Abstract
Object
The goal of this study was to compare cortical sensorimotor adaptations associated with neurological deterioration and then recovery following surgical decompression for cervical spondylotic myelopathy (CSM).
Methods
Eight patients with CSM underwent functional MR (fMR) imaging during wrist extension and the 3-finger pinch task, along with behavioral assessments before and 3 and 6 months after surgery. Six healthy control volunteers were scanned twice.
Results
Cervical spine MR imaging demonstrated successful cord decompression. The patients improved after surgery on the modified Japanese Orthopaedic Association score for the upper extremity, which correlated with the changes in task-associated activation in specific sensorimotor regions of interest. Pinch-related activation in sensorimotor cortex contralateral to the movement paradigm was reduced before surgery then increased toward the extent of healthy controls after surgery. Before surgery, patients showed broader activation in ipsilateral sensorimotor cortex during wrist extension than during pinch, but activations became similar to those of healthy controls after surgery. Pinch-related activation volume in the ipsilateral sensorimotor cortex and the magnitude of activation in the contralateral dorsal premotor cortex evolved linearly across time after surgery, along with wrist extension–related activation magnitude in the contralateral supplementary motor area.
Conclusions
Serial fMR imaging studies in CSM can capture the adaptations in specific sensorimotor cortices that accompany clinical deterioration and postsurgical improvement in sensorimotor function associated with damage and partial recovery of conduction in corticospinal pathways. These adaptive regions can be monitored by serial fMR imaging to detect a critical loss of supraspinal reserve in compensatory plasticity, which might augment clinical information about the need for surgical decompression.
Keywords: cervical spondylotic myelopathy, functional magnetic resonance imaging, motor cortex, rehabilitation, spinal cord injury, surgical decompression
In incomplete SCI, cerebral reorganization and playsiological changes in spinal motor neuron pools play a role in maintaining residual motor function.5,7,27 The modification of preexisting connections (synaptic plasticity) and the development of new circuitry through sprouting of axons and dendrites (anatomical plasticity) are the major adaptive processes that evolve within cortical and subcortical motor centers and the spinal cord after SCI.27 Nishimura and colleagues24 demonstrated that functional reorganization in bilateral M1, followed by contralesional primary motor and bilateral premotor areas, occurred after lateral corticospinal tract transection at the C4–5 segment in the macaque. This evolving pattern of cortical motor activation was associated with the restoration of skilled finger movements. Furthermore, the contribution of an activation increase in motor cortices over time depended on the recovery stage of dexterity of the hand, which suggests that different neural mechanisms may be involved in early versus late recovery after SCI. In patients with cervical myelitis, robust adaptations of cortical sensorimotor recruitment were inversely correlated to the severity of spinal cord damage.29 Thus, compensatory reorganization in sensorimotor cortices induced by SCI may play an important role in limiting the deterioration of motor function as well as contributing to sensorimotor recovery if disease progression is halted.
Cervical spondylotic myelopathy is one of the most common spinal cord disorders causing motor and sensory deficits of the upper and lower extremities in adults.39 The predominant pathological mechanisms in CSM include demyelination of the ascending and descending corticospinal fibers and destruction of anterior horn neurons and of dorsolateral axons in advanced stages.10 The progressive loss of axonal conduction due to ischemia, edematous changes, and demyelination within the compromised spinal cord segments may be partially reversible following surgical decompression. This potential for reversibility allowed us to explore the plasticity of supraspinal structures resulting from SCI during symptom progression, then to monitor subsequent adaptive responses following surgical decompression, as corticospinal conductivity and relearning of skilled hand movements evolved.
Activity-triggered muscle fatigability, weakness of wrist extensors and intrinsic hand muscles, and impaired finger coordination are common motor deficits of the distal upper extremity in patients with symptomatic CSM. Prior to decompressive spinal surgery, we used fMR imaging during both wrist extension and 3-finger pinch to investigate cortical sensorimotor reorganization associated with clinical deterioration of hand function in patients with symptomatic CSM. We assessed subsequent cortical adaptations in response to partial recovery of corticospinal tract conduction following surgical decompression and rehabilitative practice.5 Our hypotheses are the following: 1) prior to surgery, patients with CSM will demonstrate significant deviations in cortical sensorimotor representational maps for dexterous hand movements compared with healthy control volunteers; 2) cortical adaptations to SCI will depend in part on the amount of residual functioning corticospinal pathways; 3) patients with CSM may have different cortical adaptations for dexterous and nondexterous hand movements; and 4) cortical sensorimotor adaptations related to wrist and finger movements will evolve gradually toward the cortical representational map of healthy controls if corticospinal conductivity and motor function of the hand improve after decompression. If the data were to support the 4 hypotheses of this study, then dynamic cerebral adaptations revealed by serial fMR imaging studies in patients with suspected CSM may also serve as an assay5 of subclinical compensatory cortical adaptations that could be useful in evaluating the need and timing for cervical decompression.
Methods
Patients and Healthy Controls
Eight patients with CSM and 6 healthy control volunteers participated. Entry criteria for patients were as follows: age 18–85 years; symptoms for at least 2 months, with recent clinical deterioration that led to the decision to perform surgical decompression; weakness of the upper extremities; spine MR imaging showing 1–2 levels of cervical spinal stenosis and cord compression; no previous cervical spine surgery; no compromise of cognitive function or other neurological or musculoskeletal diseases; and ability to tolerate and meet the safety criteria for repetitive fMR imaging studies. Before enrollment, all participants signed the consent form that had been approved by the University of California at Los Angeles institutional review board. All surgical procedures were performed by the same surgeon (L.T.H.). Patient information is summarized in Table 1.
TABLE 1.
Characteristics, surgical procedure, and behavioral measurements in 8 patients with CSM*
| mJOA-Upper |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cord Diam (mm) |
Total Score |
Motor Score |
||||||||||||
| Case No. |
Age (yrs), Sex |
Symptom Duration |
Handed-ness | Task Hand |
LCCC | Preop | Postop | Op Procedure | Preop | Postop 1 |
Postop 2 |
Preop | Postop 1 |
Postop 2 |
| 1 | 69, M | 2 yrs | It | It | C5–7 | 4.6 | 5.6 | ACDF | 7 | 7 | 8 | 4 | 4 | 5 |
| 2 | 50, F | 2 yrs | rt | It | C4–6 | 4.0 | 4.6 | CLF | 6 | 7 | 7 | 4 | 4 | 4 |
| 3 | 66, M | 4mos | rt | rt | C4–5 | 7.4 | 9.3 | ACDF | 7 | 8 | 8 | 4 | 5 | 5 |
| 4 | 68, F | 2 yrs | rt | rt | C4–5 | 5.0 | 7.2 | CLF | 5 | 6 | 7 | 4 | 5 | 5 |
| 5 | 47, F | 2mos | rt | rt | C5–7 | 4.4 | 6.0 | laminoplasty | 6 | 8 | 8 | 4 | 5 | 5 |
| 6 | 83, M | 6mos | rt | It | C5–7 | 5.5 | 6.9 | laminectomy | 4 | 6 | 6 | 2 | 3 | 3 |
| 7 | 40, F | 2mos | rt | rt | C5–6 | 5.9 | 5.9 | ACDF | 5 | 7 | 7 | 4 | 5 | 5 |
| 8 | 66, M | 1 yr | rt | rt | C4–5 | 4.9 | 5.9 | ACF | 5 | 6 | 8 | 3 | 4 | 5 |
ACDF = anterior cervical discectomy and fusion; ACF = anterior corpectomy and fusion; CLF = cervical laminectomy and fusion; diam = diameter; LCCC = level of cervical cord compression; postop 1 = 3 months postsurgery; postop 2 = 6 months postsurgery; task hand = the hand used to perform the motor activation tasks.
Clinical and Behavioral Assessments
Patients underwent neurological examination and behavioral assessment within 1 month before and 3 and 6 months after decompressive surgery. Cervical spine MR images were acquired shortly before surgery and between 1 and 4 months after the operation by using a Siemens Sonata 1.5-T MR imaging unit. The cervical spinal cord diameter served as a relative quantitative measure of the severity of spinal cord compression. The midline anterior-posterior spinal cord diameter of the most severely compressed segment, which was determined from examining both the sagittal and axial T2-weighted views, was measured with digital calipers. This width was compared with the diameter of the spinal cord located one segment rostral and caudal to the defined stenosis. The Edinburgh Handedness Inventory was used to determine the handedness of the participants.25 The mJOA-upper was the primary clinical outcome measure.38 The scale for the mJOA-upper has a total score of 8 (5 points are allocated to motor function and 3 to sensation of the hand and proximal arm).
Motor Activation Tasks on fMR Imaging
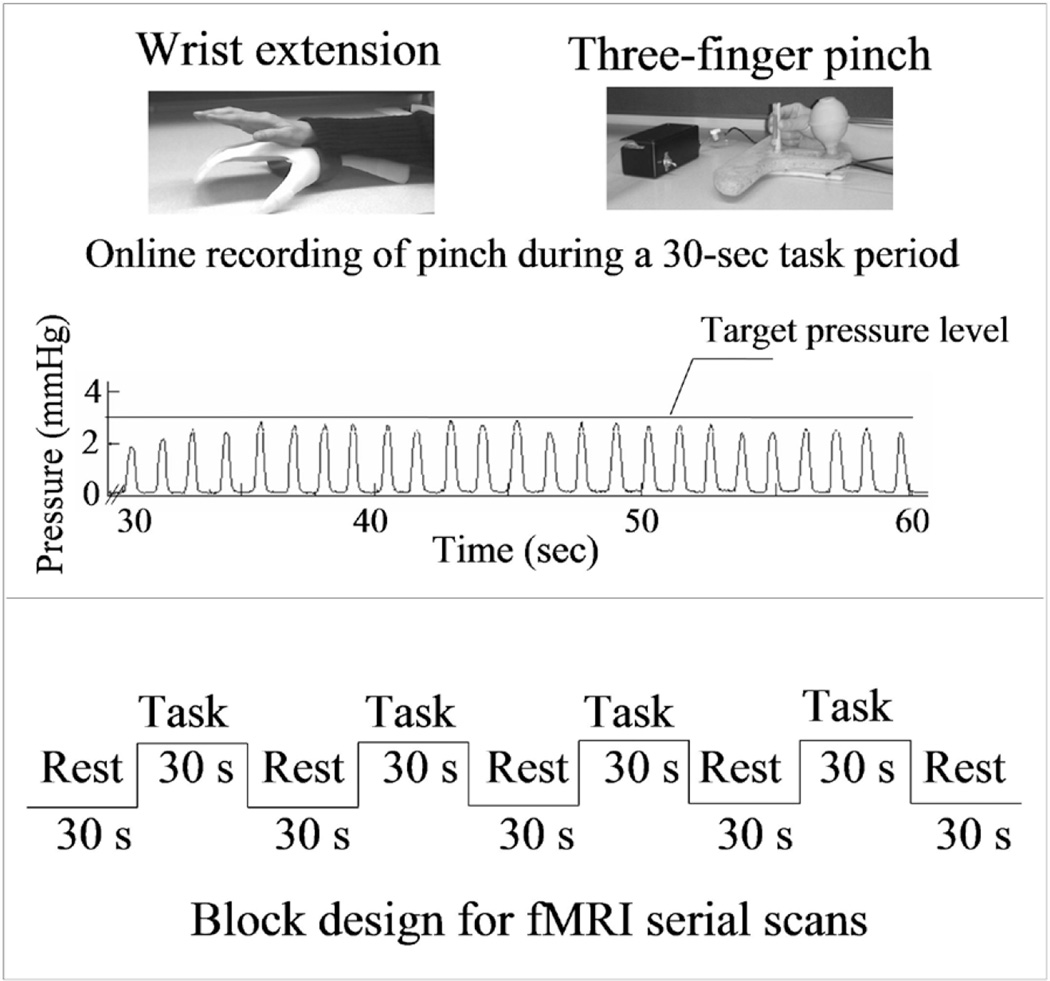
Wrist extension and 3-finger pinch were used as the fMR imaging activation tasks (Fig. 1 upper).8 For wrist extension, a block design with 30 seconds of rest and 30 seconds of wrist extension alternating for a total scan time of 4.5 minutes was used. Participants performed 10 movements per block that allowed 1 second for extension, 1 second to return to the neutral position, and 1 second of rest between each voluntary movement (Fig. 1lower). The palm and forearm rested on an orthotic mold and the participant’s wrist extended ~ 15° until the dorsum of the hand touched a barrier, which provided tactile feedback to stop the extension. Visual cues informed the participant when to extend and when to relax the wrist.
Fig. 1.
Photographs showing the apparatus for the fMR imaging activation tasks, an illustration of pinch movement recording (upper panels), and a schema showing the experimental design (lower panel) Upper: For the wrist extension task, the participants extended the wrist following visual cues while the hand and forearm rested on top of the plastic mold in the scanner. For the 3-finger pinch task, the participants squeezed the center of the plastic tube with the index and middle fingers against the thumb following a metronome sound. Lower: Conventional block design was used in the study. Each fMR imaging session started from a 30-second rest period, followed by four 30-second task periods alternating with four 30-second rest periods. During each task period, the patients performed either wrist extension or 3-finger pinch by following visual or auditory cues (see Methods for details).
The pinch task required a 3-finger (thumb-indexmiddle fingers) coordinated squeeze of a thin vertical plastic tube, which was connected to a pressure transducer through a hollow tube (Fig. 1upper). A block design of five 30-second rest periods alternating with four 30-second task periods of repetitive pinch was used (Fig. 1 lower). During scanning, a feedback display was fed to the goggle worn by the participant. The participants saw a horizontal red target line (set to 50% of the individual’s maximal pinch pressure) and a black waveform of their real-time pinch pressure. An ideal performance made the real-time pinch peaks rise to the target line (Fig. 1 upper). Pinch rate (0.5 Hz) was cued by metronomic sound through the earphone. A DAQ Card 500 (National Instruments) and customized software were used to record the pinch pressure and rate for offline analysis. Although both dominant and nondominant hands were affected, patients performed the tasks with the weaker one (Table 1). The healthy control volunteers used each hand for the 3-finger pinch in separate fMR imaging sessions, but used only the dominant hand for wrist extension.
Structural and fMR Imaging Acquisition
Patients underwent a baseline scan within 1 month before surgery, and 2 follow-up scans were obtained, one at 3 and the other at 6 months after surgery. Healthy controls were scanned twice, ~ 2 weeks apart. Brain MR images were collected using a Siemens Sonata 1.5-T scanner optimized for head imaging at the Brain Mapping Center, University of California at Los Angeles. First, a high-resolution 3D T1-weighted MR image covering the whole brain was acquired using a gradient-echo sequence (TR 1970 msec, TE 4.38 msec, TI 1100 msec, flip angle 15°, matrix 256 × 256). Second, a set of high-resolution T1-weighted spin echo images were acquired with the following parameters: 25 contiguous axial slices parallel to the anterior commissure-posterior commissure plane; 4-mm thickness with 1-mm gap; TE 15 msec; TR 600 msec; flip angle 90°; matrix 256 × 256. Last, a set of functional volumes was acquired using an echo planar T2*-weighted sequence with blood oxygenation level–dependent contrast material. The parameters for functional images were as follows: 25 axial slices with 4-mm thickness and 1-mm gap; TR 2500 msec; TE 60 msec; flip angle 80°; in-plane resolution 3 × 3 × 4 mm; matrix 64 × 64. A total of 108 volumes was collected during each fMR imaging session.
Behavioral Data and fMR Imaging Analysis
The FMRIB Software Library (version 3.3; http://www.fmrib.ox.ac.uk/fsl) software package was used to analyze functional time series.34 The preprocessing steps included the following: 1) the fMR imaging time series was corrected for head motion by using MCFLIRT software (FMRIB’s Linear Image Registration Tool); 2) all functional volumes were aligned to the middle volume to correct for misalignment and residual head motion; 3) the realigned and motion-corrected functional volumes were spatially smoothed using a Gaussian kernel of 6 mm full-width-half-maximum; 4) the fMR imaging time series was decomposed using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components program to identify components clearly related to motion artifact and physiological noise, which were then excluded from further analysis;2 and 5) functional volumes were transformed to the standard Montreal Neurological Institute space by using FMRIB’s Linear Image Registration Tool. A task-specific effect was estimated using FMRIB’s Improved Linear Model with autocorrelation correction.17 A t-test was performed to construct a task-specific voxel-by-voxel activation map (activation vs rest). The statistical threshold was set at a cluster level of z > 3.1 with spatial extent at p < 0.01 (corrected for multiple comparisons according to random field theory). Furthermore, an ROI analysis was conducted using in-house software modified from Featquery. The anatomical regions included bilateral M1 and S1, PMd, SMA, Cm, and cerebellum. They were defined on the T1-weighted MR imaging template in Montreal Neurological Institute space. These ROIs were used as masks for computing VC and %SC from the thresholded z statistical maps and the contrast of parameter estimation images, respectively. The VC and %SC are fMR imaging variables used to represent task-associated activation volume and magnitude, respectively, within a specified brain region. Moreover, locations of the peak activation (maximum z score) and the average activation corresponding to the geometric center of all activated voxels within each ROI were computed from suprathresholded z statistical images. A 2-sample t-test was applied for comparisons of activation (measured using VC and %SC) in the aforementioned ROIs between patients and healthy controls and for between-task comparisons of activation in patients. A paired t-test was used for between-session comparisons of activation in healthy controls.
Statistical Analysis
Pinch pressure was analyzed using DataWizard, a custom MATLAB (The MathWorks) software tool developed at the University of California at Los Angeles to compare peak to target pressure in each movement trial. All the peaks from the four 30-second task periods were extracted and averaged to generate a mean pressure (± standard error) for each fMR imaging session. Constant error and variable error were used to represent accuracy and consistency of pinch performance during scanning. The linear mixed model was applied for comparisons of %SC and VC, and pinch parameters (constant error, variable error, pinch rate, and peak pinch pressure) and mJOA-upper across time from preoperatively to 3 and 6 months postsurgery (SAS version 9.1 for Windows; SAS Institute, Inc.). A paired t-test was used to compare spinal cord diameter before and after surgery. The Spearman correlation coefficient by rank (SPSS version 11.5 for Windows; SPSS, Inc.) was used to test correlations among pre- and postsurgery changes in task-associated activation (measured using VC and %SC), mJOA-upper, and spinal cord diameter.
Results
Patients showed a significant increase in spinal cord diameter after surgical decompression (presurgery 5.2 ± 0.4 mm; postsurgery 6.4 ± 0.5 mm; p = 0.002; Table 1). All patients had improved in strength of muscle groups below the level of SCI by 3 months after decompression.Among the 8 patients, 2 had much less and 6 no longer exhibited fatigability immediately after 10 repetitive lightly resisted muscle contractions.6 Clinical examination and patient self-reports confirmed postsurgery improvement in use of the hands and walking. As a group, the patients’ mJOA-upper increased linearly across time, indicating significant improvement of upper-extremity function after surgical decompression (p = 0.00001 for total score; p = 0.0006 for motor score).
Task-Associated Cerebral Activation Patterns in Healthy Controls and Patients
The patients in Cases 1–7 accomplished both tasks, but the one in Case 8 did only the wrist extension task. For the wrist extension task, movement rate and the degree of wrist extension were consistent across sessions in healthy controls and patients. For the pinch task, no between-session differences in pinch pressure and rate, variable error, and constant error were found in healthy controls and patients (p > 0.1), indicating consistent task performance over time. In healthy controls, the 3-finger pinch and wrist extension with the dominant (right) hand activated the expected cortical sensorimotor network (Fig. 2). Activation volume (measured using VC) and magnitude (measured using %SC) during 3-finger pinch and wrist extension in bilateral M1, S1, PMd, SMA, Cm, and cerebellum were reproducible between repetitive fMR imaging sessions in healthy controls (p > 0.1; data not shown). No significant differences in activation volume and magnitude in the selected regions between wrist extension and 3-finger pinch were found in healthy controls (p > 0.1), although the pinch task produced slightly more activation (larger VC and higher %SC) in bilateral M1 and S1 than did wrist extension.
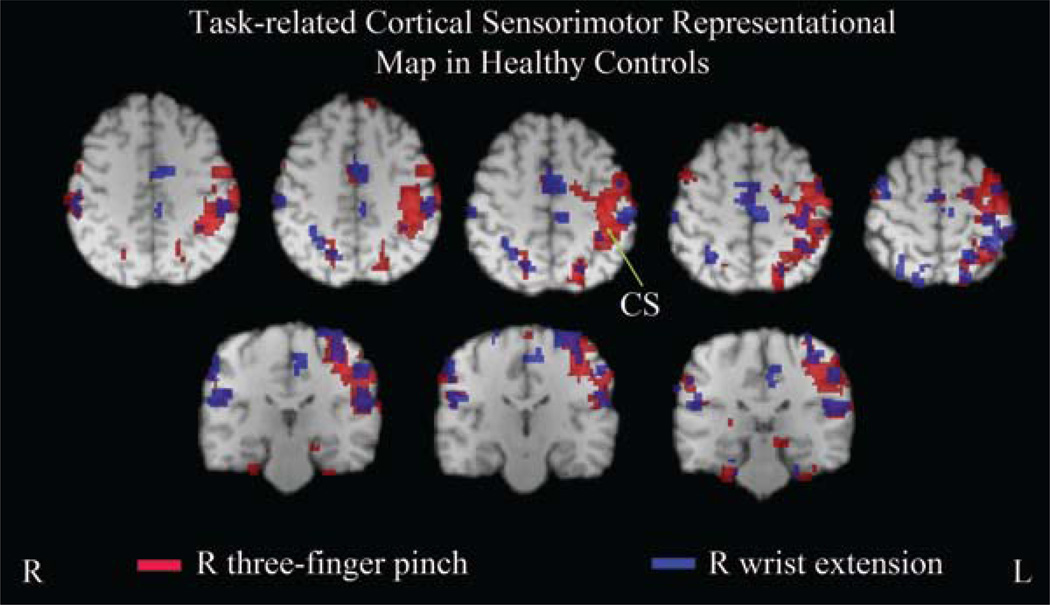
Fig. 2.
Functional MR images depicting task-related cortical sensorimotor representational maps for the motor tasks performed with the dominant hand (R) in healthy controls. Both wrist extension and the 3-finger pinch produced left-lateralized activation in the M1, S1, PMd, and parietal cortex, which was widespread for pinch relative to wrist extension, although no statistical significance was found at the group level. A small amount of activation was also detected in the ipsilateral sensorimotor cortices for both tasks. Bilateral SMA was mostly activated during wrist extension compared with the 3-finger pinch. CS = central sulcus.
Before surgery, patients showed a smaller activation volume in the contralateral S1, M1, and PMd during pinch than did healthy controls (S1: p = 0.03; M1: p = 0.07; PMd: p = 0.07), but had the same amount of activation volume as healthy controls in these regions during wrist extension (p > 0.1; Fig. 3). Three months after surgery, a lower activation magnitude (measured using %SC) in the contralateral PMd during pinch (p = 0.04) and a smaller activation volume in bilateral Cm and ipsilateral SMA during wrist extension were found in patients than in healthy controls (ipsilateral Cm: p = 0.003; contralateral Cm: p = 0.002; ipsilateral SMA: p = 0.04). Six months after surgery, similar sensorimotor activation patterns were found between patients and healthy controls, except for a lower than control level of activation magnitude in the contralateral M1 during pinch (p = 0.03), suggesting some degree of abnormal axon conduction or axon loss within corticospinal fibers for fine finger movements after surgical decompression.
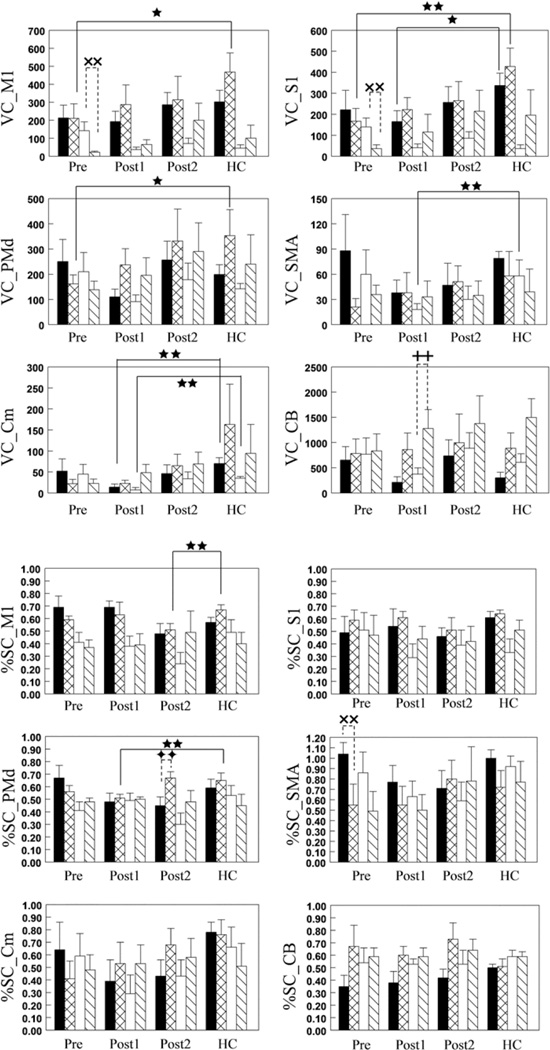
Fig. 3.
Bar graphs showing task-related activation volume and magnitude (measured using VC and %SC) in sensorimotor cortices in patients with CSM in comparison with those values in healthy controls (HCs). Before surgery, the 3-finger pinch produced a smaller activation volume in the contralateral M1, S1, and PMd in patients than in healthy controls. Three months after surgery, wrist extension yielded a smaller amount of activation in contralateral S1, ipsilateral SMA, and bilateral Cm in patients than in healthy controls. A lower than normal level of pinch-related activation magnitude was found in contralateral PMd 3 months postsurgery and in contralateral M 1 6 months postsurgery Before surgery, wrist extension produced more activation volume in ipsilateral M1 and S1 and higher activation magnitude in contralateral SMA than did pinch, whereas pinch yielded more activation volume in ipsilateral cerebellum and higher activation magnitude in contralateral PMd than did wrist extension at 3 and 6 months postsurgery, respectively. ■ and  = contralateral activation during wrist extension and pinch;
= contralateral activation during wrist extension and pinch;  and
and 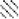 = ipsilateral activation during wrist extension and pinch; ⋆ = 0.05 < p < 0.09; ⋆ ⋆ = p < 0.05 between patients and healthy controls; xx = p < 0.05 between tasks before surgery; ++ = p < 0.05 between tasks 3 months after surgery; ♦♦ = p < 0.05 between tasks 6 months after surgery. CB = cerebellum; Post1 = 3 months after surgery; Post2 = 6 months after surgery; Pre = before surgery.
= ipsilateral activation during wrist extension and pinch; ⋆ = 0.05 < p < 0.09; ⋆ ⋆ = p < 0.05 between patients and healthy controls; xx = p < 0.05 between tasks before surgery; ++ = p < 0.05 between tasks 3 months after surgery; ♦♦ = p < 0.05 between tasks 6 months after surgery. CB = cerebellum; Post1 = 3 months after surgery; Post2 = 6 months after surgery; Pre = before surgery.
A between-task comparison revealed a smaller activation volume in the ipsilateral M1 and S1, along with higher activation magnitude in the contralateral SMA during 3-finger pinch than during wrist extension (M1: p = 0.04; S1: p = 0.05; SMA: p = 0.05). These between-task differences gradually disappeared after surgery. The pinch task, however, produced a larger activation volume in the ipsilateral cerebellum (right side; p = 0.03) at 3 months postsurgery and a higher activation magnitude in the contralateral PMd (left side; p = 0.03) at 6 months postsurgery than was found for wrist extension (Fig. 3), suggesting a greater role of these regions for coordinated finger movements following spinal cord decompression.
Evolution of Cortical Sensorimotor Activation
There was a linear increase in activation volume (measured using VC) in the ipsilateral M1 and S1 during the 3-finger pinch, and a continuous decrease of activation magnitude (measured using %SC) in the contralateral SMA during wrist extension from presurgery to 3 and 6 months postsurgery (Fig. 4A and C). The activation magnitude in contralateral PMd during the pinch task increased significantly from 3 to 6 months postsurgery (Fig. 4B). In addition, a linear dorsal shift of the center of ipsilateral PMd activation during pinch (a linear increase in Z coordinate; Fig. 4D) and linear lateral shifts (X coordinate) of the center of bilateral S1 activation during wrist extension were detected (Fig. 4E).
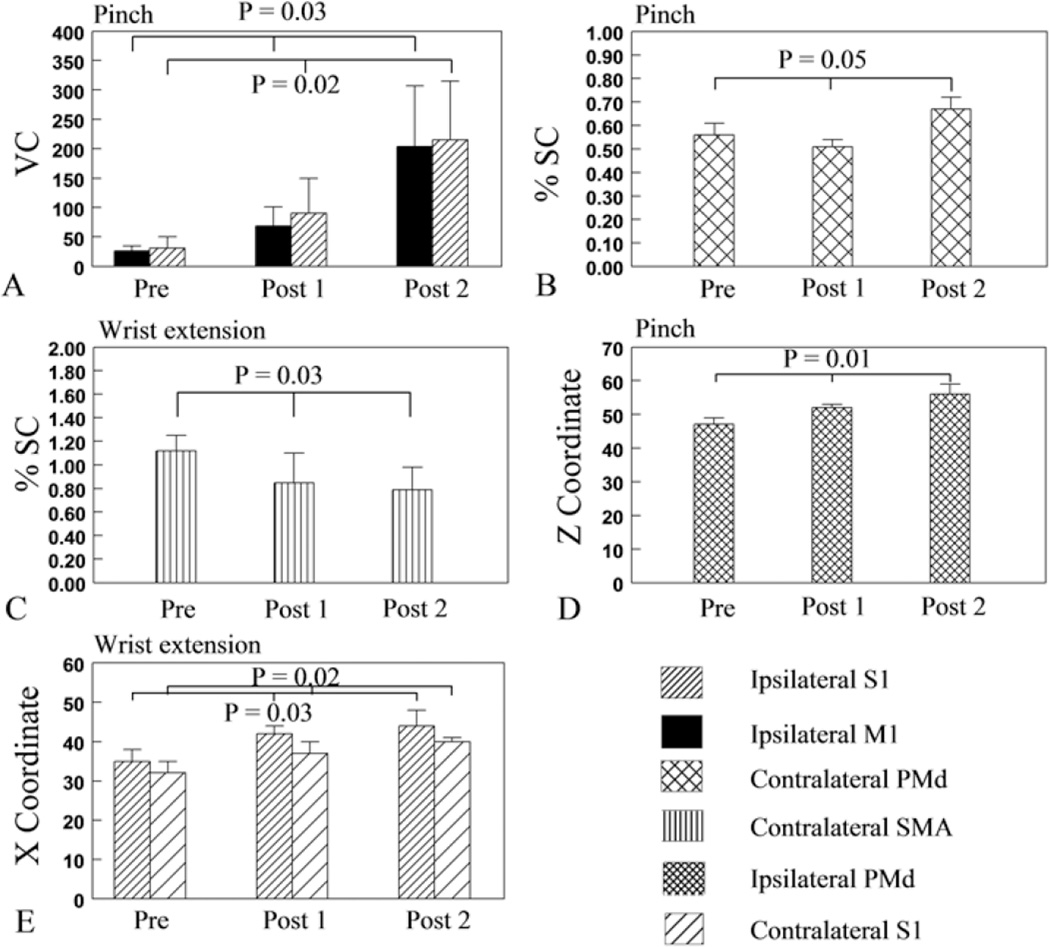
Fig. 4.
Bar graphs showing the evolution of task-related activation volume and magnitude, and locations of the maximum activation from pre- to postsurgery. Activation volume (measured using VC) in ipsilateral M1 and S1 (A) and activation magnitude (measured using %SC) in contralateral PMd (B) during pinch increased linearly across time. The linear increase of %SC in the contralateral PMd was attributed to the significant increase from 3 to 6 months postsurgery. Activation magnitude in contralateral SMA during wrist extension decreased linearly across time (C). The center of ipsilateral PMd activation during pinch shifted dorsally (D). The center of bilateral S1 activation during wrist extension shifted laterally (E).
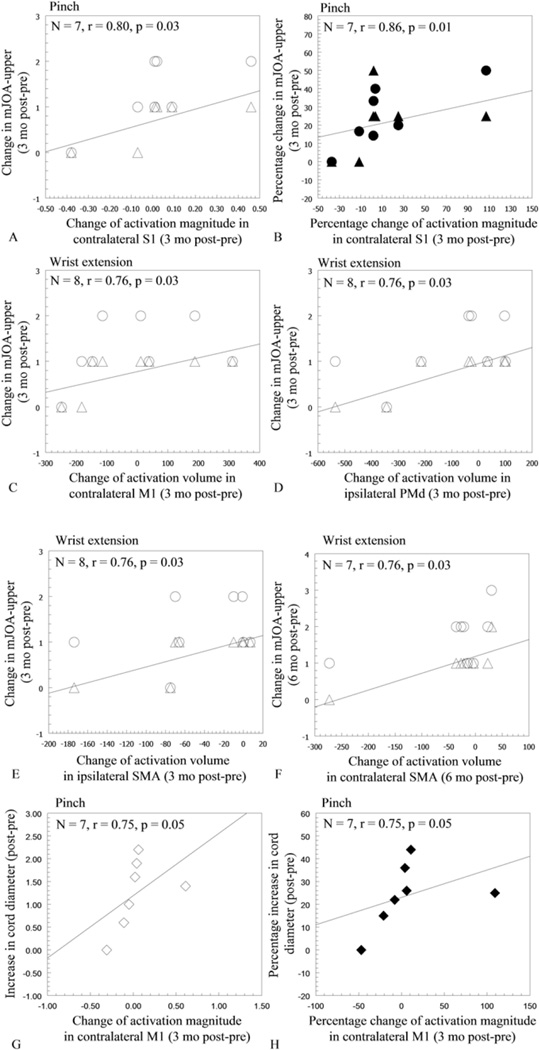
Associations Between Changes in Cortical Sensorimotor Activation, Improvement in Upper-Extremity Function, and Spinal Cord Expansion
Significant improvement in the mJOA-upper occurred at 3 months postsurgery compared with presurgery. Five of 8 patients showed no further changes from 3 to 6 months after surgery. Significant correlations were found between the 3-month postsurgery to presurgery change in pinch-related activation magnitude (measured using %SC) in the contralateral S1 and the increase in the mJOA-upper (Fig. 5A and B). The increase in the mJOA-upper motor score from presurgery to 3 months postsurgery correlated positively with the changes of wrist extension–related activation volume (measured using VC) in the contralateral M1, ipsilateral PMd, and ipsilateral SMA (Fig. 5C – E), respectively. At 6 months postsurgery, the increase in mJOA-upper motor score correlated positively with the wrist extension–related changes in activation volume in the contralateral SMA (Fig. 5F). In addition, the 3-month post- to presurgery change of pinch-related activation magnitude in the contralateral M1 correlated positively with the increase in spinal cord diameter (Fig. 5G and H). The relationship among pre- and postsurgery changes in cortical sensorimotor activation, behavioral improvement, and spinal cord expansion in a representative patient (Case 4) is illustrated in Fig. 6.
Fig. 5.
Scatterplots showing correlations between pre- and postsurgery changes in cortical sensorimotor activation, behavioral improvements, and the level of cervical cord expansion. The greater change in pinch-related activation magnitude (measured using %SC) in the contralateral S1 from pre- to 3 months postsurgery correlated with the greater increase in the mJOA-upper motor score (△), but not with the increase in total score (○) (A). The greater percentage change in %SC in contralateral S1 correlated with the greater percentage increase in mJOA-upper total score (●), but not with motor score (▲) (B). The greater increase in mJOA-upper motor score from pre- to 3 months postsurgery correlated with the greater change of wrist extension-related activation volume (measured using VC) in contralateral M1 (△; C), ipsilateral PMd (△; D), and ipsilateral SMA (△; E), respectively. The change in wrist extension-related activation volume in contralateral SMA from pre- to 6 months postsurgery correlated positively with the increase in mJOA-upper motor score (△; F). The greater increase of spinal cord diameter correlated with the greater increase in pinch-related %SC in contralateral M1 at 3 months postsurgery (G and H). △ and ▲ = absolute and percentage change in mJOA-upper motor score; ○ and ● = absolute and percentage change in mJOA-upper total score; ◊- and ♦ = absolute and percentage change in spinal cord diameter. Percentage change = [(post - pre)/pre] × 100.
Fig. 6.
Neuroimages and bar graphs revealing the evolution of cortical sensorimotor representational maps in relation to spinal cord expansion and behavioral improvement from pre- to postsurgery in a representative patient (Case 4). As illustrated on the T2-weighted MR images of the spine (A, left panels), moderate C4–5 spinal cord compression was evident before surgery, and the cord reexpanded after surgical decompression (cord diameter changed from 5.0 mm presurgery to 7.2 mm postsurgery). Cortical representational maps obtained during pinch and during wrist extension with the weaker hand (R) before and after surgery were rendered on the patient’s T1-weighted MR images (A, right panels). Activation volume (measured using VC) and magnitude (measured using %SC) in bilateral M1, S1, and PMd during wrist extension and the 3-finger pinch in comparison with those of healthy controls were plotted (B). Before surgery, both pinch and wrist extension tasks yielded a smaller amount of activation in the M1, S1, and PMd than was seen in healthy controls. Pinch-related activation in these areas increased continuously from pre- to postsurgery, whereas wrist extension-related activation in the same areas evolved multidirectionally. The progressive increase in pinch-related cortical sensorimotor activation is in accordance with the improvements in dexterity function, an increase in mJOA-upper score, and the degree of spinal cord expansion. ■ and  = contralateral activation during wrist extension and pinch;
= contralateral activation during wrist extension and pinch;  and
and  = ipsilateral activation during wrist extension and pinch.
= ipsilateral activation during wrist extension and pinch.
Discussion
This is the first serial fMR imaging study of cerebral reorganization provoked by progressive CSM, the evolution of cortical sensorimotor adaptations following surgical decompression, and direct associations between pre- and postsurgery changes in cortical sensorimotor activation in relation to behavioral improvement and expansion of the compressed spinal cord segments. Patients with CSM had altered cortical sensorimotor recruitment patterns during wrist and finger movements prior to surgery, which evolved toward the recruitment maps of healthy controls as they gained upper-extremity function following surgical decompression. Patients showed significantly less recruitment of the ipsilateral M1 and S1 during the pinch task than during wrist movements prior to surgery. This between-task difference in the ipsilateral cortical sensorimotor recruitment pattern gradually disappeared after surgery. Movement-associated activation volume in ipsilateral M1 and S1 and activation magnitude in contralateral PMd and SMA changed linearly across time. In addition, pre- to postoperative changes of activation in particular sensorimotor cortices were associated with behavioral improvement and the extent of spinal cord expansion.
Although complicated and still incompletely understood, the recovery processes after SCI depend in part on reorganization occurring in cortical and subcortical motor centers and the spared spinal cord fiber tracts connecting these centers.27,32 Incomplete SCI may evoke multilevel cerebral adaptations largely depending on the percentage of preserved axons within ascending and descending pathways connecting sensorimotor cortices and the compromised cord segments. For example, cortical remapping that included an expansion and a shift of the motor representational maps for the upper and lower extremities was observed in patients with chronic SCI during recovery facilitated by rehabilitation interventions.7,15,19 Due to the progressive axonal deterioration and gradual development of potentially reversible conduction blockage of the corticospinal tract, patients with symptomatic CSM may manifest comparable sensorimotor reorganization patterns and share similar compensatory mechanisms identified in stroke and in SCI in which corticospinal integrity is compromised.4,19,27 The sensorimotor areas of adaptation detected in our patients prior to and following surgical decompression may also serve as important ROIs for monitoring subclinical deterioration over time in patients with progressing CSM.
We had reported changes on cortical representational maps for ankle and wrist movement following decompressive surgery in a preliminary study, in which progressive focusing of cortical sensorimotor activation associated with wrist extension and ankle dorsiflexion toward the patterns of healthy controls occurred, along with postsurgery improvements in hand function and walking speed.15 The current study quantitatively captured reduced activation in the contralateral M1, S1, and PMd for dexterous finger movements, but not for less complex wrist movements before surgery, followed by a progressive increase toward the activation pattern of healthy controls, as functional gains in the upper extremity proceeded after surgical decompression. The reduced pinch-related activation and the normal amount of wrist extension–related activation in contralateral M1 and S1 before surgery suggest that the coordinated finger pinch task demands greater integrity of the corticospinal tract. Behavioral studies in monkeys with an induced lesion of one pyramidal tract revealed severe impairment of independent finger movements despite recovery of grasp.20,21 On the other hand, experimental data from monkeys and patients with stroke and SCI suggest that indirect corticospinal pathways routed via subcortical or spinal interneuronal relays, such as the corticorubrospinal and corticoreticulospinal tracts, are involved not only in mediating nondexterous movements, but also play an important role in regaining skilled dexterous function.16,23,33 It is likely that for our patients, the residual direct and indirect corticospinal pathways were insufficient to mediate cortical sensorimotor commands for the control of skilled finger movements due to axonal damage resulting from spinal cord compression.
The dynamic pre- to postsurgery changes in contralateral sensorimotor representational maps (M1 and S1 activation) for dexterous pinch movements suggest that some nonpermanently damaged axons within descending motor and ascending sensory pathways had the potential for reversibility of a conduction block. Thus, more cortical sensorimotor neurons were recruited postsurgery as a result of enhanced corticospinal conductivity. It is also possible that by halting the progression of corticospinal tract injury, cortical sensorimotor reorganization evolved to compensate for lost axons. Indeed, greater synaptic efficacy, as indexed by lower than normal activation magnitude in the contralateral PMd despite improved dexterity after decompressive surgery, is typical of learning-related plasticity in the presence of a reduced number of intact corticospinal axons.5
Ipsilateral M1 and S1 tend to be extensively recruited as the difficulty of a motor task increases in healthy controls.36 Enhanced recruitment of ipsilateral M1 and S1 has been reported in functional imaging studies of patients with multiple sclerosis,26,30 stroke,4,8 peripheral denervation,28 and isolated myelitis.29,31 The contribution of ipsilateral M1 to improved dexterity varied depending on the recovery stage in monkeys with a C4–5 spinal cord lesion affecting the lateral corticospinal pathway.24 Specifically, inactivity in ipsilateral M1 was not associated with a dexterous hand deficit prior to inducing a C4–5 lesion, but was associated with a deficit in the early recovery stage at 1 month after a hemisection, but not at 3 months when finger dexterity was unchanged. This finding provides evidence for a time-dependent role of ipsilateral M1 for the recovery of dexterous function after SCI, possibly via reduced interhemispheric inhibition and the uncrossed descending pathway. We found a progressive increase in ipsilateral M1 activation during the pinch task, along with postsurgery gains in hand function in our patients with CSM, probably due to an increased conductivity in both crossed and uncrossed descending pathways.24 Moreover, the overrecruitment of ipsilateral sensorimotor cortices during nondexterous relative to dexterous movements before surgery, together with the postsurgery progressive increase of dexterity-related activation, supports the notion that functional recovery of nonpermanently damaged corticospinal projections and cessation of further cord damage may enable the recruitment of ipsilateral M1 and S1 when needed for the control of coordinated finger movements.
Contralateral PMd is involved in the recovery of dexterity after stroke and SCI, possibly through corticospinal and corticorubral projections to spinal motor neurons in the midcervical segments.18,24 The significant increase of neuronal activity in contralateral PMd associated with finger pinch between 3 and 6 months after surgery suggests that a postoperative increase in the recruitment of higher-order motor cortex might emerge relatively late and contribute to continuous recovery. Accordingly, nonprimary motor areas such as the PMd, together with the M1 and S1, might be interesting to monitor over a period of clinical observation in the patients who are suspected of having progression of spinal cord compression prior to surgery, to capture evolving cortical adaptations. Certain patterns of cortical sensorimotor response could be compensatory for progressive loss of axons to help maintain hand function, as well as indicate the limitation of neuronal reserve for further functional reorganization and, thus, the need for surgical decompression. The pre- to postsurgery remapping of the center of task-related activation in PMd and S1, which was observed in our patients, may be another one of these cortical adaptive patterns that is worth monitoring.
The SMA and the Cm are involved in preparation and execution of practiced movement sequences and are strongly influenced by attention, performance, and other components of the movement.11,14 Bilateral axonal terminals in the cervical spinal cord have origins in the Cm.9 Studies in primates suggest that the contribution of SMA to movement generation and control occurs indirectly through spinal interneurons in the intermediate zone.3,22 The observed postsurgery decrease of neural activity in the SMA and Cm during nondexterous wrist movement most likely reflects changes in compensatory recruitment, because more efficient direct corticospinal projections from the primary sensorimotor cortices regained conductivity postoperatively.
Longitudinal studies of the dynamic evolution of cortical sensorimotor representational maps and functional gains after stroke and SCI suggest that extensive activation in secondary sensorimotor cortices is related to poor motor performance, whereas a progressive increase in contralateral M1 activation corresponds to improved motor function.19,35 In the current study, for the first time, we have demonstrated direct associations between changes in contralateral cortical sensorimotor activation and the improvements of upper-extremity function following spinal cord decompression in symptomatic CSM. A plausible explanation for the correlation between a postsurgical increase in S1 activity during dexterous movements and behavioral improvement might be the relative preservation of corticospinal axons originating from S1,12,13 because its descending fibers are more posterior and medial than those of M1 and less vulnerable to compression. Several cortical motor areas, including M1, PMd, and SMA, showed changes in wrist extension–related activation correlated with postsurgery behavioral improvements, which suggests that adaptations of corticospinal projections from these areas may play an important role in maintaining and mediating recovery of wrist movement important for reaching and grasping.
In summary, the observed “brain–behavior” associations provide evidence that neuronal plasticity in particular sensorimotor cortices that is provoked by spinal cord decompression plays an important role in driving postoperative functional recovery of the upper extremity. In addition, the association between a postoperative increase in neuronal activity in the contralateral M1 and the degree of focal expansion of the compressed cord segment suggests a close relationship between reversibility of the nonpermanently damaged axons within the descending pathway and pyramidal neuronal activity in M1.
Several limitations are worth mentioning. We examined only the weaker one of the bilaterally affected hands due to the cost of imaging time and the burden on patients in the MR imaging unit. Bilateral and asymmetrical compromise of ascending and descending fibers at the level of spinal cord compression may increase the complexity and variability of movement-related fMR imaging activation patterns. We believe that any differences in cortical representational maps related to the side of the task-performing hand (left or right) had an insignificant effect on longitudinal comparisons, because the fMR imaging data within the selected brain regions were generated from the same task-performing hand across time in the same individual before group analysis. In addition, our ability to monitor carefully each participant’s task performance parameters assured us that the activation tasks were highly reproducible over time.
Because no significant difference in pinch-related cortical activation at submaximal force (25% of maximum) was detected between dominant and nondominant hands in healthy controls, we assumed that any variation in activation was negligible and could be omitted in between-group comparisons. However, it would be optimal to compare cortical sensorimotor activation during dominant and nondominant hand movements separately in a future study with a sample size of at least 15 patients, if the objective was to monitor patients for serial sensori motor remapping associated with progressive cervical cord compromise. Given the small sample size and the ceiling effect of the mJOA score at 6 months postsurgery, the “brain–behavior” associations found in our patients need to be replicated for generalizability in a larger sample of patients with CSM who are prospectively monitored with serial imaging and concordant clinical assessments. A better impairment and functional outcome measure than the mJOA-upper may be the British Medical Research Council Scale of motor strength tested for each root level below the lesion, combined with the Wolf Motor Function Test.37 Advanced imaging techniques such as MR spectroscopy for better characterization of spinal cord damage, as well as finer-tuned assessments of upper-extremity function, may further elucidate the relationships between cerebral adaptations, degree of spinal cord compressive injury, sensorimotor function, and time of progression.
Conclusions
Patients with CSM may compensate for the loss of axonal function through adaptations among the neuronal assemblies representing simple nondexterous and complex dexterous movements of the hand. Our findings of dynamic cortical reorganization and its correlation with postsurgery behavioral improvement may be useful for clinicians to predict short- and long-term behavioral outcomes, as well as to design appropriate rehabilitation approaches to manipulate the neurophysiological reserve of supraspinal centers for maximal sensorimotor recovery. The observed sensorimotor areas of dynamic adaptation may also serve as the focus for future serial fMR imaging studies to monitor cerebral reorganization while corticospinal tracts are gradually compromised in insidiously progressive CSM. An fMR imaging index of cerebral reorganization may then provide useful information beyond clinical examinations and structural scans1 about the physiological reserve of supraspinal structures that are continuously compensating for sensorimotor function until their maximal capacity has been reached. Thus, future studies may aim to examine dynamic cerebral adaptation as an early indicator of the need for surgical decompression as well as the optimal timing.
Acknowledgments
The authors thank Dr. Tom Rockwell for his generous financial support, and Dr. Allan Wu for his help with customizing the DataWizard software for force data analysis.
Disclosure
This study was supported by the Cervical Spine Research Society Award and Stein-Oppenheimer Endowment awarded to Dr. Langston Holly and the Adelson Program in Neural Repair and Rehabilitation of the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. The imaging portion was made possible by the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund and RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR).
Abbreviations used in this paper
- Cm
anterior cingulate motor cortex
- CSM
cervical spondylotic myelopathy
- fMR
functional MR
- FMRIB
Oxford Centre for fMR Imaging of the Brain
- mJOA-upper
modified Japanese Orthopaedic Association score for the upper extremity
- PMd
dorsal premotor area
- ROI
region of interest
- SCI
spinal cord injury
- SMA
supplementary motor area
- S1
primary sensory cortex
- %SC
percentage signal change
- VC
voxel count.
References
- 1.Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17:315–322. doi: 10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 3.Boudrias MH, Belhaj-Saif A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex. 2006;16:632–638. doi: 10.1093/cercor/bhj009. [DOI] [PubMed] [Google Scholar]
- 4.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 5.Dobkin BH. Confounders in rehabilitation trials of task-oriented training lessons from the designs of the EXCITE and SCILT multicenter trials. Neurorehabil Neural Repair. 2007;21:3–13. doi: 10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobkin BH. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Repair. 2008;22:105–110. doi: 10.1177/1545968308315046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobkin BH. Spinal, supraspinal plasticity after incomplete spinal cordinjury correlations between functional magnetic resonance imaging and engaged locomotor networks. Prog Brain Res. 2000;128:99–111. doi: 10.1016/S0079-6123(00)28010-2. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 9.Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neuro-physiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- 12.Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical motor reorganization after paraplegia an EEG study. Neurology. 1999;53:736–743. doi: 10.1212/wnl.53.4.736. [DOI] [PubMed] [Google Scholar]
- 13.Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury an electroencephalographic study. Neurology. 1998;50:1115–1121. doi: 10.1212/wnl.50.4.1115. [DOI] [PubMed] [Google Scholar]
- 14.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe motor areas on the medial surface of the hemisphere. J Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dob-kin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2007;6:544–551. doi: 10.3171/spi.2007.6.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isa T, Ohki Y, Alstermark B, Pettersson LG, Sasaki S. Direct and indirect cortico-motoneuronal pathways and control of hand/arm movements. Physiology (Bethesda) 2007;22:145–152. doi: 10.1152/physiol.00045.2006. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 18.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 19.Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527–538. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 22.Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons an anatomical and electro-physiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- 23.Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Pantano P, Iannetti GD, Caramia F, Mainero C, Di Legge S, Bozzao L, et al. Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain. 2002;125:1607–1615. doi: 10.1093/brain/awf164. [DOI] [PubMed] [Google Scholar]
- 27.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 28.Reddy H, Floyer A, Donaghy M, Matthews PM. Altered cortical activation with finger movement after peripheral denervation: comparison of active and passive tasks. Exp Brain Res. 2001;138:484–491. doi: 10.1007/s002210100732. [DOI] [PubMed] [Google Scholar]
- 29.Rocca MA, Agosta F, Martinelli V, Falini A, Comi G, Filippi M. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. Neuroimage. 2006;30:879–884. doi: 10.1016/j.neuroimage.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Rocca MA, Gavazzi C, Mezzapesa DM, Falini A, Colombo B, Mascalchi M, et al. A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage. 2003;19:1770–1777. doi: 10.1016/s1053-8119(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 31.Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Agosta F, Martinelli V, et al. Cord damage elicits brain functional reorganization after a single episode of myelitis. Neurology. 2003;61:1078–1085. doi: 10.1212/01.wnl.0000086821.49353.40. [DOI] [PubMed] [Google Scholar]
- 32.Roelcke U, Curt A, Otte A, Missimer J, Maguire RP, Dietz V, et al. Influence of spinal cord injury on cerebral sensorimotor systems: a PET study. J Neurol Neurosurg Psychiatry. 1997;62:61–65. doi: 10.1136/jnnp.62.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neuro-physiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Beh-rens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Ward NS. Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol. 2004;17:725–730. doi: 10.1097/00019052-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Wexler BE, Fulbright RK, Lacadie CM, Skudlarski P, Kelz MB, Constable RT, et al. An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging. 1997;15:385–396. doi: 10.1016/s0730-725x(96)00232-9. [DOI] [PubMed] [Google Scholar]
- 37.Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, et al. The EXCITE trial: attributes of the Wolf motor function test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 38.Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the Japanese Orthopaedic Association Scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26:1890–1895. doi: 10.1097/00007632-200109010-00014. [DOI] [PubMed] [Google Scholar]
- 39.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064–1070. 1073. [PubMed] [Google Scholar]