Abstract
Objective
To evaluate the efficacy and safety of passive cooling during transport of asphyxiated newborns.
Study Design
Retrospective medical record review of newborns with perinatal asphyxia transported for hypothermia between July 2007 and June 2010.
Results
Forty-three newborns were transported, 27 of whom were passively cooled. Twenty (74%) passively cooled newborns arrived with axillary temperature between 32.5 and 34.5 °C. One newborn (4%) arrived with a subtherapeutic temperature, and 6 (22%) had temperatures >34.5 °C. Time from birth to hypothermia was significantly shorter among passively cooled newborns compared with newborns not cooled (215 vs. 327 minutes, p<0.01), even though time from birth to arrival was similar (252 vs. 259 minutes, p=0.77). There were no significant adverse events related to passive cooling.
Conclusions
Exclusive passive cooling for hypoxic-ischemic encephalopathy results in significantly earlier achievement of effective therapeutic hypothermia without significant adverse events.
Keywords: hypoxic-ischemic encephalopathy, therapeutic hypothermia, amplitude-integrated electroencephalography
Introduction
Hypoxic-ischemic encephalopathy (HIE) occurs in 1 to 3 of every 1,000 live term births in the United States. A significant proportion of newborns with HIE will manifest later neurologic sequelae including intellectual, sensory, and motor impairments resulting from brain injury. Four clinical trials have demonstrated that moderate hypothermia reduces rates of death and disability and improves neurologic function among survivors of neonatal HIE.1–4 Although the American Academy of Pediatrics has not yet endorsed therapeutic hypothermia as standard of care, many specialists in newborn intensive care now offer this therapy in their clinical practice.5–7
In order to achieve the greatest benefit for newborns with HIE, hypothermia needs to be initiated prior to the onset of the secondary energy failure that contributes to hypoxic-ischemic brain injury.8–10 Animal data strongly support the initiation of hypothermia within 6 hours after birth,11–13 posing a challenge to caregivers to stabilize and transport critically ill newborns to a hospital offering the therapy within this 6-hour time frame. Remote hospitals and settings with limited infrastructure and resources may not be able to transport newborns to a hypothermia center within 6 hours, which could result in the exclusion of newborns who may benefit from this therapy. In order to initiate hypothermia therapy earlier, some centers have implemented passive cooling (removal of external heat sources to the newborn) prior to initiation of active cooling, particularly since many hospitals and transport teams are unable to offer active, controlled hypothermia during transport.
Several reports have described results of cooling both at referral hospitals and during transport to a hypothermia center.14–17 Notably, referral centers practiced varied procedures during transport, including both passive and active cooling (in the latter, applying cold packs or ice to the newborn’s body), with overcooling in several cases.15, 16
The aim of this study was to determine whether passive cooling on transport would improve delivery of therapeutic hypothermia, by comparing temperature and safety data from asphyxiated newborns transported before and after adoption of passive cooling during transport. We hypothesized that passive cooling of asphyxiated newborns would reduce the time to goal temperature, would be safe, and that there would be a low rate of overcooling.
Patients and Methods
We retrospectively reviewed the medical records of all newborns with perinatal asphyxia who were admitted to Children’s Hospital Boston (CHB) for evaluation for treatment with therapeutic hypothermia. The Newborn Intensive Care Unit (NICU) at CHB began offering therapeutic hypothermia for newborns with HIE in July 2007; referral nurseries were required to transport newborns who met criteria for the therapy to have evaluation and amplitude-integrated electroencephalography (aEEG) performed by pediatric neurology at CHB prior to initiation of active cooling procedures. Newborns were considered for hypothermia if they met a combination of diagnostic criteria established by the first two large published trials of therapeutic hypothermia for neonatal HIE, including clinical, laboratory, and amplitude-integrated electroencephalography (aEEG) findings.1, 2 Criteria for treatment included gestational age > 36 weeks, evidence of both fetal and neonatal distress, and encephalopathy diagnosed by both neurologic examination and aEEG on admission to CHB. All newborns admitted to CHB are outborn; referring physicians initiate requests for transport through the CHB Communication Center, and all cases are managed in consultation with a CHB intensive care unit fellow by recorded telephone calls including the dispatched transport team.
For quality improvement purposes, a database of all newborns transported for potential treatment with hypothermia was maintained to track short- and long-term outcomes and adverse events. The database includes perinatal history, clinical characteristics of the transport, hospital course at CHB, results of laboratory studies, and neurophysiologic and neuroimaging data. We conducted a retrospective review of the database for all newborns admitted since initiation of the therapeutic hypothermia protocol. The Institutional Review Board of CHB approved the study and publication of de-identified data.
The CHB transport team transports on average 1,000 patients per year, 35% of whom are newborns. The transport team records vital signs for each patient upon initial assessment and at 30-minute intervals for stable patients (i.e. patients not on inotropic medications) or intervals of 15 minutes or less for unstable patients. Temperature is measured in the axilla of the newborn using the Allegiance Dual Scale Digital Thermometer. The CHB transport team does not record skin probe temperature measurements.
In the first year during which therapeutic hypothermia was offered at CHB, many newborns arrived close to or just over the 6-hour time limit to initiate hypothermia. The practice of exclusive passive cooling for newborns with perinatal asphyxia before and during transport was therefore established in July 2008 to ensure that newborns would reach target temperature (33.5 °C) by 6 hours of age. To achieve lower body temperature by passive cooling, referring hospitals were asked to turn off all external heat sources and record axillary or rectal temperature with routine vital signs. The transport team either maintained or initiated passive cooling by placing the newborn in an isolette without active heating. If a newborn’s axillary temperature dropped below 33°C, the transport nurse would turn the isolette on and set the temperature at 34°C using servo-controlled warming, monitored by continuous skin probe measurements. The nurse would then record the infant’s axillary temperature 15 minutes after warming was initiated until it was > 33°C. No active cooling was performed during transport to CHB.
For this retrospective review, times and results of all documented vital signs from birth to admission to CHB were analyzed. These measurements included axillary and rare rectal temperatures, as well as esophageal temperatures once active cooling procedures were initiated. Time to target temperature range was defined as the time from birth to reach an axillary temperature of 32.5–34.5°C. The target temperature range for hypothermia was based on data from whole body hypothermia trials, with a goal temperature of 33.5°C.1, 2 The range 32.5–34.5 was chosen because core temperature is almost always higher than axillary temperature and because newborns in this study were monitored using axillary measurements during transport to CHB.18, 19 The American Academy of Pediatrics guidelines were used to define the range of normal newborn body temperature of 36.5–37.4°C, measured in the axilla.20 In our NICU and on newborn transports, the protocol is to monitor temperature in the axilla rather than the rectum unless there is a significantly abnormal measurement by axilla that requires verification. This policy is due to the risk for rectal perforation,21, 22 particularly in the setting of the high incidence of coagulopathy and potential for bleeding in hypothermic newborns who have sustained a systemic hypoxic-ischemic insult.23, 24
We collected clinical data regarding each newborn’s medical history and birth events prior to transport, including gestational age, Apgar scores, birth weight, first recorded pH, severity of illness score (SNAPPE-II), intubation, seizures, and need for inotropic support. Statistical analysis was performed using PASW Statistics 18 (IBM, Chicago, Illinois). Independent samples t-tests were used to compare demographic characteristics and details of transport and admission between newborns who were not passively cooled and those who were passively cooled. Wilcoxon-Mann-Whitney tests were used to compare demographic data that were not normally distributed. Fisher’s Exact Test was used to compare gender between the two groups. Analysis of variance was used to test whether time to target temperature differed by severity of encephalopathy. Multiple linear regression analysis was used to determine the adjusted effects of factors that might influence time to target temperature, such as birth weight, transport distance, and time from birth to admission to CHB. A p- value of 0.05 was considered significant.
Results
From July 2007 to June 2010, 52 newborns with perinatal asphyxia were referred for possible treatment with therapeutic hypothermia. We did not include in our analysis 9 newborns transported by referring hospitals for whom temperature data prior to admission could not be obtained. Data analysis was therefore restricted to 43 newborns for whom complete temperature measurements prior to CHB admission were available. As passive cooling was initiated one year after initiation of the therapeutic hypothermia protocol, 16 newborns were not passively cooled whereas 27 were passively cooled by the referring hospital and the transport team.
Descriptive data regarding the characteristics of the newborns are shown in Table 1. There was no statistically significant difference with respect to demographics or markers of illness severity (Score for Neonatal Physiology-Perinatal Extension-II [SNAPPE-II]) between newborns not cooled during transport and those who were passively cooled. The distribution of severity of encephalopathy did not differ significantly between the two groups (Table 1).
Table 1.
Clinical Characteristics of Newborns with Perinatal Asphyxia
| No Passive Cooling (n=16) | Passive Cooling (n=27) | P-value | |
|---|---|---|---|
|
| |||
| Gender 1,4: | |||
| Male | 8 (50%) | 18 (67%) | |
| Female | 8 (50%) | 9 (33%) | 0.34 |
|
| |||
| Birth weight 2,5 (grams) | 3118 ± 465 (2300–3890) | 3271 ± 522 (2050–4180) | 0.34 |
|
| |||
| 5-minute Apgar 2,5 | 3.6 ± 2.7 (0–9) | 2.4 ± 1.8 (0–6) | 0.09 |
|
| |||
| First recorded pH 2,5 | 7.0 ± 0.2 (6.6–7.3) | 7.0 ± 0.2 (6.6–7.4) | 0.76 |
|
| |||
| SNAPPE-II 2,5,7 | 33.3 ± 9.9 (18–47) | 38.3 ± 17.6 (12–83) | 0.30 |
|
| |||
| Gestational age 3,6 (weeks) | 39.0 ± 3.0 (35, 42) | 39.0 ± 1.0 (36, 41) | 0.99 |
|
| |||
| Severity of HIE 1,8: | |||
| None/mild | 3 (19%) | 6 (22%) | |
| Moderate | 9 (56%) | 10 (37%) | 0.45 |
| Severe | 4 (25%) | 11 (41%) | |
Expressed as n (%)
Expressed as mean ± SD (range)
Expressed as median ± IQR (range)
P-value obtained from a Fisher’s Exact Test
P-value obtained from an independent samples t-test
P-value obtained from Wilcoxon-Mann-Whitney test
SNAPPE-II, Score for Neonatal Acute Physiology-Perinatal Extension-II
P-value obtained from Chi-square Test
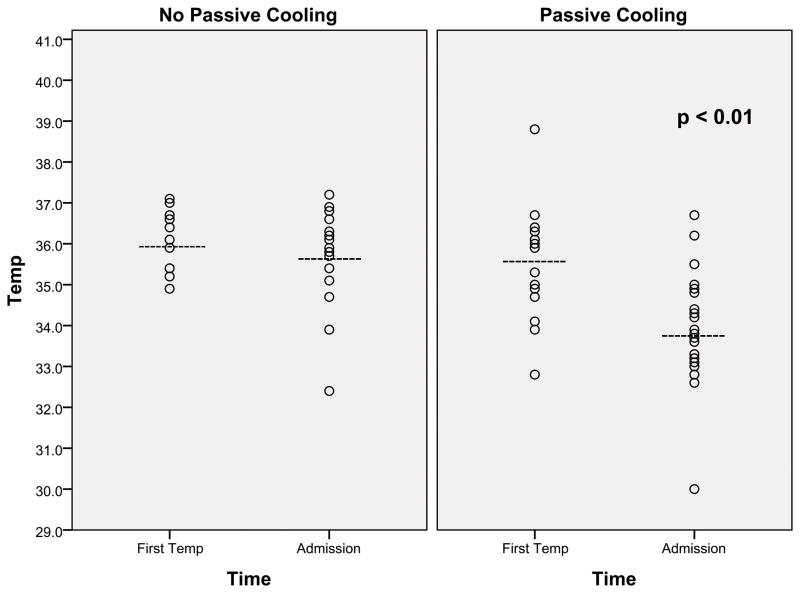
Temperature on admission to CHB was significantly lower among newborns who were passively cooled during transport (33.8 ± 1.3 vs. 35.7 ± 1.3 °C; p <0.01, Figure 1). In the passively cooled group, 20 of 27 newborns (74%) had an admission temperature in the target range, 32.5–34.5°C. Six newborns (22%) with temperatures higher than 34.5°C (range 34.8–36.7°C) on admission had been transported shorter distances (mean of 8 miles) and one newborn (4%) had a temperature on admission of 30°C. The newborn with the admission temperature of 30°C had only one temperature documented at the birth hospital prior to admission to CHB. This severely asphyxiated newborn underwent a prolonged resuscitation and two emergent diagnostic procedures at the referring hospital, which may explain the infrequent temperature monitoring. For almost all newborns, temperature was stable during transport without significant instability or need for intervention. One newborn was warmed during transport because of a recorded temperature of 32.9°C; the temperature 15 minutes later was 33.5°C and remained within target range until admission. Newborns were not treated with sedatives or narcotics for at least 30 minutes prior to arrival at CHB for neurologic evaluation (by neurologic examination and aEEG recording), and a similar percentage in each group received such medications prior to or during transport (38% of non-passively cooled, 33% of passively cooled). Newborns with no or mild encephalopathy by exam and aEEG, i.e., not qualifying for hypothermia, were re-warmed without any complications (n=6). Among newborns not passively cooled on transport, admission temperatures ranged from 32.4 to 37.2°C. The newborn with an admission temperature of 32.4°C also had severe asphyxia.
Figure 1.
Scatter plot showing temperature (°C) recorded after birth and on admission to CHB, before (n=16) and after (n=27) implementation of passive cooling during transport. The dashed lines indicate the mean temperature, and p<0.01 indicates significant difference in mean temperature on admission between cooled and non-cooled groups.
Newborns who were passively cooled achieved the target temperature range significantly earlier than those who were not passively cooled (215 ± 78 vs. 327 ± 88 minutes; p <0.01). Notably, there was no difference in time from birth to admission (259 ± 67 vs. 252 ± 83 minutes; p=0.77) or distance traveled to CHB (3.0 ± 18.0 vs. 7.5 ± 21.0 miles; p=0.22) between newborns who were passively cooled and those who were not cooled during transport. The severity of encephalopathy was not significantly associated with time to reach target temperature range in either group of newborns (p=0.71). After adjusting for factors that could influence rapidity of cooling during transport such as birth weight, distance traveled, and time from birth to admission to CHB, only time from birth to admission significantly increased time to reach target temperature (p=0.001).
Data were also analyzed to determine whether efficacy of passive cooling varied with seasonal temperature. No significant difference was found; newborns transported during the warmer months (May–October) arrived with a mean temperature of 33.7 ± 1.5°C compared with 34.1 ± 0.77°C (p = 0.43) for newborns transported during colder months (November–April). To increase the contrast in environmental temperature, arrival temperatures were compared between the warmest and coldest months of the year (May–August vs. November–February), and there was no difference between the groups (data not shown).
Since temperature was measured in the axilla during transport, the correlation between axillary and esophageal temperature measurements was examined both before and after initiation of active cooling procedures. Five passively cooled newborns had axillary and esophageal temperatures measured simultaneously on admission to CHB. All 5 had higher esophageal temperatures, and the measurements were very similar, with a mean difference (esophageal – axillary) of 0.3°C (range 0.1–0.6°C). Additional simultaneous measurements were recorded among both groups of newborns after admission and once active cooling procedures were initiated. Twelve newborns had multiple simultaneous axillary and esophageal temperature measurements during the first hours of active cooling. Esophageal temperature was higher than axillary in 29 of 31 observations, with a mean difference between esophageal and axillary temperatures of 0.37°C (range −0.3°C to +0.9°C). The 2 observations of higher axillary than esophageal temperature occurred in one non-passively cooled patient whose esophageal temperature plummeted quickly from 36.1°C on admission to 33.2°C after just 20 minutes of active cooling. These two measures of higher axillary temperatures were recorded soon after this rapid 20-minute cooling period - at 31 and 48 minutes after admission - after which all axillary temperatures were lower than esophageal temperatures.
Finally, medical records were reviewed to assess for adverse events related to passive cooling including intubation, initiation of inotropic or antiepileptic medications, as well as mean heart rate and blood pressure. There were no adverse events during transport in either group. In the non-cooled group, 14 of 16 (88%) were intubated for transport and all newborns in the passively cooled group were intubated. Six newborns in the non-cooled cooled group and 5 in the passively cooled group had suspected seizures either at the referral hospital or during transport and were treated with phenobarbital and in one case, a second anti-epileptic medication (fosphenytoin). For evaluation of hemodynamic data, we reviewed the transport records for vital signs and need for inotropic medications for 8 non-passively cooled newborns and 18 passively cooled newborns transported >1 mile for whom we had detailed transport data. There was no difference between newborns who were not passively cooled and those who were cooled with respect to heart rate or mean arterial blood pressure measured at the end of transport (134 ± 20 vs. 121 ± 22 beats per minute; p=0.16 and 48 ± 4 vs. 46 ± 9 mmHg; p=0.41). Four newborns in each group were already receiving inotropic support prior to transport. Three newborns required initiation of inotropic support during transport; one newborn who was not passively cooled and 2 who were passively cooled.
Discussion
These data demonstrate that passive cooling of newborns during transport resulted in earlier achievement of effective hypothermia, with the majority (74%) of newborns arriving within 1 degree of goal temperature (33.5 °C). To our knowledge, this report is the first to compare data before and after initiation of exclusive passive cooling during transport to a hypothermia center. These data show that newborns who were passively cooled during transport achieved target temperature range nearly 2 hours (mean of 112 minutes) earlier than newborns who were not cooled, even though time from birth to arrival at CHB did not differ between groups. Since regression analysis showed that only time from birth to admission to CHB was associated with time to reach target temperature, passive cooling is most important for newborns born furthest from hypothermia centers. Experimental data show that therapeutic hypothermia is more effective with improved outcome when implemented earlier than 6 hours after an hypoxic-ischemic insult.11, 13 Our data show that passive cooling at the birth hospital and during transport allows for achievement of effective hypothermia well before the 6-hour mark used in published trials of therapeutic hypothermia. Further analysis of neurodevelopmental follow-up data will be needed to determine any effect of earlier hypothermia on outcome.
Our data also support the safety of passive cooling during transport, with a low rate of overcooling and no serious adverse events. Among newborns passively cooled during transport, overcooling was observed in only one newborn with severe perinatal asphyxia, a phenomenon described in earlier reports.24–26 Although we did not find a statistically significant association between severity of encephalopathy and rapidity of cooling in our cohort, we did observe overcooling in one severely asphyxiated newborn in each group, confirming the impaired ability to maintain normal body temperature in newborns with severe asphyxia, even in the absence of passive cooling. The low rate of overcooling in this study is likely the result of exclusive use of passive cooling procedures; no active cooling procedures were utilized until each newborn arrived at our hospital. In addition, frequent communication between referring centers, transport team members, and CHB NICU physicians and nurses ensured that passive cooling was performed in a controlled manner with close monitoring.
Several studies have evaluated cooling during transport of asphyxiated newborns, in most cases with both passive and active cooling procedures.15–17 In one study, passive cooling was used exclusively in just 2 of 35 newborns transported, and both newborns had rectal temperatures at the referring hospital and on admission to the hypothermia center of 34–35°C.16 In another study, 6 of 18 (33%) newborns who were cooled before and during transport arrived with temperatures in the sub-therapeutic range.15 In that study, temperature was not monitored vigilantly in all cases and temperature data were not differentiated between newborns who received both passive and active cooling from those who were only passively cooled during transport.15 Both of these studies also reported a higher risk of overcooling among newborns with greater severity of asphyxia.15, 16 Indeed, active cooling without a servo-controlled device appears more likely to result in overcooling, especially for newborns with severe asphyxia, based on data from studies in which both active and passive cooling were employed during transport.15, 16 In a third study, 4 of 37 newborns (11%) who underwent exclusive passive cooling arrived with rectal temperatures below the target range of 33–34°C.17 If we used the goal core temperature range (33–34°C by rectal or esophageal probe) used by the other published reports, 7 (26%) passively cooled newborns in our cohort would have had sub-therapeutic temperatures on admission. However, since the CHB transport team uses only axillary temperature measurements, we extended our range down to 32.5°C, since axillary temperatures tend to be lower than core temperatures. This decrease of the low end of the goal temperature range for axillary temperature on transport was supported by the finding that axillary temperatures were on average 0.37°C lower than esophageal temperatures in our patients.
Indeed, a key difference between the data presented in this report and those from other published reports is the site at which temperature was measured during transport. It is well known that rectal and esophageal measures are both closer approximations of core body temperature than axillary measures and are therefore optimal for determination of core temperature.17 However, it is not always feasible or safe to have continuous rectal or esophageal temperature monitoring during transport. Although simultaneous axillary and rectal measurements in infants may yield slightly different results, axillary measurements are consistently lower than rectal measurements.18, 19 A review of 16 studies comparing simultaneous axillary and rectal temperature measurements in newborns and infants demonstrated that rectal-axillary difference was smaller among newborns than among older infants and children, with a mean difference of just 0.17°C (95% limits of agreement, −0.15°C–0.5°C) in newborns vs. 0.92°C (−0.15°C–1.98°C) in infants.28 A recent study of passive cooling during transport noted poor correlation of simultaneous skin probe and rectal temperatures, but good agreement between rectal and axillary temperatures (mean difference 0.1°C in 61 paired measurements).17 The somewhat larger mean difference of 0.37°C between esophageal (not rectal) and axillary temperatures in the current study may reflect a lower esophageal than rectal temperature and/or a greater difference that may occur during the process of cooling rather than with normothermia or stable temperatures. Nonetheless, the current data demonstrate that frequent axillary temperature measurements are feasible and sufficient for monitoring a newborn’s temperature during passive cooling.
Our data support the continued use of passive cooling procedures during transport to hypothermia centers, despite ongoing development of protocols and equipment for precise measurement of core temperature and delivery of hypothermia.27 Although no significant adverse events occurred, the small sample size of 27 passively cooled newborns in our study is not sufficient to determine the safety of this practice. Despite the limited sample size, our data support the approach of exclusive passive cooling with frequent temperature monitoring and a procedure to correct subtherapeutic temperatures as a safe approach to deliver hypothermia without overcooling during transport. Ongoing data collection and refinement of passive cooling procedures during transport remain important to ensure efficacious and safe delivery of hypothermia to asphyxiated newborns.
Conclusion
Passive cooling of asphyxiated newborns during transport to a hypothermia center results in significantly earlier achievement of therapeutic hypothermia and appears to be generally effective and safe. Frequent axillary temperature monitoring and correction of subtherapeutic temperatures are needed to ensure that passive cooling is both effective and safe during transport. Passive cooling presents an inexpensive, easily applied alternative to using a servo-controlled cooling device to ensure that newborns requiring transport can benefit from this therapy. Protocols should be developed and standardized to ensure timely and safe application of passive cooling during transport particularly for those centers and transport teams where asphyxiated newborns may be transported long distances and servo-controlled devices for hypothermia are not available. Earlier achievement of therapeutic hypothermia may improve its efficacy and hence may improve long-term neurologic outcome for newborns with HIE.
Acknowledgments
This work was supported in part by a grant from the Charles H Hood Foundation. We thank Shaye Moore and Elizabeth Jarvis for their assistance with the preparation of this manuscript.
Footnotes
Financial Disclosure/Conflict of Interest: None of the authors have any conflicts of interest or financial relationships that would compromise the integrity of this research. This work was supported in part by a grant from the Charles H Hood Foundation.
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO network RCT. Pediatrics. 2010;126(4):e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 5.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(3 Pt 2):S28–33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- 6.Hoehn T, Hansmann G, Buhrer C, Simbruner G, Gunn AJ, Yager J, et al. Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation. 2008;78(1):7–12. doi: 10.1016/j.resuscitation.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Gunn AJ, Hoehn T, Hansmann G, Buhrer C, Simbruner G, Yager J, et al. Hypothermia: an evolving treatment for neonatal hypoxic ischemic encephalopathy. Pediatrics. 2008;121(3):648–9. doi: 10.1542/peds.2007-3310. author reply 9–50. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt JS, Edwards AD, Azzopardi D, Reynolds EO. Magnetic resonance and near infrared spectroscopy for investigation of perinatal hypoxic-ischaemic brain injury. Arch Dis Child. 1989;64(7 Spec):953–63. doi: 10.1136/adc.64.7_spec_no.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39(11):718–25. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 10.Gunn AJ, Battin M, Gluckman PD, Gunn TR, Bennet L. Therapeutic hypothermia: from lab to NICU. J Perinat Med. 2005;33(4):340–6. doi: 10.1515/JPM.2005.061. [DOI] [PubMed] [Google Scholar]
- 11.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99(2):248–56. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102(5):1098–106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 13.Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46(3):274–80. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Anderson ME, Longhofer TA, Phillips W, McRay DE. Passive cooling to initiate hypothermia for transported encephalopathic newborns. J Perinatol. 2007;27(9):592–3. doi: 10.1038/sj.jp.7211781. [DOI] [PubMed] [Google Scholar]
- 15.Hallberg B, Olson L, Bartocci M, Edqvist I, Blennow M. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. 2009;98(6):942–6. doi: 10.1111/j.1651-2227.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 16.Fairchild K, Sokora D, Scott J, Zanelli S. Therapeutic hypothermia on neonatal transport: 4-year experience in a single NICU. J Perinatol. 2010;30(5):324–9. doi: 10.1038/jp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ on behalf of the Cooling on Retrieval Study G. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010 doi: 10.1136/adc.2010.187211. [DOI] [PubMed] [Google Scholar]
- 18.Falzon A, Grech V, Caruana B, Magro A, Attard-Montalto S. How reliable is axillary temperature measurement? Acta Paediatr. 2003;92(3):309–13. doi: 10.1080/08035250310009220. [DOI] [PubMed] [Google Scholar]
- 19.Morley CJ, Hewson PH, Thornton AJ, Cole TJ. Axillary and rectal temperature measurements in infants. Arch Dis Child. 1992;67(1):122–5. doi: 10.1136/adc.67.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics, Committee on Fetus and Newborn. Hospital stay for healthy term newborns. Pediatrics. 2010;125(2):405–9. doi: 10.1542/peds.2009-3119. [DOI] [PubMed] [Google Scholar]
- 21.Frank JD, Brown S. Thermometers and rectal perforations in the neonate. Arch Dis Child. 1978;53(10):824–5. doi: 10.1136/adc.53.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenbaum EI, Carson M, Kincannon WN, O’Loughlin BJ. Rectal thermometer-induced pneumoperitoneum in the newborn. Report of two cases. Pediatrics. 1969;44(4):539–42. [PubMed] [Google Scholar]
- 23.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32(1):11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Thoresen M. Supportive care during neuroprotective hypothermia in the term newborn: adverse effects and their prevention. Clin Perinatol. 2008;35(4):749–63. vii. doi: 10.1016/j.clp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Burnard ED, Cross KW. Rectal temperature in the newborn after birth asphyxia. Br Med J. 1958;2(5106):1197–9. doi: 10.1136/bmj.2.5106.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahm LS, James LS. Newborn temperature and calculated heat loss in the delivery room. Pediatrics. 1972;49(4):504–13. [PubMed] [Google Scholar]
- 27.Johnston ED, Becher JC, Mitchell AP, Stenson BJ. Provision of servo-controlled cooling during neonatal transport. Arch Dis Child Fetal Neonatal Ed. 2011 doi: 10.1136/fetalneonatal-2011-211649. [DOI] [PubMed] [Google Scholar]
- 28.Craig JV, Lancaster GA, Williamson PR, Smyth RL. Temperature measured at the axilla compared with rectum in children and young people: systematic review. Bmj. 2000;320(7243):1174–8. doi: 10.1136/bmj.320.7243.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]