Abstract
The exquisite architecture of cortex incorporates a myriad of inhibitory interneuron types. Until recently, the dearth of techniques for cell type identification in awake animals has made it difficult to link interneuron activity with circuit function, computation and behavior. This situation has changed dramatically in recent years with the advent of novel tools for targeting genetically distinct interneuron types so their activity can be observed and manipulated. The association of different interneuron subtypes with specific circuit functions, such as gain modulation or disinhibition, is starting to reveal canonical circuit motifs conserved across neocortical regions. Moreover, it appears that some interneuron types are recruited at specific behavioral events and likely control the flow of information among and within brain areas at behavioral time scales. Based on these results we propose that interneuron function goes beyond network coordination and interneurons should be viewed as integral elements of cortical computations serving behavior.
Introduction
Devoted to the idea that “nature delights in repeating itself”, Cajal developed the notion that cerebral cortex may be composed of stereotypic patterns, repeated with a large diversity of specific variations [1,2]. His research initiated the search for canonical circuit motifs: cortical sub-networks that are repeated across areas and presumably support similar computational functions. This line of research led to the discovery of the “cortical column”, a vertical structure of neurons sharing similar receptive field properties in sensory cortices [3,4] and its proposed anatomical substrate, the “cortical module” [5]. The perplexing variety of cell types within cortex long appeared an “impenetrable jungle” [1] until recently developed technologies for cell-type-specific targeting enabled the field to probe how distinct interneuron types participate in cortical circuits and what computations these circuits support during behavior.
The main focus of our review will be on recent work that uses genetic targeting to access specific cortical interneuron subtypes. First, we will provide a brief historical overview of research leading to the conclusion that interneurons are central to cortical computation. Next, we discuss two faces of interneuron function; under what conditions are they activated (“recruitment”) and how do they affect the local circuit (“impact”). Novel techniques for cell type identification and manipulation have finally enabled the investigation of these questions and begun to reveal the function of interneurons in cortical computations and behavior.
Do interneurons compute? Insights from hippocampus and visual cortex
The neuronal operations that transform the inputs to a cortical area into its outputs are referred to as ‘cortical computations’ and were traditionally investigated in terms of principal cell function, leaving open questions about the role of interneurons. The potential involvement of inhibitory neurons in computations has been investigated and debated mainly in the hippocampus and the primary visual cortex (V1), two regions with well-established single neuronal tuning properties: place cells (i.e., cells that fire in a particular physical location) in the hippocampus and orientation and direction tuned cells of V1. In these studies, interneuron identity was mostly inferred from high firing rate and narrow spike width, features likely corresponding to parvalbumin (Pv) expressing basket cells [6–8].
Most place cells are sharply tuned to one or a few locations of the environment, while inhibitory cells often have more complex, multimodal tuning properties [9,10]. The spatial firing maps of hippocampal interneurons were initially interpreted as mere reflections of their local presynaptic pyramidal inputs [11–13], arguing against computational roles. Later it was discovered that hippocampal interneurons have both “on” and “off” fields, spatially localized increases and decreases in activity, with information content comparable to that of principal cells [9,10]. Furthermore, interneurons not only exhibit positive spatial correlation with place cell firing, suggestive of a place cell to interneuron direction of information flow, but sometimes also strong negative correlations [14]. Thus interneurons could contribute to place-specific firing with “on” fields that suppress out-of-field excitation [10] and “off” fields that allow spatially restricted excitatory input [9]. These results lead to the suggestion that hippocampal interneurons play critical roles in determining the spatial tuning of principal cell [10].
A parallel line of studies attempted to elucidate whether and how interneurons in sensory cortices influence receptive field properties of principal cells. Interneurons in the visual cortex exhibit heterogeneous tuning properties; many show broad or even no tuning, whereas other inhibitory cells are as narrowly tuned as pyramidal cells [15–18]. Most of the principal cells receive inhibition tuned to their preferred orientation, but in a large subset the inhibitory input is tuned to non-preferred orientations [19]. Whether inhibitory interneurons actually participate in shaping tuning in V1 in specific ways can be probed using optogenetic manipulations. Two recent studies showed that Pv interneurons provide different forms of gain control: Atallah et al. found Pv cells perform a linear transformation on pyramidal cell input-output curves involving both subtractive and divisive components [20], whereas Wilson et al. found Pv cells primarily divisive [21]. In contrast, Lee et al. showed that Pv cells sharpen tuning and thus improve perceptual discrimination [22]. These and other studies also probed the role of somatostatin (Som) expressing interneurons in V1. They showed that Som interneurons provide subtractive inhibition, shifting the tuning curves of pyramidal cells [21]. In addition, Som interneurons appear to be involved in surround suppression, the attenuation of responses at the center of a neuron’s receptive field by stimulation of the receptive field surround [23,24].
In summary, a new consensus is emerging according to which interneurons actively participate in cortical computations by influencing the receptive field properties of principal neurons [20–25]. However, determining which specific transformations are performed by which interneuron types will require further investigation.
What are the canonical inhibitory circuit motifs?
Cortical interneurons differ in the expression of protein markers (e.g. parvalbumin), in the neuromodulators they co-release (e.g. somatostatin), in their firing patterns in response to current injections and in many other ways [26,27]. While a discrete classification of interneurons based on any single marker is not possible, many markers do map to anatomically relatively homogeneous neuronal classes and can provide systematic access to genetically homogeneous populations [26]. The identity of cells recorded in vitro was traditionally revealed only post hoc in the course of morphological or immunocytochemical evaluation. This made studying interneuron types tedious and characterizing rare subtypes remained a subject of a great deal of serendipity. Recently, targeted in vitro recordings, enabled by cell type specific expression of fluorescent markers in new transgenic rodent models [28], allowed high-yield and more easily repeatable experiments on interneuron connectivity. Furthermore, bidirectional optogenetic manipulations provided a powerful tool for probing circuit functions of various interneuron types. These technological improvements were exploited by a series of novel studies, greatly advancing our understanding of cortical interneuron circuits.
Cortical inhibitory interneurons are classically divided into two major categories. Peri-somatic interneurons synapse on the somata and proximal dendrites of pyramidal cells and are thus strategically positioned to control their output. Dendrite-targeting interneurons, on the other hand, send projections to the distal dendrites of the pyramidal cells, thus gating the incoming information [27,29]. The two most prominent representatives of these classes are the Pv and Som expressing interneurons (Fig. 1a–b). Perisomatic Pv cells are heavily interconnected by chemical synapses and electric coupling promoting synchronous activity [8,30–33]. Pv-expressing interneurons with basket morphology form recurrent loops with pyramidal neurons, thought to be important substrates of feedback inhibition [34]. A recent study showed that the other major basket cell type, interneurons that express cholecystokinin (Cck), provide strong feed-forward inhibition recruited by incoming fibers in the hippocampus [35]. A third type of perisomatic interneurons, the chandelier cells, are defined by their extreme target specificity [36]. Because they exclusively target the spike initiation zone of pyramidal cells they were long proposed to serve to ‘veto’ output spikes. However, recent studies showed that their effect on pyramidal neurons may be excitatory [37]. Determining the exact area-specific contingencies under which they provide inhibition, excitation or shunting [37–40] will require further studies. A novel developmental genetic approach to selectively target chandelier cells holds great promise for better understanding their network and behavioral function [41]. As opposed to Pv neurons, the dendrite-targeting Som interneurons largely lack within-type synaptic connections providing more asynchronous parallel pathways onto other interneuron types as well as pyramidal cells [8,31,32,42]. A subset of Som interneurons, Martinotti cells projecting to layer 1, participate in local pyramidal cell–interneuron–pyramidal cell circuits by mediatinh disynaptic inhibition from one principal cell to its excitatory neighbors [43,44]. Som interneurons were also shown to be capable of exerting highly focal, compartmentalized control over individual dendritic spines [45]. A subpopulation of Som expressing interneurons in layer 4 mediates disinhibition of local principal cells via Pv interneurons [46]. While these studies suggest specific connectivity patterns within the cortical circuit, a recent report found non-selective, nearly all-to-all connectivity from Som interneurons to local pyramidal cells in the mouse frontal cortex [47].
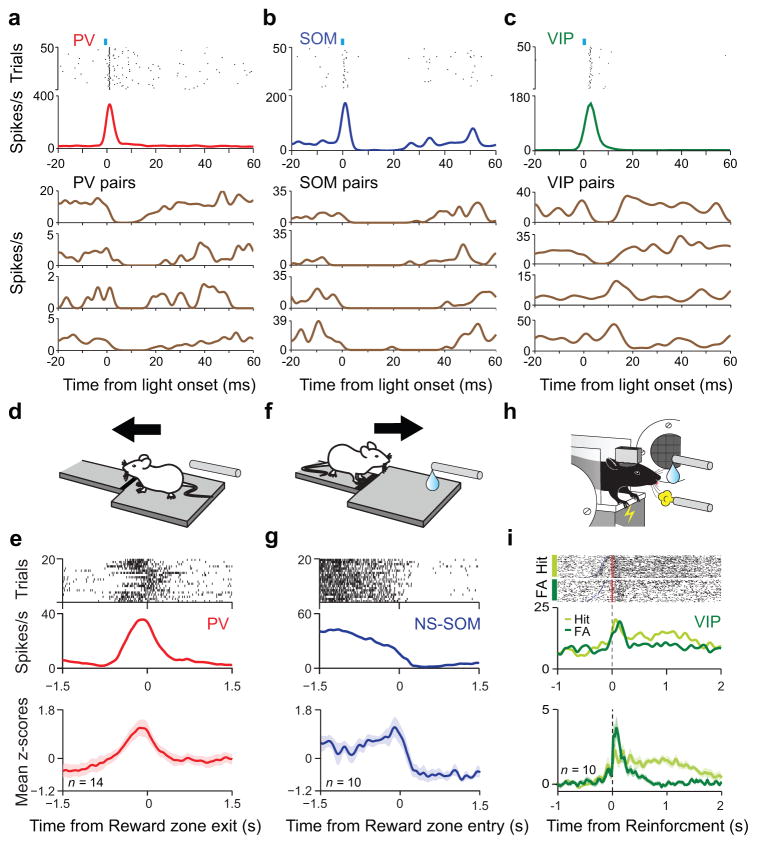
Figure 1. Network effects and behavioral correlates of identified interneuron classes.
ab,c, Top, spike raster and peri-stimulus time histogram (PSTH) of Pv (a), Som (b) and Vip (c) interneurons aligned to light pulses. Bottom, PSTH of four simultaneously recorded unidentified neurons (Pv pairs, Som pairs and Vip pairs). Pv and Som pairs are inhibited after light pulses; Vip pairs are either inhibited (first and second PSTH) or show delayed activation (third and fourth PSTH), indicating disinhibition. d, Schematic depicting a mouse leaving the reward zone in a foraging task. e, Top two panels, spike raster and peri-event time histogram (PETH) of a Pv interneuron aligned to reward zone exit. Bottom, mean z-scored response of 14 PV neurons (shaded area indicates s.e.m.). f, Schematic depicting a mouse entering the reward zone in a foraging task. g, Top panels, spike raster and PETH of a NS-Som interneuron aligned to reward zone entry. Bottom, mean z-scored responses of 10 NS-Som neurons (shaded area indicates s.e.m.). h, Schematic of auditory go/no-go discrimination task. i, Top two panels, raster plots and PETHs of a Vip interneuron aligned to reinforcement (light green, reward; dark green, punishment; FA, false alarm). Bottom panel, mean z-scored responses of 10 Vip interneurons. Modified from refs 8 and 50.
Interneurons make up almost all the neurons in layer 1 and recent in vivo work demonstrated that a major fraction disinhibits layer 2/3 pyramidal cells via Pv neurons in auditory cortex [48]. In vitro work showed that anatomically defined cell types of layer 1 differentially affect layer 5 pyramidal cells in sensorimotor cortices: neurogliaform cells inhibited whereas single-bouquet cells disinhibited them [49].
Recently, three papers converged on a circuit motif controlled by cells expressing vasoactive intestinal polypeptide (Vip; Fig. 1c) [32,50,51]. Vip expression demarcates a small population of interneurons (~10–15%), located mostly in the supragranular cortical layers, that are distinct from the two major interneuron populations defined by Pv and Som expression. These neurons – as also suggested by earlier anatomical studies [52–54] – preferentially target other types of inhibitory neurons, potentially providing disinhibitory control by releasing pyramidal cells from inhibition. All three studies agreed that the major target of Vip interneurons are the Som-expressing interneurons. VIP inhibition onto Pv-expressing interneurons was found to be either comparable to [32], stronger [50] or weaker [51] than that onto pyramidal cells but always weaker than onto Som interneurons, suggesting that the strength of these connections may vary slightly across different cortical areas. The Vip to Pv connection in the hippocampus and motor cortex was shown to undergo substantial experience-related plasticity, which likely increases the variability of connection strength [55]. Importantly, Pi et al. provided the first in vivo demonstration that Vip interneurons generated disinhibition, impacting a functionally defined, strongly tone-responsive subset of pyramidal cells in the auditory cortex. Taken together, the three studies described very similar connectivity patterns for Vip interneurons in four functionally and cytoarchitectonically different regions of the neocortex: three sensory areas, the auditory [50], visual [32] and somatosensory [51] cortices and the prefrontal cortex [50]. Thus, the Vip-controlled disinhibitory circuit appears to define a canonical cortical circuit motif (Fig. 2).
Figure 2. Disinhibitory microcircuit controlled by Vip interneurons.
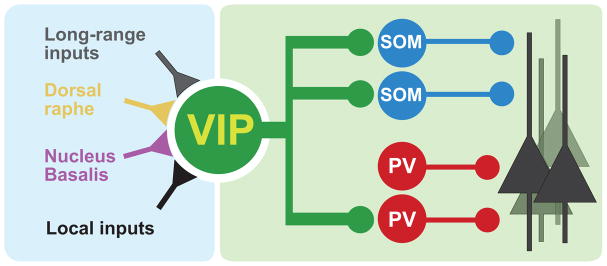
Vip interneurons receive long-range and local excitatory inputs as well serotonergic and cholinergic neuromodulatory projections from the dorsal raphe and nucleus basalis, respectively. They control the activity of a cortical pyramidal cell subpopulation by disinhibiting them mostly through Som interneurons.
Mapping the behavioral repertoire of cortical interneurons
Cortical neurons tend to show great heterogeneity in response properties during behavior (e.g. [56–59]). For instance, even neighboring neurons in prefrontal cortex encode distinct combinations of sensory, motor and other features with unique temporal dynamics, resulting in the vexingly complex “cortical response zoo”, which greatly complicates the interpretation of extracellularly recorded neural activity during behavior [56,59,60]. Similarly puzzling response diversity has been reported in sensory and motor cortices [57,58]. Indeed, such representational heterogeneity can have computational benefits [60–63]. However, it is not known whether response diversity is a property of a defined cortical population, or if part of this heterogeneity can be attributed to cell-type diversity within the recorded population. This question is particularly relevant for inhibitory interneurons because of the large diversity of subtypes each with distinct connectivity and intrinsic properties [27,64].
In the previous section we reviewed evidence for well-defined network functions associated with interneuron classes; however, network function does not necessarily imply behavioral correlates. It is conceivable, that interneuron function can only be understood with reference to the circuit they are embedded in; for instance, by referencing interneuron activity to local principal cells using a cross-correlation approach or registering interneuron spiking to local field potential oscillations [29,65–68]. In this case, aligning interneuron activity to behavioral events may not uncover specific moments of recruitment.
Recent technical developments have enabled testing these ideas [8,69–76]. For instance, optogenetics-assisted identification of genetically defined cell types enables mapping the behavioral correlates of rare neuron types [8,75,76]. Briefly, a defined population of neurons is rendered light-sensitive by cell-type specific expression of a light-sensitive cation channel (variants of channelrhodopsin; [77]) via viral delivery or transgenic approaches [28]. Neurons from the targeted cell type are then identified in extracellular recordings based on their short latency light-evoked responses.
Applying this optogenetic tagging method, Pv- and Som-expressing interneurons were recently investigated in the anterior cingulate cortex (ACC) of freely behaving mice (Fig. 1d–g) [8]. Mice were trained to perform a foraging task in which they had to shuttle back and forth between a distant trigger location and a dedicated reward zone. Pv and a subset of Som interneurons, characterized by narrow action potentials (NS-Som), showed strong behavioral correlates of the foraging decisions: NS-Som neurons uniformly suppressed their activity when the mice entered the reward zone (Fig. 1f–g), whereas Pv neurons, again as a homogenous population, became phasically active when the animal left the reward zone (Fig. 1d–e). In addition, the activity increase of Pv cells was correlated with the time the animal had spent in the reward zone before deciding to leave. In another study, prefrontal Pv-expressing interneurons were investigated in mice during auditory fear conditioning [78]. Pv neurons were suppressed during freezing and fear expression was causally dependent on this effect.
Pv-expressing interneurons have been also recorded with the juxtacellular technique in head restrained behaving rats [74]. This study showed that while pyramidal neurons in the forelimb area of the motor cortex have diverse behavioral correlates in relation to motor preparation, initiation and execution, identified Pv neurons are mostly active during the expression of voluntary movements, constituting a homogeneous group. Similarly, Lapray et al. demonstrated that hippocampal Pv-expressing basket cells are mostly active during movement as opposed to quiet wakefulness in freely moving rats [71].
The responses of Vip-expressing interneurons were probed in auditory cortex during an auditory discrimination task [50]. Many Vip neurons showed auditory tuning but the surprising observation was that they were most strongly and uniformly recruited by reinforcement signals: with rapid phasic activation after punishment (air puff or foot shock) and somewhat weaker but more sustained response after water reward (Fig. 1h–i) [50]. Vip interneurons have similar circuit functions across four distinct cortical regions, which shows they play analogous roles in distinct cortical circuits [32,50,51]; therefore, it will be interesting to determine if they also have similar behavioral correlates across regions.
These new data, combined with our knowledge of cortical interneuron circuits, allows one to speculate about the network and behavioral function of interneuron types. Pv neurons are likely to control the output of their cortical area. This implies that Pv neurons should be active when this output is formed, which is strongly region-specific. This may explain their activation during foraging decisions in the ACC (Fig. 1d–g) [8] and their elevated firing during movement expression in the motor cortex [74]. On the other hand, Som interneurons are in a position to control the input to cortical pyramidal cells. Interestingly, NS-Som cells are uniformly silent in the ACC during the exact period when incoming information might be integrated to form a leaving decision in the foraging task [8]. In line with this proposed role in inhibitory gating, Som neurons in the barrel cortex are suppressed during passive and active whisker sensing [51,79]. Vip interneurons express fast ionotropic receptors for serotonin and acetylcholine [80–83], putting them in an optimal position to rapidly relay long-range neuromodulatory signals [84] as well as other long-range input [51]. By disinhibiting pyramidal cells via Som interneurons, they provide a switch by which other cortical and subcortical areas can engage cortex. These data support a new model of interneuron function: interneurons may exert a precise control over cortical information flow by selectively gating distinct input and output channels governed by the requirements of ongoing behavior.
Concluding remarks
Until recently, it has been unknown whether interneurons form canonical circuit motifs across different cortical areas. Similarly, it has been unclear whether specific types of interneurons have signature behavioral correlates or their function can only be explored in terms of circuit function and oscillations. New transgenic mouse lines, optogenetic tagging and awake juxta/intracellular recording techniques enabled a series of recent studies that have already revealed a great deal about interneuron network and behavioral functions. The contours of a canonical cortical microcircuit are already becoming visible, revealing different cortical interneuron subtypes in critical positions. Moreover, it appears that interneurons have functions beyond network coordination and certain subtypes can be recruited at specific behavioral events. As the behavioral repertoire of different interneurons is becoming clearer it appears that they serve to control the flow of information. These recent breakthroughs foreshadow a deeper understanding of the logic of the cortical network guided by studies of identified cortical cell types.
Highlights.
Inhibitory interneurons actively participate in cortical computations
Cortical interneurons form canonical circuits
Cortical interneurons are recruited under specific behavioral contingencies
Acknowledgments
This work was supported by grants from NIH NINDS R01NS075531, the Klingenstein, John Merck, Sloan and Whitehall Foundations to A.K. B.H. received support from the Swartz Foundation and Marie Curie International Outgoing Fellowship within the EU Seventh Framework Programme for Research and Technological Development. D.K. received support from The Robert Lee and Clara Guthrie Patterson Trust Postdoctoral Fellowship and Human Frontier Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Balázs Hangya, Email: bhangya@cshl.edu.
Hyun-Jae Pi, Email: hjpi@cshl.edu.
Duda Kvitsiani, Email: kvitsian@cshl.edu.
Sachin P. Ranade, Email: ranades@cshl.edu.
Adam Kepecs, Email: kepecs@cshl.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cajal SRy. Recollections of My Life [traslated by E.H. Craigie and J. Cano, 1989] MIT Press; 1937. [Google Scholar]
- 2.Douglas RJ, Martin KA. Mapping the matrix: the ways of neocortex. Neuron. 2007;56 :226–238. doi: 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–34. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–50. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- 5.Szentagothai J. The neuron network of the cerebral cortex_: a functional interpretation. 1977;201:219–248. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- 6.Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex. 1997;7:534–45. doi: 10.1093/cercor/7.6.534. [DOI] [PubMed] [Google Scholar]
- 7.Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 8**.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–6. doi: 10.1038/nature12176. Pv and Som interneurons of mouse anterior cingulate cortex were investigated in a foraging task. Pv and a narrow spiking subpopulation of Som interneurons formed functionally homogeneous populations responding to specific behavioral events and exerted differential inhibitory impact on principal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilent WB, Nitz DA. Discrete place fields of hippocampal formation interneurons. J Neurophysiol. 2007;97:4152–61. doi: 10.1152/jn.01200.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ego-Stengel V, Wilson MA. Spatial Selectivity and Theta Phase Precession in CA1 Interneurons. Hippocampus. 2007;174:161–174. doi: 10.1002/hipo.20253. [DOI] [PubMed] [Google Scholar]
- 11.Kubie JL, Muller RU, Bostock E. Spatial firing properties of hippocampal theta cells. J Neurosci. 1990;10:1110–23. doi: 10.1523/JNEUROSCI.10-04-01110.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall L, Henze DA, Hirase H, Leinekugel X, Dragoi G, Buzsaki G. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J Neurosci. 2002;22:RC197. doi: 10.1523/JNEUROSCI.22-02-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J Neurosci. 2006;26:13485–92. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hangya B, Li Y, Muller RU, Czurkó A. Complementary spatial firing in place cell-interneuron pairs. J Physiol. 2010;588:4165–75. doi: 10.1113/jphysiol.2010.194274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch Ja, Martinez LM, Pillai C, Alonso J-M, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nat Neurosci. 2003;6:1300–8. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- 16.Runyan Ca, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67:847–57. doi: 10.1016/j.neuron.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zariwala Ha, Madisen L, Ahrens KF, Bernard A, Lein ES, Jones AR, Zeng H. Visual tuning properties of genetically identified layer 2/3 neuronal types in the primary visual cortex of cre-transgenic mice. Front Syst Neurosci. 2011;4:162. doi: 10.3389/fnsys.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly Tuned Response Properties of Diverse Inhibitory Neuron Subtypes in Mouse Visual Cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–80. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 20.Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–70. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–8. doi: 10.1038/nature11347. Using cell-type-specific optogenetic activation, the authors show that Pv and Som interneurons perform essentially arithmetic operations: division and subtraction from excitatory responses, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S-H, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–83. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–31. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nienborg H, Hasenstaub A, Nauhaus I, Taniguchi H, Huang ZJ, Callaway EM. Contrast dependence and differential contributions from somatostatin- and parvalbumin-expressing neurons to spatial integration in mouse V1. J Neurosci. 2013;33:11145–54. doi: 10.1523/JNEUROSCI.5320-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33:13713–23. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascoli Ga, Alonso-Nanclares L, Anderson Sa, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28*.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. The authors present a rich repertoire of genetically engineered knock-in mouse lines enabling reliable targeting of genetically and developmentally specified interneuron populations. This genetic toolkit has enabled a slew of studies of interneuron function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–7. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Ma Y, Agmon A. Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J Neurosci. 2011;31:3351–61. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–76. doi: 10.1038/nn.3446. This study determined in great detail the synaptic connectivity patterns among and within different genetic subtypes of interneurons in visual cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamás G, Buhl EH, Lörincz a, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–71. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 34.Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–21. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somogyi P. A specific “axo-axonal” interneuron in the visual cortex of the rat. Brain Res. 1977;136:345–50. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 37.Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–5. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 38.Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–3. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson Sa, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31:17872–86. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci. 2013;16:1802–11. doi: 10.1038/nn.3550. Chandelier cells of the rat CA3 were found to be inhibited during sharp wave ripples, fire rhythmically during theta oscillations anti-phase with pyramidal neurons and receive medial septal inhibitory input. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–4. doi: 10.1126/science.1227622. A groundbreaking developmental characterization and genetic targeting of cortical chandelier interneurons that specialize in inhibiting the axon initial segment of pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cottam JCH, Smith SL, Hausser M. Target-Specific Effects of Somatostatin-Expressing Interneurons on Neocortical Visual Processing. J Neurosci. 2013;33:19567–19578. doi: 10.1523/JNEUROSCI.2624-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–46. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–53. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–62. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu H, Jeong H-Y, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–67. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–203. doi: 10.1016/j.neuron.2011.02.025. The authors found that Som interneurons are connected to nearly all nearby pyramidal neurons in mouse frontal cortex, suggesting non-selective inhibitory connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–5. doi: 10.1038/nature10674. An auditory disinhibitory circuit controlled by layer 1 interneurons and recruited by cholinergic inputs is necessary for associative fear learning. [DOI] [PubMed] [Google Scholar]
- 49*.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–8. doi: 10.1038/nn.3305. Using quadruple-octuple in vitro and dual in vivo whole-cell recordings, two previously unknown interneuronal circuits were identified in rat sensorimotor cortex, linking L1-3 interneurons to L5 pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. Vip-expressing interneurons were found to generate disinhibition by inhibiting most Som and a fraction of Pv interneurons in auditory and prefrontal cortices. Vip interneurons in auditory cortex were recruited by reinforcement signals during behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–70. doi: 10.1038/nn.3544. Vip-expressing interneurons in superficial layers of somatosensory cortex were found to be strongly recruited by long-range input from motor cortex. In turn Vip interneurons predominantly inhibited Som interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acsády L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73:317–34. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 53.Hajos N, Acsady L, Freund TF. Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur J Neurosci. 1996;8:1415–31. doi: 10.1111/j.1460-9568.1996.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 54.Dávid C, Schleicher A, Zuschratter W, Staiger JF. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur J Neurosci. 2007;25:2329–40. doi: 10.1111/j.1460-9568.2007.05496.x. [DOI] [PubMed] [Google Scholar]
- 55.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 56.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hromádka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Churchland MM, Shenoy KV. Temporal complexity and heterogeneity of single-neuron activity in premotor and motor cortex. J Neurophysiol. 2007;97:4235–57. doi: 10.1152/jn.00095.2007. [DOI] [PubMed] [Google Scholar]
- 59.Machens CK, Romo R, Brody CD. Functional, but not anatomical, separation of “what” and “when” in prefrontal cortex. J Neurosci. 2010;30:350–60. doi: 10.1523/JNEUROSCI.3276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mante V, Sussillo D, Shenoy KV, Newsome WT. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature. 2013;503:78–84. doi: 10.1038/nature12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maass W, Natschläger T, Markram H. Real-time computing without stable states: a new framework for neural computation based on perturbations. Neural Comput. 2002;14:2531–60. doi: 10.1162/089976602760407955. [DOI] [PubMed] [Google Scholar]
- 62.Sussillo D, Abbott LF. Generating coherent patterns of activity from chaotic neural networks. Neuron. 2009;63:544–57. doi: 10.1016/j.neuron.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chelaru MI, Dragoi V. Efficient coding in heterogeneous neuronal populations. Proc Natl Acad Sci U S A. 2008;105:16344–9. doi: 10.1073/pnas.0807744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- 67.Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–8. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 68.Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–33. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Harvey CD, Collman F, Dombeck Da, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–6. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15:1265–71. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Long Ma, Lee AK. Intracellular recording in behaving animals. Curr Opin Neurobiol. 2012;22:34–44. doi: 10.1016/j.conb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sullivan D, Ozen S, Buzsaki G. Juxtacellular recording and labeling in the hippocampal dentate gyrus of freely moving rats. In SFN abstract. 2008:435.2/H7. [Google Scholar]
- 74*.Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009;12:1586–93. doi: 10.1038/nn.2431. Pv interneurons in the rat motor cortex are specifically recruited during the execution of self-initiated voluntary movement as opposed to excitatory neurons that exhibit diverse behavioral correlates. [DOI] [PubMed] [Google Scholar]
- 75.Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–8. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–57. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TCM, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2013 doi: 10.1038/nature12755. Pv interneurons in the prefrontal cortex are inhibited during freezing in mice subjected to auditory fear conditioning and fear expression is causally related to this effect. [DOI] [PubMed] [Google Scholar]
- 79**.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CCH. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–12. doi: 10.1038/nn.3051. Som interneurons in layer 2/3 of the mouse somatosensory cortex are active during quiet wakefulness; on the other hand, they are hyperpolarized and show reduced firing during both passive and active whisking. [DOI] [PubMed] [Google Scholar]
- 80.Paspalas CD, Papadopoulos GC. Serotoninergic afferents preferentially innervate distinct subclasses of peptidergic interneurons in the rat visual cortex. Brain Res. 2001;891:158–67. doi: 10.1016/s0006-8993(00)03193-0. [DOI] [PubMed] [Google Scholar]
- 81.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–7. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 82.Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–64. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci. 2012;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–53. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]