Abstract
Portal fibroblasts, the resident fibroblasts of the portal tract, are found in the mesenchyme surrounding the bile ducts. Their roles in liver homeostasis and response to injury are undefined and controversial. Although portal fibroblasts almost certainly give rise to myofibroblasts during the development of biliary fibrosis, recent lineage-tracing studies suggest that their contribution to fibrogenesis is limited compared to that of hepatic stellate cells. Other functions of portal fibroblasts include participation in the peri-biliary stem cell niche, regulation of cholangiocyte proliferation, and deposition of specific matrix proteins. Portal fibroblasts synthesize elastin and other components of microfibrils; these may serve structural roles, providing stability to ducts and the vasculature under conditions of increased ductal pressure, or could regulate the bioavailability of the fibrogenic transforming growth factor β in response to injury. Viewing portal fibroblasts in the context of fibroblast populations throughout the body and studying their niche-specific roles in matrix deposition and epithelial regulation could yield new insights into their contributions in the normal and injured liver. Understanding the functions of portal fibroblasts will require us to view them as more than just an alternative to hepatic stellate cells in fibrosis.
Keywords: Liver fibrosis, hepatic stellate cell, portal tract, bile duct, cholangiocyte, elastin, elastic fiber, NTPDase 2
Portal fibroblasts, the resident fibroblasts of the portal tract, are found in the mesenchyme surrounding the bile ducts. Fibroblasts in the portal mesenchyme were first described more than 50 years ago, in rat livers after bile duct ligation.1 These cells have recently come to prominence as possible precursors of the contractile, matrix-depositing, α smooth muscle actin (αSMA)-positive myofibroblasts that promote fibrosis.2–4 Portal fibroblasts have been proposed to deposit matrix specifically in biliary fibrosis, and to have a role in place of, or in addition to, hepatic stellate cell-derived myofibroblasts.5, 6 Their contribution to matrix deposition in fibrosis remains controversial, however, and the definitive experiments necessary to determine their role have not been carried out. Similarly, functions of portal fibroblasts that are independent of fibrogenesis are poorly understood. We propose that viewing portal fibroblasts through a broad lens, in the context of other fibroblast populations rather than primarily in comparison to hepatic stellate cells, may yield important insights into their roles in the liver.
Portal fibroblasts are heterogeneous and represent 1 of several fibroblast populations in the liver. In this review, the term portal fibroblast refers to any fibroblast in the portal region, and portal myofibroblast to any myofibroblast that originates in the portal area and is not derived from hepatic stellate cells. There are fibroblasts surrounding the central vein (second layer cells) and in the liver capsule;7, 8 many, if not all, are derived from a common mesothelial or submesothelial progenitor cell that also gives rise to hepatic stellate cells.9 Additionally, there are fibroblasts in the connective tissue surrounding the extrahepatic bile ducts that may be distinct from portal fibroblasts. None of these populations have been well defined or studied, and little is known about their contribution to fibrosis, epithelial maintenance, and other functions essential to the health of the liver and bile ducts. Although portal fibroblasts are the subject of this review, it is likely that these other fibroblasts have similar functions.
Defining the roles of portal fibroblasts in the normal and injured liver has been hampered by the lack of reliable markers to distinguish them from hepatic stellate cells. Markers reported to identify portal fibroblasts include fibulin 2, elastin, interleukin 6, cofilin 1, and the ectonucleotidase NTPDase 2. Markers of stellate cells include vitamin A-containing lipid droplets, desmin, cytoglobin, b2 macroglobulin and Hand2. There is significant variability in marker expression among species and between stages of myofibroblastic differentiation, however, and none of the markers listed has been validated as both sensitive and specific. Detailed discussions of the use and value of these markers are found in the literature.4, 10, 11 Two recent papers making use of genetically modified mice to trace or delete hepatic stellate cells suggest that expression of the platelet-derived growth factor (PDGF) receptor β subunit and lecithin-retinol acetyltransferase is specific to hepatic stellate cells in the mouse liver. These markers might prove useful in studies comparing the function of stellate cells and portal fibroblasts.12, 13
Fibroblasts and Local Niches
Fibroblasts establish and maintain the connective tissue stroma. They are defined morphologically as cells with an elongated, flat, and spindle shape in culture. Functionally, they secrete, organize, and remodel type I collagen and other extracellular matrix (ECM) components and communicate with nearby vascular and epithelial cells.14 There is no single marker used to identify fibroblasts. Depending on the tissue, combinations of markers, including the cell-surface protein Thy1, the intermediate filament vimentin, the collagen receptor DDR2, and the calcium-binding protein S100A4, as well as reactivity to the monoclonal antibody TE-7, may be used to mark fibroblasts, although none are specific.14, 15 In the liver, hepatic stellate cells as well as portal fibroblasts express many of these markers.
As a class of cells, fibroblasts have important roles in matrix homeostasis, wound healing and the response to injury, and epithelial patterning and fate determination. Beyond these common functions, however, fibroblasts are highly heterogeneous. Elegant research over the last decade has shown that they display “topographic differentiation”, such that patterns of gene expression cluster fibroblasts according to their site of origin (anterior–posterior, rostral–caudal, and dermal vs non-dermal); individual populations of fibroblasts have distinct transcriptomes, which can be used to determine their position in the body.16 Expression of HOX genes, in particular, varies among different fibroblasts and may have an important role in site-specific epithelial differentiation.17 These patterns are determined during development but persist in adults, providing positional memory.17 They also persist in cells in culture—findings from in vitro studies of portal fibroblasts are likely to provide information about their site-specific roles in vivo.17
Expression patterns of large clusters of genes can be used to differentiate fibroblast populations based on their functions.18 One such function is site-specific ECM synthesis; fibroblasts differ in the expression of specific types of collagens, genes that regulate elasticity, and collagen cross-linking proteins such as lysyl oxidases. Genes that regulate epithelial pattern formation encode transcription factors, growth factors, and growth-factor receptors (including transforming growth factor β [TGFβ] family members, Wnt proteins, or G-protein–coupled receptor pathway proteins); their expression also varies among different fibroblast populations. Finally, fibroblasts express genes that regulate cell migration guidance signals (for example, semaphorins, ephrins, and Slit proteins) in a site-specific way.18 All told, these findings indicate that fibroblast populations occupy highly specific local niches.19 It may be helpful to understand the function of portal fibroblasts not only by considering their role in fibrosis and wound healing, but also by examining their potentially unique functions in matrix deposition, epithelial patterning, and cell migration guidance.
Portal Fibroblasts as Myofibroblast Precursors in Fibrosis
Fibroblasts throughout the body participate in wound healing and fibrosis in response to injury. Interest in the role of portal fibroblasts in liver fibrosis came to the fore 10–20 years ago, when several studies demonstrated that liver myofibroblasts were heterogeneous and that myofibroblasts embedded in matrix deposits around the biliary tree expressed markers not consistent with a stellate cell origin.10, 20, 21 By virtue of their location in the portal stroma and their close proximity to cholangiocytes, portal fibroblasts were prime candidates to be the cells of origin for these new myofibroblasts.
Other publications implicated a second cell population in fibrogenesis, demonstrating that hepatic stellate cells were often absent from fibrotic regions, especially in biliary fibrosis. Tuchweber et al. showed that portal fibroblasts began to proliferate immediately after bile duct ligation, doing so with different kinetics than hepatic stellate cells, and that they were the precursors of a desmin-negative, αSMA-positive population of cells adjacent to proliferating bile ducts and connective tissue stroma.22 Experiments with rat precision-cut liver slices showed that portal fibroblasts proliferated selectively after exposure to bile acids, although the identity of these cells as portal fibroblasts, rather than hepatic stellate cells, was not definitively established given the hand2 used.23 Beaussier et al. demonstrated that most myofibroblasts accumulating at early and late time points in 2 models of biliary injury (bile duct ligation and arterial ischemia) were desmin negative and therefore not hepatic stellate cells.24 Notably, although these studies provided evidence for a non-stellate cell-derived population of myofibroblasts, they did not show that these cells originated specifically from portal fibroblasts, as opposed to other mesenchymal cells in the liver.
In vivo and in vitro studies have shown that portal fibroblasts deposit the fibrillar and basement membrane collagens typical of liver fibrosis. Immunohistochemical analysis of liver tissue demonstrated that a peri-portal population of cells lacking stellate cell markers deposited collagens I, III, and IV soon after bile duct ligation (before expressing αSMA).25 Portal myofibroblasts in culture, isolated by outgrowth and perfusion methods, secrete collagens,26, 27 and freshly isolated portal fibroblasts, which clearly undergo myofibroblastic differentiation in culture, also express mRNAs encoding collagens I and III.28 Interestingly, a proteomic analysis comparing myofibroblastic stellate cells and portal myofibroblasts in culture did not identify any matrix proteins among the set of proteins with statistically different expression patterns.29 Portal myofibroblasts expressed higher levels of mRNAs encoding fibronectin and collagen I than myofibroblastic stellate cells, although these differences may have been related to time in culture.30
Based on 2 decades of data, it appeared that portal fibroblasts gave rise to a second myofibroblast population distinct from hepatic stellate cell myofibroblasts, and that these myofibroblasts had a particularly significant role in biliary fibrosis. In 2002, Kinnman and Housset proposed that portal fibroblasts and myofibroblasts were the first responders after biliary injury, with hepatic stellate cells migrating to the point of injury as a secondary population.6 More recently, these authors proposed that stellate cells and portal fibroblasts occupy different niches, with the stellate cell niche in the injured liver induced by hypoxia and the portal fibroblast niche induced by the ductular reaction. According to the model, these niches yield myofibroblasts with specialized functions in liver injury: stellate cell-derived myofibroblasts mediate hepatocellular healing, and portal fibroblast-derived myofibroblasts regulate scar formation.4
Lineage-tracing studies
Previous studies were limited by a lack of definitive markers for hepatic stellate cells or portal fibroblasts; a recent lineage-tracing study using one such marker calls into question the role of portal fibroblasts as fibrogenic precursors in biliary fibrosis. Mederacke et al. developed mice carrying a bacterial artificial chromosome with a Cre reporter driven by lecithin-retinol acetyltransferase.12 In these mice, hepatic stellate cells were specifically and efficiently labeled (more than 99%), whereas portal fibroblasts were not. The investigators found that hepatic stellate cells gave rise to 82%–96% of fibrogenic myofibroblasts in 7 models of fibrosis, including toxic and biliary injury and fatty liver disease. Although there was a small population of unlabeled portal myofibroblasts that appeared to derive from portal fibroblasts, these cells expressed significantly lower levels of ECM proteins, including collagen I, than hepatic stellate cells.12 Hepatic stellate cells therefore appear to give rise to the dominant myofibroblast population in all forms of fibrosis, including biliary fibrosis, and the myofibroblast response does not seem to be disease specific.
This study by Mederacke et al. was rigorously performed, and the data convincingly support the conclusion that stellate cells give rise to most fibrogenic myofibroblasts, regardless of the model. Nonetheless, there are caveats. The investigators did not use an inducible Cre recombinase, so they could not rule out the possibility that portal fibroblasts were marked by Cre late in the response to injury, although the morphology of the labeled cells (stellate shaped, with retinoid-containing lipid droplets) indicates that this is unlikely. Additionally, most of the models assessed in this study were analyzed at a single, late time point, and the contribution of non-stellate cell-derived (portal) myofibroblasts to early stages of fibrosis was not examined. This is particularly important given that portal myofibroblasts may have important roles as first responders after injury.6
Mederacke et al. showed that a small subpopulation of myofibroblasts is derived from a source other than hepatic stellate cells, particularly in biliary fibrosis. Although these observations indicated a role for portal fibroblasts, they were at odds with findings from other investigators, particularly those of Beaussier et al., who reported detecting large numbers of desmin-negative myofibroblasts in models of biliary fibrosis.24 The function of these desmin-negative myofibroblasts, regardless of their numbers, is not known—it is possible that the collagen I and other matrix proteins they deposit, although in low amounts compared to hepatic stellate cells, has a particularly important role in fibrosis, for example in initiating or driving the progression of fibrosis. Because Mederacke et al. did not delete portal fibroblasts in their study, they could not rule out the possibility that portal fibroblasts are necessary but not sufficient for some forms of fibrosis. The possibility that portal fibroblasts and myofibroblasts are early responders after biliary injury and recruit or otherwise interact with fibrogenic stellate cells is particularly intriguing and warrants further study.
The role of portal fibroblasts in fibrosis, biliary or otherwise, is therefore still a subject of debate. Findings from multiple in vivo and in vitro studies, carried out over previous decades, suggest that portal myofibroblasts participate in biliary fibrosis. Their contribution to matrix deposition, however, is almost certainly minor compared to that of hepatic stellate cells, and their primary role in fibrosis remains undetermined.
Portal myofibroblasts and bridging fibrosis
Myofibroblasts are notable for their contractility and matrix deposition; they may, via their contractile properties, mediate matrix remodeling associated with healing. Contraction is produced by αSMA, organized in stress fibers, and can be manifest as strong (μN) contractions transmitted over long (tens of μm) distances.31 Groups of contractile cells placed in collagen I matrices compact and align the collagen between them, appearing remarkably similar to liver tissue with bridging fibrosis.32, 33 Hinz et al. proposed that advanced liver fibrosis, in particular the large-scale remodeling and distortion associated with bridging fibrosis and cirrhosis, is secondary to myofibroblast contractility.31 This could be an important role for portal myofibroblasts. αSMA-expressing portal cells distinct from stellate cells were evident even in the rigorous lineage-tracing study discussed above,12 and we have observed that portal myofibroblasts are highly contractile in culture (unpublished findings). Given that bridging fibrosis connects fibroblast-rich regions such as the portal tract and the central vein, it is plausible that portal myofibroblasts promote the process.
Potential Niche-specific Functions of Portal Fibroblasts
Fibroblast populations are heterogeneous, occupying specific anatomic niches where they regulate matrix production and epithelial patterning, and guide cell migration.18 Even so-called quiescent fibroblasts are highly metabolically active, and may have functions that affect the overall health of their niche.34
Neither the location-specific functions of portal fibroblasts nor the heterogeneity within the population has been delineated. Based on studies of large functional groups of fibroblasts, however, we can identify several areas that generally define unique functions of portal fibroblasts.
Site-specific ECM synthesis
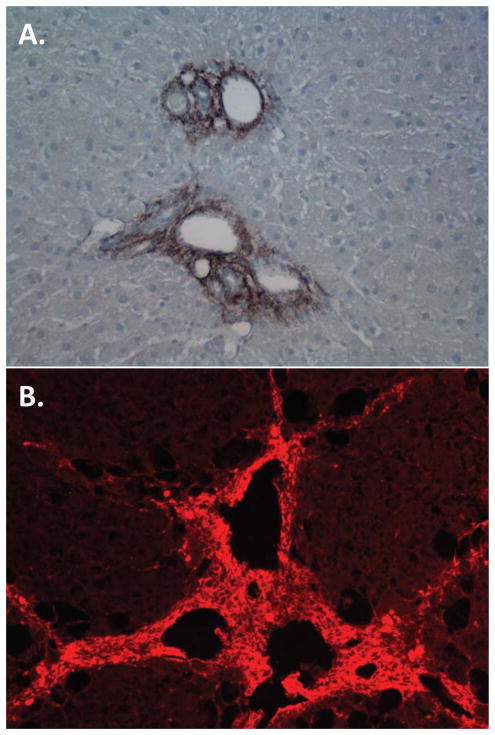
Although all fibroblasts deposit matrix, the ECM proteins produced by fibroblast populations vary with their site of origin.18 In the liver, portal fibroblasts and myofibroblasts are the source of elastic fibers. These fibers are composed of a cross-linked elastin core surrounded by fibrillin-rich microfibrils.35 Portal fibroblasts and myofibroblasts are the major, if not the only, cells in the liver that produce elastin (hepatic stellate cells do not); elastin is prominent in the portal region of the fibrotic liver (Fig. 1A).21, 25, 27 Portal myofibroblasts, distinct from hepatic stellate cells, were first defined by their production of fibulin 2,30 a linker protein occupying the interface between microfibrils and their elastin core.36 Fibulin 2 is a multi-domain protein with numerous functions, including sequestration of latent TGFβ binding proteins and regulation of smooth muscle cell migration.36–38 Portal fibroblasts also produce lysyl oxidase (LOX) and the related protein LOXL1, which cross-link elastin; expression of elastin and the LOX enzymes increase as portal fibroblasts become myofibroblastic.27, 28 In the vasculature, elastic fibers provide resilience and enable cycles of extension and recoil. Their function in the portal tract is less clear. They might provide mechanical stability to the bile ducts and the portal vasculature.12 Increased elastin deposition after bile duct obstruction might also be part of a mechanically oriented response to limit damage caused by increased ductal pressure. Alternatively, elastic fibers regulate bioavailability of the fibrogenic growth factor TGFβ by associating with latent TGFβ binding proteins,35 and might thereby mediate the progression of fibrosis.
Figure 1. Specialized periportal matrix deposition in fibrosis.
A) Portal region of rat liver 4 weeks after bile duct ligation, stained with antibody against elastin. B) Rat liver after 6 weeks of CCl4 intoxication, showing prominent periportal deposition of hyaluronic acid, which is visualized using hyaluronic acid binding protein.
Interactions with the epithelium
Fibroblasts have an important role in epithelial patterning, providing positional information that establishes and maintains epithelial cell phenotypes.17 In the fetal liver, there is evidence that progenitors of hepatic stellate cells and portal fibroblasts (defined by expression of the neurotrophin receptor p75) express the laminin α1 subunit, which, along with laminin α5 produced by cholangiocytes, interacts with the β1 integrin subunit to maintain the polarity and lumen of the bile duct.39 There is also evidence that portal mesenchymal cells (not always specifically identified as portal fibroblasts) express TGFβ2 and other TGFβ-family members,40 hedgehog ligands, and Jagged during development, and that these ligands bind to receptors on hepatoblasts, inducing their differentiation to cholangiocytes.5, 41 Portal fibroblasts may be active participants in the peribiliary stem cell niche and similar pathways may be involved in liver regeneration and repair in chronic liver disease.42, 43 Bi-potential progenitor cells, characterized by Foxl1 expression, for example, were shown to be surrounded by portal fibroblasts in injured livers.44 In addition to growth factor secretion, observed during development, portal myofibroblasts might deposit matrix required for progenitor cell expansion.45
Jhandier et al. have proposed an elegant model in which interactions between portal fibroblasts and cholangiocytes regulate cholangiocyte proliferation, which could account for the expansion of cholangiocytes observed after bile duct obstruction. Portal fibroblasts in the normal liver express the ecto-nucleotidase NTPDase 2, which hydrolyzes extracellular nucleotides, making them unable to interact with P2Y-class receptors on cholangiocytes and stimulate cholangiocyte proliferation.46 Portal myofibroblasts, however, do not express NTPDase 2, so bile duct obstruction (from stones, tumors, or experimental bile duct ligation), which leads to the appearance of portal myofibroblasts, effectively increases the concentration of extracellular nucleotides, resulting in increased activation and signaling of P2Y receptors and increased cholangiocyte proliferation.46 This mechanism may be an important regulatory component of the ductular reaction observed in some forms of biliary fibrosis.47
Portal fibroblasts and myofibroblasts could also regulate cholangiocyte proliferation via their production of hyaluronic acid. Hyaluronic acid, which increases proliferation of cholangiocytes in culture, is deposited in large amounts in the portal tract in biliary fibrosis (although a portal fibroblast source has not been established) and interacts primarily with the receptor CD44 (Fig. 1B).48 Expression of CD44 increases under conditions of cholestasis, with the highest levels in cholangiocytes, providing another portal fibroblast-mediated mechanism for increased cholangiocyte mass in the injured liver.
Many other potential interactions could occur between cholangiocytes and mesenchymal cells under conditions of biliary injury. In many cases, the 2 cell types have reciprocal growth factor and growth factor receptor expression.5, 41 However, interactions between cholangiocytes and portal fibroblasts and myofibroblasts have not yet been specifically studied.
Cell migration guidance signals
A third significant category that defines fibroblast specificity encompasses cell migration guidance signals, especially as applied to nerves and the vasculature. We know of no data implicating portal fibroblasts in nerve cell migration, although it is intriguing that portal fibroblast precursors express the p75 neurotrophin receptor, which is involved in neuronal development during embryology, and that the biliary tree is richly innervated.49 Future research into the patterning of the peribiliary plexus should examine the potential role of portal fibroblasts.
Angiogenesis also has a significant role in the development and maintenance of fibrosis. Hepatic stellate cells, as pericytes, are thought to have important functions in the liver vasculature.2 Angiogenesis in the portal region is prominent during the development of biliary fibrosis, and portal fibroblasts might contribute. Lemoine et al., in a review article, report that portal mesenchymal cells are involved in angiogenesis, but these findings have not been published.4
Other potential functions of portal fibroblasts
Portal fibroblasts could have interesting activities in the lymphatic system. Most studies of fibroblasts and lymphatic tissues have been carried out in fibrotic lung. Meinecke et al. demonstrated that during the development of pulmonary fibrosis, lymphatics are covered by αSMA-positive mural cells, resulting in increased accumulation of hyaluronic acid.50 This process is driven by PDGF produced by lymphatic endothelial cells. Portal fibroblasts and myofibroblasts might serve similar functions in the liver. Portal fibroblasts and myofibroblasts proliferate significantly in response to PDGF, and the portal tract is filled with lymphatics, which drain the space of Disse. Additionally, hyaluronic acid accumulates significantly in the portal tract (Fig. 1B); hyaluronic acid signaling to the biliary tree might be involved in development of cholestatic liver disease.48 It will be important to evaluate the role of lymphatics in the portal tract and the role of portal myofibroblasts in their fibrosis-associated expansion and dysfunction.
Conclusions
Portal fibroblasts are a poorly understood population of non-parenchymal cells in the liver. Their contribution to the development of biliary fibrosis is undefined, although recent evidence indicates that they are a minor fibrogenic population compared with hepatic stellate cells, regardless of the type of hepatic injury. Re-evaluating portal fibroblasts not as myofibroblast precursors but rather as a location-specific population of fibroblasts may lead to the identification of new functions for these cells in maintaining the health of the liver and biliary tree and in responding to injury (Fig. 2). In particular, the role of portal fibroblasts as immune modulators is a relatively untapped area of research that may yield important insights into the pathogenesis of cholangiopathies.41, 47
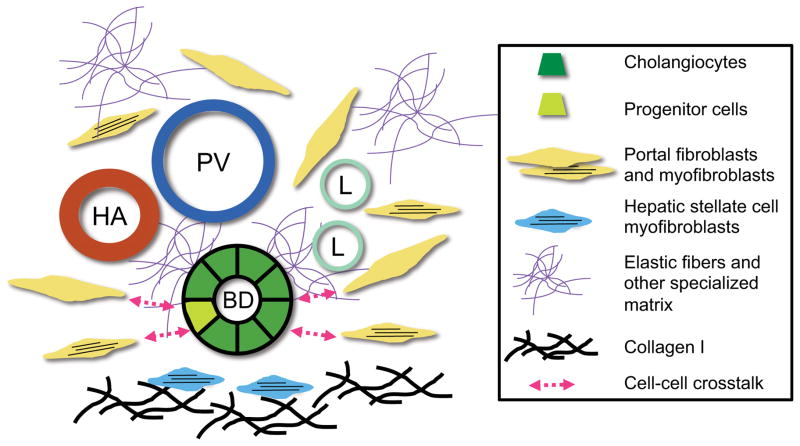
Figure 2. Potential functions of portal fibroblasts.
Although hepatic stellate cells are likely to be the source of most fibrillar collagen, portal fibroblasts and myofibroblasts deposit elastic fibers and possibly other specialized matrix components. Portal fibroblasts and myofibroblasts also potentially mediate the phenotype of cholangiocytes and liver progenitor cells via bidirectional cross-talk. HA, hepatic artery; PV, portal vein; BD, bile duct; L, lymphatic.
It is important for researchers to move beyond 2-dimensional (2D) culture models. There are significant differences in fibroblast behavior in 2D vs 3D culture, and improved in vitro systems, including cell-deposited matrices, could identify important new functions of portal fibroblasts.19 In vivo studies are needed, including portal fibroblast-specific lineage tracing experiments, to define the heterogeneity of the population and its response to liver damage. Precision-cut liver slices provide a culture system that lies between isolated cells and animal models. It has already been used to study the divergent functions of hepatic stellate cells and portal fibroblasts.11
Hepatic stellate cells have been called “protean, multifunctional, and enigmatic.”51 Although portal fibroblasts lag far behind as research subjects, they might one day be recognized to be as interesting as their more famous non-parenchymal colleagues.
Acknowledgments
This work was supported by NIH R01 DK-058123 to RGW.
Rosalyn Diaz and Maryna Perepelyuk are gratefully acknowledged for providing photographs. This work was supported in part by the NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30DK050306) and its core facilities.
Footnotes
No conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steiner JW, Carruthers JS. Studies on the fine structure of proliferated bile ductules. II. Changes of the ductule-connective tissue envelope relationship. Can Med Assoc J. 1961;85:1275–87. [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol. 2011;25:207–17. doi: 10.1016/j.bpg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27 (Suppl 2):65–8. doi: 10.1111/j.1440-1746.2011.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemoinne S, Cadoret A, El Mourabit H, et al. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948–54. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–44. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:d496–503. doi: 10.2741/A790. [DOI] [PubMed] [Google Scholar]
- 7.Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:1452–73. doi: 10.1002/hep.1840160623. [DOI] [PubMed] [Google Scholar]
- 8.Chapman GB, Eagles DA. Ultrastructural features of Glisson’s capsule and the overlying mesothelium in rat, monkey and pike liver. Tissue Cell. 2007;39:343–51. doi: 10.1016/j.tice.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Asahina K, Zhou B, Pu WT, et al. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–95. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassiman D, Libbrecht L, Desmet V, et al. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–9. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 11.Guyot C, Combe C, Clouzeau-Girard H, et al. Specific activation of the different fibrogenic cells in rat cultured liver slices mimicking in vivo situations. Virchows Arch. 2007;450:503–12. doi: 10.1007/s00428-007-0390-y. [DOI] [PubMed] [Google Scholar]
- 12.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster T, Legesse-Miller A, Hameed MR, et al. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–58. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinn JL, Bondre C, Gladstone HB, et al. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinn JL, Wang JK, Allen N, et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 2008;22:303–7. doi: 10.1101/gad.1610508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschumperlin DJ. Fibroblasts and the ground they walk on. Physiology (Bethesda) 2013;28:380–90. doi: 10.1152/physiol.00024.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knittel T, Kobold D, Piscaglia F, et al. Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochem Cell Biol. 1999;112:387–401. doi: 10.1007/s004180050421. [DOI] [PubMed] [Google Scholar]
- 21.Lorena D, Darby IA, Reinhardt DP, et al. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest. 2004;84:203–12. doi: 10.1038/labinvest.3700023. [DOI] [PubMed] [Google Scholar]
- 22.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, et al. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–78. [PubMed] [Google Scholar]
- 23.Clouzeau-Girard H, Guyot C, Combe C, et al. Effects of bile acids on biliary epithelial cell proliferation and portal fibroblast activation using rat liver slices. Lab Invest. 2006;86:275–85. doi: 10.1038/labinvest.3700386. [DOI] [PubMed] [Google Scholar]
- 24.Beaussier M, Wendum D, Schiffer E, et al. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 25.Desmouliere A, Darby I, Costa AM, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765–78. [PubMed] [Google Scholar]
- 26.Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–73. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Dranoff JA, Chan EP, et al. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–56. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 28.Perepelyuk M, Terajima M, Wang AY, et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605–14. doi: 10.1152/ajpgi.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosselut N, Housset C, Marcelo P, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–28. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 30.Knittel T, Kobold D, Saile B, et al. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–21. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 31.Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Schickel ME, Stevenson MD, et al. Fibers in the extracellular matrix enable long-range stress transmission between cells. Biophys J. 2013;104:1410–8. doi: 10.1016/j.bpj.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Q, Ghosh RP, Engelke H, et al. Rapid disorganization of mechanically interacting systems of mammary acini. Proc Natl Acad Sci U S A. 2014;111:658–63. doi: 10.1073/pnas.1311312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemons JM, Feng XJ, Bennett BD, et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 36.Argraves WS, Greene LM, Cooley MA, et al. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono RN, Sengle G, Charbonneau NL, et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem. 2009;284:16872–81. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strom A, Olin AI, Aspberg A, et al. Fibulin-2 is present in murine vascular lesions and is important for smooth muscle cell migration. Cardiovasc Res. 2006;69:755–63. doi: 10.1016/j.cardiores.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Tanimizu N, Kikkawa Y, Mitaka T, et al. alpha1- and alpha5-containing laminins regulate the development of bile ducts via beta1 integrin signals. J Biol Chem. 2012;287:28586–97. doi: 10.1074/jbc.M112.350488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–10. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 41.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 44.Greenbaum LE, Wells RG. The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol. 2011;43:222–9. doi: 10.1016/j.biocel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Hul NK, Abarca-Quinones J, Sempoux C, et al. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–35. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]
- 46.Jhandier MN, Kruglov EA, Lavoie EG, et al. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–92. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 47.Fausther M, Lavoie EG, Dranoff JA. Contribution of Myofibroblasts of Different Origins to Liver Fibrosis. Curr Pathobiol Rep. 2013;1:225–230. doi: 10.1007/s40139-013-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Wu GD, Sadahiro T, et al. Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. Am J Physiol Gastrointest Liver Physiol. 2008;295:G305–12. doi: 10.1152/ajpgi.90229.2008. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K, Tanaka M, Watanabe N, et al. p75 Neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology. 2008;135:270–281. e3. doi: 10.1053/j.gastro.2008.03.075. [DOI] [PubMed] [Google Scholar]
- 50.Meinecke AK, Nagy N, Lago GD, et al. Aberrant mural cell recruitment to lymphatic vessels and impaired lymphatic drainage in a murine model of pulmonary fibrosis. Blood. 2012;119:5931–42. doi: 10.1182/blood-2011-12-396895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]