Abstract
We investigated age-related changes in frontal and parietal scalp event-related potential (ERP) activity during bottom-up and top-down attention. Younger and older participants were presented with arrays constructed to induce either automatic “pop-out” (bottom-up) or effortful “search” (top-down) behavior. Reaction times (RTs) increased and accuracy decreased with age, with a greater age-related decline in accuracy for the search than for the pop-out condition. The latency of the P300 elicited by the visual search array was shorter in both conditions in the younger than in the older adults. Pop-out target detection was associated with greater activity at parietal than at prefrontal locations in younger participants and with a more equipotential prefrontal-parietal distribution in older adults. Search target detection was associated with greater activity at prefrontal than at parietal locations in older relative to younger participants. Thus, aging was associated with a more prefrontal P300 scalp distribution during the control of bottom-up and top-down attention. Early latency extrastriate potentials were enhanced and N2-posterior-contralateral (N2pc) was reduced in the older group, supporting the idea that the frontal enhancements may be due to a compensation for disinhibition and distraction in the older adults. Taken together these findings provide evidence that younger and older adults recruit different frontal-parietal networks during top-down and bottom-up attention, with older adults increasing their recruitment of a more frontally distributed network in both of these types of attention. This work is in accord with previous neuroimaging findings suggesting that older adults recruit more frontal activity in the service of a variety of tasks than younger adults.
Keywords: Aging, Event-related potentials (ERPs), Visual pop-out, Visual search, Control of attention, P300, N2pc
1. Introduction
Visual attention is needed to deal with the brain’s limited processing capacity in the face of the abundant amount of visual information in the environment. Visual attention is controlled by both top-down cognitive and bottom-up sensory factors, which have been investigated in a variety of functional imaging, neurophysiological, and neuropsychological studies (Corbetta and Shulman, 2002; Knudsen, 2007). These studies provide compelling evidence that frontoparietal networks play important roles in both types of attention control (Bledowski et al., 2004a, 2004b; Giesbrecht et al., 2003; Husain and Nachev, 2007; Kastner and Ungerleider, 2000; Mesulam, 1998; Sarter et al., 2001). Recent work in monkey (Buschman and Miller, 2007) and human (Li et al., 2010) electrophysiology suggests that the frontal and parietal cortices are differentially engaged by bottom-up and top-down control of visual search, with frontal cortex more involved in top-down search processes and parietal cortex more involved in bottom-up search processes.
Normal aging is associated with changes in neural structure (gray and white matter volumes; Gordon et al., 2008; Raz et al., 2005) and function (including both hyper- and hypoactivation; Grady, 2008; Kramer et al., 2006) and declines in the performance of a variety of cognitive functions, with especially prominent effects observed in the control of attention (Fabiani and Gratton, in press; Fabiani, 2012). Older adults often perform more poorly than younger adults in tasks involving both bottom-up, involuntary attention and top-down, voluntary attention, with a more prominent decline in tasks emphasizing top-down attention control (Greenwood and Parasuraman, 1999; Greenwood et al., 1993, 1997; Kok, 2000; Lien et al., 2011; Madden, 2007; Madden et al., 2005).
1.1. Age-related fronto-parietal activation
There is substantial neuroimaging evidence for shifts in the brain networks used by older as compared with younger adults across a variety of tasks, including tasks involving visual attention (Anderson et al., 2000; Cabeza et al., 2004; Lorenzo-López et al., 2008; Madden et al., 2004, 2005; McIntosh et al., 1999). A recent study examined the neural correlates of top-down visual search in aging in a combined functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) study. In this study, older adults showed increased magnitude and spread of activity in frontoparietal regions compared with younger adults (Madden et al., 2007). In older adults, this frontoparietal activity was related to increased performance on the task, whereas for younger adults increased activity in fusiform regions was positively related to task performance. This suggests that the increased frontoparietal activation in older adults during top-down visual search may represent either (1) compensation for a decline in overall bottom-up sensory input (dedifferentiation, Madden et al., 2007; see review Reuter-Lorenz and Park, 2010), or (2) an increase in neural noise/decrease in the differentiation of top-down goal-directed representations resulting in the release from inhibition of sensory areas (Fabiani et al., 2006; Gazzaley et al., 2005, 2008; Miller et al., 2011).
This fits with evidence from other aging studies of attention reporting increased activity in frontal regions associated with improved performance in older adults (Grady et al., 2002; Lorenzo-López et al., 2008; Madden et al., 2004; McIntosh et al., 1999; Vallesi et al., 2010). However, activity decreases in frontal cortex (Anderson et al., 2000; Johnson et al., 2004; Milham et al., 2002) or activity increases in frontal cortex associated with decreased performance have also been reported in certain tasks (Fabiani and Friedman, 1995; Madden et al., 2005). The “Compensation Related Utilization of Neural Circuits Hypothesis” (CRUNCH) represents 1 framework within which to interpret these contradictory results, whereby older adults use more or new neural circuits to accomplish the same tasks as young adults in an effort to compensate for neural decline in various brain systems (Reuter-Lorenz and Cappell, 2008; Reuter-Lorenz and Lustig, 2005). In visual attention older adults may rely more on control processes mediated by the frontal cortex to compensate for the loss of efficient sensory, or bottom-up, processing compared with young adults. However, this increased reliance on frontal cortex is some-what counterintuitive, given findings of increased structural and functional deterioration in these areas (Raz et al., 2005; West, 1996). Alternative interpretations also include less efficient allocation of attentional resources (Lorenzo-López et al., 2008) or a reduction in inhibitory control functions in attention in older adults (Andrés et al., 2006; Colcombe et al., 2003; Fabiani, 2012; Hasher et al., 2008; Madden and Whiting, 2003).
1.2. The P300 component
Electroencephalographic (EEG) recordings provide a way to examine bottom-up and top-down attention with high temporal resolution. A P300 (or P3b) event-related potential (ERP) with a latency of 300–600 ms from target onset, is elicited during successful target detection (Li et al., 2010; Picton, 1992; Polich, 2003; Polich and Criado, 2006). The P300 is sensitive to aging effects in visual detection tasks (Kutas et al., 1994; Lorenzo-López et al., 2008). P300 scalp distributions are typically parietally maximal in young adults (Fabiani et al., 2007; Polich, 2007), but intracranial recordings (Baudena et al., 1995; Halgren et al., 1995a,1995b), functional imaging studies (Clark et al., 2000; Linden et al., 1999; McCarthy et al., 1997), and studies with lesion patients (Knight, 1997) demonstrate that multiple neural sources, including the prefrontal cortex, temporal-parietal junction, lateral parietal cortex, and anterior cingulate contribute to P300 generation. Across a variety of tasks the P300 has been demonstrated to be smaller, have a longer latency, and a more frontal scalp distribution in older relative to younger adults (reviewed by Friedman, 2008; Polich, 2007). These shifts in distribution have been interpreted as indicative of age-related changes in the utilization of frontal lobe function (Fabiani and Friedman, 1995; Friedman et al., 1997; Nielsen-Bohlman and Knight, 1995; Yamaguchi and Knight, 1991). A recent study used sLORETA standardized low resolution brain electromagnetic tomography to examine the neural sources contributing to the age-related changes in the scalp distribution of the P300 in a top-down visual search task (Lorenzo-López et al., 2008). They found differences in the neural sources that contributed to the P300 between younger and older subjects. In older adults, the P300 was generated most prominently by bilateral activation of prefrontal cortex across a range of conditions, with individual conditions less differentiated from one another. Furthermore, older adults showed weakened contributions from occipitotemporal areas (Lorenzo-López et al., 2008). These results suggest, consistent with the neuroimaging findings, that in aging top-down visual search is associated with increased involvement on frontal regions in the presence of less reliable signals from more posterior, sensory regions.
There is, however, relatively little evidence directly investigating aging-related network shifts in bottom-up attention. Some research suggests that there may be age-related increases in the capture of involuntary attention by irrelevant distracters, which has been interpreted as a failure of the frontal cortex to properly engage in inhibitory processes (Andrés et al., 2006; Lien et al., 2011). However, little research has investigated the neural underpinnings of these changes (however, see Kok, 2000 for a review of findings of involuntary attention and ERP amplitude changes, including decreases in P300 amplitude with aging in involuntary attention tasks).
1.3. Experiment outline
In both the neuroimaging and EEG literature, brain networks involved in bottom-up and top-down attention during visual search are often examined in separate experiments, and there is a paucity of research directly comparing these networks in older individuals. Past evidence in young individuals suggests that these 2 types of attention are associated with different control networks (Corbetta and Shulman, 2002), with frontal regions contributing more to top-down attention and parietal regions contributing more to bottom-up attention (Li et al., 2010). In this study, we aimed to determine whether these same network dissociations are present in older subjects as well. We used a paradigm based on a study by Buschman and Miller (2007) to investigate top-down and bottom-up control in younger adults. In the task, a target appeared among 3 distracters in separate “pop-out” and “search” conditions. In the pop-out condition, the distracters differed from the target in both color and orientation, such that the target drew attention automatically and search was influenced primarily by bottom-up processes. In the search condition, the target differed from distracters only in orientation, requiring a more effortful serial search process controlled primarily by top-down attention. The purpose of the present study was to examine the effects of aging in target detection during visual search and simultaneously compare the contributions of frontal and parietal regions during top-down and bottom-up attention. We hypothesized that, although the two different conditions elicit differential parietal versus frontal engagement in young adults, both conditions will show a shift toward frontal activation in older adults, as indexed by changes in the scalp distribution of the P300 ERP.
2. Methods
2.1. Subjects
Fourteen younger and 14 older subjects participated in the study for monetary compensation. One younger and 1 older subject were excluded from analyses because of excessive blinks (fewer than 3% of trials were left), leaving reliable recordings from 13 young subjects (6 females, mean age = 23.9 ± 4.3; range, 18 to 35 years old) and 13 older subjects (6 females, mean age = 63.1 ± 6.2; range, 52 to 75 years old). The mean numbers of years of education for the younger and older subjects were 15.9 ± 2.5 and 16.0 ± 2.2 respectively. All the subjects were right-handed, had normal color vision, and had no history of neurological problems. None of the subjects were taking any psychotropic, neurological, or psychiatric medications at the time of testing. Informed written consent was obtained from all subjects prior to being tested. The study was approved by the Committee for the Protection of Human Subjects for the University of California, Berkeley.
2.2. Stimuli and procedure
A 500-ms fixation cross signaled the start of each trial. After the cross, a target triangle (with a specific color and orientation) appeared in the center of the screen for 1000 ms and was followed by a short 500-ms delay screen with a fixation cross. After the delay, a 4-stimulus array was presented, consisting of the target and 3 distracter items. The 4 stimuli were divided such that one appeared in each of the quadrants of the screen and the target was randomly presented in one of these locations. Participants were required to find the target and indicate whether it was on the left or the right side of the screen, irrespective of the vertical position. The stimuli remained on the screen until a response was selected and were followed by a 1000-ms fixation cross to signal the end of the trial. Subjects sat in an experimental booth 110 cm from a 21-inch computer screen.
Experimental stimuli were made up of acute isosceles triangles, each with a particular color (red or green) and orientation (1 of 8, [i−1] × 45°, i = 1, 2, 3, 4, 5, 6, 7, 8). Each color-orientation combination was used, for a total of 16 possible triangles. The triangles had 2 equal sides 6.5 cm in length and a third side 5.5 cm long, with an area of 16.20 cm2. The position of triangles in the 4 quadrants (upper-left, lower-left, upper-right, and lower-right) did not change across the course of the experiment. The center of the triangle was 6.2 cm vertical (either up or down) from the horizontal midline and 8.2 cm lateral (either right or left) from the vertical midline, resulting in stimuli at a visual angle of 5.34 degrees from the fixation cross.
An example of the two conditions can be seen in Fig. 1 (Li et al., 2010). Distractor triangles were chosen to create the two main attention conditions in the experiment: “pop-out” and “search.” The bottom-up, pop-out, condition was created using distracters that differed from the target in both color and orientation (Treisman and Gelade, 1980). The top-down, search condition was created by using distracters that differed from the target only in orientation. Half of the trials in the experiment were in the pop-out condition and half were in the search condition (each trial included a target). Half of the targets were presented in left visual field and half were in right visual field. Subjects were asked to maintain fixation on the central cross throughout the experimental trial and to indicate as quickly and as accurately as possible whether the target was to the left or right of fixation. Participants used their right hand for responding and selected either 1 for left or 2 for right from a computer key pad.
Fig. 1.
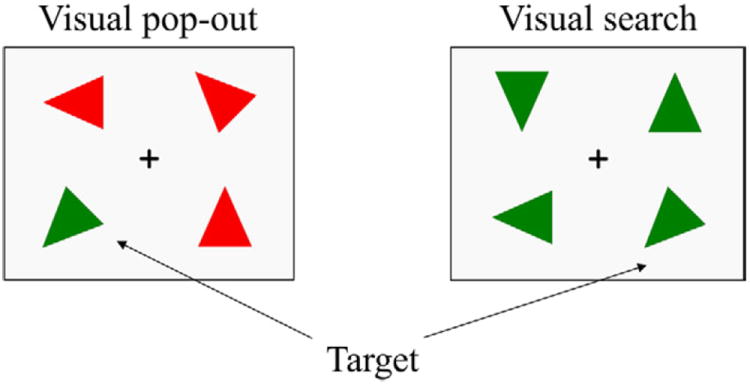
An example of the stimulus array in the 2 experimental conditions. Each trial includes a target. In the left panel, the distracters differ from the target in both color and orientation, so that target detection is highly efficient and easy. In this pop-out condition, search is influenced primarily by the bottom-up attention. In the right panel, the distracters differ from the target only in orientation, so that target detection is less efficient and difficult. In this search condition, target detection is controlled mainly by top-down attention.
Participants performed 2 practice blocks before starting the experiment. Practice blocks were exactly like experimental blocks, except that the first practice included feedback. Extra practice blocks were given as required until subjects were able to reach a mean accuracy of 80% in the task. After the practice, participants completed 12 experimental blocks comprising 32 trials each, with each block lasting about 2.5 minutes. Participants were given 1–2 minute breaks between blocks, with longer breaks every 3 blocks. E-prime 1.1 (Psychology Software Tools, Pittsburgh, PA, USA) was used to present the stimuli and analyze the behavioral results.
2.3. EEG recording
An ActiveTwo system (Biosemi, The Netherlands) with a 64-channel electrode cap was used to record EEG. In addition, 6 other electrodes (right and left earlobes and 4 electro-oculogram [EOG]) were simultaneously recorded. Channels were off-line referenced to the average of the right and left earlobes. EOG was measured from an electrode above and below the right eye to record vertical eye movements and electrodes on the outer canthus of each eye to measure horizontal eye movements. All channels were amplified with an analog bandpass filter of 0.06–208 Hz and were digitized at 1024 Hz.
2.4. Data analysis
Matlab 7.2 (The MathWorks, Inc., Natick, Ma, USA) was used for all data processing and ERP analysis. Data processing began by off-line rereferencing of the raw signals to the averaged earlobes. This was followed by filtering from 0.5 to 55 Hz with a two-way FIR bandpass filter (eegfilt.m from EEGLAB toolbox, Delorme and Makeig, 2004) and segmenting from 200 ms before the onset of the stimulus (visual array) to 1000 ms after the stimulus. Trials were rejected if they had an incorrect response or lacked a button press between 200 and 1500 ms (younger adults) or 200–2000 ms (older adults) after the onset of the stimulus array.
EOG artifacts were excluded in two steps. First, epochs were removed if there was a difference in amplitude between the two vertical EOG of greater than 100 μV. Second, epochs were removed if there was a difference in amplitude of the two horizontal EOG of greater than 100 μV or 3 standard deviations from the mean of the EOG difference wave. Linear drift was removed from each epoch and the data were then baseline corrected using the 200 ms prestimulus onset period. Finally, any epoch with a channel containing amplitudes of more than 4 standard deviations from the epoch mean was rejected. Following these steps, 1017 trials remained for pop-out targets and 903 trials remained for visual search targets in the young participants, and 819 trials remained for pop-out targets and 676 trials remained for visual search targets for the older participants, which were used for further ERP analysis. For each subject, at least 25 trials were included in the average for each condition.
Single epochs for the pop-out and search conditions were averaged to the onset of the visual search array. The largest positive point within the 300–600 ms time window was labeled as the P300 peak. P300 peak amplitudes and latencies were measured in both groups in both attention conditions at 4 medial electrodes (prefrontal [Fpz], frontal [Fz], central [Cz], and parietal [Pz]). In addition, N1 peak amplitudes and latencies were measured during the 50–200 ms time window at the PO7 and PO8 electrodes in order to compare early perceptual processing in younger and older participants. Normalized amplitudes across all 64 electrodes were plotted in topographical maps for each condition at the latencies of the peak N1 and P300 components for comparison of scalp distributions (Li et al., 2010). P300 amplitudes and latencies were assessed using a 2-way analysis of variance (ANOVA) with condition (pop-out and search) and age (young and old) as factors. A 3-way ANOVA was performed with electrode (Fz and Pz, as well as Fpz and Pz), condition (pop-out and search) and age (young and old) as factors. A linear regression analysis was performed to compare the relationship between reaction times and P300 latencies and amplitudes at the Fpz, Fz, Cz, and Pz electrodes.
The N2-posterior-contralateral (N2pc) was measured by the difference between the contralateral and ipsilateral ERP waveforms at lateral parietal-occipital electrodes (PO7 and PO8). The contralateral waveform was the mean of the ERPs at PO7 for right visual field targets and that at PO8 for left visual field targets. In contrast, the ipsilateral waveform was the mean of the ERPs at PO8 for right visual field targets and that at PO7 for left visual field targets. The difference waveforms for each subject were then filtered with a low-pass filter at 10 Hz to reduce high frequencies noise. The amplitude of the N2pc was the mean amplitude of this difference waveform during a 200–275 ms time window for the pop-out condition and a 350–450 ms time window for the search condition. The N2pc amplitudes were analyzed with a repeated measure ANOVA with condition (pop-out and search) as the within-subject factor and age group (young and old) as the between-subject factor.
3. Results
3.1. Behavioral results
Mean reaction times (RTs) and accuracy rates are summarized in Table 1 (mean ± standard deviation [SD] for this and all following results). There was a main effect of age (young and old) and condition (pop-out and search) on mean RTs [age effect: F(1,24) = 70.74; p < 0.001; condition effect: F(1,24) = 130.03; p < 0.001, ANOVA], with slower RTs in the older subjects and in the search condition. However, there was no significant interaction in RTs between age and condition. Fig. 2 illustrates the accuracy rates for both conditions and groups. A main effect of age and condition was also observed on accuracy rates [age effect: F(1,24) = 24.42; p < 0.001; condition effect: F(1,24) = 89.42; p < 0.001], with higher accuracy overall in the younger group and in the pop-out condition. There was a significant interaction in accuracy between age and condition [F(1,24) = 15.20; p < 0.001], showing an increased decline in accuracy for the older compared with the younger subjects in the search condition compared with the pop-out condition.
Table 1.
Behavioral results
| Condition | RT
|
Accuracy (%)
|
||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Visual pop-out | 481.94 ± 99.41 | 691.40 ± 96.13 | 99.12 ± 0.54 | 97.96 ± 1.34 |
| Visual search | 772.18 ± 115.09 | 1016.81 ± 74.31 | 92.94 ± 4.07 | 83.10 ± 6.76 |
Data are given as mean ± SD. Mean reaction times (RTs; ms) and accurate rates (%) and their corresponding standard deviations as a function of condition in younger and older subjects.
Fig. 2.
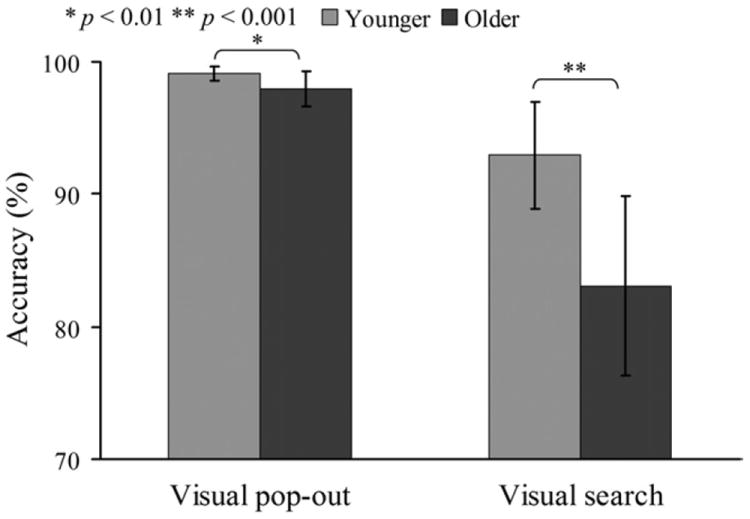
Accuracy rates for visual pop-out and search targets in younger and older subjects. There was significant interaction on accuracy rates between age and condition. A stronger decline in accuracy was observed in the search condition than pop-out condition in older subjects [search: F(1,24) = 20.19; p < 0.001; pop-out: F(1,24) = 8.46; p < 0.01, 1-way analysis of variance (ANOVA)].
3.2. ERP results
3.2.1. P300 component
Fig. 3 presents the grand-average ERPs at the Fpz, Fz, Cz, and Pz electrodes for the pop-out and search conditions in younger and older participants. Table 2 summarizes the P300 latency and amplitude measurements from waveforms shown in Fig. 3. As can be seen in Table 2 and Fig. 3, P300 latency was prolonged in older compared with younger adults [age effect: Fpz: F(1,24) = 27.06; p < 0.001; Fz: F(1,24) = 44.02; p < 0.001; Cz: F(1,24) = 22.66; p < 0.001; Pz: F(1,24) = 23.59; p < 0.001, 2-way ANOVA]. There was a main effect of condition for P300 latency at the Fpz [F(1,24) = 4.36; p = 0.042] and Fz [F(1,24) = 8.37; p = 0.005] electrodes. In younger participants, latency in the pop-out condition was significantly shorter than in the search condition at electrode Fz [paired t tests, t(12) = 3.44; p = 0.005], but no such effect was present in the elderly group [t(12) = 1.97; p = 0.07]. There was no overall interaction between condition and age for P300 latency.
Fig. 3.
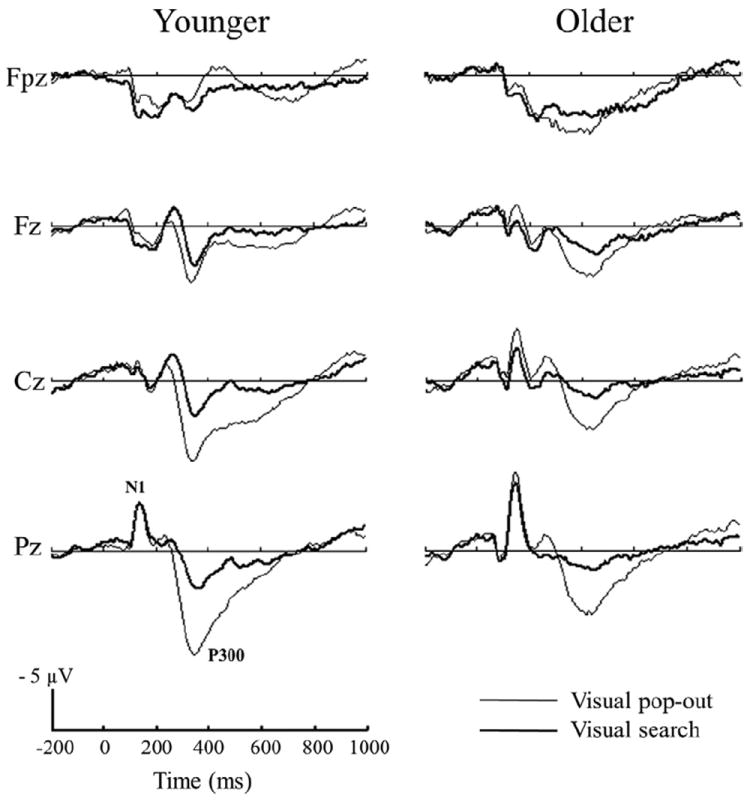
Grand average event-related potentials (ERPs) elicited by visual pop-out and search targets in the young and elderly subjects at Fpz, Fz, Cz, and Pz electrode sites.
Table 2.
P300 latency and amplitude
| Condition | P300 latency
|
P300 amplitude
|
||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Fpz | ||||
| op-out | 320.06 ± 26.05 | 386.07 ± 47.94 | 4.58 ± 4.03 | 9.53 ± 4.65 |
| Search | 341.89 ± 28.72 | 446.18 ± 86.92 | 5.66 ± 4.03 | 7.85 ± 3.16 |
| Fz | ||||
| Pop-out | 337.11 ± 24.18 | 405.77 ± 35.38 | 8.21 ± 3.82 | 7.93 ± 4.20 |
| Search | 360.47 ± 19.16 | 452.42 ± 73.58 | 6.05 ± 3.99 | 5.31 ± 1.92 |
| Cz | ||||
| Pop-out | 348.83 ± 26.65 | 419.60 ± 42.17 | 11.30 ± 4.19 | 7.41 ± 5.51 |
| Search | 369.62 ± 30.22 | 416.36 ± 67.24 | 5.95 ± 4.19 | 3.96 ± 2.41 |
| Pz | ||||
| Pop-out | 356.94 ± 20.39 | 430.33 ± 50.77 | 14.44 ± 4.97 | 9.52 ± 5.93 |
| Search | 369.86 ± 24.92 | 415.38 ± 64.64 | 6.83 ± 3.83 | 4.32 ± 3.59 |
Data are given as mean ± SD. P300 latency (ms) and amplitude (μV) and their corresponding standard deviations as a function of condition in younger and older subjects.
A 2-way ANOVA revealed a significant main effect of condition and age in the peak amplitude of the P300 waveform at the Cz [age effect: F(1,24) = 6.32; p = 0.015; condition effect: F(1,24) = 14.12; p < 0.001] and Pz electrodes [age effect: F(1,24) = 8.37; p = 0.005; condition effect: F(1,24) = 24.68; p < 0.001], and condition [F(1,24) = 5.73; p = 0.02] but not age [F(1,24) = 0.26; p = 0.61] effect at the Fz electrode, and an age [F(1,24) = 10.37; p = 0.002] but not a condition effect [F(1,24) = 0.07; p = 0.79] at the Fpz electrode. In younger adults, the average peak P300 amplitude in the pop-out condition increased from anterior to posterior electrodes (see Table 2). P300 amplitude was significantly smaller for older compared with younger adults at central and posterior electrodes, but larger for older than younger adults at the prefrontal electrode Fpz. There was no overall interaction between condition and age for P300 amplitude.
A 3-way ANOVA was performed on P300 amplitude with electrode location (Fpz and Pz), condition (pop-out and search) and age (younger and older) as factors. There was a main effect of electrode location [F(2,48) = 4.80; p = 0.03], and condition [F(2,48) = 15.41; p < 0.001]. A significant interaction between electrode location and age [F(2,48) = 18.20; p < 0.001] indicated that the P300 amplitude distribution across prefrontal and parietal locations differed between the younger (Fpz: 5.13 ± 3.98 μV; Pz: 10.63 ± 5.83 μV) and older (Fpz: 8.69 ± 3.99 μV; Pz: 6.92 ± 5.50 μV) groups. In younger adults, P300 amplitudes over the parietal electrode were larger than over the prefrontal electrode in both conditions [F(1,24) = 14.30; p = 0.001 between Pz and Fpz, Bonferroni corrected], but no such effect was present in the older adults [F(1,24) = 1.28; p = 0.27 between Pz and Fpz, Bonferroni corrected]. There was a significant interaction between electrode location and condition [F(2,48) = 12.78; p = 0.001], with pop-out having higher amplitudes for both groups over the parietal electrode than over the prefrontal electrode [F(1,48) = 12.88; p = 0.001 between Pz and Fpz, Bonferroni corrected] in the pop-out condition, but not in the search condition [F(1,48) = 1.35; p= 0.25 between Pz and Fpz, Bonferroni corrected].
Fig. 4A shows the topography of the peak P300 across 64 electrodes. There was a smaller peak amplitude at the parietal location in the older compared with the younger group in both conditions. Pop-out target detection generated larger activity at parietal locations than at frontal locations in both groups, as can be seen in the top row of Fig. 4A. Search target detection generated different distributions in both younger and older participants, with a frontocentral distribution in the younger group and a frontal distribution in the older group, as can be seen in the bottom row of Fig. 4A. In addition, P300 amplitude across Fpz and Pz revealed not only a main effect of electrode [pop-out condition: F(1,24) = 12.88; p = 0.001], but also an interaction between electrode and age in each condition [pop-out condition: F(1,24) = 12.93; p = 0.001; search condition: F(1,24) = 5.35; p = 0.025]. Younger adults had larger amplitudes at parietal compared with prefrontal locations in the pop-out condition, while older adults did not. However, older adults showed larger amplitudes at prefrontal locations relative to parietal locations in the search condition, while younger adults did not.
Fig. 4.
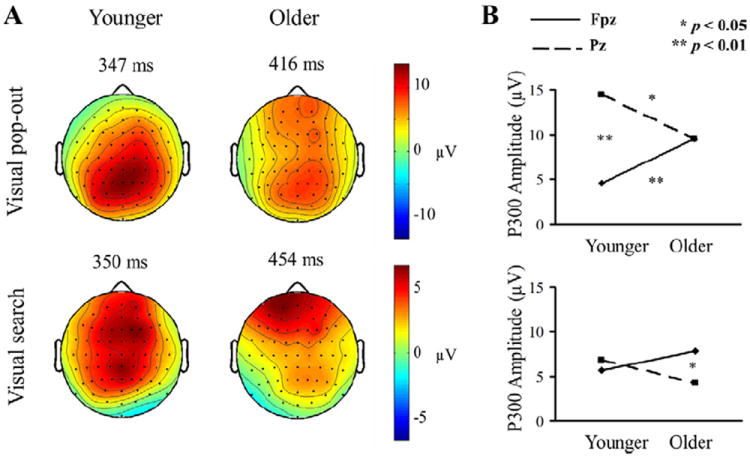
(A) The distribution of the P300 effect across all 64 channels for the pop-out (upper) and search (bottom) condition in young (left) and elderly subjects (right), at the latency of the peak amplitude of the component. There was no interaction effect in pop-out targets, but there was interaction effect in search condition between electrode location and age. The pop-out P300 was more parietally distributed for both groups, whereas the search P300 in young group was more medial central-frontally distributed and the search P300 in elderly group was more frontally distributed. (B) P300 amplitudes of pop-out (upper) and search (bottom) conditions in both groups in prefrontal and parietal regions. There was interaction between age and location in visual pop-out and search conditions. Parietal activation evoked by pop-out targets was larger than prefrontal activation for younger adults, and prefrontal activation evoked by search targets was larger than parietal activation for older adults. Prefrontal activation evoked by pop-out targets was smaller for younger than for older adults and parietal activation evoked by pop-out targets was larger for younger than for older adults. Search targets evoked similar parietal and frontal activation in both groups.
Fig. 4B shows the P300 amplitudes for the pop-out (top) and search (bottom) conditions for both groups in prefrontal and parietal regions. Parietal activation evoked by pop-out targets was larger than prefrontal activation for younger adults [F(1,24) = 30.89; p < 0.001], and prefrontal activation evoked by search targets was larger than parietal activation for older adults [F(1,24) = 7.10; p = 0.01]. Fig. 4B also shows that at the prefrontal electrode (Fpz), pop-out targets evoked greater activation in older than younger adults in the pop-out condition [pop-out condition: F(1,24) = 8.44; p = 0.008, search condition: F(1,24) = 2.39; p = 0.14]. Search targets evoked similar parietal activation for both groups [F(1,24) = 2.98; p= 0.10], but parietal activation evoked by pop-out targets was larger for younger than older adults [F(1,24) = 5.22; p = 0.03].
Fig. 5 illustrates the presence of significant positive linear relationship within the older adults between P300 latency and RT in the visual pop-out condition at the Fz electrode [Pearson correlation coefficient (r) = 0.609; p = 0.027]. A multiple regression was used to test whether a quadratic relationship was also contributing to the variance, but it was not significant [r = 0.610; p= 0.098]. For the younger adults, the correlation between P300 latency and RT at the Fz electrode was not significant (r = 0.309; p = 0.304) in the pop-out condition. All correlations in the visual search condition at the Fz electrode were also not significant (p > 0.05).
Fig. 5.
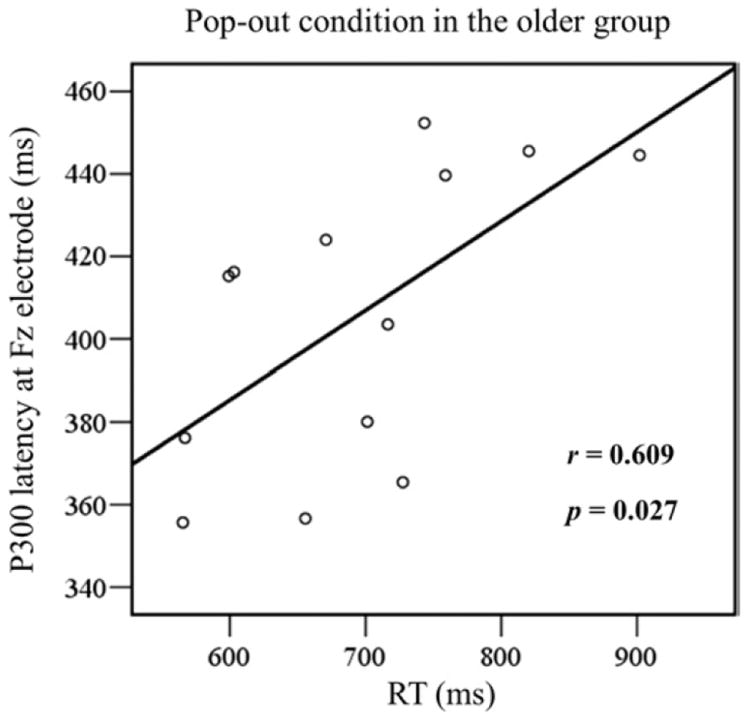
Linear regression between mean reaction time (RT) and P300 latency for the pop-out condition in frontal area (Fz) in the older group. Each subject is marked as a circle, with 13 circles in total. Pearson correlation coefficient (r = 0.609) was significant (p = 0.027), indicating a positive linear correlation between RT and P300 latency in this condition.
3.2.2. N1 component
The peak amplitudes of the N1 (50–200 ms) and N2 (200–400 ms) components were analyzed by a 1-way ANOVA in younger and older subjects separately (see Fig. 3). There was a main effect of age for the N1 peak amplitude at the Pz electrode [F(1,24) = 5.72; p = 0.02], with lower amplitudes in the younger group (pop-out: −7.45 ± 3.99 μV, search: −7.69 ± 4.33 μV) than in the older group (pop-out: −11.31 ± 5.66 μV, search: −10.03 ± 4.54 μV) (see Fig. 3). There were no significant difference in N1 and N2 amplitudes at the Fpz, Fz, Cz, and Pz electrodes between the pop-out and search conditions in either group (p > 0.05).
Fig. 6A shows the grand-average waveforms at the PO8 electrode, superimposed for each group and condition; Fig. 6B shows the scalp topographies within the N1 time window for both groups and conditions. To analyze the difference in N1 peak amplitude and latency at lateral occipital sites, a 3-way ANOVA was performed with electrode (PO7 and PO8), condition (pop-out and search), and group (young and old) as factors. There was a main effect of electrode on the N1 peak amplitude [F(2,48) = 7.59; p = 0.007]. Mean N1 peak amplitude at the PO7 electrode (young: pop-out: −11.37 ± 4.48 μV, search: −11.96 ± 4.26 μV; older pop-out: −11.49 ± 6.90 μV, search: −11.33 ± 5.97 μV) was smaller than that of the PO8 electrode (young: pop-out: −16.32 ± 6.44 μV, search: −16.91 ± 5.61 μV; old: pop-out: −13.45 ± 8.99 μV, search: −13.89 ± 8.66 μV) in both groups. However, there was no other significant main effect of condition, electrode, and group, or interactions among electrode, group and condition in amplitude or latency. The topographic maps show a robust occipital N1 component for both conditions and groups, with the distribution skewed toward the right hemisphere.
Fig. 6.
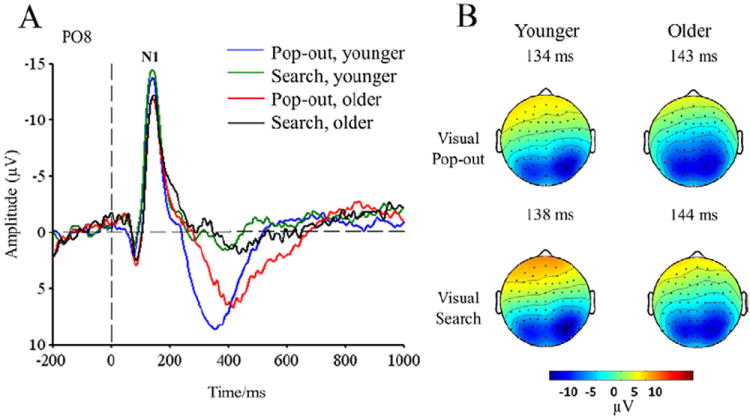
(A) N1 event-related potentials (ERPs) in the PO8 electrode for both groups and conditions. (B) Scalp topographies of the peak amplitude over the N1 time-window.
3.2.3. N2pc component
The N2pc waveforms are illustrated in Fig. 7. For the pop-out condition, the N2pc during the 200–275 ms time window was smaller in the older (−12.35 ± 1.99 μV) than in the younger group (−21.74 ± 2.61 μV) [F(1,24) = 8.1; p = 0.009]. For the search condition there was also a smaller N2pc amplitude during the 350–450 ms time window in the older (−1.72 ± 0.61 μV) than in the younger group (−5.00 ± 0.46 μV) [F(1,24) = 18.15; p = 0.0003]. When we enlarged the time window from 200–275 ms to 200–400 ms for the pop-out condition, younger subjects still had a significantly larger N2pc. Similarly, when we expanded the time window from 350–450 ms to 300–500 ms for the search condition, younger subjects had a larger N2pc. Finally, although we used different time windows for each condition, it was clear that the N2pc was smaller in the search condition than in the pop-out condition for both the younger [F(1,24) = 39.95; p < 0.00001] and older adults [F(1,24) = 25.87; p < 0.0001].
Fig. 7.
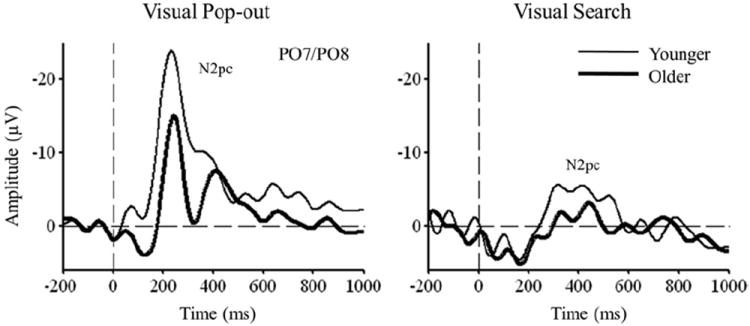
N2pc event-related potentials (ERPs) in the PO7/PO8 electrodes for both groups and conditions.
4. Discussion
We manipulated the context of target detection in a visual search array to induce either top-down or bottom-up attention in a single experiment. Aging had prominent effects on both behavioral and electrophysiological measures under these two types of attention. Specifically, aging led to slowed performance and decreased accuracy, especially for the top-down condition, and older adults showed a frontal shift in the topography of target detection (as measured by the P300) in both top-down and bottom-up attention. In addition, the N100 ERP was larger in older subjects. This supports the idea that frontal increases in aging are not due to a loss of sensory flow, but may be due to a disinhibition of sensory information and increased distractibility (Fabiani, 2012; Kok and Talsma, 1999; Scheibel and Scheibel, 1975; Woodruff, 1982). In support of this, the N2pc ERP was reduced in older subjects in both conditions, indicating that the attention shift indexed by the N2pc was reduced with age (Lorenzo-López et al., 2008).
4.1. Behavioral responses
Older adults were slower than younger adults in both conditions but the relative changes in RTs across tasks were comparable for both groups. Although a decline in accuracy in the older subjects was also observed in both conditions, a greater age-related decline was present in the search condition (Fig. 2). Age-related delays in RTs and reductions in accuracy rates for both types of target detection are in line with previous visual search studies showing declines in the performance levels of older relative to younger participants. We found that age-related reductions in accuracy in the search condition were greater than those in the pop-out condition, suggesting additional attentional demands for older subjects in top-down attention tasks. This finding is consistent with research indicating that older adults often have substantially more difficulty than younger adults in target detection with increasing complexity (Greenwood and Parasuramen, 1999; Greenwood et al., 1993, 1997; Hommel et al., 2004; Madden and Whiting, 2003; Madden et al., 2007).
4.2. P300 latency and amplitude
In both conditions, the latency of the P300 was longer in the older participants, in accord with their longer reaction times, supporting the idea that there is a slowing of cognitive performance with advancing age (Salthouse, 1996). P300 latency over frontal regions in the pop-out condition was shorter than in the search condition for the younger group, but no such effect was present in the older group. However, P300 latency over frontal electrodes in the pop-out condition showed a significant positive linear correlation with reaction time in the older group (Fig. 5), whereas no such effect was present in the younger participants.
Aging was associated with a reduced P300 amplitude over parietal regions in the pop-out condition, which is in accord with numerous previous studies that have reported that parietal P300 responses decrease in amplitude with normal aging (Fabiani and Friedman, 1995; Friedman, 2008; Lorenzo-López et al., 2008; Pfefferbaum and Ford, 1988; Polich, 1997). Because the P300 component has often been considered to index the attention and working memory capacity (Kok, 1997), a decrease in P300 amplitude with age suggests a possible decline in the attentional resources of older subjects in the visual pop-out condition. In contrast, aging did not affect neural activity over parietal regions in the visual search condition, which at these regions had similar activity for both groups. These findings suggest that changes in the timing and magnitude of target detection signals, especially in the pop-out condition, index shifts in the mechanisms that older adults use to accomplish visual search tasks.
4.3. P300 frontoparietal distribution
The distribution of P300 amplitude (Fig. 4) showed that there was an interaction between location (prefrontal and parietal regions) and age group (younger and older) in both bottom-up and top-down attention. Studies of age-related changes in P300 amplitude distribution across the scalp have consistently reported that the P300 becomes more anteriorly oriented, and more uniformly distributed over the scalp (Fabiani et al., 1998; Fjell and Walhovd, 2001; Friedman, 2008). In the pop-out condition, the distribution of the P300 shifts toward prefrontal sites with age, which suggests that different neural network mechanisms modulate the scalp distribution of the P300 in older and younger subjects during bottom-up attention. It has been suggested that the prefrontal shift of the P300 complex in aging may be related to structural age-related phenomenon, such as the thickness of the cerebral cortex or the degree of myelinization (Fjell et al., 2005), and that increased use of prefrontal regions may be a compensatory functional mechanism as described in the CRUNCH (Reuter-Lorenz and Cappell, 2008; Reuter-Lorenz and Lustig, 2005). Another hypothesis suggests that increased activation of frontal regions may be a signature of age-related decline in the ability to properly inhibit activity (Greenwood and Parasuraman, 1999; Kok, 2000; Madden, 2007; Madden et al., 2005). Our behavioral results together with our N1, N2pc, and P300 ERP results favor aspects of both ideas revealing prefrontal compensation for inhibitory dysfunction with aging.
Similarly, in the search task the P300 had a more prefrontal scalp distribution in older adults (with a frontocentral distribution in the younger group and a more frontopolar distribution in the older group). Again this suggests that, as with bottom-up attention, top-down attention in older adults is subserved by different neural mechanisms than in younger adults (Greenwood and Parasuraman, 1999; Greenwood et al., 1993, 1997; Kok, 1997, 2000; Madden, 2007; Madden et al., 2005).
Previous findings from lesion studies have consistently identified contributions of both parietal and frontal cortices to the target detection P300 network (Knight, 1997; Knight et al., 1989a, 1989b; Linden et al., 1999; McCarthy et al., 1997; Mulert et al., 2004; Verleger et al., 1994; Yamaguchi and Knight, 1991). Consistent with this evidence, in our study the target P300 response had contributions from two different neural systems, corresponding to bottom-up and top-down attention. In this model, one system is associated with saliency maps for bottom-up parietal-dependent selection, and the other system is linked to a more frontally-dependent goal-directed system for the detection and distinguishing of a target from distracters (Constantinidis and Steinmetz, 2005). With top-down attention processing of task-relevant information in frontal cortex has been found to precede information processing in parietal cortex, whereas the reverse order of activation has been found under the bottom-up attention control (Buschman and Miller, 2007). This is in accord with the distribution of the P300 shown in Fig. 4A, which shows a more parietal distribution for pop-out trials and a more frontal distribution in search trials in both younger and older adults. In an EEG study involving younger subjects we found significantly increased power from 4 to 24 Hz in parietal areas for pop-out targets and over frontal regions for search targets during the P300 time-window (Li et al., 2010), providing corroborating evidence for the relative differences in frontal and parietal networks in top-down and bottom-up attention. In the present study, however, the shift in distribution also interacted with age. Older subjects showed a more prefrontal P300 distribution than younger subjects in both conditions, suggesting that the networks used for both top-down and bottom-up attention shift to a greater involvement of frontal network regions in older adults.
4.4. N1 and N2pc
The N1 extrastriate ERP increased in amplitude with age (Fig. 3). This supports the idea that frontal increases in aging are not due to loss of sensory flow but may be due to disinhibition of sensory information and increased distractibility (Fabiani, 2012; Kok and Talsma, 1999; Scheibel and Scheibel, 1975; Woodruff, 1982). Because there was no significant effect of attentional condition on N1 amplitude or latency at either of the parieto-occipital electrodes, our findings suggest that the difference between pop-out and visual search conditions is the result of cognitive rather than perceptual changes (Hillyard and Kutas, 1983). Finally, the N2pc ERP was reduced with aging in both conditions, indicating that the attention shift indexed by the N2pc is greater in younger than in older adults independently of attention condition (Lorenzo-López et al., 2008). Thus, evidence from both the N1 and N2pc components support the idea that frontal increases in aging are related to dealing with disinhibition of sensory flow and subsequent increased behavioral distractibility.
4.5. Conclusions
In the current study, we found evidence for age-related changes in the activity of parietal and frontal regions during the control of top-down and bottom up attention. Together with evidence from past literature on the sources of the P300 ERP (Knight, 1997) and animal work on the networks contributing to top-down and bottom-up attention (Buschman and Miller, 2007), these results suggest that bottom-up and top-down targets lead to differential prefrontal and parietal activations in younger and older adults. In younger adults, target detection with top-down attention is associated with frontal activity and bottom-up attention is associated with more parietal activity. However, older adults showed a prominent frontal shift for both top-down and bottom-up attention, as measured with scalp-recorded EEG. This suggests that the underlying neural mechanisms for both of these two different types of attentional control may shift toward the use of frontal networks in older adults, perhaps in an effort to compensate for decreases in inhibitory control with aging.
Acknowledgments
This research was supported by grants from the NINDS NS21135, PO 40813, NSFC (30800242, 91120016), NSF (graduate fellowship to CG, 2008069381), Program for Changjiang Scholars and Innovative Research Team in University, and the Fundamental Research Funds for the Central Universities.
The study was approved by the Committee for the Protection of Human Subjects for the University of California, Berkeley. Informed written consent was obtained from all subjects prior to being tested.
Footnotes
Disclosure statement
The authors disclose no conflicts of interest.
References
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. J Cogn Neurosci. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Andrés P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr Clin Neurophysiol. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Linden DEJ. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004a;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004b;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Colcombe AM, Kramer AF, Irwin DE, Peterson MS, Colcombe S, Hahn S. Age-related effects of attentional and oculomotor capture by onsets and color singletons as a function of experience. Acta Psychol. 2003;113:205–225. doi: 10.1016/s0001-6918(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Posterior parietal cortex automatically encodes the location of salient stimuli. J Neurosci. 2005;25:233–238. doi: 10.1523/JNEUROSCI.3379-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–21. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: The novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Federmeier K. Event related brain potentials. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. third ed. Cambridge University Press; New York, NY: 2007. pp. 85–119. [Google Scholar]
- Fabiani M, Gratton G. Aging, working memory and attention control: a tale of two processing streams? In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. 2. New York: Oxford University Press; in press. [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J Cogn Neurosci. 2006;18:637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Fabiani M. It was the best of times, it was the worst of times: a psychophysiologist’s view of cognitive aging. Psychophysiology. 2012;49:283–304. doi: 10.1111/j.1469-8986.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I. Age-dependent changes in distribution of P3a/P3b amplitude and thickness of the cerebral cortex. Neuroreport. 2005;16:1451–1454. doi: 10.1097/01.wnr.0000177011.44602.17. [DOI] [PubMed] [Google Scholar]
- Friedman D. The components of aging. In: Luck SL, Kappenman ES, editors. Oxford Handbook of Event-Related Potential Components. Oxford University Press; New York, NY: 2008. pp. 513–536. [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol. 1997;104:498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Scale of attentional focus in visual search. Percept Psychophys. 1999;61:837–859. doi: 10.3758/bf03206901. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Controlling the focus of spatial attention during visual search: Effects of advanced aging and Alzheimer disease. Neuropsychology. 1997;11:3–12. doi: 10.1037//0894-4105.11.1.3. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Haxby JV. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31:471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol. 1995a;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995b;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks R. Inhibitory mechanisms and the control of attention. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. Oxford University Press: New York; 2008. pp. 227–249. [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annu Review Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual Search Across the Life Span. Dev Psychol. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, Greene EJ. An age-related deficit in prefrontal cortical function associated with refreshing information. Psychol Sci. 2004;15:127–132. doi: 10.1111/j.0963-7214.2004.01502009.x. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Review Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Knight RT. Distributed cortical network for visual attention. J Cogn Neurosci. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989a;504:338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Res. 1989b;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kok A, Talsma D. Aging and inhibition: multiple inhibitory systems. In: Falkenstein M, Hohnsbein J, Ullsperger P, editors. Cognitive Changes due To Aging and Fatigue as Revealed in Electrical Brain Activity. BAUA; Dortmund: 1999. pp. 100–111. [Google Scholar]
- Kramer AF, Fabiani M, Colcombe SJ. Handbook of the Psychology of Aging. sixth ed. Academic Press; New York: 2006. Contributions of Cognitive Neuroscience to the Understanding of Behavior and Aging; pp. 57–83. [Google Scholar]
- Kutas M, Iragui V, Hillyard SA. Effects of aging on event-related brain potentials (ERPs) in a visual detection task. Electroencephalogr Clin Neurophysiol. 1994;92:126–139. doi: 10.1016/0168-5597(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Li L, Gratton C, Yao D, Knight RT. Role of frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Res. 2010;1344:173–184. doi: 10.1016/j.brainres.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien MC, Gemperle A, Ruthruff E. Aging and involuntary attention capture: electrophysiological evidence for preserved attentional control with advanced age. Psychol Aging. 2011;26:188–202. doi: 10.1037/a0021073. [DOI] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Völlinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Lorenzo-López L, Amenedo E, Pascual-Marqui RD, Cadaveira F. Neural correlates of age-related visual search decline: a combined ERP and sLORETA study. Neuroimage. 2008;41:511–524. doi: 10.1016/j.neuroimage.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and visual attention. Curr Dir Psychol Sci. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL. Age-related changes in visual attention. Adv Cell Aging Gerontol. 2003;15:41–88. [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. University Press; Oxford: 2005. Age-related changes in neural activity during visual perception and attention; pp. 157–185. [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related changes in neural activity during visual target detection measured by fMRI. Cereb Cortex. 2004;14:143–155. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, Bennett PJ. Recruitment of unique neural systems to support visual memory in normal aging. Curr Biol. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional Control in the Aging Brain: Insights from an fMRI Study of the Stroop Task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller BT, Vytlacil J, Fegen D, Pradhan S, D’Esposito M. The prefrontal cortex modulates category selectivity in human extrastriate cortex. J Cogn Neurosci. 2011;23:1–10. doi: 10.1162/jocn.2010.21516. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Schmitt R, Bussfeld P, Pogarell O, Möller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. NeuroImage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalogr Clin Neurophysiol. 1988;71:55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Electroencephalogr Clin Neurophysiol. 1997;104:244–56. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of Change:Event-Related Potential and fMRI Findings. Kluwer Academic Press; Boston: 2003. pp. 83–98. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Structural changes in the aging brain. In: Brody H, Harmon D, Ordy JM, editors. Aging. Vol. 1. Raven Press; New York, NY: 1975. pp. 11–37. [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Hasher L, Stuss DT. Age-related changes in transfer costs: evidence from go/nogo tasks. Psychol Aging. 2010;25:963–967. doi: 10.1037/a0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R, Heide W, Butt C, Kompf D. Reduction of P3b potentials in patients with temporo-parietal lesions. Cognitve Brain Res. 1994;2:103–116. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Woodruff DS. Advances in the psychophysiology of aging. In: Craik FIM, Trehub S, editors. Aging and Cognitive Processes. Plenum Press: New York; 1982. pp. 29–53. [Google Scholar]
- Yamaguchi S, Knight R. Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci. 1991;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


