Abstract
Cardiovascular imaging is an important part of procedural planning and safety for catheter ablation of atrial fibrillation (AF). However, the costs of imaging surrounding catheter ablation of AF have not been described. Medicare fee-for-service data were used to evaluate Medicare expenditures before, during, and after catheter ablation for AF from July 2007 to December 2009. Among 11,525 patients who underwent catheter ablation for AF, the mean overall expenditure on the day of the procedure was $14,455 (SD $7,441). The mean imaging expenditure in the periprocedural period, which included the 30 days before the catheter ablation and the day of the ablation itself, was $884 (SD $455). Periprocedural imaging expenditures varied by the imaging strategy used, ranging from a mean of $557 (SD $269) for patients with electroanatomic mapping only to $1,234 (SD $461) for patients with electroanatomic mapping, transesophageal echocardiogram, and computed tomography or magnetic resonance imaging. Mean patient-level imaging expenditures varied by provider (mean $872, SD $249). Periprocedural imaging expenditures also varied by patient risk, with mean expenditures of $862 (SD $444) for patients with a CHADS2 score of ≥2 compared with $907 (SD $466) for CHADS2 score <2 (p <0.001). In conclusion, peri-procedural imaging accounts for approximately 6% of mean Medicare expenditures for catheter ablation of AF. The expenditures for periprocedural imaging vary both at the patient and at the provider level and they are inversely related to stroke risk by CHADS2 score.
The use of cardiovascular imaging increased dramatically over the last 15 years. From 1999 to 2004, cardiovascular imaging in Medicare patients increased by 14% a year with expenditures doubling from $1.6 billion to $3.2 billion.1 Medicare has tried to slow the growth of imaging by reducing reimbursement.2,3 Based on the increasing use of atrial fibrillation (AF) ablation and the role of periprocedural imaging, it is important to understand the impact of imaging on the cost of AF ablation in the current cost-conscious health-care environment. This analysis examines the expenditures for periprocedural imaging in AF ablation and how they contribute to overall expenditures for AF ablation within the Medicare program.
Methods
We received research identifiable files from the US Centers for Medicare & Medicaid Services for all patients who underwent intracardiac ablation of supraventricular tachycardia (Healthcare Common Procedure Coding System [HCPCS] code 93651) in 2007, 2008, and 2009. Medicare denominator files identified demographic data, enrollment information, and dates of death. Medicare inpatient, outpatient, and carrier claims files described costs, imaging received, and co-morbid conditions.
Patients underwent ablation between July 1, 2007 and December 31, 2009, were aged ≥65 years, and were enrolled in Medicare fee-for-service program at the time of and for the 6 months before the ablation. Not all cardiac catheter ablations were included.4 We required that the catheter ablation claim include an associated primary diagnosis code for AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 427.31) and an HCPCS code for electronatomic mapping (HCPCS 93613). Patients with a history of atrioventricular node ablation (HCPCS 93650), anomalous atrioventricular excitation (ICD-9-CM 426.7), or paroxysmal supraventricular tachycardia (ICD-9-CM 427.0) were excluded to improve specificity. If a patient had >1 eligible ablation over the study period, only the earliest was included.4
Diagnosis codes from all claims in the 6-month period before ablation were used to identify co-morbid conditions. Previously validated coding algorithms were used to identify diabetes mellitus, ischemic heart disease, peripheral vascular disease, heart failure, hypertension, chronic pulmonary disease, chronic kidney disease, dementia, cancer, valvular heart disease, and previous stroke or transient ischemic attack.5,6 Atrial flutter was identified by ICD-9-CM diagnosis code 427.32.
HCPCS codes in carrier claims identified imaging procedures. We searched for preablation transthoracic echo-cardiogram, transesophageal echocardiogram (TEE), chest or cardiac computed tomography (CT), and chest or cardiac magnetic resonance imaging (MRI) in the 4 weeks before the ablation procedure date; intraprocedural intracardiac echo-cardiography (ICE) and electroanatomic mapping (EAM) on the same date as the ablation procedure; and postprocedural chest or cardiac CT, chest or cardiac MRI, transthoracic echocardiogram, and lung perfusion scanning (V/Q) in the 6 months after the ablation procedure. We assumed that TEE procedures on the day of the ablation were done before the ablation, and these TEE procedures were considered pre-procedural for the purpose of this analysis. Rotational angiography was not included in the analysis, as there is no HCPCS code for this imaging technique.
We defined Medicare expenditures as the amount paid by both the Medicare program and the Medicare beneficiaries (or their supplemental insurance) for care received. Expenditures included payments on inpatient, outpatient, and carrier claims. Imaging expenditures were identified using specific line items from the outpatient and carrier claims. Expenditures were adjusted to 2009 United States dollars using the consumer price index medical care component.
We calculated overall expenditures in the 6 months before ablation (preprocedural), on the day of ablation (day-of-procedure), and in the 6 months after ablation (post-procedural). For patients who received their ablation as an inpatient, the institutional costs for the entire stay were considered to have occurred on the day of the ablation. We calculated imaging expenditures in the 30 days before ablation (preprocedural), during the ablation (intraprocedural), and in the 6 months after ablation (postprocedural). Only EAM and ICE were considered to have occurred during the ablation (intraprocedural). All other imaging procedures on the day of ablation were considered to have occurred during the preprocedural period. For imaging costs, the periprocedural period included both the preprocedural and intra-procedural periods. Only patients who were alive and enrolled in fee-for-service Medicare for the entire 6 months after their ablation were included in calculations of post-procedural overall and imaging expenditures.
To describe patient baseline characteristics, we use means with SDs for continuous variables and frequencies with percentages for categorical variables. We summarized expenditures using both means with SDs and medians with interquartile ranges. When comparing expenditures between groups defined by imaging strategy and CHADS2 score, we tested for differences using Kruskal-Wallis tests. Providers were identified by 5-digit zip code and were only included in the variation analysis if they had at least 5 patients in the study population. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, North Carolina).
Results
There were 11,525 Medicare patients who underwent ablation for AF. Patients had a mean age of 71.6 years, and their mean CHADS2 score was 1.7 (Table 1). Most AF ablations were inpatient procedures (57.2%).
1.
Table Baseline characteristics
| Variable | Overall (n = 11,525) |
|---|---|
| Age, mean (SD) | 71.6 (4.9) |
| Men | 6,826 (59.2%) |
| White patients | 11,130 (96.6%) |
| Black patients | 155 (1.3%) |
| Atrial flutter | 4,449 (38.6%) |
| Cancer | 1,259 (10.9%) |
| Chronic kidney disease | 830 (7.2%) |
| Chronic pulmonary disease | 2,784 (24.2%) |
| Dementia | 55 (0.5%) |
| Diabetes mellitus | 2,576 (22.4%) |
| Heart failure | 2,999 (26.0%) |
| Hypertension | 9,260 (80.3%) |
| Ischemic heart disease | 6,131 (53.2%) |
| Peripheral vascular disease | 2,002 (17.4%) |
| Stroke/TIA | 921 (8.0%) |
| Valvular heart disease | 5,345 (46.4%) |
| CHADS2 score | |
| Mean (SD) | 1.7(1.1) |
| Score ≥2 | 5,909 (51.3%) |
| Inpatient procedure | 6,593 (57.2%) |
| Procedure year | |
| 2007 | 1,818 (15.8%) |
| 2008 | 4,257 (36.9%) |
| 2009 | 5,450 (47.3%) |
SD = standard deviation.
The median overall Medicare expenditure for patients on the day of the procedure was $13,283 (Q1 $11,670; Q3 $16,574), and the mean was $14,455 (SD $7,441; Table 2). Median total expenditures in the 6 months before the ablation was $5,358 (Q1 $2,643; Q3 $11,742) and the mean was $9,201 (SD $11,023). Total expenditures in the 6 months after ablation (median $4,146 [Q1 $1,952; Q3 $12,443] and mean $9,713 [SD $15,595]) were lower than before ablation.
2.
Table Medicare expenditures, overall and for imaging studies
| Variable | Overall (n = 11,525) | |
|---|---|---|
| Mean (SD) | Median (Q1; Q3) | |
| Overall expenditures | ||
| Pre-procedure, 6m prior | 9,201 (11,023) | 5,358 (2,643; 11,742) |
| Day-of-procedure | 14,455 (7,441) | 13,283 (11,670; 16,574) |
| Post-procedure, 6m following* | 9,713 (15,595) | 4,146 (1,952; 12,443) |
| Imaging expenditures | ||
| Peri-procedure, 30d prior + day-of | 884 (455) | 747 (546; 1,111) |
| Pre-procedure, 30d prior† | 375 (403) | 223 (88; 577) |
| Intra-procedure‡ | 509 (191) | 527 (420; 552) |
| Post-procedure, 6m following* | 206 (334) | 0 (0; 306) |
d = day; m = month; Q = quartile; SD = standard deviation.
Only reported for 8792 patients with complete 6m follow-up.
Includes day-of-ablation transesophageal echocardiogram (TEE)/trans- thoracic echocardiogram (TTE) procedures.
Includes intracardiac echocardiography (ICE) and electroanatomic mapping (EAM).
Imaging expenditures in the periprocedural period had a median of $747 (Q1 $546; Q3 $1,111) and mean of $884 (SD $455) per patient. These imaging expenditures represent approximately 6% of the median and mean overall expenditures for the day of the procedure. The intra-procedural and preprocedural imaging expenditures were about 4% and 2%, respectively, of the overall expenditures on the day of the procedure, using both median and mean values (Table 2). Among ablation procedures, TEE, CT, MRI, and ICE were used in 53% (median $117), 44% (median $414), 6% (median $434), and 67% (median $152), respectively (Table 3).
3.
Table Medicare expenditures for peri-ablation imaging, by modality
| Imaging Modality | Mean (SD) 2009 US $ | Median (Q1; Q3) 2009 US $ | Periprocedural Utilization (n = 11,525) |
|---|---|---|---|
| Transthoracic echocardiogram (TTE) | 159 (166) | 71 (47; 250) | 2,584 (22.4%) |
| Transesophageal echocardiogram (TEE) | 231 (230) | 117 (113; 135) | 6,060 (52.6%) |
| Cardiac/chest CT/MRI | 424 (273) | 415 (204; 533) | 5,724 (49.7%) |
| Cardiac/chest CT | 418 (263) | 414 (251; 515) | 5,065 (43.9%) |
| Cardiac/chest MRI | 463 (346) | 434 (142; 672) | 670 (5.8%) |
| Intracardiac ultrasound (ICE) | 163 (129) | 152 (148; 159) | 7,715 (66.9%) |
| Electroanatomic mapping system (EAM) | 404 (88) | 391 (378; 420) | 11,525 (100%) |
Q = quartile; SD = standard deviation.
Periprocedural imaging expenditures vary substantially by the imaging strategy chosen, with the lowest expenditures for EAM only and the highest for EAM with TEE and CT or MRI (Table 4). Despite the wide range of peri-procedural imaging expenditures, the imaging expenditures for the 6 months after the procedure had a narrow range. The postprocedural imaging expenditures did not differ, regardless of the periprocedural imaging strategy.
4.
Table Medicare expenditures by peri-procedural imaging groups
| Time Period | EAM Only (n = 3,238) | EAM + TEE (n = 2,563) | EAM + CT/MRI (n = 2,227) | EAM + CT/MRI + TEE (n = 3,497) | p |
|---|---|---|---|---|---|
| Overall expenditures | |||||
| Pre-procedure, 6m prior | |||||
| Mean (SD) | 8,996 (11,952) | 9,566 (11,490) | 9,200 (10,582) | 9,125 (10,005) | <.001 |
| Median (Q1; Q3) | 4,892 (2,305; 11,321) | 5,313 (2,529; 12,001) | 5,626 (2,732; 12,021) | 5,742 (3,007; 11,860) | |
| Day-of-procedure | |||||
| Mean (SD) | 13,805 (7,310) | 15,019 (7,733) | 13,928 (6,150) | 14,980 (8,002) | <.001 |
| Median (Q1; Q3) | 12,647 (11,179; 16,206) | 13,574 (11,854; 16,912) | 13,178 (11,590; 16,555) | 13,806 (12,151; 16,593) | |
| Post-procedure, 6m following* | |||||
| Mean (SD) | 10,244 (17,283) | 9,875 (15,028) | 8,894 (13,708) | 9,614 (15,430) | .07 |
| Median (Q1; Q3) | 4,027 (1,862; 13,215) | 4,658 (1,994; 12,690) | 3,861 (1,907; 11,754) | 4,225 (2,028; 12,052) | |
| Imaging expenditures | |||||
| Peri-procedure, 30d prior + day-of | |||||
| Mean (SD) | 557 (269) | 725 (281) | 994 (392) | 1,234 (461) | <.001 |
| Median (Q1; Q3) | 531 (414; 596) | 657 (548; 765) | 963 (752; 1,150) | 1,155 (874; 1,521) | |
| Pre-procedure, 30d prior† | |||||
| Mean (SD) | 61 (145) | 233 (241) | 468 (289) | 712 (442) | <.001 |
| Median (Q1; Q3) | 0 (0; 47) | 123 (114; 229) | 446 (280; 618) | 611 (331; 1,022) | |
| Intra-procedure‡ | |||||
| Mean (SD) | 496 (222) | 492 (130) | 526 (258) | 522 (138) | <.001 |
| Median (Q1; Q3) | 514 (391; 545) | 521 (400; 546) | 533 (438; 555) | 532 (509; 554) | |
| Post-procedure, 6m following* | |||||
| Mean (SD) | 205 (328) | 192 (317) | 198 (318) | 223 (360) | .22 |
| Median (Q1; Q3) | 0 (0; 288) | 0 (0; 278) | 0 (0; 314) | 26 (0; 353) |
d = day; EAM = electroanatomical mapping; ICE = intracardiac echocardiography; m = month; Q = quartile; SD = standard deviation; TEE = transesophageal echocardiogram; TTE = transthoracic echocardiogram.
Only reported for patients with complete 6m follow-up.
Includes day-of-ablation TEE/TTE procedures.
Includes ICE (when used) and EAM.
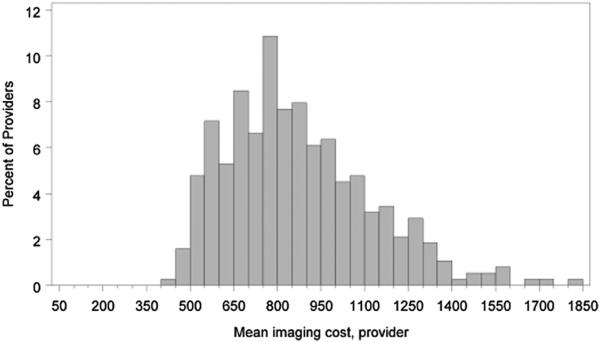
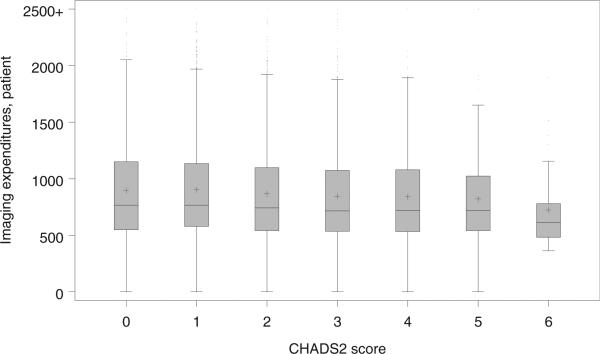
There were substantial variations in the mean imaging expenditures by provider (median $828 [Q1 $685; Q3 $1,019]; Figure 1). Small but statistically significant regional differences in periprocedural imaging were seen with the highest expenditures in the northeast (mean $943 [SD $477]) and the lowest in the south (mean $858 [SD $451], p <0.001). There were considerable variations at the patient-level. Patients with CHADS2 score ≥2 had higher mean and median overall expenditures in the 6 months before ablation and the 6 months after ablation relative to the patients with CHADS2 score <2 (Table 5). Conversely, patients with CHADS2 score ≥2 had lower mean and median ex penditures for periprocedural imaging. There was an inverse relation between imaging expenditures and the CHADS2 score (Figure 2). Mean imaging expenditures for CHADS2 scores of 0, 2, 4, and 6 were $900 (SD $464), $874 (SD $446), $845 (SD $437), and $724 (SD $359), respectively.
Figure 1.
Provider variation in periprocedural imaging expenditures.
5.
Table Medicare expenditures by CHADS2 score
| Time Period | CHADS2 <2(n = 5,616) | CHADS2 ≥2(n = 5,909) | p | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (Q1; Q3) | Mean (SD) | Median (Q1; Q3) | ||
| Overall expenditures | |||||
| Pre-procedure, 6m prior | 6,590 (7,424) | 3,811 (2,131; 8,363) | 11,683 (13,115) | 7,246 (3,491; 14,863) | <.001 |
| Day-of-procedure | 13,981 (6,123) | 13,272 (11,614; 16,435) | 14,906 (8,482) | 13,288 (11,720; 16,766) | .004 |
| Post-procedure, 6m followinga | 7,714 (11,467) | 3,371 (1,646; 9,657) | 11,633 (18,521) | 5,255 (2,342; 14,584) | <.001 |
| Imaging expenditures | |||||
| Peri-procedure, 30d prior + day-of | 907 (466) | 766 (564; 1,137) | 862 (444) | 728 (540; 1,090) | <.001 |
| Pre-procedure, 30d prior† | 391 (413) | 240 (99; 596) | 360 (393) | 207 (70; 561) | <.001 |
| Intra-procedure‡ | 516 (202) | 529 (427; 554) | 502 (180) | 523 (406; 546) | <.001 |
| Post-procedure, 6m followinga | 205 (336) | 0 (0; 312) | 207 (332) | 29 (0; 299) | .006 |
d = day; EAM = electroanatomical mapping; ICE = intracardiac echocardiography; m = month; Q = quartile; SD = standard deviation; TEE = trans esophageal echocardiogram; TTE = transthoracic echocardiogram.
Only reported for patients with complete 6m follow-up.
Includes day-of-ablation TEE/TTE procedures.
Includes ICE (when used) and EAM.
Figure 2.
Variation in periprocedural imaging expenditures by CHADS2 score, presented as a schematic box plot. The box represents the inter-quartile range (IQR), the internal horizontal line represents the median, and the + symbol represents the mean. Whiskers are drawn from the box to a distance or 1.5 IQR or to the most extreme value observed within this range.
Discussion
There are 2 main findings in this nationwide analysis of catheter ablation in patients with AF aged ≥65 years. First, periprocedural imaging expenditures represent a modest fraction of overall catheter ablation expenditures, although imaging accounts for a substantial absolute amount of money spent. Second, there is considerable variation in the periprocedural imaging expenditures by provider, patient, and patient co-morbidity.
Despite routine use of periprocedural imaging, there are no published analyses of overall periprocedural imaging expenditures for AF ablation. The clinical impact and cost-effectiveness of TEE before AF ablation has been evaluated with a decision model,7 and preablation imaging expenditures have been included in cost-effectiveness analyses for AF ablation.8 Our analysis shows that periprocedural imaging expenditures are a modest 6% of the overall Medicare expenditures related to AF ablation. Compared with the cost of procedural equipment, imaging costs are slightly less than the list price of a transseptal steerable sheath, approximately 1/4 the list price of an open irrigated–tip navigational ablation catheter, and about 1/8 the list price of a cryoablation balloon.9 Periprocedural imaging remains an important issue because there is substantial variation in expenditures by provider, and a subset of patients have periprocedural imaging expenditures that are 2 to 3 times those of the average AF ablation patient.
There is also overlap between the diagnostic abilities of the various periprocedural imaging tests. TEE,10 MRI,11 CT,12 and ICE13,14 are all able to identify left atrial appendage thrombus. Similarly, CT,15,16 MRI,17 ICE,18 and EAM19 can all play an important role in defining anatomic structures for AF ablation. Three-dimensional rotational angiography is an emerging technology that is also able to assist with defining left atrial and pulmonary vein anatomy at the time of the procedure.20,21 Refinements in adjunctive imaging used with EAM (e.g., ultrasound, acoustic radiation force impulse imaging, and rotational angiography) may also provide improvements in the efficiency of periprocedural imaging. The data are inconclusive about whether image integration during the ablation procedure improves long-term maintenance of sinus rhythm.17,22,23
We found that periprocedural imaging expenditures varied by patient co-morbidity and stroke risk. Patients with CHADS2 score <2 had a statistically significant lower expenditure of periprocedural imaging than the patients with CHADS2 score ≥2. There was an inversely linear association between CHADS2 score and periprocedural imaging expenditures. This represents a risk-imaging paradox, because the patients at the highest risk of stroke and complications receive less imaging, despite having the most to benefit from pre- or intra-procedural identification of thrombus.
A similar paradox has been noted for oral anticoagulant use in patients with AF, where the patients at highest risk of stroke have the lowest rates of oral anticoagulant use. The explanation for the anticoagulation paradox is often that patients at higher risk of stroke are also at higher risk of bleeding. Although the risk profile with imaging is different from that with oral anticoagulants, the same risk-versus-reward decision making may be resulting in lower imaging expenditures in patients with higher CHADS2 scores. For example, CT imaging requires contrast dye, which puts patients at risk for acute kidney injury.
The United States health-care system is changing in the setting of a more cost-conscious environment. Payment for AF ablation has changed over recent years with a billing code being established for a complex AF ablation instead of itemized charges for the case. Cardiac CT and/or MRI and TEE are not part of the AF ablation billing code, but as of January 1, 2013, EAM and ICE are included in the AF ablation billing code. More accurate cost and outcomes data are needed to understand the clinical benefit of various periprocedural imaging strategies. Additional cost data on periprocedural imaging may be available after the Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation trial has finished, as there will be a cost-effectiveness analysis component to the trial.24
Our study has important limitations. This analysis was based on observational, administrative claims data and is subject to coding and reporting bias. The methods used to identify patients with AF ablation have been previously used and validated, but we are unable to exclude the possibility that some ablation procedures were for treatment of non-AF arrhythmias. We are unable to determine if the patients included in the analysis had previous ablations before 2007. Expenditures are the Medicare and Medicare beneficiary payments, and they do not reflect costs to the institutions or the health-care system.
Cardiovascular imaging studies around the time of AF ablation do not substantially impact the overall expenditures of AF ablation. The expenditures for periprocedural imaging vary at the patient and the provider level. An imaging paradox exists, in which patients at higher procedural risk have lower expenditures on periprocedural imaging.
Acknowledgments
Dr. Pokorney receives modest research grant support from AstraZeneca (London, United Kingdom) and modest consultant/advisory board support from Janssen Pharmaceuticals (Titusville, New Jersey). Dr. Steinberg was funded by NIH T-32 training grant #5 T32 HL 7101-38. Dr. Piccini receives significant research grant support from ARCA biopharma (Westminster, Colorado), Boston Scientific (Natick, Massachusetts), GE Healthcare (Milwaukee, Wisconsin), Johnson & Johnson/Janssen Pharmaceuticals (Titusville, New Jersey), and ResMed (San Diego, California); modest consultant/advisory board support from Forest Laboratories (New York, New York), Medtronic (Fridley, Minnesota), and Spectranetics (Colorado Springs, Colorado); and significant consultant/advisory board support from Johnson & Johnson/Janssen Pharmaceuticals (Titusville, New Jersey).
Footnotes
Disclosures
No other authors have disclosures.
References
- 1.Pearlman AS, Ryan T, Picard MH, Douglas PS. Evolving trends in the use of echocardiography: a study of Medicare beneficiaries. J Am Coll Cardiol. 2007;49:2283–2291. doi: 10.1016/j.jacc.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 2.Iglehart JK. Health insurers and medical-imaging policy—a work in progress. N Engl J Med. 2009;360:1030–1037. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 3.MedPAC Report to the Congress. Medicare and the Health Care Delivery System. Available at: http://www.medpac.gov/documents/June13_EntireReport.pdf. Washington DMJ. Accessed on October 28, 2013.
- 4.Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, Ellinor PT, Hernandez AF, Walkey AJ, Heckbert SR, Benjamin EJ, Curtis LH. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. doi: 10.1161/CIRCULATIONAHA.112.109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 6.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–1074. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 7.Gula LJ, Massel D, Redfearn DP, Krahn AD, Yee R, Klein GJ, Skanes AC. Impact of routine transoesophageal echocardiography on safety, outcomes, and cost of pulmonary vein ablation: inferences drawn from a decision analysis model. Europace. 2010;12:1550–1557. doi: 10.1093/europace/euq306. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:362–369. doi: 10.1161/CIRCEP.108.837294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Physician-controlled costs: the choice of equipment used for atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36:157–165. doi: 10.1007/s10840-013-9782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomstrom Lundqvist C, Auricchio A, Brugada J, Boriani G, Bremerich J, Cabrera JA, Frank H, Gutberlet M, Heidbuchel H, Kuck KH, Lancellotti P, Rademakers F, Winkels G, Wolpert C, Vardas PE. The use of imaging for electrophysiological and devices procedures: a report from the first European Heart Rhythm Association Policy Conference, jointly organized with the European Association of Cardiovascular Imaging (EACVI), the Council of Cardiovascular Imaging and the European Society of Cardiac Radiology. Europace. 2013;15:927–936. doi: 10.1093/europace/eut084. [DOI] [PubMed] [Google Scholar]
- 11.Rathi VK, Reddy ST, Anreddy S, Belden W, Yamrozik JA, Williams RB, Doyle M, Thompson DV, Biederman RW. Contrast-enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm. 2013;10:1021–1027. doi: 10.1016/j.hrthm.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Hur J, Pak HN, Kim YJ, Lee HJ, Chang HJ, Hong YJ, Choi BW. Dual-enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2013;112:238–244. doi: 10.1016/j.amjcard.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Baran J, Stec S, Pilichowska-Paszkiet E, Zaborska B, Sikora-Frac M, Krynski T, Michalowska I, Lopatka R, Kulakowski P. Intracardiac echocardiography for detection of thrombus in the left atrial appendage: comparison with transesophageal echocardiography in patients undergoing ablation for atrial fibrillation: the Action-Ice I Study. Circ Arrhythm Electrophysiol. 2013;6:1074–1081. doi: 10.1161/CIRCEP.113.000504. [DOI] [PubMed] [Google Scholar]
- 14.Saksena S, Sra J, Jordaens L, Kusumoto F, Knight B, Natale A, Kocheril A, Nanda NC, Nagarakanti R, Simon AM, Viggiano MA, Lokhandwala T, Chandler ML, Group I-CIS A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the Intracardiac Echocardiography Guided Cardioversion Helps Interventional Procedures study. Circ Arrhythm Electrophysiol. 2010;3:571–577. doi: 10.1161/CIRCEP.110.936161. [DOI] [PubMed] [Google Scholar]
- 15.Dong J, Calkins H, Solomon SB, Lai S, Dalal D, Lardo AC, Brem E, Preiss A, Berger RD, Halperin H, Dickfeld T. Integrated electro-anatomic mapping with three-dimensional computed tomographic images for real-time guided ablations. Circulation. 2006;113:186–194. doi: 10.1161/CIRCULATIONAHA.105.565200. [DOI] [PubMed] [Google Scholar]
- 16.Martinek M, Nesser HJ, Aichinger J, Boehm G, Purerfellner H. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:1215–1223. doi: 10.1111/j.1540-8159.2007.00843.x. [DOI] [PubMed] [Google Scholar]
- 17.Caponi D, Corleto A, Scaglione M, Blandino A, Biasco L, Cristoforetti Y, Cerrato N, Toso E, Morello M, Gaita F. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome? A randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010;12:1098–1104. doi: 10.1093/europace/euq107. [DOI] [PubMed] [Google Scholar]
- 18.Daccarett M, Segerson NM, Gunther J, Nolker G, Gutleben K, Brachmann J, Marrouche NF. Blinded correlation study of three-dimensional electro-anatomical image integration and phased array intra-cardiac echocardiography for left atrial mapping. Europace. 2007;9:923–926. doi: 10.1093/europace/eum192. [DOI] [PubMed] [Google Scholar]
- 19.Estner HL, Deisenhofer I, Luik A, Ndrepepa G, von Bary C, Zrenner B, Schmitt C. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX). Europace. 2006;8:583–587. doi: 10.1093/europace/eul079. [DOI] [PubMed] [Google Scholar]
- 20.Knecht S, Wright M, Akrivakis S, Nault I, Matsuo S, Chaudhry GM, Haffajee C, Sacher F, Lellouche N, Miyazaki S, Forclaz A, Jadidi AS, Hocini M, Ritter P, Clementy J, Haissaguerre M, Orlov M, Jais P. Prospective randomized comparison between the conventional electro-anatomical system and three-dimensional rotational angiography during catheter ablation for atrial fibrillation. Heart Rhythm. 2010;7:459–465. doi: 10.1016/j.hrthm.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Kriatselis C, Tang M, Nedios S, Roser M, Gerds-Li H, Fleck E. Intraprocedural reconstruction of the left atrium and pulmonary veins as a single navigation tool for ablation of atrial fibrillation: a feasibility, efficacy, and safety study. Heart Rhythm. 2009;6:733–741. doi: 10.1016/j.hrthm.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Della Bella P, Fassini G, Cireddu M, Riva S, Carbucicchio C, Giraldi F, Maccabelli G, Trevisi N, Moltrasio M, Pepi M, Galli CA, Andreini D, Ballerini G, Pontone G. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J Cardiovasc Electrophysiol. 2009;20:258–265. doi: 10.1111/j.1540-8167.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- 23.Bertaglia E, Bella PD, Tondo C, Proclemer A, Bottoni N, De Ponti R, Landolina M, Bongiorni MG, Coro L, Stabile G, Dello Russo A, Verlato R, Mantica M, Zoppo F. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the Carto-Merge Italian Registry. Europace. 2009;11:1004–1010. doi: 10.1093/europace/eup152. [DOI] [PubMed] [Google Scholar]
- 24.Packer D. Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) Available at: http://clinicaltrials.gov/ct2/show/NCT00911508. Accessed March 5, 2013.