Abstract
Cocaine abuse and addiction remain great challenges on the public health agendas in the U.S. and the world. Increasingly sophisticated perspectives on addiction to cocaine and other drugs of abuse have evolved with concerted research efforts over the last 30 years. Relapse remains a particularly powerful clinical problem as, even upon termination of drug use and initiation of abstinence, the recidivism rates can be very high. The cycling course of cocaine intake, abstinence and relapse is tied to a multitude of behavioral and cognitive processes including impulsivity (a predisposition toward rapid unplanned reactions to stimuli without regard to the negative consequences), and cocaine cue reactivity (responsivity to cocaine-associated stimuli) cited as two key phenotypes that contribute to relapse vulnerability even years into recovery. Preclinical studies suggest that serotonin (5-hydroxytryptamine; 5-HT) neurotransmission in key neural circuits may contribute to these interlocked phenotypes well as the altered neurobiological states evoked by cocaine that precipitate relapse events. As such, 5-HT is an important target in the quest to to understand the neurobiology of relapse-predictive phenotypes, to successfully treat this complex disorder and improve diagnostic and prognostic capabilities. This review emphasizes the role of 5-HT and its receptor proteins in key addiction phenotypes and the implications of current findings to the future of therapeutics in addiction.
Keywords: Addiction, Cocaine, Cue Reactivity, Dependence, Impulsivity, Serotonin
1. Introduction
Thirty years of intensive study have elucidated molecular mechanisms underlying the in vivo effects of abused substances, the impressive, distributed network of circuitry at the heart of addictive processes, and have made inroads into the complex polygenetic nature of addiction (Ducci and Goldman, 2012; Kalivas and Volkow, 2005; Koob and Volkow, 2010). These 30 years have produced increasingly sophisticated perspectives on addiction as a chronic, relapsing brain disorder that engages reward function and an `expanding cycle of dysfunction' in cognition, learning and emotional functions (Volkow, et al., 2010). This trajectory from drug use to addiction begins against a background of vulnerability based upon genetic and environmental factors, and progresses as neuronal plasticity in key brain circuits entrains addictive behaviors in concert with experiential learning and the added pharmacological impact of the abused substance. The means to reverse causal neuroplasticity and therapeutically improve function in the addicted brain is an important quest and one ripe with near-term therapeutic potential with positive outcomes. Although surprisingly less is known about its influence compared to key players dopamine (DA) and glutamate (Kalivas and Volkow, 2005), serotonin (5-hydroxytryptamine; 5-HT) neurotransmission in key neural circuits may contribute to inherent states of vulnerability as well as the altered neurobiological states that drive the transition from drug use to abuse to dependence. This review emphasizes the role of 5-HT in key addiction phenotypes and the implications of current findings to the future of therapeutics in addiction.
There are a multitude of factors that contribute to the initial decision to experience an abused drug, to the continued maintenance of drug use and the ultimate development of dependence or addiction. The same or even different factors may contribute to vulnerability to withdrawal-related syndromes, difficulty achieving abstinence, and sensitivity to relapse during abstinence. Relapse is a particularly challenging clinical problem as, even though a drug abuser may initiate termination of drug use and commit to abstinence, the recidivism rates can be very high (Brandon, et al., 2007). Relapse is defined as reversion to drug-using behavior which interrupts the progress of abstinence and can be seen as a dynamic process rather than a single, discrete event (Maisto and Connors, 2006; Miller, et al., 1996). Vulnerability to endogenous factors (e.g., craving, stress, withdrawal) are interwoven with responsiveness to exogenous stimuli (e.g., drug-associated cues) which can serve as immediate antecedents to relapse (Hendershot, et al., 2011). Efficacious relapse prevention programs emphasize cognitive-behavioral skills and coping responses with an accent on environmental stimuli and cognitive processes as triggers (Hendershot, et al., 2011). Medications are also useful to suppress relapse which requires reestablishment of normal brain function consequent to long-term, drug-induced neuroplasticity and diminishment of the power of relapse triggers (Modesto-Lowe and Kranzler, 1999; Paterson, 2011). Currently, medications for treatment of opioid (heroin, morphine) and alcohol addiction which help to suppress relapse in the context of behavioral therapy have been developed. However, although nicotine replacement therapy, buproprion and varenicline are effective therapeutics for nicotine addictions, medication development efforts have not yet yielded effective pharmacotherapies for cocaine and other abused psychostimulants. A great deal of interest remains in filling this gap to maximize the probability of treatment success by minimizing lapses to drug use (Hendershot, et al., 2011;Volkow and Skolnick, 2012).
2. Cocaine
The illicit abuse of psychostimulants is a problem that includes a myriad of abused chemical substances. The present review will focus on cocaine for several reasons. First, cocaine remains one of the greatest challenges on the public health agendas in the U.S. and the world. Indicators of the extent of the cocaine problem in the U.S. (e.g., forensic seizures, treatment, mortality and emergency department admissions) continue to dominate the landscape; serious health and social consequences of cocaine use extensively impact families and communities and drain their resources (Community Epidemiology Work Group, 2005). There were ~1.4 million current cocaine users aged 12 or older in the U.S. in 2011 (SAMHSA, 2012); 50% of the four million drug-related emergency department visits in 2010 involved illicit drug use and cocaine was cited as the abused drug most commonly-involved (DAWN: Drug Abuse Warning Network, 2013). While treatment has been shown to decrease morbidity and mortality associated with this disorder, only ~11% of those who needed treatment received care in 2009, with cost and inaccessibility cited as primary barriers (Center on Addiction and Substance Abuse at Columbia University, 2012). Second, there is a wealth of epidemiological knowledge that attests to the tenacity of cocaine abuse in those who suffer its vagaries (Degenhardt and Hall, 2012; Schulden, et al., 2009) as well as basic and translational research in both clinical populations and animal models (Kalivas and Volkow, 2005; Koob and Volkow, 2010). Lastly, this rich database provides a knowledge scaffold upon which to disentangle the role of 5-HT in key facets of cocaine addiction and to ultimately integrate this knowledge into the dominant theoretical constructs of addiction (Kalivas and Volkow, 2005; Koob and Volkow, 2010). Thus, the impact of cocaine abuse and dependence on the quality of health and society is massive, and the lack of useful therapeutic medications to suppress intake and enhance recovery in cocaine dependence is considered a major unaddressed gap (Hendershot, et al., 2011;Volkow and Skolnick, 2012).
The cycling course of cocaine intake, abstinence and relapse is tied to a multitude of behavioral and cognitive processes with impulsivity and cue reactivity cited as two key phenotypes that set up vulnerability to relapse even years into recovery (Drummond, 2001; Koob and Volkow, 2010; Moeller, et al., 2001b; O'Brien, et al., 1998; Saunders, et al., 2013). Impulsivity has been defined clinically as a predisposition toward rapid unplanned reactions to stimuli without regard to the negative consequences (Moeller, et al., 2001a), while cue reactivity is conceptualized as the sensitivity to cues linked with the drug-taking experience that are known to trigger craving in humans (Carter and Tiffany, 1999; O'Brien, et al., 1988; O'Brien, et al., 1998). Inherent (trait) impulsivity and the incentive motivation for drug and drug-associated cues are woven in a complex web of cause and effect. Cocaine-dependent subjects are more impulsive than non-drug using subjects on both self-report questionnaires and laboratory measures of impulsivity (Moeller, et al., 2001a; Moeller, et al., 2001b). Furthermore, greater impulsivity predicts reduced retention in outpatient treatment trials for cocaine dependence (Green, et al., 2009; Moeller, et al., 2001b; Moeller, et al., 2007; Patkar, et al., 2004; Schmitz, et al., 2009). High impulsivity has been noted in cocaine-dependent subjects who express high cocaine cue reactivity (Liu, et al., 2011) and a similar relationship has been observed in cigarette smokers (Doran, et al., 2007; Doran, et al., 2008). Individuals with poor impulse control may be more vulnerable to drug-associated stimuli, and less capable of engaging processes that override heightened attentional biases for cues (Liu, et al., 2011). Interestingly, acute amphetamine administration decreased impulsive choice when the delay to obtain the reinforcer was signaled by a discrete cue (light/tone), but increased impulsive choice in the absence of a paired cue (Cardinal, et al., 2000), further suggesting that impulsivity and cue-associated events are intertwined. Individual differences in reactivity to cues could also be caught in this web, although it is difficult to assess whether there may be “trait” sensitivity to reward-related cues (Mahler and de Wit, 2010) that predates chronic cocaine use. Phenotypic differences in sensitivity to reward-related cues are reported in rats such that the propensity to approach a cue predictive of reward availability (“sign-tracking”) predicts the motivation to self-administer cocaine and to reinstate drug-seeking after extinction from cocaine self-administration relative to rats that preferentially approach the location of the reward delivery (“goal-trackers”) (Saunders and Robinson, 2013; Yager and Robinson, 2013). Interestingly, sign-trackers exhibit higher impulsivity relative to goal-trackers (Flagel, et al., 2010; Tomie, et al., 2008). Thus, there is a growing appreciation that impulsivity and cocaine cue reactivity are interlocked contributors to relapse, a cardinal facet of addiction and may be mediated by shared neurobiology and neuroanatomy.
3. Addiction Neurocircuitry
Addiction is now recognized as a disordered integration of cognitive and motivational aspects of reward-directed behavior involving higher order limbic-corticostriatal circuit structures (Kalivas and Volkow, 2005). This circuit controls both impulsivity and cue reactivity and neuroadaptations in limbic-corticostriatal subnuclei are noted as particularly relevant in addiction (Feil, et al., 2010; Fineberg, et al., 2010; Kalivas and Volkow, 2005; Koob and Volkow, 2010). Several key regions implicated include: (1) ventral tegmental area (VTA), initiation of sensitization (enhanced behavioral responsivity), reward-mediated behaviors, anhedonia of acute withdrawal; (2) medial prefrontal cortex (mPFC), inhibitory control, site of action for abused drugs to alter executive function and decision-making; (3) nucleus accumbens (NAc), reward, maintenance of sensitization, persistent drug-induced behaviors; (4) amygdala, memory for motivational impact of drug-associated stimuli) (Everitt and Wolf, 2002; Kalivas and Volkow, 2005; Kelley, 2004).
The complexity of the limbic-corticostriatal circuitry involved in addictive process should not be underestimated, particularly with innervation from all monoamine efferents and extensive reciprocal and looping interconnections with other cortical, limbic, and thalamic nodes. Functional imaging studies conducted in humans are consistent in citing corticostriatal connectivity as critical in impulsivity and cocaine cue reactivity (Chase, et al., 2011; Chikazoe, 2010; Wager, et al., 2005). Exposure to drug-associated cues activates cortical regions such as the anterior cingulate cortex, the orbitofrontal cortex, and the insula in humans (Bechara, 2005; Childress, et al., 1999; Goldstein, et al., 2007; O'Brien, et al., 1988). Further, studies indicate that the inferior, middle and superior frontal gyri in the PFC, orbitofrontal cortex and ventral striatum [caudate, NAc] share increased activation during impulsive action tasks and in response to drug-associated cues (Chase, et al., 2011; Chikazoe, 2010; Wager, et al., 2005). The fact that the impulsive behavior in cocaine-dependent subjects resembles that of patients with PFC lesions (Bechara, et al., 1997) is interesting in light of observations that the PFC has been implicated in cue-elicited cocaine-seeking behaviors (McLaughlin and See, 2003). The mPFC imposes “top down” control of subcortical structures, especially the ventral striatum (NAc) (Dalley, et al., 2011). Compromised signaling between the mPFC and basal ganglia are thought to underlie the cognitive inability to withhold prepotent responses (impulsivity) (Dalley, et al., 2011; Fineberg, et al., 2010), and to maintain abstinence in the face of drug-associated cues in rodents (Kalivas, 2008). Recently, Moeller and colleagues demonstrated differential frontostriatal connectivity features in cocaine-dependent versus non-drug using controls (Ma, et al., 2014). Further, the dorsal prelimbic projection to the NAc core and the ventral infralimbic mPFC projection to the NAc shell (Vertes, 2004) oppositionally control drug-seeking (Di Ciano and Everitt, 2004; Kalivas, 2008; Kalivas and McFarland, 2003; Koya, et al., 2009; McLaughlin and See, 2003; Stefanik, et al., 2013), while the infralimbic mPFC is implicated in impulsive action (Chudasama, et al., 2003; Dalley, et al., 2011; Fineberg, et al., 2010; Murphy, et al., 2012). The normal function of the mPFC microcircuitry is driven by the excitatory and inhibitory balance engendered by glutamate pyramidal neurons and γ-aminobutyric acid (GABA) interneurons, respectively; alterations in the excitatory/inhibitory balance in the mPFC are hypothesized to drive cognitive deficits in addiction and psychiatric disorders such as schizophrenia (O'Donnell, 2011). An elegant study employing novel optogenetic tools recently found that selective activation of glutamate pyramidal neurons in mouse mPFC resulted in deficits in cognitive function while selective activation of GABA interneurons minimally affected behavior; however, activation of GABA interneurons could partially restore the deficits due to activation of glutamate pyramidal neurons (Yizhar, et al., 2011). Thus, there is considerable evidence in support of an imbalance in the limbic-corticostriatal function that may serve an integrating mechanism underlying impulsive action and cue reactivity. While the a priori decision to focus on this circuitry in the present review does not exclude the involvement of a more encompassing neural network over these behaviors, this focus is appropriate for the discussion of 5-HT involvement given its control over critical limbic-corticostriatal circuits (for reviews) (Alex and Pehek, 2006; Bubar and Cunningham, 2008).
4. Serotonin
The 5-HT neurotransmitter system is integral in motor, cognitive, reward and affective function (Jacobs and Fornal, 1995; Lucki, 1998; Soubrié, 1986; Tops, et al., 2009), processes that are under the control of dense 5-HT afferent input to the limbic-corticostriatal circuit afforded by 5-HT neurons originating in the dorsal (DRN) and medial (MRN) raphe nuclei (Kosofsky and Molliver, 1987; Lidov, et al., 1980; Vertes and Linley, 2008). To understand the detailed interactions of 5-HT, 5-HT receptors and drugs of abuse, the picture is astonishingly complex. Serotonin functions within the synapse are controlled by the 5-HT transporter (SERT) while the actions of 5-HT are mediated by 14 genetically-encoded subtypes of 5-HT receptors (5-HTXR), which are grouped into seven families (5-HT1R – 5-HT7R) according to their structural and functional characteristics (for reviews) (Bockaert, et al., 2006; Hoyer, et al., 2002). The 5-HT receptor family is comprised of 13 distinct G protein-coupled receptors (GPCR) and one ligand gated ion channel (5-HT3R), providing a diverse landscape of available signaling cascades and mechanisms unparalleled in any other neurotransmitter system. The 5-HT1R (5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1ER, 5-HT1FR) couple principally to Gαi/o, the 5-HT2 (5-HT2AR, 5-HT2BR, 5-HT2CR) to Gαq/11, the 5-HT4R, 5-HT6R, and 5-HT7R couple to Gαs while 5-HT5AR couples to Gαi/o; the specific G protein isoform(s) which couples to 5-HT5BR is unknown. An appreciation of the impact of cocaine on 5-HT function must take into consideration the fact that increased synaptic efflux of 5-HT consequent to cocaine-evoked inhibition of 5-HT reuptake would result in 5-HT available for interaction with the regionally-localized receptors, thus, the role of 5-HT in the in vivo effects of cocaine is heavily dependent upon the receptor subtype(s) activated and downstream signaling webs triggered. Experimental approaches that largely stimulate or block multiple 5-HT receptors affords a limited appreciation of the role of 5-HT in neurobiology because these diverse receptors are distributed unevenly in the brain and affect many neural circuits. In fact, we would argue that confusion in our understanding of the relative roles of 5-HT receptors in neurobiology has been perpetrated by the employment of non-selective 5-HT receptor ligands in behavioral and mechanistic studies. A primary consideration is the role of individual 5-HT-receptive proteins in a given brain region, and the balance between facilitatory and inhibitory mechanisms that govern neuronal function and which, in turn, can be altered in long-term cocaine exposure. Serotonin neurotransmission is important in the tonic and phasic control over the limbiccorticostriatal circuit via regulation of both DA and glutamate neurotransmission (for review) (Alex and Pehek, 2006) and perturbations in the balance of its control may contribute to basal states of vulnerability as well as the altered neurobiological states that drive the transition from cocaine use to abuse to dependence.
The current literature supports a role for serotonergic control over impulsivity and cocaine cue reactivity, although there are decided gaps in appreciating how 5-HT systems interdigitate with other neurotransmitters (e.g., dopamine, glutamate) involved in these phenotypes. Here, we review the results of studies which employed global 5-HT manipulations and relatively selective 5-HT receptor ligands across assays that tap into behavioral expression of these constructs. Ultimately, we focus on the involvement of 5-HT2AR and 5-HT2CR systems due to the consistency in experimental outcomes (Table 1). This 5-HT2R family is comprised of the 5-HT2AR, 5-HT2BR and 5-HT2CR that share sequence homology, similar pharmacological characteristics and signaling pathways (Bockaert, et al., 2006; Hoyer, et al., 2002). The ultimate level of functionality of the 5-HT2R is determined by a culmination of factors, including availability of active pools of receptors and effective coupling to and activation of downstream signaling components. Upon the initial event of receptor activation by agonist, both 5-HT2AR and 5-HT2CR interact primarily with the heterotrimeric G protein Gaq/11 to activate the enzyme phospholipase Cβ which generates intracellular second messengers inositol-1,4,5-trisphosphate and diacylglycerol, leading to increased calcium release from intracellular stores (Hannon and Hoyer, 2008; Millan, et al., 2008). Both receptors also activate phospholipase A2 and generate arachidonic acid through an (unidentified) pertussis toxin-sensitive G protein (Felder, et al., 1990) as well as phospholipase D (McGrew, et al., 2002; Moya, et al., 2007). Lastly, the 5-HT2AR and 5-HT2CR directly associate with β-arrestin2 to control downstream signaling (Abbas, et al., 2009; Labasque, et al., 2008), a G protein-independent process which has profound behavioral relevance (Schmid, et al., 2008). The degree to which these downstream signaling pathways are recruited varies between the receptors, both at the level of agonist-dependent (“ligand-directed signaling”, “biased agonism”, “functional selectivity”) and agonist-independent activation (“constitutive activity”) of each pathway (Berg, et al., 2005; Berg, et al., 1998). Such features are likely to distinguish the functional effects of the 5-HT2R subtypes and their roles in behaviors key in addictive processes.
Table 1.
Effects of 5-HT manipulations on impulsivity, cocaine reward, and cocaine-seeking in animals
| 5-HT Manipulation | Action | Impulsive Action* | Cocaine Self-Administration* | ||
|---|---|---|---|---|---|
| Reward | Cocaine-Seeking | ||||
| Cue | Cocaine | ||||
| 5,7-DHT1–6 | 5-HT neurotoxin |

|

|

|

|
| PCPA6–8 | Tryptophan hydroxylase inhibitor |

|

|
||
| Fenfluramine9–11 | 5-HT releaser |

|

|
NE | |
| Fluoxetine10,12–19 | SSRI |

|

|

|
NE |
| 8-OH-DPAT8,17,21–25 | 5-HT1AR agonist |

|

|
||
| WAY1006358,11,22,23,26 | 5-HT1AR antagonist |
 /NE /NE
|
NE |

|
|
| RU249698,23,27 CP9425328 |
5-HT1BR agonist |
 /NE /NE
|

|

|

|
| SB216641, GR12793529 | 5-HT1BR antagonist |

|

|
||
| --- | 5-HT1BR overexpression in NAc 30 |

|

|

|
|
| DOI8,21,23,31–35 | 5-HT2AR agonist |

|
|||
| M1009072,36–40 | 5-HT2AR antagonist |

|
NE |

|

|
| RO60-017536,40,49–50 WAY16390941–42,44–45 MK 21249lorcaserin43 |
5-HT2CR agonist |

|

|

|

|
| SB242084^36,40,46, 48-51, 55 | 5-HT2CR antagonist |

|
 /NE /NE
|

|
|
| --- | 5-HT2CR knockdown in mPFC 47 |

|

|
||
| SB27014652–54 Ro04-679054 |
5-HT6R antagonist | NE |
 /NE /NE
|

|

|
“Impulsive Action” refers to studies that assayed “action restraint” measures of impulsive action, (see text). “Reward” refers to studies that evaluated effects of manipulations predominantly on intake of cocaine (reinforcing effects) and/or breakpoints (motivational effects). “Cocaine-seeking” refers to behavioral output reinforced by contextual and/or discrete drug-associated cues during withdrawal or following repeated extinction training which occurred in the absence (“cue”) or presence of investigator-delivered cocaine (“Cocaine”). Greyed cells indicate that no publications for these manipulations were identified. The red and blue arrows indicate an increase ( ) or decrease (
) or decrease ( ), respectively, in cocaine intake or breakpoint (Cocaine Reward), operant responses following extinction training or forced abstinence in the absence (Cue-Evoked Cue Reactivity) or presence of pretreatment with investigator-delivered cocaine (Cocaine-Primed Cue Reactivity). “NE” indicates that no effect was observed under the treatment conditions employed.
), respectively, in cocaine intake or breakpoint (Cocaine Reward), operant responses following extinction training or forced abstinence in the absence (Cue-Evoked Cue Reactivity) or presence of pretreatment with investigator-delivered cocaine (Cocaine-Primed Cue Reactivity). “NE” indicates that no effect was observed under the treatment conditions employed.
SB242084 alone supported self-administration in squirrel monkeys (Manvich et al., 2012).
2001;
2004;
2003;
2011;
2012;
Recent research emphasizes that the 5-HT system exhibits a multitude of distinctive properties relative to other monoaminergic systems. A new conceptual understanding of 5-HT systems was born with the recent discovery that the synthetic enzyme for 5-HT in brain (tryptophan hydroxylase-2) is structurally distinct from that in peripheral tissues (tryptophan hydroxylase-1) (Walther and Bader, 2003). Not one, but two, serotonergic pathways innervate cortical and subcortical structures from their parent neurons of origin in the raphe nuclei with one set of serotonergic fibers (fine fibers from DRN) exquisitely sensitive and the other (beaded fibers from MRN) relatively insensitive to neurotoxic damage by substituted amphetamines (Kosofsky and Molliver, 1987). The 5-HT2CR is the only GPCR to known to undergo pre-RNA editing such that the 5-HT2CR can exist in 32 predicted mRNA isoforms that could encode up to 24 different receptor protein isoforms in human (Gurevich, et al., 2002) or rat brain (Anastasio, et al., 2014). Constitutive activity for some 5-HT receptors is prominent and the regulation of the 5-HT2R family is unique in that both agonists and antagonists have been noted to downregulate/desensitize receptors, an outcome of chronic treatment which is inconsistent with classical pharmacological thought (Bockaert, et al., 2006). Even in cases of very high structural homology between receptor subtypes, the functional impact of stimulation may be notably oppositional (Bockaert, et al., 2006; Bubar and Cunningham, 2008) and the molecular characteristics of the protein complex may overlap, but are not identical (Becamel, et al., 2004). The significance of these distinct aspects of the 5-HT system is largely a mystery, primarily because of our overall lack of knowledge. However, the more we learn about the biomachinery of the 5-HT system, the more these processes will yield to a greater understanding and recognition of its importance in cocaine dependence as well as overall brain function.
5. Cocaine Reward and Serotonin
Food, water, sex or an abused drug can serve as a rewarding stimulus that positively reinforces, and increases the probability of, behavior leading to its consumption (Morse and Skinner, 1958). Multiple, somewhat distinguishable, facets of reward include hedonic value (`liking'), the organism's motivation to approach and consume rewards (incentive salience; `wanting'), and the learning of predictive associations between the reward and allied internal/external stimuli (drug-associated stimuli) (Berridge, et al., 2009). The activity of 5-HT neurons that innervate limbic-corticostriatal circuit controls the hedonic value of natural rewards (Nakamura, et al., 2008; Ranade and Mainen, 2009) and the motivational aspects of reward evoked by intracranial self-stimulation (Miliaressis, 1977; Simon, et al., 1976; Van der Kooy, et al., 1978). Electrophysiological studies in monkeys suggest that neurons in the DRN encode reward-seeking behaviors, and that DRN 5-HT neurons broadcast reward-related information to the forebrain (Bromberg-Martin, et al., 2010; Inaba, et al., 2013; Miyazaki, et al., 2011a; Miyazaki, et al., 2011b). Utilizing optogenetic technologies, the selective stimulation of mPFC neurons that project directly to the DRN resulted in a profound influence over a motivated behavior (Warden, et al., 2012) which is likely due to control of neuronal firing and 5-HT efflux in the DRN (Celada, et al., 2001). While these data strongly support the involvement of 5-HT innervation of forebrain in reward processing, there is little known about how 5-HT neurons coordinate the hedonic, motivational and learned aspects of cocaine reward. It is clear that cocaine binds to SERT, inhibits reuptake and increases extracellular 5-HT efflux (Koe, 1976), actions that have profound implications for the cellular activity of 5-HT raphe neurons (Cunningham and Lakoski, 1988; Cunningham and Lakoski, 1990; Pitts and Marwah, 1986) and 5-HT efflux and neuronal signaling in the terminal regions of raphe 5-HT neurons, including cortical and striatal regions (Bradberry, et al., 1993; Cameron, et al., 1997; Parsons and Justice, Jr., 1993; Pum, et al., 2007). Thus, cocaine exposure significantly alters the function of 5-HT neurons and 5-HT signaling in downstream targets in limbic-corticostriatal circuitry, and, as such, would likely impact the control of 5-HT neurons over reward processing.
The hedonic aspects of cocaine reward as well as the incentive motivational value attributed to cocaine reward and cocaine-associated cues are studied in the self-administration assay under controlled conditions in animals. Elegant self-administration assays have also been developed in humans (Comer, et al., 2008; Matuskey, et al., 2012), and applied to study cocaine reward (Fischman and Foltin, 1992; Fischman and Schuster, 1982), although reports in humans predominantly rely on self-report questionnaires to explore the euphorigenic and subjective effects of cocaine. In drug self-administration, the presentation of an abused drug as an appetitive “reward” contingent upon a behavioral response increases the probability that that behavior will reoccur. There are multiple self-administration paradigms which are employed to study drug reward processing in animals. Evaluation of active drug self-administration under experimental manipulations allows deductions about the role of a specific signaling pathway in the rewarding (reinforcing) effects of the self-administered drug. The progressive ratio self-administration variant in which the number of responses required for each reinforcer increases progressively during the session taps into the motivation to approach and consume rewards (incentive salience); the breakpoint is used as a measure of the motivation to take cocaine (Roberts, et al., 2007). The predictive associations between cocaine reward and allied internal/external stimuli are frequently assessed by measuring the learned behavioral output (“drug-seeking”) within the drug-taking context and/or in the presence of discrete drug-associated cues during forced abstinence or following repeated extinction training (Marchant, et al., 2013; Pickens, et al., 2011). There is a great deal of intriguing information emerging concerning the control of cues that are discrete (e.g., light or tone delivered with the drug) (Cunningham, et al., 2012; Meil and See, 1996; Nic Dhonnchadha, et al., 2009), contextual (environment is which drug self-administration occurs) (Crombag, et al., 2008; Hearing, et al., 2011), discriminative (predictive of drug availability) (Bradberry and Rubino, 2004; Kallupi, et al., 2013; Weiss, et al., 2000) or provoked by external stimuli such exposure to the drug itself (de Wit and Stewart, 1981) or stressful circumstances (Ahmed and Koob, 1997; Goeders and Guerin, 1994). The propensity for cocaine-associated cues to trigger relapse-like behavior in animals (return to attempts to deliver cocaine) (Kalivas and McFarland, 2003) as well as craving and relapse in humans and to activate extended limbic-corticostriatal circuitry (Childress, et al., 1999; Kosten, et al., 2006; Sinha and Li, 2007), elevate the need to understand the mechanisms through which cocaine and cocaine-associated cues so powerfully control behavior. The dominant hypothesis in the field is that the neural circuitry generating the reinforcing effects of abused drugs converge with those that serve as the substrate for engendering drug-taking (Kalivas and McFarland, 2003). Animal models provide the opportunity to address hypotheses employing selective ligands and manipulations of the 5-HT system, many of which are not available for human research. Although several additional models are employed (e.g., conditioned place preference, conditioned hyperactivity, etc.), here we focus predominantly upon the results derived from self-administration paradigms.
Cocaine is a robust reinforcer in self-administration assays in animals and humans, and its hedonic value is under the control of 5-HT as assessed by studies of rate of responding for cocaine and/or breakpoints. A summary of studies across rodents and monkeys is shown in Table 1 (Cocaine Reward; please note that Table 1 collapses observations across multiple types of paradigms and the reader is directed to the actual publications to gain a complete appreciation of the methodologies employed and the resulting observations). The potency of cocaine analogues to support self-administration correlated negatively with potency to bind to SERT (Ritz, et al., 1987), an observation which suggests that 5-HT may contribute an inhibitory role in cocaine reward. Notably, increased breakpoints for cocaine seen following depletion of central 5-HT after intracerebroventricular (ICV) infusion of the neurotoxin 5,7-dihydroxytryptamine (5,7-DHT) correlated with decreases in 5-HT in midbrain, but not NAc or amygdala (Roberts, et al., 1994). Localized depletion of 5-HT in the medial forebrain bundle or amygdala resulted in increased breakpoints (Loh and Roberts, 1990) suggesting that forebrain 5-HT innervation is critical to cocaine reward. Systemic administration of l-tryptophan to increase central 5-HT via synthesis (Carroll, et al., 1990b; McGregor, et al., 1993), the 5-HT releaser fenfluramine (Glowa, et al., 1997) or the selective 5-HT reuptake inhibitor (SSRI) fluoxetine (Carroll, et al., 1990a; Oleson, et al., 2011; Peltier and Schenk, 1993; Richardson and Roberts, 1991) suppressed the reinforcing and motivational properties of cocaine. In sum, increased and decreased central 5-HT tracked with blunted and augmented reinforcing properties of cocaine, respectively. There are no published reports of which we are aware that have evaluated the influence of 5-HT manipulations on cocaine self-administration in humans directly. However, in self-report analyses, fluoxetine suppressed cocaine-induced subjective effects and drug “liking” (Walsh, et al., 1994) while tryptophan depletion augmented cocaine-induced drug “wanting” (Cox, et al., 2011) but suppressed the “high” induced by intravenous cocaine (Aronson, et al., 1995). These few results in humans must be interpreted with caution as it is unclear whether self-report of drug-induced state reflects sensitivity to the reinforcing effects of the drug (Matuskey, et al., 2012), however they provide support to the general concept that 5-HT may regulate the reward value of cocaine.
Further clarity in understanding this question is provided by analyses of cocaine reward following pretreatment with a selective 5-HT receptor ligand, however, no such compounds are yet available for analyses in humans (see 9. Implications for Treatment of Cocaine Addiction). Table 1 summarizes animal studies with available compounds with the best selectivity for a given 5-HT receptor. These data suggest that the 5-HT1AR (Czoty, et al., 2002; Muller, et al., 2007; Nader and Barrett, 1990; Peltier and Schenk, 1993) and 5-HT2CR (Cunningham, et al., 2011; Fletcher, et al., 2002; Grottick, et al., 2000; Neisewander and Acosta, 2007) provide inhibitory tone over cocaine reward while an excitatory role for the 5-HT1BR (Parsons, et al., 1998; Pentkowski, et al., 2009; Pentkowski, et al., 2012; Przegalinski, et al., 2007; Przegalinski, et al., 2008) is supported; the 5-HT2AR appears to uninvolved in the reinforcing or motivational effects of cocaine (Filip, 2005; Fletcher, et al., 2002; Nic Dhonnchadha, et al., 2009), and there are conflicting data as to the involvement of the 5-HT6R (Valentini, et al., 2013; van Gaalen, et al., 2010) while the 5-HT2BR, 5-HT4R and 5-HT5R remain to be evaluated. These data support a role for 5-HT in the control of reward processing for cocaine and one which is dependent upon involvement of specific receptor subtypes.
Serotonin innervation (Kosofsky and Molliver, 1987; Lidov, et al., 1980; Vertes and Linley, 2008) and distribution of SERT (Maloteaux, 1986; Pickel and Chan, 1999) are not uniform across forebrain and the magnitude and temporal attributes of the effects of cocaine on 5-HT efflux is region-specific (Bradberry, et al., 1993; Cameron, et al., 1997; Parsons and Justice, Jr., 1993; Pum, et al., 2007). Systemic cocaine administration elevates 5-HT efflux in mesocorticostriatal regions, including the VTA (Chen and Reith, 1994; Parsons and Justice, Jr., 1993), NAc (Andrews and Lucki, 2001; Parsons and Justice, Jr., 1993) as well as PFC and other neocortical areas (Mangiavacchi, et al., 2001; Pum, et al., 2007). Localized microinjections of 5-HT2R ligands significantly alter cocaine-evoked behaviors (i.e., hyperactivity, discriminative stimulus effects) (Bubar and Cunningham, 2008), however, only a handful of published studies have manipulated 5-HT receptors in discrete brain nuclei and assessed the reinforcing or motivational effects of cocaine in self-administration paradigms. These few studies focused on the VTA (5-HT2CR) (Fletcher, et al., 2004), the NAc (5-HT1BR) (Pentkowski, et al., 2012) or mPFC (5-HT2AR, 5-HT2CR) (Anastasio, et al., 2014; Pentkowski, et al., 2010; Pockros, et al., 2011). Intra-VTA activation of 5-HT2CR attenuated cocaine intake on both fixed and progressive ratio schedules (Fletcher, et al., 2004), matching directionally the results following systemic administration of a selective 5-HT2CR agonist (Cunningham, et al., 2011; Fletcher, et al., 2002; Grottick, et al., 2000; Neisewander and Acosta, 2007). Overexpression of the 5-HT1BR in the medial NAc shell resulted in a leftward/upward shift in the dose-effect curve and increased breakpoint for cocaine delivery (Pentkowski, et al., 2012), consistent with observations seen after systemic 5-HT1BR agonists (Parsons, et al., 1998). Microinfusion of a selective 5-HT2AR antagonist into ventral mPFC (Pockros, et al., 2011) or a 5-HT2CR agonist into the prelimbic, infralimbic mPFC or the neighboring cingulate cortex were ineffective at altering cocaine intake (Pentkowski, et al., 2010). Thus, based upon this limited data set, the NAc 5-HT1BR and VTA 5-HT2CR exert an excitatory and inhibitory role, respectively, to control the reinforcing and motivational properties of cocaine.
6. Impulsivity and Serotonin
Impulsivity is not a unitary construct, but can be considered a “trait” comprised of several components that contribute to its characteristic pattern of poorly conceived, disadvantageous decisions and behaviors (Evenden, 1999a; Moeller, et al., 2001a). Some of the components of impulsivity as a cognitive construct include maladaptive inhibitory control, failure to consider the consequences of behaviors, and a preference for immediate rather than delayed rewards (Evenden, 1999a; Moeller, et al., 2001a). Impulsive behavior is not always aberrant as there are ecological advantages for the decision to act versus to reflect. In the United States, the lifetime prevalence of self-reported impulsive behavior is ~17% and within this population ~83% report a lifetime history of at least one major psychiatric disorder including addiction (Chamorro, et al., 2012). As impulsivity is a multi-dimensional construct, at present, the rank order and importance of each dimension within any one psychiatric disorder is unclear. Impulsivity is highly associated with “externalizing” psychiatric disorders such as attention-deficit hyperactivity disorder, bipolar disorder and drug dependence (Chamorro, et al., 2012). It is likely that impulsive behavior contributes significantly to several components of the addiction cycle, including initial choice to use drugs, the establishment of repetitive patterns of drug intake, the transition from casual to compulsive use to dependence and cue-evoked relapse events as proposed by the results of animal studies (Anastasio, et al., 2014; Belin, et al., 2008; de Wit and Richards, 2004). Thus, the convergence of the impulsivity trait with the dynamic state of cocaine dependence may contribute to greater vulnerability to relapse.
The multidimensional nature of impulsivity is reflected in the numerous measurement tools developed for laboratory assessments of impulsivity in humans and animals (Winstanley, 2011). These models are highly translational in that the operant tasks are employed across species (human, primate, and rodent) with appropriate alterations in design. Although no current preclinical model successfully measures all facets of impulsivity within individual subjects, animal models enrich and foster investigations into the neuromolecular underpinnings of disorders characterized by aberrant impulsivity enabling conclusions to be drawn across preclinical and clinical studies. Generally divided into three categories (Table 2), each task is designed to measure a different dimension of impulsivity: (1) measures of decision-making, in which impulsivity is defined as preference for a smaller-sooner reward over a larger-later reward; (2) behavioral disinhibition, in which impulsivity is assessed as premature or disinhibited responses; and (3) punishment/extinction, in which impulsivity is defined as the perseveration of a response which is punished or not reinforced (Table 2) (for reviews) (Cardinal, et al., 2004; Robbins, 2002; Winstanley, 2011). [We present animal models as measures of “impulsive action” or “impulsive choice” to avoid using anthropomorphic labels for animal behaviors.]
Table 2.
Comparison of behavioral laboratory measures of impulsivity
| Rats | Humans |
|---|---|
| Impulsive Choice Tasks | Reward-Directed Paradigms |
| Delay Discounting | Delay Discounting (Delayed Reward) |
| Iowa Gambling Task | |
| Impulsive Action Tasks | Rapid-Decision Paradigms |
| Stop Signal Task | Stop Signal Reaction Time Task |
| Go-NoGoTask | Go-NoGoTask |
| One/Five-Choice Serial Reaction Time Task | Continuous Performance Task; Immediate and Delayed Memory Task |
| Differential Reinforcement of Low-Rate | Single Key Impulsivity Paradigm |
| Fixed Consecutive Number Task | |
| Punishment/Extinction Tasks | Punishment/Extinction Paradigms |
| Fixed-Interval Extinction | lntra-/Extra-dimensional Set Shifting |
| Maze-based Set Shifting |
Impulsive action (behavioral disinhibition; rapid-response impulsivity; difficulty in withholding a prepotent response) and impulsive choice (decision-making; delayed reward measures) are two primary dimensions of impulsivity that have been associated with cocaine dependence in humans (Moeller, et al., 2001a; Moeller, et al., 2001b; Patkar, et al., 2004) and in rodent models of cocaine addiction (Anastasio, et al., 2014; Belin, et al., 2008; Perry, et al., 2005). The results of these studies suggest that inherent levels of impulsive behavior are positively correlated with the sensitivity to the effects of psychostimulants. Further, acute or chronic exposure to psychostimulants changes the probability of impulsive behaviors. In general, cocaine has been noted to increase impulsive action (Anastasio, et al., 2011; Paine and Olmstead, 2004; Stoffel and Cunningham, 2008) and impulsive choice (Logue, et al., 1992; Paine, et al., 2003), although there is not an absolute consensus with regard to the outcome of investigator-delivered cocaine administration across tasks (Paine, et al., 2003). Studies in healthy controls indicate a relationship between individual differences in response inhibition and activated neural circuits (Congdon, et al., 2010), however, the specific neural substrates that underlie these individual differences remain relatively unexplored.
Behavioral characteristics common to impulsivity have been linked to various aspects of serotonergic function within the corticostriatal circuit. There is extensive evidence that 5-HT manipulations alter performance in impulsivity tasks in humans and in animals (Table 1) (for reviews) (Dalley and Roiser, 2012; Fineberg, et al., 2010; Pattij and Vanderschuren, 2008; Winstanley, et al., 2006). Maladaptive impulsive behaviors have been correlated with diminished 5-HT levels in plasma and 5-HT metabolite (5-hydroxyindoleacetic acid) levels in cerebrospinal fluid (Brown and Linnoila, 1990; Stein, et al., 1993; Virkkunen and Narvanen, 1987), suggesting that an imbalance in 5-HT function may underlie impulsive behaviors. Depletion of 5-HT globally by 5,7-DHT (Winstanley, et al., 2004a) or PCPA (Evenden, 1998a; Masaki, et al., 2006) as well as 5,7-DHT into the DRN increased impulsive action (Harrison, et al., 1997). In healthy volunteers, tryptophan depletion has been reported to increase impulsivity in laboratory tasks (Booij, et al., 2006; Dougherty, et al., 2007; LeMarquand, et al., 1999; Walderhaug, et al., 2002; Walderhaug, et al., 2007), as does tryptophan challenge, but only in those subjects with high trait impulsivity (Markus and Jonkman, 2007; Young, et al., 1988). The 5-HT releaser fenfluramine and the SSRI fluoxetine suppressed impulsive action in rodents (Baarendse and Vanderschuren, 2012; Humpston, et al., 2013; Wolff and Leander, 2002), observations mirrored in humans (Manuck, et al., 1998; Moeller, et al., 2007; Patkar, et al., 2004; Soloff, et al., 2003). While the hypothesis is that decrements in 5-HT function may underlie impulsive behaviors, its validity has been challenged by recent findings that ablation of 5-HT terminals in the frontal cortex or the NAc did not alter impulsive actions in rats (Fletcher, et al., 2009) and that high levels of impulsive action are associated with elevated 5-HT release in the mPFC (Dalley, et al., 2002; Puumala and Sirviö, 1998). The inconsistent effects of global 5-HT manipulations are best explained by differences between tasks employed across studies and underscores not only the multidimensionality of impulsivity, but also that different tasks tap into distinct aspects of impulsive behavior that are engendered through discrete brain circuits and neural substrates. Furthermore, the complexity of impulsivity is highlighted by findings that the 5-HT system selectively regulates “action restraint” measures of impulsive action, but not “action cancellation” measures or impulsive choice (Winstanley, et al., 2004b). These last observations suggest that 5-HT plays a nuanced role in impulsivity, perhaps driven at the level of the 5-HT-receptive proteins and interactions with the limbic-corticostriatal DA and glutamate systems (Dalley and Roiser, 2012; Winstanley, et al., 2006).
Preclinical laboratories have pioneered elucidation of the pivotal roles for specific 5-HTXR in impulsive behaviors using the most selective pharmacologic tools; Table 1 summarizes studies with selective 5-HT receptor ligands upon systemic administration and virally-mediated gene transfer methods to manipulate expression of specific receptor proteins in subregions of the limbic-corticostriatal circuit in adult rats. Mixed results with 5-HT1AR ligands (Blokland, et al., 2005; Carli and Samanin, 2000; Evenden, 1998b; Evenden, 1999b) and incomplete pharmacological and/or genetic assessments of the 5-HT1BR complicates identification of a role for the 5-HT1R family in the emergence of impulsive action while the 5-HT6R does not appear to play a primary role in impulsive action (de Bruin, et al., 2013; Talpos, et al., 2006). A selective 5-HT2AR antagonist (e.g., M100907) (Anastasio, et al., 2011; Fletcher, et al., 2007; Fletcher, et al., 2011; Winstanley, et al., 2004b) or a selective 5-HT2CR agonist (e.g., Ro 60-0175, WAY163909) (Cunningham, et al., 2012; Fletcher, et al., 2007; Fletcher, et al., 2011; Navarra, et al., 2008; Winstanley, et al., 2004b) consistently reduced impulsive action. Systemic administration of the preferential 5-HT2AR agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) increased impulsivity (Blokland, et al., 2005; Carli and Samanin, 2000; Evenden, 1998b; Evenden, 1999b; Evenden and Ryan, 1999; Hadamitzky and Koch, 2009; Koskinen, et al., 2000a; Koskinen, et al., 2000b; Koskinen, et al., 2003) and the 5-HT2CR antagonist SB242084 produced qualitatively similar results to DOI (Anastasio, et al., 2013; Winstanley, et al., 2004b; Young, et al., 2011). Thus, there is an almost-perfect oppositional control of impulsive action observed in preclinical studies upon systemic administration of 5-HT2R ligands. Lastly, the possibility that the 5-HT2AR and 5-HT2CR may act in concert to control impulsivity is suggested by the discovery that impulsive action was synergistically suppressed by very low, ineffective doses of a selective 5-HT2AR antagonist (M100907) plus a selective 5-HT2CR agonist (WAY163909) (Cunningham, et al., 2012).
7. Cocaine Cue Reactivity and Serotonin
Drug-associated cues have long been hypothesized to be an essential driver in the cycle of addiction, relapse and recovery (Wikler, 1948), and psychological theorists have greatly enriched our understanding of the intertwined natures of drug reward and drug-related stimuli at behavioral and neuromolecular levels (Crombag, et al., 2008; Kelley, 2004; Richard, et al., 2013; Saunders and Robinson, 2013). Physiological responses (Robbins, et al., 1997), and altered brain activation patterns in limbic-corticostriatal circuits are observed upon exposure to cocaine-associated cues in cocaine-dependent subjects (Carpenter, et al., 2006; Goldstein, et al., 2007; Hester and Garavan, 2009; Potenza, et al., 2012). Attentional bias toward cocaine-associated cues measured in the cocaine-word Stroop task correlates positively with the severity of cocaine craving (Copersino, et al., 2004; Field, et al., 2009) and is also predictive of treatment outcomes in cocaine-dependent subjects (Carpenter, et al., 2006). Given their importance in addictive disorders, individual differences in sensitivity to reward-associated cues are now under study as a factor predictive of the propensity for drug use and/or relapse events in humans (Mahler and de Wit, 2010) and animals (Flagel, et al., 2010; Liu, et al., 2013; Saunders and Robinson, 2013; Tomie, et al., 2008; Yager and Robinson, 2013).
The phrase “cue reactivity” is a descriptor employed here to designate the sensitivity to drug-associated stimuli conditioned to the drug-taking experience regardless of the clinical scale or procedure employed to analyze this behavioral construct in humans or animals. However, there is not a consensus as to the operational definition of cue reactivity in experimental studies nor as to the protocols that provide the most translational applicability to the human situation. Of relevance to the present analysis of 5-HT control over drug-seeking, the experimental design of most pharmacological and genetic manipulations includes acquisition and maintenance of a short or long-access cocaine self-administration followed by extinction and subsequent reinstatement sessions in the presence of the drug-associated environment (Cunningham, et al., 2012; Fletcher, et al., 2007; Nic Dhonnchadha, et al., 2009; See, 2005; Shaham, et al., 2003). An alternative is to employ a self-administration assay followed by assessment of cocaine-seeking during an imposed withdrawal period (forced abstinence) (Anastasio, et al., 2014; Fuchs, et al., 1998; Grimm, et al., 2001; Liu, et al., 2013; Panlilio and Goldberg, 2007). The operant responses (e.g., lever presses) during the reinstatement session are recorded, but may or may not result in the delivery of cocaine-associated cues. Each variable of the protocol employed is ultimately important in dissecting the neurobiology of drug-seeking, however, the neuroadaptations that underlie withdrawal-related sequela determined following extinction training versus during forced abstinence are not identical (Di Ciano and Everitt, 2002; Schmidt, et al., 2001; Self, et al., 2004; Sutton, et al., 2003). Because of the limited number of studies that have evaluated the effects of selective 5-HT manipulations across impulsive action, cocaine reward and cocaine-seeking (Table 1), we have summarized results of studies in which cocaine-seeking was assessed during forced abstinence or following extinction training and under conditions in which the cocaine-seeking response was/was not reinforced by discrete drug-associated cues.
Depletion of forebrain 5-HT (Tran-Nguyen, et al., 1999; Tran-Nguyen, et al., 2001) and drugs which promote increased central 5-HT (fenfluramine, fluoxetine) (Baker, et al., 2001; Burmeister, et al., 2003; Burmeister, et al., 2004)) suppressed cue-evoked drug-seeking(Table 1). Cocaine-primed drug-seeking was, in contrast, elevated under conditions of 5-HT depletion (Tran-Nguyen, et al., 1999) and unaffected by fenfluramine and fluoxetine (Burmeister, et al., 2003). The outcomes suggest that 5-HT plays differential roles over cocaine-seeking versus cocaine reward (above), and between drug-seeking generated in the absence or presence of cocaine itself (Table 1). This pattern of observations is mirrored in humans as tryptophan depletion suppressed cue-evoked craving in the absence of cocaine administration (Satel, et al., 1995) and augmented cocaine-primed craving (Cox, et al., 2011) suggesting that cue- and cocaine-primed craving are under the regulation of 5-HT neurotransmission. A defining characteristic of these studies is whether cocaine is present at time of study, however, it is necessary to consider the small number of studies and that the neuroadaptations associated with repeated cocaine exposure are superimposed upon the overlapping, widespread changes of central 5-HT levels evinced by these 5-HT manipulations (see 8. Intersection of Impulsivity and Cue Reactivity in Cocaine Dependence).
The employment of pharmacological and genetic tools in animals allows insight into the controlling features of 5-HT neurotransmission over cocaine-seeking. A perusal of Table 1 indicates that, while mixed results obfuscate the role of 5-HT1AR (Czoty, et al., 2002; Muller, et al., 2007; Nader and Barrett, 1990; Peltier and Schenk, 1993), blockade of the 5-HT6R suppresses both cue- and cocaine-primed cocaine-seeking (Valentini, et al., 2013; van Gaalen, et al., 2010). The 5-HT2BR, 5-HT4R and 5-HT5R remain to be evaluated, but there is strong support for a regulatory role of the 5-HT1BR, 5-HT2AR and 5-HT2CR in cocaine cocaine-seeking. With regard to the 5-HT1BR, Neisewander and colleagues have shown that a selective 5-HT1BR agonist suppresses cue- and cocaine-primed drug-seeking (Acosta, et al., 2005; Pentkowski, et al., 2009), although conflicting data are reported (for a discussion, see (Przegalinski, et al., 2008). As predicted by the suppressive effects of a systemically-administered 5-HT1BR agonist (Acosta, et al., 2005; Pentkowski, et al., 2009), overexpression of the 5-HT1BR in the NAc (Pentkowski, et al., 2012) reduced both cue- and cocaine-evoked drug-seeking. Interestingly, a diametrically-opposite role for the 5-HT1BR is seen for cocaine intake (Parsons, et al., 1998; Pentkowski, et al., 2009; Pentkowski, et al., 2012; Przegalinski, et al., 2007). These data highlight that a given 5-HT receptor can play a differential role in cocaine reward versus responsivity to cocaine-conditioned stimuli; in the case of the 5-HT1BR, the differential role likely reflects the status of the 5-HT1BR expression and function which is persistently altered during withdrawal from cocaine self-administration (O'Dell, et al., 2006). This series of studies highlights the need to understand the dynamic neuroadaptation of 5-HT systems consequent to repeated cocaine self-administration to best comprehend the mechanisms underlying cue reactivity.
The most consistent evidence of a role for 5-HT receptors in cocaine cue reactivity has emerged from evaluation of 5-HT2AR and 5-HT2CR manipulations. In all studies conducted to date, a selective 5-HT2AR antagonist (M100907) (Fletcher, et al., 2002; Nic Dhonnchadha, et al., 2009) or a selective 5-HT2CR agonist (Ro 60-0175, WAY163909) share efficacy to suppress cue- and cocaine-primed drug-seeking (Cunningham, et al., 2011; Fletcher, et al., 2002; Grottick, et al., 2000; Neisewander and Acosta, 2007). The selective 5-HT2CR antagonist SB242084 increased cocaine-seeking in most studies (Burbassi and Cervo, 2008; Burmeister, et al., 2004; Fletcher, et al., 2002; Manvich, et al., 2012; Neisewander and Acosta, 2007; Pelloux, et al., 2012) and actually exhibited stimulant-like properties and supported self-administration in non-human primates (Manvich, et al., 2012). The possibility that the 5-HT2AR and 5-HT2CR may act in concert is suggested by the fact that both cue- and cocaine-primed drug-seeking were synergistically suppressed by very low, ineffective doses of a selective 5-HT2AR antagonist (M100907) plus a selective 5-HT2CR agonist (WAY163909) (Cunningham, et al., 2012). Because the 5-HT2AR and 5-HT2CR are oppositional in vivo (Bubar and Cunningham, 2008), results observed following administration of nonselective 5-HT2A/2CR antagonists are confounded by a concurrent blockade of the 5-HT2CR which would be expected to have oppositional actions to those of blockade of the 5-HT2AR, therefore, it is perhaps not surprising that non-selective 5-HT2A/2CR antagonists failed to attenuate cocaine-seeking in animals (Burmeister, et al., 2004; Filip, 2005; Schenk, 2000) or humans (Ehrman, et al., 1996) or influence self-reported euphoric effects of cocaine (Newton, et al., 2001) or craving (De La Garza, et al., 2005; Loebl, et al., 2008). Thus, once distinguished pharmacologically, the 5-HT2AR and 5-HT2CR plays an excitatory and inhibitory role, respectively, in cue reactivity.
8. Intersection of Impulsivity and Cue Reactivity in Cocaine Dependence
High impulsivity and cue reactivity are linked to an increased propensity to relapse (Moeller, et al., 2001b; O'Brien, et al., 1998) while studies suggest that these are interlocked phenotypes in humans (Liu, et al., 2011) and animals (Anastasio, et al., 2014; Belin, et al., 2008; Perry, et al., 2005). Serotonin is a sensitive homeostatic regulator of these interlocked phenotypes as measured following 5-HT manipulations which disrupt the 5-HT balance in the entire brain (e.g., 5,7-DHT, fluoxetine; Table 1). Serotonin neurons cast a wide net to all corners of the brain and their influence is “implicated in virtually everything, but responsible for nothing” (Jacobs and Fornal, 1995). Thus, 5-HT acts not so much as a sole mediator of specific behaviors, but rather as an elemental rheostat of “higher-order” neural circuits. Serotonin functions to constrain the response to arousing, rewarding and motivating stimuli while reduced 5-HT potentiates the impact of such stimuli (Izquierdo, et al., 2012; Lucki, 1998; Soubrié, 1986) and disengages stimuli from their emotional significance (Beevers, et al., 2009; Deakin, 1998). Within this construct, 5-HT flexibly modulates the organism's “drive to withdraw” from aversive or intense stimulation relative to the “withdrawn” state of low reactivity to stimulation (Tops, et al., 2009). When a global deficiency of 5-HT (e.g., 5,7-DHT) predicts the loss of restraint over arousing stimuli, measures of impulsive action, cocaine reward and cocaine-primed cocaine-seeking were elevated; under conditions of tonic elevation of 5-HT efflux (e.g., fluoxetine), impulsive action, cocaine reward and cue-evoked cocaine-seeking were diminished (Table 1). Such data give overall credence to the important role of 5-HT to control fundamental facets of the stimulus-response relationships that drive these behaviors. Increased firing of 5-HT neurons has been associated with “waiting” when the possibility of future reward is high (Miyazaki, et al., 2011a; Miyazaki, et al., 2011b; Miyazaki, et al., 2012b), a mechanism suggested to underlie serotonergic control over impulsive behavior (Miyazaki, et al., 2012a) and 5-HT can influence cognitive processing through both tonic and phasic mechanisms (Ranade and Mainen, 2009). The role of specific 5-HT receptor populations (e.g., 5-HT2AR and 5-HT2CR) as a nexus connecting these two phenotypes and the drive to relapse versus to maintain abstinence may be dependent upon tonic and phasic mechanisms which dictate serotonergic modulation over the limbic-corticostriatal circuit (Figure 1).
Figure 1. Serotonin serves as a key nexus for vulnerability to relapse.
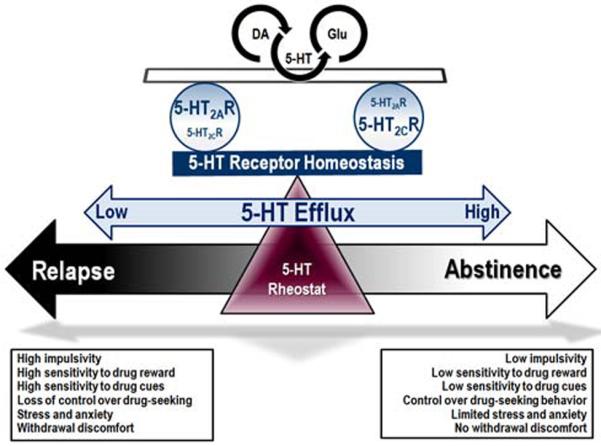
The 5-HT neurotransmitter system regulates higher order neural circuits that ultimately control expression of the impulsivity and cue reactivity phenotypes that contribute to relapse to drug-seeking. Alterations in the level of 5-HT efflux and associated disruptions in 5-HT receptor homeostasis shifts the balance of 5-HT:DA:glutamate neurotransmission that govern impulsivity, sensitivity to reward and cues, and control over drug seeking. A global deficiency of 5-HT, such as that observed during withdrawal from cocaine administration, is associated with loss of restraint over arousing stimuli, such that measures of impulsive action, cocaine reward and cocaine-primed cocaine-seeking are elevated. These factors, coupled with elevated stress, anxiety and withdrawal discomfort set the stage for enhanced vulnerability to relapse. As such, medications that act to restore 5-HT rheostatic control may be effective in promoting abstinence. This may best be accomplished by targeting specific 5-HT receptors of which the 5-HT2AR and 5-HT2CR, which consistently exert oppositional influence upon impulsivity and cue reactivity, have emerged as key targets mediating the intersection of these interlocked phenotypes.
It is quite possible that impulsivity and related irregularities of the 5-HT system set the stage for neurochemical and behavioral vulnerability to cocaine-associated stimuli (Anastasio, et al., 2014). The mPFC has received the most attention as a site at which the 5-HT2AR and 5-HT2CR evoke oppositional control over behavior based upon the results of intracranial microinfusion studies. Localized infusion of a 5-HT2AR-selective antagonist into the ventral mPFC suppressed impulsive action (Winstanley, et al., 2003) and cue-evoked cocaine-seeking (Pockros, et al., 2011). Microinfusion of a 5-HT2CR agonist into the prelimbic or infralimbic mPFC, but not the neighboring anterior cingulate cortex, suppressed both cue- and cocaine-primed cocaine-seeking (Pentkowski, et al., 2010). Most recently, we found that virally-mediated 5-HT2CR knockdown in the mPFC resulted in both elevated impulsive action and cocaine-seeking relative to controls (Anastasio, et al., 2014). Thus, 5-HT control of impulsivity and cocaine cue reactivity (but not cocaine reward) intersects within the mPFC with oppositional roles for mPFC 5-HT2AR and 5-HT2CR.
A 5-HT2AR:5-HT2CR balance within the mPFC may serve as a neurobiological rheostat over the mechanisms underlying impulsivity and sensitivity to cues through a highly organized interaction between 5-HT, DA, and glutamate systems within the limbic-corticostriatal circuit (Figure 2). Serotonin neurotransmission through its cognate 5-HT2AR and 5-HT2CR is important in establishing the excitatory/inhibitory balance in the mPFC microciruitry, although the specific regulatory forces involved are decidedly understudied. Rich 5-HT innervation to the mPFC arises from 5-HT neurons of the DRN and MRN and the mRNA and/or protein for the 5-HT2AR and 5-HT2CR are found in both primary neuronal types in the mPFC (Burnet, et al., 1995; Pompeiano, et al., 1994). A quantitative analysis indicated that 50–66% of pyramidal neurons express 5-HT2AR mRNA; within layers II–V, nearly every glutamate neuron expressed 5-HT2AR mRNA (de Almeida J. and Mengod, 2007). The great majority of 5-HT2AR labeling in mPFC is postsynaptic, although some localization in presynaptic terminals has been reported (Miner, et al., 2003). A more modest expression of 5-HT2AR mRNA is found in GABA interneurons (~13–46%), most notably parvalbumin- and calbindin-positive cells (de Almeida J. and Mengod, 2007). By comparison, lower overall expression of 5-HT2CR mRNA (Pompeiano, et al., 1994) and protein are observed relative to 5-HT2AR in the mPFC (de Almeida J. and Mengod, 2007); we have reported prominent expression of 5-HT2CR immunoreactivity in parvalbumin-positive interneurons in the mPFC (Liu, et al., 2007) consistent with previous observations (Burnet, et al., 1995). Activation of either receptor can result in depolarization of pyramidal neurons or GABA interneurons which regulate pyramidal outflow (Alex and Pehek, 2006; Araneda and Andrade, 1991; Beique, et al., 2007; Puig, et al., 2010). Importantly, the net consequence of 5-HT2AR and 5-HT2CR function in mPFC appears to be interactive; there is evidence that constitutive knockout of the 5-HT2AR results in upregulation of 5-HT2CR control over the excitability of mPFC pyramidal neurons (Beique, et al., 2007). This interaction between the 5-HT2AR and 5-HT2CR in mPFC is supported by our observation that, consequent to knockdown of the 5-HT2CR in mPFC, 5-HT2AR control over impulsive action is augmented (Anastasio and Cunningham, unpublished). These data suggest that a functional disruption of the cortical 5-HT2AR:5-HT2CR balance may be a key neurobiological trigger that sets the stage for enhanced impulsivity and sensitivity to cues. There is now evidence that 5-HT receptor control of PFC in humans remains balanced across development into adulthood (Lambe, et al., 2011) and that dysregulation of individual receptor subtypes during development (such as consequent to maternal deprivation) (Benekareddy, et al., 2010) could have long-lasting repercussions for 5-HT receptor control of the mPFC microcircuitry. Such developmental disruptions in 5-HT control of mPFC communications could set up conditions conducive to generation of inherent vulnerability traits such as impulsivity (Lambe, et al., 2011) or generalized cue reactivity (de Wit and Richards, 2004) that could promote initial drug use, trigger addictive and/or relapse processes. The magnitude and nature of the cellular response to 5-HT, however, are heavily dependent upon the cortical subregion, cellular morphology and laminar position of the neurons (Beique, et al., 2007; Gonzalez-Burgos, et al., 2005; Liu, et al., 2007). Similarly, the 5-HT2AR and 5-HT2CR could control mPFC output through actions in the same or different, interacting neurons or neuronal networks (Araneda and Andrade, 1991; Puig, et al., 2010). Nonetheless, we propose that an imbalance in the mPFC 5-HT2AR:5-HT2CR interaction disrupts the cortical microcircuitry resulting in profound neuroplastic consequences to overlapping and interlooping subcortical structures and shifts the 5-HT:DA:glutamate homeostatic framework within the limbic-corticostriatal circuit.
Figure 2. The PFC 5-HT2AR and 5-HT2CR is an important site of action for the intersection of impulsivity and cue reactivity.
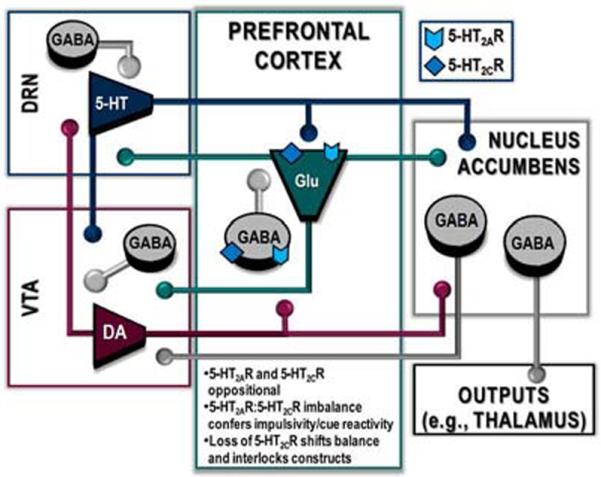
A schematic overview of the overlapping and reciprocal interactions between 5-HT, DA and glutamate neurotransmission is illustrated within the limbic-corticostriatal circuit. Serotonin neurons (blue) from the dorsal raphe nucleus (DRN) innervate the ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC); DA neurons (red) from the VTA project to the DRN, NAc and PFC; pyramidal glutamate neurons (green) from the PFC innervate the DRN, VTA and NAc; GABA medium spiny projection neurons (gray) from the NAc project to the VTA and other primary output systems (e.g., thalamus). The microcircuitry within each brain region is comprised of the respective 5-HT, DA, or glutamate projection neurons and GABA interneurons. Within the PFC 5-HT2AR (chevron) and 5-HT2CR (diamond) are each localized to GABA interneurons and pyramidal glutamate projection neurons, and thus, are perfectly situated to tightly control the PFC microcircuitry. We theorize that disruption of the oppositional 5-HT2AR:5-HT2CR balance within the PFC shifts the 5-HT, DA and/or glutamate homeostatic framework within the limbic-corticostriatal circuitry and sets the stage for enhanced impulsivity and sensitivity to cues in cocaine dependence.
Studies to date support the overarching hypothesis that connections between impulsivity, cocaine cue reactivity and a dysregulated 5-HT system exist. However, whether dysfunction in 5-HT neurotransmission which promote impulsivity and cue reactivity (Table 1), contribute to or result from, repeated cocaine self-administration is not known. An important consideration in understanding the mechanistic roles of 5-HT is the fact that repeated cocaine self-administration evokes significant neuroadaptations that are likely to be entangled with critical addictive processes. The neuroplastic changes in DA and glutamate subsystems associated with cocaine self-administration are well-characterized and integrated into dominant theories of addiction (Kalivas and Volkow, 2005; Koob and Volkow, 2010), but the features of serotonergic neuroplasticity have only marginally been evaluated in appropriate animal models. For example, termination of cocaine self-administration resulted in decreased basal levels of extracellular 5-HT in the NAc that persisted for 6–10 hours (Parsons, et al., 1995; Weiss, et al., 2001), although other timepoints and regions of the limbic-corticostriatal circuit have not been evaluated in this regard. Changes in the functional status of the 5-HT2R systems appear to follow repeated cocaine exposures as well, however, the use of widely variant doses and regimens of cocaine administration (predominantly, investigator-delivered), and 5-HT2R ligands which lack specificity are likely to contribute to the mixed directional changes. Serotonergic receptor function is reportedly altered in rodents during withdrawal from repeated cocaine exposure (Baumann and Rothman, 1996; Darmani, et al., 1992; Filip, et al., 2006; Levy, et al., 1994) and in abstinent human cocaine abusers (Ghitza, et al., 2007; Handelsman, et al., 1998; Lee and Meltzer, 1994; Patkar, et al., 2006). Response-independent chronic cocaine exposure regimens investigated to date did not indicate robust effects on either 5-HT2AR or 5-HT2CR mRNA or protein expression (Javaid, et al., 1993; Johnson, et al., 1993; Neisewander, et al., 1994), however, long-term cocaine self-administration was associated with increased 5-HT2AR availability in the frontal cortex of monkeys (Sawyer, et al., 2012). These findings suggest that the altered responsiveness of 5-HT2AR or 5-HT2CR seen following repeated cocaine administration may be related to direct alterations in receptor expression.
There is also evidence that changes in expression, cellular localization and/or function of downstream effectors that couple to 5-HT2AR or 5-HT2CR may account in part for cocaine-associated sequela. For example, 5-HT2AR supersensitivity of some behavioral and neuroendocrine effects seen during withdrawal from repeated, investigator-delivered cocaine administration was associated with an increase in membrane-associated Gαq/11 protein expression in the hypothalamus and amygdala, rather than a specific increase in 5-HT2AR expression (Carrasco, et al., 2003; Carrasco, et al., 2004). Of note, the postsynaptic scaffolding protein PSD95 plays a key role in the surface expression and agonist-mediated internalization of 5-HT2AR (Abbas, et al., 2009; Xia, et al., 2003) and promotes desensitization of the 5-HT2CR (Abbas, et al., 2009; Gavarini, et al., 2006). Thus, despite the fact that the 5-HT2AR and 5-HT2CR share a great degree of homology (Bockaert, et al., 2006; Hoyer, et al., 2002), the regulation of these receptors are differentially and profoundly regulated by PSD95 (Abbas, et al., 2009; Becamel, et al., 2004; Xia, et al., 2003). Decreased expression of PSD95 was observed in mice treated chronically with cocaine, while mice with a targeted deletion of PSD95 exhibited enhanced cocaine-induced locomotor hyperactivity (Yao, et al., 2004). It is probable that the 5-HT2R:PSD95 interaction is essential to normal 5-HT neurobiology and that cocaine-induced neuroplasticity may engage such processes, however, few studies have critically evaluated the status of 5-HT2AR or 5-HT2CR function during phases of cocaine self-administration nor linked 5-HT neuroadaptations with impulsive action or cocaine-seeking. We have recently discovered that rats with the highest level of impulsive action or cocaine cue reactivity displayed the lowest levels of mPFC 5-HT2CR protein in mPFC, and were the least responsive to 5-HT2CR ligands (Anastasio, et al., 2014). Further, we found that synaptosomal PSD95 protein in the mPFC associated with the 5-HT2CR to a greater extent in high impulsive rats versus low impulsive rats (Anastasio, et al., 2014). This augmented 5-HT2CR:PSD95 association would be expected to desensitize and internalize the 5-HT2CR (Anastasio, et al., 2014). Given the potential interactive nature of 5-HT2AR and 5-HT2CR function in the mPFC (Anastasio and Cunningham, unpublished) (Beique, et al., 2007), we propose that dysregulation of cortical 5-HT2AR and 5-HT2CR may be a neurobiological mechanism underlying the intersection of impulsive action and cocaine cue reactivity.
9. Implications for Treatment of Cocaine Addiction
Relapse is a major challenge to achievement of successful remission of cocaine addiction, and predictors of relapse and treatment success would be helpful to control addictive behaviors. Progress in understanding the neurobiology of vulnerability factors for relapse and identifying predictors of individual vulnerability are important steps in increasing opportunities for prevention of cocaine addiction. For example, the use of screening to identify maladaptive impulsivity could be helpful to identify individuals at risk for drug use (Perry, et al., 2011). Identification and validation of single nucleotide polymorphisms (SNPs) that predict impulsivity, cue reactivity or treatment success could also be useful in guiding prevention efforts (Ducci and Goldman, 2012). Impulsivity has been associated with genotypes that predict reduced 5-HT function (Bevilacqua, et al., 2010; Bevilacqua and Goldman, 2013; Bjork, et al., 2002; Stoltenberg, et al., 2012) and we have recently demonstrated that cocaine-dependent subjects who carry a SNP in the HTR2C (rs6318) (Lappalainen, et al., 1995) predicted to alter 5-HT2CR functionality (Brasch-Andersen, et al., 2011; Kuhn, et al., 2004; Okada, et al., 2004; Walstab, et al., 2011), exhibit significantly higher attentional bias on the cocaine-word Stroop task. As convergent evidence links genotypes to phenotypes important in addictive processes, it will be possible to best understand the shared genetic liability for impulsivity and cue reactivity, and to integrate this information into more effective clinical care for afflicted patients.
The development of biomarkers or predictors for vulnerability to addictive disorders will be of immense help in facilitation of treatment goals. Importantly, the advancement toward an armamentarium of medications that are effective and accessible means to suppress relapse (Volkow, et al., 2009) is necessary to improve the health outcomes of cocaine-dependent individuals. There has been little attention to the possibility that selective serotonergic medications may be of use in this regard, in large part because of the lack of such compounds for clinical trials in cocaine dependence. The SSRIs (e.g., fluoxetine) have been most studied and are largely ineffective as treatment in outpatient cocaine-dependent subjects as measured by outcomes of treatment retention, cocaine-positive urines or reductions in craving (Covi, et al., 1995; Grabowski, et al., 1995). However, Moeller and colleagues found that the SSRI citalopram in combination with cognitive behavioral therapy and contingency management significantly reduced cocaine-positive urines in citalopram-treated subjects who exhibited high baseline impulsivity scores (Green, et al., 2009; Moeller, et al., 2007; Schmitz, et al., 2009); these subjects remained in the study significantly longer than those that received placebo, reversing the negative correlation between the degree of baseline impulsivity and time in retention (Green, et al., 2009; Moeller, et al., 2001b; Moeller, et al., 2007; Patkar, et al., 2004; Schmitz, et al., 2009). These findings are consistent with a shared role for serotonergic substrates in impulsivity and cocaine relapse phenomenon and suggest that serotonergic strategies may be particularly effective in the impulsive subpopulation of cocaine addicts.
Several explanations are plausible for the inconsistency in the observations seen with citalopram versus other SSRIs in cocaine dependence. First, the SSRIs are a heterogeneous group of drugs that exhibit notable affinity for SERT and potently inhibit 5-HT reuptake, but differ with regard to relative affinities at the DA transporter (DAT) and norepinephrine (NE) transporter (NET), onset of therapeutic actions and half-life, and pharmacokinetic and metabolic profiles (Hiemke and Hartter, 2000; Vaswani, et al., 2003). Second, the fact that citalopram is a highly selective SSRI (3,400-fold higher affinity for SERT over NET or DAT) (Owens, et al., 1997) may contribute to the improved outcomes in cocaine-dependent subjects (Green, et al., 2009; Moeller, et al., 2001b; Moeller, et al., 2007; Patkar, et al., 2004; Schmitz, et al., 2009). The pharmacological profiles of SSRIs also includes moderate to high affinity for some 5-HT receptors. In particular, fluoxetine binds to the 5-HT2CR and acts as a potent 5-HT2CR antagonist (Ni and Miledi, 1997; Palvimaki, et al., 1996). Given that a selective 5-HT2CR antagonist (SB242084) enhances impulsive action (Fletcher, et al., 2007; Winstanley, et al., 2004b; Young, et al., 2011), cocaine-seeking (Burbassi and Cervo, 2008; Burmeister, et al., 2004; Fletcher, et al., 2002; Manvich, et al., 2012; Neisewander and Acosta, 2007; Pelloux, et al., 2012) and supports self-administration in non-human primates (Manvich, et al., 2012), fluoxetine actions as a 5-HT2CR antagonist may contribute to its failure to reduce craving and increase retention in treatment (Covi, et al., 1995; Grabowski, et al., 1995). Citalopram exhibits in vivo effects consistent with prominent actions as a 5-HT2CR agonist which are dose-dependently blocked by selective 5-HT2CR antagonists (Millan, et al., 1999; Palvimaki, et al., 2005). Third, a curious consequence of citalopram exposure may also contribute to its prominent actions as a 5-HT2CR agonist in vivo. A single injection of citalopram (20 mg/kg) was shown to rapidly upregulate 5-HT2CR binding sites in rat choroid plexus, an effect which is even more pronounced after chronic citalopram treatment (Palvimaki, et al., 2005; Palvimaki, et al., 1996). An increased density of 5-HT2CR after citalopram would allow 5-HT itself to exert greater actions via the 5-HT2CR and might account for the observations that the behavioral effects of citalopram fit the profile of a 5-HT2CR agonist moreso than do those of other SSRIs (Palvimaki, et al., 2005). In keeping with this hypothesis, fluoxetine does not alter the density of 5-HT2CR possibly due to its actions as a 5-HT2CR antagonist (Palvimaki, et al., 2005). Therefore, we propose that the treatment successes observed with citalopram (Green, et al., 2009; Moeller, et al., 2007; Schmitz, et al., 2009) over fluoxetine (Covi, et al., 1995; Grabowski, et al., 1995) in cocaine-dependent subjects may be related to the efficacy of citalopram to mimic selective 5-HT2CR agonists to suppress impulsivity (and perhaps cue reactivity) (Table 1).
There are no FDA-approved medications with the profile of a selective 5-HT2AR antagonist or a selective 5-HT2CR agonist which would be predicted to suppress drug-seeking promoted by impulsive action and/or cocaine cue reactivity administered either alone (Table 1) or in combination (Cunningham, et al., 2012). Preclinical and early clinical evaluations of selective 5-HT2AR antagonists support their potential efficacy as therapeutics in sleep disorders (Ancoli-Israel, et al., 2011), psychosis (Weiner, et al., 2001), psychosis in Parkinson's disease (Meltzer, et al., 2012) and other psychological disorders (Roberts, 2006). The 5-HT2AR antagonist (inverse agonist) pimavanserin has been shown to be effective in suppressing L-DOPA-induced psychosis in Parkinson's disease and clinical trials are ongoing to determine long-term safety and tolerability of pimavanserin in patients (www.clinicaltrials.gov) (Meltzer, et al., 2012). M100907 (volinanserin) and other selective 5-HT2AR antagonists promote the maintenance of sleep in animals (Griebel, et al., 2013) and M100907 has been evaluated in several clinical trials for treatment of insomnia (www.clinicaltrials.gov). It is noteworthy that sleep disorders (Morgan, et al., 2010) and psychosis (Peer, et al., 2009) are frequently co-morbid with cocaine addiction and that recurrent side effects of dopamine replacement therapy in Parkinson's Disease are increased impulsivity, compulsivity, gambling and risk-taking behaviors (Park and Stacy, 2011). Given the preclinical and clinical data summarized here, a compelling case can be made that a selective 5-HT2AR antagonist will prove effective to suppress relapse and also relieve concomitant sleep and psychiatric disorders seen in cocaine addiction. However, volinanserin and related compounds (e.g., eplivanserin) are not under study for this indication and are not readily available for proof-of-concept clinical research or outpatient trials.
The selective, high-efficacy 5-HT2CR agonist lorcaserin (APD-356, Belviq®) has been approved by the FDA and will be marketed soon for patients with a body-to-mass (BMI) index of >30 or with a BMI >27 comorbid with type-2 diabetes, hypertension or dyslipidemia (www.us.eisai.com/). Several clinical trials conducted with lorcaserin demonstrated significant reductions in weight, insulin resistance, serum lipids and inflammatory markers as well as blood pressure in obese subjects (Fidler, et al., 2011; Smith, et al., 2009; Smith, et al., 2010). Lorcaserin is a mild and tolerable medication with side effects noted as transient headache, nausea and dizziness predominantly (Fidler, et al., 2011; Smith, et al., 2009; Smith, et al., 2010). In the trials conducted to date, there is no evidence that heart valve or pulmonary problems were increased upon lorcaserin treatment relative to baseline values (Fidler, et al., 2011; Smith, et al., 2009; Smith, et al., 2010). In healthy recreational polydrug users, the subjective effects of a therapeutic dose of lorcaserin (20 mg) were similar to placebo while supratherapeutic doses (40, 60 mg) were associated with negative subjective effects; there was no evidence of lorcaserin-induced perceptual distortion or abuse potential noted (Shram, et al., 2011). Given the potential of a selective 5-HT2CR agonist to suppress impulsive action, cocaine-taking and cocaine-seeking (Table 1) as well as recent observations that lorcaserin suppressed nicotine-induced impulsive action, nicotine-taking and - seeking (Higgins, et al., 2012; Levin, et al., 2011), the ultimate marketing of lorcaserin will provide the opportunity to test the hypothesis that lorcaserin will be efficacious in extending abstinence in psychostimulant addiction.
Serotonin neurotransmission through the 5-HT2CR suppressed impulsive action, cocaine-taking and cocaine-seeking in animal models (Table 1). The consistency of these observations suggests that additional drug discovery opportunities in addition to 5-HT2CR agonists. Two such opportunities are being exploited by our group (Anastasio, et al., 2013; Ding, et al., 2012). The sequence of ligand binding to a GPCR and subsequent activation of downstream signaling can be positively or negatively modulated by binding of ligands at allosteric sites topographically-distinct from the orthosteric ligand binding site (Conn, et al., 2009; Melancon, et al., 2012). In addition, allostery could also be promoted through protein:protein interactions at the 5-HT2CR cytosolic face (Becamel, et al., 2002; Labasque, et al., 2008), however, the functional impact of such interactions over 5-HT2CR signaling remains to be thoroughly explored. There are theoretical reasons (i.e., increased selectivity, upper ceiling effects, separate control of affinity and efficacy) as to why allosteric ligands may be preferred therapeutic chemical targets (Kenakin, 2010). Therefore, 5-HT2CR allosteric modulators present a novel drug design strategy (Anastasio, et al., 2013; Ding, et al., 2012; Im, et al., 2003) to selectively tune up (or dampen) signaling in response to endogenous 5-HT in a site- and event-specific manner (Conn, et al., 2009; Melancon, et al., 2012). The observation that decreased extracellular levels of 5-HT were observed in the NAc following termination of cocaine self-administration (Parsons, et al., 1995; Weiss, et al., 2001) as well as our observations that rats with the highest level of impulsive action (Anastasio, et al., 2014) or cocaine-seeking (Liu, et al., 2013) displayed the lowest levels of mPFC 5-HT2CR protein in mPFC suggest that means to restore function through positive allosteric 5-HT2CR modulation may provide alternative approaches to treatment of cocaine addiction.
10. Future Directions
Many questions remain concerning the explicit role of 5-HT receptors and transduction systems within key neural circuits in mediating impulsivity, cocaine reward and cocaine-associated cue reactivity. There are several, overlapping mechanisms that could underlie the efficacy of either global changes in 5-HT efflux (after manipulation of 5-HT levels in brain) or following direct 5-HT receptor ligands which are incompletely understood, and here we illustrate several future investigations of interest to gaining a full appreciation of how 5-HT influences these processes. First, 5-HT is likely to impact the hedonic value of cocaine (`liking'), the motivation to take cocaine (`wanting'), and also stimulus-response learning that link cocaine with cocaine-associated stimuli (Berridge, et al., 2009). Given the role of 5-HT to control responsiveness to multiple types of external and internal stimuli (Izquierdo, et al., 2012; Lucki, 1998; Soubrié, 1986), future efforts will be need to deconstruct its involvement in key aspects of impulsivity, cocaine reward and cue reactivity. One aspect that warrants attention is the extent to which 5-HT-mediated reductions in the reinforcing effects of cocaine could be due to the alleviation of simultaneously-experienced aversive effects (Ettenberg, et al., 2011; Riley, 2011). The distinction between these aspects of how 5-HT influences reward versus aversion may be important when considering recent findings that SSRIs limit the neural activation observed upon presentation of either rewarding or aversive stimuli (Kranz, et al., 2010; McCabe, et al., 2011). Second, conditioned associations to cues previously associated with drug-taking are a formidable combination of factors that become encoded into memory and 5-HT neuroplasticity is a component of these processes. This memory becomes stabilized through a process of consolidation, dependent on protein synthesis, wherein short-term memory traces are transformed into stable long-term memories (Davis and Squire, 1984; Dudai and Eisenberg, 2004). Serotonin is described as necessary to establish normal long-term potentiation (LTP) in invertebrate and mammalian models (Kandel, 2004; Kim, et al., 2006), and is thought to be responsible for inhibitory control in the establishment of LTP in hippocampal-PFC circuits (Ohashi, et al., 2003). At present, a role is suggested for the 5-HT2R family in cortical LTP (Edagawa, et al., 2000; Inaba, et al., 2009) and in the acquisition, consolidation and retrieval of learned behaviors, especially in those engendered in simple memory models (e.g., autoshaping tasks) (Meneses, 2007; Prado-Alcala, et al., 2003). Serotonin is in fact required to recruit protein synthesis and synaptic growth thought to underlie long-term memory (Bailey, et al., 2000). However, there are no explicit studies to demonstrate that 5-HT is involved in the learning, retrieval and/or reconsolidation of cocaine-associated memories or to investigate the exact 5-HT circuitry involved. These processes may be particularly important in the learning and memory involved in cocaine cue reactivity given that patterns of 5-HT involvement in cue- and cocaine-primed drug-seeking depend in part upon specific aspects of the task employed to evaluate these behaviors (see 7. Cocaine Cue Reactivity and Serotonin; Table 1). Understanding these neural systems and their special properties vis-à-vis drug-associated cues will open the door to pharmacological or genetic means to selectively abolish cocaine cue reactivity and its control over drug-seeking (Everitt and Wolf, 2002). This situation reflects the complex nature of serotonergic processing of information in brain, the lack of selective molecular and pharmacological manipulations available until recently, as well as the stage of learning and memory investigated.
11. Conclusions
We have entered the world of personalized medicine in which a patient's unique genetic and phenotypic profiles will ultimately provide the signposts to tailor diagnoses, maximize treatment success and reduce off-target and side effect profiles of treatments. The science in chronic diseases such as cancer is rapidly advancing in these goals to provide new targeted diagnostics and medications for treatment tailored to provide the best clinical response in an individual (Hamburg and Collins, 2010). This vision is also within reach of addiction scientists. We now appreciate the involvement of disruptions in brain DA and glutamate and homeostasis involved in cocaine addiction (Kalivas and Volkow, 2005; Koob and Volkow, 2010) and new therapeutic approaches to forestall relapse are emerging from this research (Olive, et al., 2011). However, we would be foolish to overlook the 5-HT neurotransmitter system as an important target in the quest to improve our diagnostic and prognostic capabilities with identified disease biomarkers, to understand vulnerability to addiction and relapse through the neurobiology of relapse-predictive phenotypes (e.g., impulsivity and cue reactivity), and treat this complex disorder. The pressing need to improve treatments for addiction demands a greater understanding of the role of 5-HT in addiction neurobiology and the integration of this knowledge into the dominant theoretical constructs of addiction. The future also holds great opportunity to establish how 5-HT signaling determines behavioral flexibility across the lifespan with the promise to discover predictors for at-risk individuals and reverse disrupted 5-HT signaling to minimize risk for addictive disorders. This information is absolutely critical to the translation from experimental analyses to improving recovery from addiction. For instance, the present review provides a framework that suggests that restoration of the 5-HT2AR:5-HT2CR balance may help to recover normal corticostriatal function and forestall relapse events. This line of investigation may lead to the targeted development of pharmacological and molecular strategies to restore 5-HT function and will provide not only insight into neural dysfunction in cocaine dependence but also into the molecular basis of normal brain function.
RESEARCH HIGHLIGHTS
Maladaptive impulsivity and cue reactivity predict relapse in cocaine addiction.
Relapse is associated with imbalanced 5-HT function in key neural circuits.
Restoration of 5-HT function may normalize impulsivity/cue reactivity to promote abstinence.
ACKNOWLEDGEMENTS
This research was supported by the National Institute on Drug Abuse grants P20 DA024157 (KAC), R01 DA006511 (KAC), K05 DA020087 (KAC), K99 DA033374 (NCA) and the Jeane B. Kempner Postdoctoral Scholar Award (NCA). The authors thank Dr. F. Gerard Moeller for his translational guidance and Dr. Marcy Bubar Jordan for helpful discussions and assistance with text and figures. We dedicate this manuscript to Mrs. Essie Anastasio who passed away during the writing of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Boynton FA, Kirschner KF, Neisewander JL. Stimulation of 5-HT1B receptors decreases cocaine- and sucrose-seeking behavior. Pharmacol Biochem Behav. 2005;80:297, 307. doi: 10.1016/j.pbb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2006;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng YJ, Bremer NM, Smith TD, Fox RG, Swinford SE, Seitz PK, Charendoff MN, Craft JW, Laezza F, Watson CS, Briggs JM, Cunningham KA. Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci. 2013;33:1615–1630. doi: 10.1523/JNEUROSCI.2656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. Serotonin (5-hydroxytryptamine) 5-HT2A receptor: Association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol. 2011;22:248–261. doi: 10.1097/FBP.0b013e328345f90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O'Neil RT, Fink LHL, Li D, Green TA, Moeller FG, Cunningham KA. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39(2):370–382. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Vanover KE, Weiner DM, Davis RE, Van Kammen DP. Pimavanserin tartrate, a 5-HT(2A) receptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Sleep Med. 2011;12:134–141. doi: 10.1016/j.sleep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews CM, Lucki I. Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:221–229. doi: 10.1007/s002130100704. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Aronson SC, Black JE, Mcdougle CJ, Scanley BE, Jatlow P, Kosten TR, Heninger GR, Price LH. Serotonergic mechanisms of cocaine effects in humans. Psychopharmacology (Berl) 1995;119:179–185. doi: 10.1007/BF02246159. [DOI] [PubMed] [Google Scholar]
- Baarendse PJ, Vanderschuren LJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER. A novel function for serotonin-mediated short-term facilitation in aplysia: conversion of a transient, cell-wide homosynaptic hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci U S A. 2000;97:11581–11586. doi: 10.1073/pnas.97.21.11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Chronic cocaine exposure potentiates prolactin and head shake responses to 5-HT2 receptor stimulation in rats. Neuropharmacology. 1996;35:295–301. doi: 10.1016/0028-3908(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Becamel C, Alonso G, Galeotti N, Demey E, Jouin P, Ullmer C, Dumuis A, Bockaert J, Marin P. Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J. 2002;21:2332–2342. doi: 10.1093/emboj/21.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci. 2010;30:12138–12150. doi: 10.1523/JNEUROSCI.3245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends Pharmacol Sci. 2005;26:625–630. doi: 10.1016/j.tips.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, Belmer A, Hodgkinson CA, Dell'Osso L, Suvisaari J, Coccaro E, Rose RJ, Peltonen L, Virkkunen M, Goldman D. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of impulsive behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120380. doi: 10.1098/rstb.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL. Serotonin 2a receptor T102C polymorphism and impaired impulse control. Am J Med Genet. 2002;114:336–339. doi: 10.1002/ajmg.10206. [DOI] [PubMed] [Google Scholar]
- Blokland A, Sik A, Lieben C. Evaluation of DOI, 8-OH-DPAT, eticlopride and amphetamine on impulsive responding in a reaction time task in rats. Behav Pharmacol. 2005;16:93–100. doi: 10.1097/00008877-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Booij L, Swenne CA, Brosschot JF, Haffmans PM, Thayer JF, Van der Does AJ. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: Microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Rubino SR. Phasic alterations in dopamine and serotonin release in striatum and prefrontal cortex in response to cocaine predictive cues in behaving rhesus macaques. Neuropsychopharmacol. 2004;29:676–685. doi: 10.1038/sj.npp.1300386. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brasch-Andersen C, Moller MU, Christiansen L, Thinggaard M, Otto M, Brosen K, Sindrup SH. A candidate gene study of serotonergic pathway genes and pain relief during treatment with escitalopram in patients with neuropathic pain shows significant association to serotonin receptor2C (HTR2C) Eur J Clin Pharmacol. 2011;67:1131–1137. doi: 10.1007/s00228-011-1056-x. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–6272. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Linnoila MI. CSF serotonin metabolite (5-HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry. 1990;51(Suppl):31–41. [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin(2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacol. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats' accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Damjanoska KJ, D'Souza DN, Zhang Y, Garcia F, Battaglia G, Muma NA, Van De Kar LD. Short-term cocaine treatment causes neuroadaptive changes in Galphaq and Galpha11 proteins in rats undergoing withdrawal. J Pharmacol Exp Ther. 2004;311:349–355. doi: 10.1124/jpet.104.069807. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Zhang Y, Damjanoska KJ, D'Souza DN, Garcia F, Battaglia G, Muma NA, Van De Kar LD. A region-specific increase in Galphaq and Galpha11 proteins in brains of rats during cocaine withdrawal. J Pharmacol Exp Ther. 2003;307:1012–1019. doi: 10.1124/jpet.103.056978. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990a;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Intravenous cocaine self-administration in rats is reduced by dietary L-tryptophan. Psychopharmacology. 1990b;100:293–300. doi: 10.1007/BF02244596. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center on Addiction and Substance Abuse at Columbia University . Addiction Medicine: Closing the Gap between Science and Practice. The National Center on Addiction and Substance Abuse at Columbia University; 2012. [Google Scholar]
- Chamorro J, Bernardi S, Potenza MN, Grant JE, Marsh R, Wang S, Blanco C. Impulsivity in the general population: a national study. J Psychiatr Res. 2012;46:994–1001. doi: 10.1016/j.jpsychires.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N-H, Reith MEA. Autoregulation and monoamine interactions in the ventral tegmental area in the absence and presence of cocaine: A microdialysis study in freely moving rats. J Pharmacol Exp Ther. 1994;271:1597–1610. [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Community Epidemiology Work Group EPIDEMIOLOGIC TRENDS IN DRUG ABUSE Advance Report and Highlights/Executive Summary: Abuse of Stimulants and Other Drugs. 2005 http://www.drugabuse.gov/PDF/CEWG/AdvReport_Vol1_105.pdf.
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Covi L, Hess JM, Kreiter NA, Haertzen CA. Effects of combined fluoxetine and counseling in the outpatient treatment of cocaine abusers. Am J Drug Alcohol Abuse. 1995;21:327–344. doi: 10.3109/00952999509002701. [DOI] [PubMed] [Google Scholar]
- Cox SM, Benkelfat C, Dagher A, Delaney JS, Durand F, Kolivakis T, Casey KF, Leyton M. Effects of lowered serotonin transmission on cocaine-induced striatal dopamine response: PET [(1)(1)C]raclopride study in humans. Br J Psychiatry. 2011;199:391–397. doi: 10.1192/bjp.bp.110.084178. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chemical Neuroscience. 2012;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Lakoski JM. Electrophysiological effects of cocaine and procaine on dorsal raphe serotonin neurons. Eur J Pharmacol. 1988;148:457–462. doi: 10.1016/0014-2999(88)90128-8. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Lakoski JM. The interaction of cocaine with serotonin dorsal raphe neurons: Single unit extracellular recording studies. Neuropsychopharmacol. 1990;3:41–50. [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacol. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Repeated administration of low doses of cocaine enhances the sensitivity of 5-HT2 receptor function. Pharmacol Biochem Behav. 1992;41:519–527. doi: 10.1016/0091-3057(92)90367-o. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- DAWN: Drug Abuse Warning Network 2013 http://www.samhsa.gov/data/2k12/DAWN096/SR096EDHighlights2010.htm.
- de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, McCreary AC, van LA, De Vries TJ, Venhorst J, van DM, Kruse CG. A novel highly selective 5-HT6 receptor antagonist attenuates ethanol and nicotine seeking but does not affect inhibitory response control in Wistar rats. Behav Brain Res. 2013;236:157–165. doi: 10.1016/j.bbr.2012.08.048. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Kalechstein AD. Risperidone diminishes cocaine-induced craving. Psychopharmacology (Berl) 2005;178:347–350. doi: 10.1007/s00213-004-2010-8. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Deakin JF. The role of serotonin in depression and anxiety. Eur Psychiatry. 1998;13:57S–63S. doi: 10.1016/S0924-9338(98)80015-1. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Ding C, Bremer NM, Smith TD, Seitz PK, Anastasio NC, Cunningham KA, Zhou J. Exploration of synthetic approaches and pharmacological evaluation of PNU-69176E and its stereoisomer as 5-HT(2C) receptor allosteric modulators. ACS Chem Neurosci. 2012;3:538–545. doi: 10.1021/cn300020x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, McChargue D, Spring B. Effect of impulsivity on cardiovascular and subjective reactivity to smoking cues. Addict Behav. 2008;33:167–172. doi: 10.1016/j.addbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology (Berl) 2007;194:279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW, Dawes MA, Bradley DM, Morgan CJ, Badawy AA. The effects of alcohol on laboratory-measured impulsivity after L: -Tryptophan depletion or loading. Psychopharmacology (Berl) 2007;193:137–150. doi: 10.1007/s00213-007-0763-6. [DOI] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor-phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000;12:1391–1396. doi: 10.1046/j.1460-9568.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW, Childress AR, O'Brien CP. Failure of ritanserin to block cocaine cue reactivity in humans. Drug Alcohol Depend. 1996;42:167–174. doi: 10.1016/s0376-8716(96)01278-1. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats II: the effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and other drugs on fixed consecutive number schedules (FCN 8 and FCN 32) Psychopharmacology (Berl) 1998a;138:283–294. doi: 10.1007/s002130050673. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats IV: the effects of selective serotonergic agents on a paced fixed consecutive number schedule 1. Psychopharmacology (Berl) 1998b;140:319–330. doi: 10.1007/s002130050773. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999a;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats VII: the effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology (Berl) 1999b;146:422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57:35–46. [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology. 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacol. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. Ciba Found Symp. 1992;166:165–173. doi: 10.1002/9780470514245.ch10. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Chambers JW, Rizos Z, Chintoh AF. Effects of 5-HT depletion in the frontal cortex or nucleus accumbens on response inhibition measured in the 5-choice serial reaction time test and on a DRL schedule. Behav Brain Res. 2009;201:88–98. doi: 10.1016/j.bbr.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacol. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacol. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology. 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Gavarini S, Becamel C, Altier C, Lory P, Poncet J, Wijnholds J, Bockaert J, Marin P. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell. 2006;17:4619–4631. doi: 10.1091/mbc.E06-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Rothman RB, Gorelick DA, Henningfield JE, Baumann MH. Serotonergic responsiveness in human cocaine users. Drug Alcohol Depend. 2007;86:207–213. doi: 10.1016/j.drugalcdep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Rice KC, Matecka D, Rothman RB. Phentermine/fenfluramine decreases cocaine self-administration in rhesus monkeys. Neurorep. 1997;8:1347–1351. doi: 10.1097/00001756-199704140-00006. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, ia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: Two placebo-controlled, double-blind trials. J Clin Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Green CE, Moeller FG, Schmitz JM, Lucke JF, Lane SD, Swann AC, Lasky RE, Carbonari JP. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: a Bayesian approach. Am J Drug Alcohol Abuse. 2009;35:95–102. doi: 10.1080/00952990802647503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Beeske S, Jacquet A, Laufrais C, Alonso R, Decobert M, Avenet P, Francon D. Further evidence for the sleep-promoting effects of 5-HT receptor antagonists and demonstration of synergistic effects with the hypnotic, zolpidem in rats. Neuropharmacology. 2013;70C:19–26. doi: 10.1016/j.neuropharm.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Koch M. Effects of acute intra-cerebral administration of the 5-HT(2A/C) receptor ligands DOI and ketanserin on impulse control in rats. Behav Brain Res. 2009;204:88–92. doi: 10.1016/j.bbr.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Kahn RS, Sturiano C, Rinaldi PJ, Gabriel S, Schmeidler JP, Bernstein DP, Siever L, Cooper TB. Hostility is associated with a heightened prolactin response to meta-chlorophenylpiperazine in abstinent cocaine addicts. Psychiatry Res. 1998;80:1–12. doi: 10.1016/s0165-1781(98)00048-1. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy. 2011;6:17. doi: 10.1186/1747-597X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacol. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Humpston CS, Wood CM, Robinson ES. Investigating the roles of different monoamine transmitters and impulse control using the 5-choice serial reaction time task 1. J Psychopharmacol. 2013;27:213–221. doi: 10.1177/0269881112466182. [DOI] [PubMed] [Google Scholar]
- Im WB, Chio CL, Alberts GL, Dinh DM. Positive allosteric modulator of the human 5-HT2C receptor. Molecular Pharmacology. 2003;64:78–84. doi: 10.1124/mol.64.1.78. [DOI] [PubMed] [Google Scholar]
- Inaba K, Mizuhiki T, Setogawa T, Toda K, Richmond BJ, Shidara M. Neurons in Monkey Dorsal Raphe Nucleus Code Beginning and Progress of Step-by-Step Schedule, Reward Expectation, and Amount of Reward Outcome in the Reward Schedule Task. J Neurosci. 2013;33:3477–3491. doi: 10.1523/JNEUROSCI.4388-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Maruyama T, Yoshimura Y, Hosoi H, Komatsu Y. Facilitation of low-frequency stimulation-induced long-term potentiation by endogenous noradrenaline and serotonin in developing rat visual cortex. Neurosci Res. 2009;64:191–198. doi: 10.1016/j.neures.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Carlos K, Ostrander S, Rodriguez D, Call-Craddolph A, Yagnik G, Zhou F. Impaired reward learning and intact motivation after serotonin depletion in rats. Behav Brain Res. 2012;233:494–499. doi: 10.1016/j.bbr.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and behavior: A general hypothesis. In: Bloom FE, Kupfer DJ, editors. Neuropsychopharmacology: The Fourth Generation of Progress. Raven Press; New York, New York: 1995. [Google Scholar]
- Javaid JI, Sahni SK, Pandey SC, Davis JM. Repeated cocaine administration does not affect 5-HT receptor subtypes (5-HT1A, 5-HT2) in several rat brain regions. Eur J Pharmacol. 1993;238:425–429. doi: 10.1016/0014-2999(93)90880-q. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Fiorella D, Rabin RA. Effects of chronic cocaine administration on the serotonergic system in the rat brain. Pharmacol Biochem Behav. 1993;46:289–293. doi: 10.1016/0091-3057(93)90355-w. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kallupi M, de GG, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2013;226:347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The Molecular Biology of Memory Storage: A Dialog Between Genes and Synapses. Bioscience Reports. 2004;V24:475–522. doi: 10.1007/s10540-005-2742-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kenakin T. G protein coupled receptors as allosteric proteins and the role of allosteric modulators. J Recept Signal Transduct Res. 2010;30:313–321. doi: 10.3109/10799893.2010.503964. [DOI] [PubMed] [Google Scholar]
- Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon HS, Jo YH, Kim MS, Rhie DJ. Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res. 2006;1103:49–55. doi: 10.1016/j.brainres.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen T, Haapalinna A, Sirvio J. Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol. 2003;92:214–225. doi: 10.1034/j.1600-0773.2003.920504.x. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT, Sirvio J. Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology. 2000a;39:471–481. doi: 10.1016/s0028-3908(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Sirvio J. The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol Biochem Behav. 2000b;66:729–738. doi: 10.1016/s0091-3057(00)00241-0. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotonergic innervation of cerebral cortex: Different classes of axon terminals aris from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Kuhn KU, Joe AY, Meyer K, Reichmann K, Maier W, Rao ML, Reinhardt MJ, Biersack HJ, Quednow BB. Neuroimaging and 5-HT2C receptor polymorphism: a HMPAO-SPECT study in healthy male probands using mCPP-challenge of the 5-HT2C receptor. Pharmacopsychiatry. 2004;37:286–291. doi: 10.1055/s-2004-832685. [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, Shannon WC. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One. 2011;6:e22799. doi: 10.1371/journal.pone.0022799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Zhang L, Dean M, Oz M, Ozaki N, Yu DH, Virkkunen M, Weight F, Linnoila M, Goldman D. Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C) Genomics. 1995;27:274–279. doi: 10.1006/geno.1995.1042. [DOI] [PubMed] [Google Scholar]
- Lee MA, Meltzer HY. Blunted oral body temperature response to MK-212 in cocaine addicts. Drug Alcohol Depend. 1994;35:217–222. doi: 10.1016/0376-8716(94)90077-9. [DOI] [PubMed] [Google Scholar]
- LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry. 1999;156:1771–1779. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011;338:890–896. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AD, Li Q, Van De Kar LD. Repeated cocaine exposure inhibits the adrenocorticotropic hormone response to the serotonin releaser d-fenfluramine and the 5-HT1A agonist, 8-OH-DPAT. Neuropharmacology. 1994;33:335–342. doi: 10.1016/0028-3908(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Lidov HGW, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat: An immunohistochemical analysis. Neuroscience. 1980;5:207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Liu S, Anastasio NC, Maili L, Hamon SC, Lane SD, Fox RG, Swinford SE, Cunningham KA, Moeller FG. Impaired serotonin 5-HT2C receptor (5-HT2CR) function is implicated in elevated cue reactivity in cocaine dependence: A translational approach. 2013. Submitted. [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin(2C) receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1667–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebl T, Angarita GA, Pachas GN, Huang KL, Lee SH, Nino J, Logvinenko T, Culhane MA, Evins AE. A randomized, double-blind, placebo-controlled trial of long-acting risperidone in cocaine-dependent men. J Clin Psychiatry. 2008;69:480–486. doi: 10.4088/jcp.v69n0321. [DOI] [PubMed] [Google Scholar]
- Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: A preliminary report. Psychopharmacology (Berl) 1992;109:245–247. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- Loh EA, Roberts DCS. Break points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology. 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG. Stochastic dynamic causal modeling of working memory connections in cocaine dependence. Hum Brain Mapp. 2014;35(3):760–778. doi: 10.1002/hbm.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ. Relapse in the addictive behaviors: integration and future directions. Clin Psychol Rev. 2006;26:229–231. doi: 10.1016/j.cpr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Maloteaux JM. Drug and transmitter receptors in human brain. Characterization and localization of serotonin, dopamine and adrenergic receptors. Acta Neurol Belg. 1986;86:61–129. [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem. 2001;79:1113–1121. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacol. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Cooper DA, Howell LL. The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 2012;342:761–769. doi: 10.1124/jpet.112.195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23(4):675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus CR, Jonkman LM. Attention switching after dietary brain 5-HT challenge in high impulsive subjects. J Psychopharmacol. 2007;21:700–708. doi: 10.1177/0269881107077354. [DOI] [PubMed] [Google Scholar]
- Masaki D, Yokoyama C, Kinoshita S, Tsuchida H, Naktomi Y, Yoshimoto K, Fukui K. Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/nogo task in rats. Psychopharmacology. 2006;V189:249–258. doi: 10.1007/s00213-006-0559-0. [DOI] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Chen JI, Wanyiri J, Nadim H, Jatlow P, Gueorguieva R, Potenza MN, Morgan PT, Bhagwagar Z, Malison RT. A single-day paradigm of self-regulated human cocaine administration. Pharmacol Biochem Behav. 2012;103:95–101. doi: 10.1016/j.pbb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011;16:592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- McGregor A, Lacosta S, Roberts DCS. L-tryptophan decreases the breaking point under a progressive ratio schedule of intravenous cocaine reinforcement in the rat. Pharmacol Biochem Behav. 1993;44:651–655. doi: 10.1016/0091-3057(93)90181-r. [DOI] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E. Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol. 2002;62:1339–1343. doi: 10.1124/mol.62.6.1339. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: An animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW, Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol. 2012;13:1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav Brain Res. 2007;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Miliaressis E. Serotonergic basis of reward in median raphe of the rat. Pharmacol Biochem Behav. 1977;7:177–180. doi: 10.1016/0091-3057(77)90204-0. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Girardon S, Dekeyne A. 5-HT2C receptors are involved in the discriminative stimulus effects of citalopram in rats. Psychopharmacology. 1999;142:432–434. doi: 10.1007/s002130050910. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, la Cour CM. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Miller WR, Westerberg VS, Harris RJ, Tonigan JS. What predicts relapse? Prospective testing of antecedent models. Addiction. 1996;91(Suppl):S155–S172. [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J Neurosci. 2011a;31:469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. The role of serotonin in the regulation of patience and impulsivity. Mol Neurobiol. 2012a;45:213–224. doi: 10.1007/s12035-012-8232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Doya K. Activation of the central serotonergic system in response to delayed but not omitted rewards. Eur J Neurosci. 2011b;33:153–160. doi: 10.1111/j.1460-9568.2010.07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Doya K. Activation of dorsal raphe serotonin neurons is necessary for waiting for delayed rewards. J Neurosci. 2012b;32:10451–10457. doi: 10.1523/JNEUROSCI.0915-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto-Lowe V, Kranzler HR. Using cue reactivity to evaluate medications for treatment of cocaine dependence: a critical review. Addiction. 1999;94:1639–1651. doi: 10.1046/j.1360-0443.1999.941116393.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001a;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001b;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing Effects of Modafinil on Sleep in Chronic Cocaine Users. Am J Psychiatry. 2010;167:331–340. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse WH, Skinner BF. Some factors involved in the stimulus control of operant behavior. J Exp Anal Behav. 1958;1:103–107. doi: 10.1901/jeab.1958.1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Barrett JE. Effects of chlordiazepoxide, buspirone, and serotonin receptor agonists and antagonists on responses of squirrel monkeys maintained under second-order schedules of intramuscular cocaine injection or food presentation. Drug Dev Res. 1990;20:5–17. [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav Brain Res. 2008;188:412–415. doi: 10.1016/j.bbr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Lucki I, McGonigle P. Time-dependent changes in sensitivity to apomorphine and monoamine receptors following withdrawal from continuous cocaine administration in rats. Synapse. 1994;16:1–10. doi: 10.1002/syn.890160102. [DOI] [PubMed] [Google Scholar]
- Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K. Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocaine. Psychiatry Res. 2001;102:227–233. doi: 10.1016/s0165-1781(01)00255-4. [DOI] [PubMed] [Google Scholar]
- Ni YG, Miledi R. Blockage of 5-HT2C serotonin receptors by fluoxetine (Prozac) Proc Natl Acad Sci USA. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress A, McLellan AT, Ehrman R, Ternes JW. Progress in understanding the conditioning aspects of drug dependence. In: Harris LS, editor. Problems of Drug Dependence 1987. U.S. Gov't Printing Office; Washington, D.C: 1988. pp. 44–61. [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. J Neurochem. 2006;99:1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Togashi H, Ueno K, Yoshioka M. The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neuroscience Letters. 2003;342:179–182. doi: 10.1016/s0304-3940(03)00293-3. [DOI] [PubMed] [Google Scholar]
- Okada M, Northup JK, Ozaki N, Russell JT, Linnoila M, Goldman D. Modification of human 5-HT(2C) receptor function by Cys23Ser, an abundant, naturally occurring amino-acid substitution. Mol Psychiatry. 2004;9:55–64. doi: 10.1038/sj.mp.4001357. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2011;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Paine TA, Olmstead MC. Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacology (Berl) 2004;175:443–50. doi: 10.1007/s00213-004-1845-3. [DOI] [PubMed] [Google Scholar]
- Palvimaki EP, Majasuo H, Syvalahti E, Hietala J. Serotonin 5-HT2C receptor-mediated phosphoinositide hydrolysis in rat choroid plexus after fluoxetine and citalopram treatments. Pharmacological Research. 2005;51:419–425. doi: 10.1016/j.phrs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Palvimaki EP, Roth BL, Majasuo H, Laakso A, Kuoppamaki M, Syvalahti E, Hietala J. Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor. Psychopharmacology (Berl) 1996;126:234–240. doi: 10.1007/BF02246453. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Stacy M. Dopamine-induced nonmotor symptoms of Parkinson's disease. Parkinsons Dis. 2011;2011:485063. doi: 10.4061/2011/485063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem. 1993;61:1611–1619. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Withdrawal from extended-access cocaine self-administration leads to decreased extracellular serotonin levels in the nucleus accumbens of the rat. In: Harris LS, editor. Problems of Drug Dependence, 1994; Proceedings of the 55th Annual Scientific Meeting; National Institute on Drug Abuse Monograph. Washington, D.C: U.S. Government Printing Office; 1995. [Google Scholar]
- Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE. Translational research in addiction: toward a framework for the development of novel therapeutics. Biochem Pharmacol. 2011;81:1388–1407. doi: 10.1016/j.bcp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Hill KP, Gopalakrishnan R, Berrettini WH. Relationship of disinhibition and aggression to blunted prolactin response to meta-chlorophenylpiperazine in cocaine-dependent patients. Psychopharmacology (Berl) 2006;185:123–132. doi: 10.1007/s00213-005-0261-7. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Peer J, Bennett ME, Bellack AS. Neurocognitive characteristics of individuals with schizophrenia and cocaine dependence: comparison of currently dependent and remitted groups. J Nerv Ment Dis. 2009;197:631–634. doi: 10.1097/NMD.0b013e3181b08bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacol. 2012;37:2505–2514. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl) 1993;110:390–394. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14:419–430. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL. Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry. 2012;72:396–404. doi: 10.1016/j.biopsych.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 2C 5-HT2C receptors attenuates cocaine-seeking behavior. Neuropsychopharmacol. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–7366. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts DK, Marwah J. Electrophysiological effects of cocaine on central monoaminergic neurons. Eur J Pharmacol. 1986;131:95–98. doi: 10.1016/0014-2999(86)90520-0. [DOI] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Alcala RA, Solana-Figueroa R, Galindo LE, Medina AC, Quirarte GL. Blockade of striatal 5-HT2 receptors produces retrograde amnesia in rats. Life Sci. 2003;74:481–488. doi: 10.1016/j.lfs.2003.06.012. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Filip M. Effects of serotonin (5-HT)(1B) receptor ligands on cocaine-seeking behavior in rats. Pharmacol Rep. 2008;60:798–810. [PubMed] [Google Scholar]
- Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol. 2007;559:165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, Muller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 2007;193:375–390. doi: 10.1007/s00213-007-0791-2. [DOI] [PubMed] [Google Scholar]
- Puumala T, Sirviö J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Neurophysiol. 2009;102:3026–3037. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci Biobehav Rev. 2013;37(9 Pt A):1919–1931. doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Roberts C. ACP-103, a 5-HT2A receptor inverse agonist. Curr Opin Investig Drugs. 2006;7:653–660. [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Loh EA, Baker GB, Vickers G. Lesions of central serotonin systems affect responding on a progressive ratio schedule reinforced either by intravenous cocaine or by food. Pharmacol Biochem Behav. 1994;49:177–182. doi: 10.1016/0091-3057(94)90473-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA . National survey of substance abuse treatment services (N-SSATS): 2011 Data on substance abuse treatment facilities. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. (BHSIS Series S-64). HHS Publication No. (SMA) 12-4730. [Google Scholar]
- Satel SL, Krystal JH, Delgado PL, Kosten TR, Charney DS. Tryptophan depletion and attenuation of cue-induced craving for cocaine. Am J Psychiatry. 1995;152:778–783. doi: 10.1176/ajp.152.5.778. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev. 2013;37(9 Pt A):1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Preclinical studies shed light on individual variation in addiction vulnerability. Neuropsychopharmacol. 2013;38:249–250. doi: 10.1038/npp.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, Rice KC, Goodman MM, Howell LL. Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacol. 2012;37:1816–1824. doi: 10.1038/npp.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol Biochem Behav. 2000;67:363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Green CE, Lane SD, Steinberg JL, Swann AC, Moeller FG. Baseline neurocognitive profiles differentiate abstainers and non-abstainers in a cocaine clinical trial. J Addict Dis. 2009;28:250–257. doi: 10.1080/10550880903028502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulden JD, Thomas YF, Compton WM. Substance abuse in the United States: findings from recent epidemiologic studies. Curr Psychiatry Rep. 2009;11:353–359. doi: 10.1007/s11920-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther. 2011;89:683–692. doi: 10.1038/clpt.2011.20. [DOI] [PubMed] [Google Scholar]
- Simon H, Le MM, Cardo B. Intracranial self-stimulation from the dorsal raphe nucleus of the rat: effects of the injection of para-chlorophenylalanine and of alpha-methylparatyrosine. Behav Biol. 1976;16:353–364. doi: 10.1016/s0091-6773(76)91486-3. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan Me, Anderson CM, Shanahan WR. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Kelly TM, Strotmeyer SJ, Malone KM, Mann JJ. Impulsivity, gender, and response to fenfluramine challenge in borderline personality disorder. Psychiatry Res. 2003;119:11–24. doi: 10.1016/s0165-1781(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Soubrié P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986;9:319–364. [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Hollander E, Liebowitz MR. Neurobiology of impulsivity and the impulse control disorders. J Neuropsychiatry. 1993;5:9–17. doi: 10.1176/jnp.5.1.9. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Christ CC, Highland KB. Serotonin system gene polymorphisms are associated with impulsivity in a context dependent manner. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:182–191. doi: 10.1016/j.pnpbp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Russo S, Boksem MA, Tucker DM. Serotonin: modulator of a drive to withdraw. Brain Cogn. 2009;71:427–436. doi: 10.1016/j.bandc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Baker DA, Grote KA, Solano J, Neisewander JL. Serotonin depletion attenuates cocaine-seeking behavior in rats. Psychopharmacology (Berl) 1999;146:60–66. doi: 10.1007/s002130051088. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Valentini V, Piras G, De Luca MA, Perra V, Bordi F, Borsini F, Frau R, Di CG. Evidence for a role of a dopamine/5-HT6 receptor interaction in cocaine reinforcement. Neuropharmacology. 2013;65:58–64. doi: 10.1016/j.neuropharm.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Van der Kooy D, Fibiger HC, Phillips AG. An analysis of dorsal and median raphe self-stimulation: effects of parachlorophenylalanine. Pharmacol Biochem Behav. 1978;8:441–445. doi: 10.1016/0091-3057(78)90083-7. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Schetters D, Schoffelmeer AN, De Vries TJ. 5-HT6 antagonism attenuates cue-induced relapse to cocaine seeking without affecting cocaine reinforcement. Int J Neuropsychopharmacol. 2010;13:961–965. doi: 10.1017/S1461145710000428. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB. In: Efferent and afferent connections of the dorsal and median raphe nuclei in the rat. Monti JM, Pandi-Erumal SR, Jacobs BJ, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects; Switzerland, Birkhäuser Verlag: 2008. [Google Scholar]
- Virkkunen M, Narvanen S. Plasma insulin, tryptophan and serotonin levels during the glucose tolerance test among habitually violent and impulsive offenders. Neuropsychobiology. 1987;17:19–23. doi: 10.1159/000118335. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacol. 2012;37:290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Lunde H, Nordvik JE, Landro NI, Refsum H, Magnusson A. Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacology (Berl) 2002;164:385–391. doi: 10.1007/s00213-002-1238-4. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Magnusson A, Neumeister A, Lappalainen J, Lunde H, Refsum H, Landro NI. Interactive effects of sex and 5-HTTLPR on mood and impulsivity during tryptophan depletion in healthy people. Biol Psychiatry. 2007;62:593–599. doi: 10.1016/j.biopsych.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- Walstab J, Steinhagen F, BRUSS M, Gothert M, BONISCH H. Differences between human wild-type and C23S variant 5-HT2C receptors in inverse agonist-induced resensitization. Pharmacol Rep. 2011;63:45–53. doi: 10.1016/s1734-1140(11)70397-8. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacol. 2004a;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004b;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Selective serotonin reuptake inhibitors decrease impulsive behavior as measured by an adjusting delay procedure in the pigeon 1. Neuropsychopharmacol. 2002;27:421–429. doi: 10.1016/S0893-133X(02)00307-X. [DOI] [PubMed] [Google Scholar]
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2013;226(2):217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN, Pihl RO, Ervin FR. The effect of altered tryptophan levels on mood and behavior in normal human males. Clin Neuropharmacol. 1988;11(Suppl 1):S207–S215. [PubMed] [Google Scholar]


