et al. investigation of 179 endometrial carcinomas with immunohistochemistry and MLH1 methylation analysis in all patients diagnosed at less than age 50 (33).
Ohio State calculation of % probable Lynch Syndrome is based on an approximate 82.7–96.9% range of MLH1 methylation rate of tumors with immunohistochemical loss of MLH1 found in the MDACC and Netherlands studies.
There are few published cost analyses that evaluate screening methodologies for Lynch Syndrome in women presenting with endometrial cancer. An advantage of the cost analysis used in this study is that calculations were derived from actual endometrial cancer patients rather than simulated patients. Kwon et al. evaluated several different screening strategies using a simulation model to evaluate the costs relative to benefits for multiple screening strategies. They found that triaging all women with endometrial cancer with immunohistochemistry who had a first-degree relative diagnosed with a Lynch Syndrome-associated cancer occurring at any age was the most cost-effective method (36). If this screening strategy was applied to this study’s existing population-based cohort, 60 (14.6%) individuals would undergo immunohistochemical analysis, with 9 of these having tumor testing suggestive of Lynch Syndrome. This leaves 34 individuals with tumor testing consistent with Lynch Syndrome that would go undiagnosed. Though determined cost effective by Kwon’s analysis, one must determine if the cost savings is worth the potential health-related implications of missing the diagnosis in 34 individuals and the impact this might have on both the patient and her first-degree relatives. Dinh et al. evaluated screening strategies for colorectal and endometrial cancer using a simulation modeled after the U.S. population starting at age 20. They found that risk-assessment of all individuals between the ages of 25–35 with PREMM1,2,6 followed by genetic testing for those with a risk score of ≥ 5 % was the most cost-effective strategy (37). In contrast, Mercado et al. found that the PREMM1,2,6 (93% sensitivity; 5% specificity), MMRPro (57% sensitivity; 85% specificity), MMRPredict proximal (71% sensitivity; 64% specificity), and MMRPredict distal (57% sensitivity; 85% specificity) algorithms do not translate well to the endometrial cancer population (8). These prediction models were attempting to predict the presence of a mismatch repair gene mutation, rather than the presence of tissue testing abnormalities suggestive of Lynch Syndrome. It is interesting to note that each of these prediction models, while initially developed to identify Lynch Syndrome-associated colorectal cancer, has a better sensitivity than SGO 5–10% (32.6% in the present work). Specificity is comparable amongst all models, except for the very low specificity of PREMM1,2,6. These comparative data are summarized in supplemental Table 2.
In the current study, the cost per probable Lynch Syndrome patient identified using a universal tumor testing strategy consisting of immunohistochemistry (and MLH1 methylation analysis when indicated) is comparable to the cost when the SGO 5–10% criteria are employed. Creating an ideal cost analysis strategy is difficult. As can be seen by both the current data and the work from others described above, there can be immense shift in costs as different assumptions and costs are added or removed from the models. For example, microsatellite instability analysis was excluded from the current tissue testing strategy in part because of previous studies documenting substantial subsets of endometrial cancers from known Lynch Syndrome patients being microsatellite stable or MSI-Low (38–40). Exclusion of microsatellite instability analysis, however, can result in potentially missing some MSI-High tumors that have intact positive immunohistochemical expression of mismatch repair proteins (15). A two antibody immunohistochemistry panel consisting of MSH6 and PMS2 has been proposed as a means to cut costs associated with tissue testing (41).
The cost-effectiveness model presented here assumes a 100% genetic counseling referral rate for endometrial cancer patients meeting SGO criteria, when published rates for such referrals vary from 17–48% (42, 43). The model also assumes that all patients either meeting SGO criteria or with tumor testing suggestive of Lynch Syndrome will accept referral for genetic counseling and/or germline testing. Compliance of endometrial cancer patients with genetic counseling referrals to evaluate for Lynch Syndrome may not be 100%. Backes et al. surveyed the 47/384 endometrial cancer patients who met institutional criteria for genetic counseling referral through a mailed questionnaire and follow-up telephone call (44). A total of 26/47 (55.3%) responded to the questionnaire, and 20/26 (77%) stated that they had been referred to see a genetic counselor. Despite referral, only 9/20 (45%) actually did see a genetic counselor, and 8/9 underwent germline testing. The two most common reasons for not seeing a genetic counselor were lack of insurance/cost and anxiety related to the results.
In summary, this is the first large, single-institution evaluation of Society of Gynecologic Oncology 5–10% clinical criteria in their utility for identifying unselected endometrial cancer patients with Lynch Syndrome. Our data show that SGO criteria identify only a small subset of probable Lynch Syndrome patients in the population-based setting. Universal tumor testing of endometrial carcinomas (immunohistochemistry and MLH1 methylation) is a cost-effective alternative that detects more individuals at elevated risk, providing more opportunity for cancer prevention among these women and their families.
Supplementary Material
Acknowledgments
Financial Support:
NIH Research Training Grant (A. Bruegl) T32 CA101642
NIH SPORE in Uterine Cancer (R. Broaddus, K. Lu): NIH 2P50 CA098258-08
Footnotes
Conflicts of Interest: none
References
- 1.Backes FJ, Cohn DE. Lynch syndrome. Clinical Obstetrics and Gynecology. 2011;54(2):199–214. doi: 10.1097/GRF.0b013e3182185a41. [DOI] [PubMed] [Google Scholar]
- 2.Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105(3):569–74. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 3.Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137(5):1621–7. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch HT, Harris RE, Lynch PM, Guirgis HA, Lynch JF, Bardawil WA. Role of heredity in multiple primary cancer. Cancer. 1977;40(4 Suppl):1849–54. doi: 10.1002/1097-0142(197710)40:4+<1849::aid-cncr2820400813>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354(26):2751–63. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296(12):1479–87. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastrinos F, Steyerberg EW, Mercado R, Balmana J, Holter S, Gallinger S, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140(1):73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercado RC, Hampel H, Kastrinos F, Steyerberg E, Balmana J, Stoffel E, et al. Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med. 2012;14(7):670–80. doi: 10.1038/gim.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107(2):159–62. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Ryan P, Mulligan AM, Aronson M, Ferguson SE, Bapat B, Semotiuk K, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118(3):681–8. doi: 10.1002/cncr.26323. [DOI] [PubMed] [Google Scholar]
- 11.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recommendations from the EGAPP Working Group genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H. Genetic testing for hereditary colorectal cancer. Surg Oncol Clin N Am. 2009;18(4):687–703. doi: 10.1016/j.soc.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Womens Health (Larchmt) 2011;20(8):1157–63. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 15.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila) 2012;5(2):320–7. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999;8(4):661–6. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 17.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352(18):1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 18.Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135(2):419–28. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Services CfMaM. Physician Fee Schedule. 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html.
- 20.Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26(36):5965–71. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–64. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 22.McCourt CK, Mutch DG, Gibb RK, Rader JS, Goodfellow PJ, Trinkaus K, et al. Body mass index: relationship to clinical, pathologic and features of microsatellite instability in endometrial cancer. Gynecol Oncol. 2007;104(3):535–9. doi: 10.1016/j.ygyno.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Schmeler KM, Soliman PT, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Endometrial cancer in young, normal-weight women. Gynecol Oncol. 2005;99(2):388–92. doi: 10.1016/j.ygyno.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks YM, Wagner A, Morreau H, Menko F, Stormorken A, Quehenberger F, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127(1):17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 25.Buttin BM, Powell MA, Mutch DG, Babb SA, Huettner PC, Edmonston TB, et al. Penetrance and expressivity of MSH6 germline mutations in seven kindreds not ascertained by family history. Am J Hum Genet. 2004;74(6):1262–9. doi: 10.1086/421332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolodner RD, Tytell JD, Schmeits JL, Kane MF, Gupta RD, Weger J, et al. Germ-line msh6 mutations in colorectal cancer families. Cancer Res. 1999;59(20):5068–74. [PubMed] [Google Scholar]
- 27.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warthin AS. Heredity with reference to carcinoma - As shown by the study of the cases examined in the Pathological Laboratory of the University of Michigan, 1895–1913. Archives of Internal Medicine. 1913;12(5):546–55. doi: 10.3322/canjclin.35.6.348. [DOI] [PubMed] [Google Scholar]
- 29.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305(22):2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Etzel CJ, Amos CI, Zhang Q, Viscofsky N, Lindor NM, et al. Genetic variants in the cell cycle control pathways contribute to early onset colorectal cancer in Lynch syndrome. Cancer Causes Control. 2009;20(9):1769–77. doi: 10.1007/s10552-009-9416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh MD, Cummings MC, Buchanan DD, Dambacher WM, Arnold S, McKeone D, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. 2008;14(6):1692–700. doi: 10.1158/1078-0432.CCR-07-1849. [DOI] [PubMed] [Google Scholar]
- 32.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009;114(3):486–90. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Leenen CH, van Lier MG, van Doorn HC, van Leerdam ME, Kooi SG, de Waard J, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer </= 70 years. Gynecol Oncol. 2012;125(2):414–20. doi: 10.1016/j.ygyno.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 34.Backes FJ, Cohn DE. Lynch syndrome. Clin Obstet Gynecol. 2011;54(2):199–214. doi: 10.1097/GRF.0b013e3182185a41. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barbera VM, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–32. e1. doi: 10.1053/j.gastro.2013.01.044. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 36.Kwon JS, Scott JL, Gilks CB, Daniels MS, Sun CC, Lu KH. Testing women with endometrial cancer to detect Lynch syndrome. J Clin Oncol. 2011;29(16):2247–52. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4(1):9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuismanen SA, Moisio AL, Schweizer P, Truninger K, Salovaara R, Arola J, et al. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Pathol. 2002;160(6):1953–8. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Berends MJ, Mensink RG, Kempinga C, Sijmons RH, van Der Zee AG, et al. Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet. 1999;65(5):1291–8. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66(15):7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 41.Shia J, Tang LH, Vakiani E, Guillem JG, Stadler ZK, Soslow RA, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol. 2009;33(11):1639–45. doi: 10.1097/PAS.0b013e3181b15aa2. [DOI] [PubMed] [Google Scholar]
- 42.Grover S, Stoffel EM, Bussone L, Tschoegl E, Syngal S. Physician assessment of family cancer history and referral for genetic evaluation in colorectal cancer patients. Clin Gastroenterol Hepatol. 2004;2(9):813–9. doi: 10.1016/s1542-3565(04)00352-0. [DOI] [PubMed] [Google Scholar]
- 43.Meyer LA, Anderson ME, Lacour RA, Suri A, Daniels MS, Urbauer DL, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115(5):945–52. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Backes FJ, Mitchell E, Hampel H, Cohn DE. Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecol Oncol. 2011;123(3):532–6. doi: 10.1016/j.ygyno.2011.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.