Abstract
Behavioral inhibition and performance monitoring are critical cognitive functions supported by distributed neural networks including the pFC. We examined neurophysiological correlates of motor response inhibition and action monitoring in patients with focal orbitofrontal (OFC) lesions (n = 12) after resection of a primary intracranial tumor or contusion because of traumatic brain injury. Healthy participants served as controls (n = 14). Participants performed a visual stop signal task. We analyzed behavioral performance as well as event-related brain potentials and oscillations. Inhibition difficulty was adjusted individually to yield an equal amount of successful inhibitions across participants. RTs of patients and controls did not differ significantly in go trials or in failed stop trials, and no differences were observed in estimated stop signal RT. However, electrophysiological response patterns during task performance distinguished the groups. Patients with OFC lesions had enhanced P3 amplitudes to congruent condition go signals and to stop signals. In stop trials, patients had attenuated N2 and error-related negativity, but enhanced error positivity. Patients also showed enhanced and prolonged post-error beta band increases for stop errors. This effect was particularly evident in patients whose lesion extended to the subgenual cingulate cortex. In summary, although response inhibition was not impaired, the diminished stop N2 and ERN support a critical role of the OFC in action monitoring. Moreover, the increased stop P3, error positivity, and post-error beta response indicate that OFC injury affected action outcome evaluation and support the notion that the OFC is relevant for the processing of abstract reinforcers such as performing correctly in the task.
INTRODUCTION
Performance monitoring and inhibition of prepotent responses are fundamental aspects of cognitive and motor control and prerequisites for dynamic context-dependent adjustments of behavior. pFC recruits and acts in concert with subcortical and posterior cortical areas in the service of higher-order control. There is increasing evidence that pFC regions, such as ventromedial, dorsomedial, and lateral pFC, contribute differentially to cognitive control processes (Sharp et al., 2010; Rubia, Smith, Brammer, & Taylor, 2003). Recent animal as well as human behavioral and neuroimaging studies suggest that OFC is concerned with monitoring and evaluation of performance outcomes (Noonan, Kolling, Walton, & Rushworth, 2012; Jung et al., 2011; Ursu & Carter, 2005), likely in cooperation with dorsomedial pFC (Rushworth, Noonan, Boorman, Walton, & Behrens, 2011). In addition, human lesion studies point to a critical role of OFC in inhibitory control and decision-making as extensive damage in this area often leads to impaired real-life decision-making, reduced concern for negative action outcomes, dysregulated affect, and decreased impulse control (Berlin, Rolls, & Kischka, 2004; Stuss & Levine, 2002; Bechara, Damasio, & Damasio, 2000). This study investigated the impact of focal OFC damage on response inhibition as well as action monitoring and evaluation using a challenging motor inhibition task.
Response inhibition is commonly studied using go/no-go or stop signal paradigms. In the go/no-go task, the participant is asked to rapidly respond to frequent go signals and to inhibit responding to low-probability no-go signals. The stop signal task (SST; Logan, Cowan, & Davis, 1984) puts a much higher load on inhibitory control as it requires the sudden cancellation of an already initiated motor response, whenever a stop signal is presented shortly after a go signal. Participants have greater difficulty cancelling their response when a stop signal is presented close to the time of response execution than when it is presented shortly after the go signal. Stop signal performance can be modeled as a race between the go and stop processes with the success or failure of response inhibition depending on the relative finishing time of these two processes. The horse race model (Logan et al., 1984) allows one to estimate the latency of the stop process, that is, the stop signal RT (SSRT; Band, van der Molen, & Logan, 2003; Logan et al., 1984). The model provides a good account for SST performance across a range of experimental contexts and patient groups (Verbruggen & Logan, 2009a). The SST has been shown to be sensitive for altered inhibitory control in clinical populations such as patients with attention deficit hyperactivity disorder (Senderecka, Grabowska, Szewczyk, Gerc, & Chmylak, 2012; Rubia, Oosterlaan, Sergeant, Brandeis, & v Leeuwen, 1998), obsessive-compulsive disorder (Krikorian, Zimmerman, & Fleck, 2004), or patients suffering from Parkinson’s disease (Gauggel, Rieger, & Feghoff, 2004).
Neuroimaging studies with healthy humans performing in the SST point to a predominantly right-lateralized network that encompasses dorsolateral and inferior frontal cortex, anterior cingulate gyrus, pre-SMA, inferior parietal lobe, and subcortical structures such as the subthalamic nucleus (STN; Chikazoe, 2010; Rubia et al., 2001, 2003). On the basis of fMRI and diffusion tensor imaging data, a three-node network involving the right inferior frontal gyrus (IFG), the pre-SMA, and the STN has been suggested as critical for response inhibition (Aron, Behrens, Smith, Frank, & Poldrack, 2007). Others have argued that signals of response conflict, uncertainty, or difficulty such as in stop trials activate ACC and pre-SMA, which then excite the STN and thereby inhibit motor output (Munakata et al., 2011). In this framework, the role of the right IFG is seen in maintaining context information about and monitoring the environment for task-relevant stimuli.
Electrophysiological studies of the SST or the go/no-go task typically found enhanced frontocentral N2 and P3 components in stop or no-go trials, but there is continuing debate about the functional significance of these effects (Huster, Enriquez-Geppert, Lavallee, Falkenstein, & Herrmann, 2013). It has been suggested that the no-go N2 indexes an early mechanism of inhibitory control (“red flag signal”) originating in pFC, which triggers the motor inhibition (Kok, 1986). One study reported a right frontal N2 that was enhanced for successful relative to unsuccessful inhibitions in the SST (Schmajuk, Liotti, Busse, & Woldorff, 2006), possibly reflecting right IFG activity. Other studies, however, observed a frontocentrally maximal N2, possibly generated in ACC or pre-SMA, that was larger on unsuccessful than successful stop trials (Ramautar, Kok, & Ridderinkhof, 2006) or did not differ between these conditions (Krämer, Knight, & Münte, 2011). It is still debated whether the stop N2 reflects an inhibitory process or a more general conflict-monitoring process (Huster et al., 2013). The above-mentioned framework of Munakata et al. might reconcile these two views on the stop N2 (Munakata et al., 2011). They argue that conflict signals activate the pre-SMA and/or ACC, possibly generating the stop N2, which then inhibit the motor system via the STN. Taken together, despite clinical observations of impulsive behavior in patients with OFC damage (Bechara et al., 2000), imaging or electrophysiological studies provide no clear evidence for a particular role of the OFC in motor inhibition.
The stop N2 is succeeded by a positive polarity P3 component typically showing greater amplitudes for no-go relative to go signals. The stop P3 has been associated with the evaluation of the outcome of the inhibitory process rather than with the response inhibition itself because it is seen after the time of failed inhibitions and the SSRT (Kok, Ramautar, De Ruiter, Band, & Ridderinkhof, 2004). The stop P3 is sometimes enhanced in successful compared with unsuccessful stop trials in healthy adults, often over frontal sites (Dimoska, Johnstone, & Barry, 2006; Kok, 1986). The stop P3 is proposed to originate in ACC and to be modulated by dopaminergic genes (Krämer et al., 2007).
In this study, we were interested in the role of the OFC not only in response inhibition but also in action monitoring and evaluation. To address this, we examined both electrophysiological and behavioral indices of action outcome evaluation and adaptive processes after errors. As noted, the stop P3 is assumed to be one such marker of evaluation of inhibitory success (Kok, 1986). Prominent neurophysiological correlates of error-monitoring are the error-related negativity (ERN) and the error positivity (Pe; Gehring, Goss, Coles, & Meyer, 1993; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1990). The ERN is a frontocentral negativity peaking around 80 msec after action errors and is proposed to be generated in ACC. The Pe occurs around 300 msec after errors and is believed to reflect awareness and affective evaluation of an error (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). Recent studies have also examined changes in oscillatory activity related to action monitoring. These studies reported an increase in the theta range after errors compared with correct responses (Marco-Pallares, Camara, Münte, & Rodriguez-Fornells, 2008; Luu & Tucker, 2001), supposedly reflecting midfrontal activity.
A behavioral index of performance monitoring and adjustment of responses after failed inhibitions in the SST is post-stop error slowing, that is, slower responses in go trials after a failed inhibition. This might reflect a response strategy adjustment after failed stop trials to increase the probability of inhibiting in the next stop trial (Verbruggen & Logan, 2009b). However, studies also indicate that stimulus repetition effects and negative priming might contribute to behavioral changes after stop trials (Verbruggen, Logan, Liefooghe, & Vandierendonck, 2008). Stimuli that have been associated with a stop signal in the previous trial will lead to slower response activation in the present trial, but no behavioral adaptation occurs when no stimulus or response repetition takes place (Beyer, Munte, Fischer, & Krämer, 2012; Verbruggen et al., 2008). Regarding neurophysiological correlates of post-error adaptation, a recent study observed a frontal beta power increase after action errors in a choice-RT task (Marco-Pallares et al., 2008), which correlated with the amount of post-error slowing. The authors interpreted this as index of an inhibitory mechanism underlying adaptive control after action errors (Marco-Pallares et al., 2008). In their view, such pFC-dependent inhibition of motor output after errors would prevent premeditated responses in the next trial and slow down RTs. A recent study replicated this post-error beta increase after failed inhibitions in an SST but did not observe any correlation with behavioral adaptations (Beyer et al., 2012). The lacking correlation with behavior was explained with longer response-stimulus intervals, but might also be attributable to the task context. Failed inhibitions in an SST cannot be corrected and participants are typically told that they cannot prevent errors as they have to trade correct performance in stop trials off against correct and fast performance in the go task.
Distinct prefrontal regions support different subprocesses required for the SST. Thus, lesions affecting various network nodes may influence performance, but for different reasons as suggested by Picton et al. (2007). In this study, we aimed to investigate the influence of the OFC on response inhibition and action monitoring by assessing behavioral and neurophysiological changes in OFC lesion patients. There are previous behavioral and EEG studies that have addressed this topic in patients with OFC lesions. Regarding performance in the SST, there is no strong evidence for impairment. A study by Aron and colleagues did not observe a significantly enhanced SSRT after damage to the right orbitofrontal gyrus (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003) but did so after right IFG damage. A study by Swick and coworkers using a go/no-go task showed that patients with injury in the left IFG and the insula had higher error rates than healthy controls, but patients with OFC lesions performed normally. These findings are in contrast to the prevalent and long standing view of patients with OFC injury as generally impulsive. In support of the emerging view that the principle role of the OFC may not be to exert inhibitory control over behavior, Rudebeck and colleagues reported that rhesus monkeys with complete bilateral exitotoxic lesions confined to the OFC, but sparing fibers passing through or near OFC, did not show deficiencies on tests of inhibitory control. However, the monkeys were impaired on a test requiring updating of object–outcome expectancies. A follow-up experiment indicated that loss of inhibitory control resulted from damage to neuronal projection axons coursing nearby or through OFC en route to other destinations. This study provided compelling evidence that inhibitory control is not the root function of the OFC but rather that it has a critical role in updating valuations according to current motivational states (Rudebeck, Saunders, Prescott, Chau, & Murray, 2013).
To our knowledge, there are no studies investigating neurophysiological correlates of response inhibition in patients with circumscribed OFC lesions. Previous results regarding error monitoring in OFC lesion patients are mixed (Turken & Swick, 2008; Ullsperger, von Cramon, & Muller, 2002). Ullsperger and colleagues reasoned that ERN or Pe should be modulated by damage to the OFC given its role in motivational and emotional control over action monitoring (Ullsperger et al., 2002). However, they found no changes in the ERN or Pe in a group of six patients with frontopolar/OFC lesions. Turken and Swick, on the other hand, found an abolished ERN in a group of four OFC patients together with reduced error correction but no changes in post-error slowing (Turken & Swick, 2008). The lesion location in the two groups differed though, as the lesions extended to the subcallosal area and pregenual ACC in the work by Turken and Swick, whereas they were located more anteriorly in the study by Ullsperger and colleagues. A modulation of error monitoring and outcome evaluation might thus be related to the rostral part of ACC, often described as the affective subdivision of ACC (Bush, Luu, & Posner, 2000).
Because OFC has been implicated in monitoring of action outcomes and the rapid reversal of behavior in response to changing circumstances, we expected that focal damage to this region would affect electrophysiological responses to both stop trial success and failure, such as the P3, ERN, and post-stop error beta increase. To the extent that patients with OFC lesions have a reduced ability for rapid and flexible modification of behavior in response to negative outcomes (Murray, O’Doherty, & Schoenbaum, 2007), it was expected that they would show less post-stop error slowing than healthy controls.
METHODS
Participants
We examined 12 patients with MRI-verified focal orbitofrontal lesions because of resection of a primary intracranial tumor (meningioma n = 8; low grade glioma n = 1) or contusion because of traumatic brain injury (n = 3) and 14 healthy control participants that were matched as closely as possible to the patients for age, sex, level of education, and IQ. Computation of full-scale IQ was based on the four subtests (Vocabulary, Similarities, Block Design, and Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Functional outcome of the patients was classified using the Extended Glasgow Outcome Scale (GOSE; Wilson, Pettigrew, & Teasdale, 1998). GOSE is a hierarchical scale in which the patient’s overall functional outcome is estimated based on the lowest item score obtained. Results of the between-group comparisons of demographic and GOSE data are presented in Table 1. Scores on the GOSE categorized the OFC group as “Moderate Recovery, Upper level,” an outcome level that characterizes patients who are capable of living an independent life despite having disabilities because of brain injury (Teasdale, Pettigrew, Wilson, Murray, & Jennett, 1998).
Table 1.
Participant Characteristics
| CTR | OFC | t Test | |
|---|---|---|---|
| n (% women) | 14 (36) | 12 (50) | |
| Age in years | 41.1 (12.4) | 48.0 (6.7) | ns |
| Education in years | 13.1 (2.5) | 13.1 (2.5) | ns |
| Total IQ | 111.4 (9.2) | 106.2 (12.5) | ns |
| GOSE | 6.3 (1.2) |
Values given are means (±SD). CTR = control group; OFC = OFC lesion group; GOSE = Extended Glasgow Outcome Scale; ns = not significant.
Patient inclusion was based on focal frontal brain lesions indicated on preexisting CT and/or MRI scans. Lesion characteristics are displayed in Table 2. Participants with a history of serious psychiatric disease, drug, or alcohol abuse requiring treatment, premorbid head injury, pre-/comorbid neurological disease, IQ < 85, substantial aphasia, visual neglect, or marked sensory impairment were excluded from participation. Testing took place at least 6 months after injury or surgery. The OFC group was tested at a mean of 29.4 (±17.4) months after injury/surgery. A neuroradiologist confirmed that the structural MRI images of the healthy control participants revealed no signs of pathology. Apart from one left-handed person in each group, the participants were right-handed. All participants reported normal acuity or vision corrected by optical lenses.
Table 2.
Lesion Characteristics of OFC Patients: Etiology, Months Post-injury, Lesion Size, and Affected Brodmann’s Areas
| Participant | Etiology | Months Post-injury | Lesion Size (cm3) | BA Left Hemisphere | BA Right Hemisphere |
|---|---|---|---|---|---|
| OFC group mean | Total: 47.0 | ||||
| RH: 25.3 | |||||
| LH: 21.7 | |||||
| 1 | Meningioma | 13 | 69.1 | 10, 11, 25, 47 | 10, 11, 25, 32, 46, 47 |
| 2 | Meningioma | 49 | 79.8 | 10, 11, 32, 46, 47 | 10, 11, 32, 46, 47 |
| 3 | Meningioma | 13 | 39.7 | 10, 11, 46, 47 | 10, 11 |
| 4 | Meningioma | 19 | 5.1 | 11 | |
| 5 | Meningioma | 43 | 134.8 | 9, 10, 11, 25, 32, 46, 47 | 9, 10, 11, 32, 45, 46, 47 |
| 6 | Meningioma | 27 | 7.2 | 11 | 11, 47 |
| 7 | Meningioma | 44 | 2.9 | 10, 11 | |
| 8 | LGG | 7 | 28.6 | 10, 11, 25 | 10, 11, 25, 32 |
| 9 | TBI | 44 | 23.6 | 11 | 10, 11 |
| 10 | TBI | 59 | 33.3 | 9, 10, 11, 32, 46 | 10, 11, 38, 46, 47 |
| 11 | TBI | 15 | 41.1 | 10, 11, 38, 45, 46, 47 | 11 |
| 12 | Meningioma | 20 | 96.8 | 10, 11, 24, 25, 32, 47 | 10, 11, 32, 46, 47 |
Lesions that comprise <0.2 cm3 in any given Brodmann’s area are not reported. BA = Brodmann’s area; RH = right hemisphere; LH = left hemisphere; TBI = traumatic brain injury; LGG = low-grade glioma.
Participants gave written informed consent before participating in the study. Healthy controls received 500 NOK (approximately 80 USD) for participation in the entire research program (neuropsychological assessment, EEG, structural and functional MRI). The study was performed in accordance with the principles stated in the Declaration of Helsinki. The experimental procedures were approved by the Norwegian Regional Committee for Medical Research Ethics, Region South.
Lesion Reconstruction
Lesion reconstructions were based on structural MRIs obtained after study inclusion. Lesions were outlined by drawing manually on Fluid Attenuated Inversion Recovery (FLAIR) images of each participant’s brain using MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron/). High-resolution T2-weighted images were used as aids to determine the borders of the lesions. The resulting lesion masks were transferred to normalized space using the Statistical Parametric Mapping software (SPM.5: www.fil.ion.ucl.ac.uk/spm/). Individual participant lesion mask, T1, and FLAIR images were first coregistered to a template T1 image and the resulting transformation parameters subsequently applied to the lesion mask. Lesions were reconstructed under the supervision of a neuroradiologist, a neurologist (RTK), and a neurosurgeon (TRM). Involved Brodmann’s areas and lesion volumes were calculated using MRIcron. Illustrations of the traced lesions are presented in Figure 1.
Figure 1.
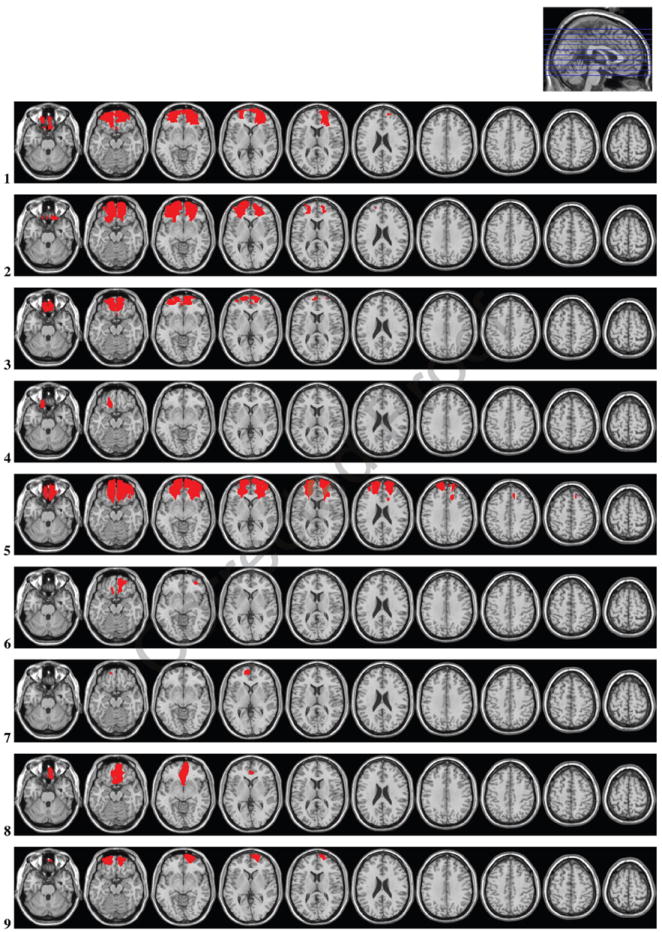
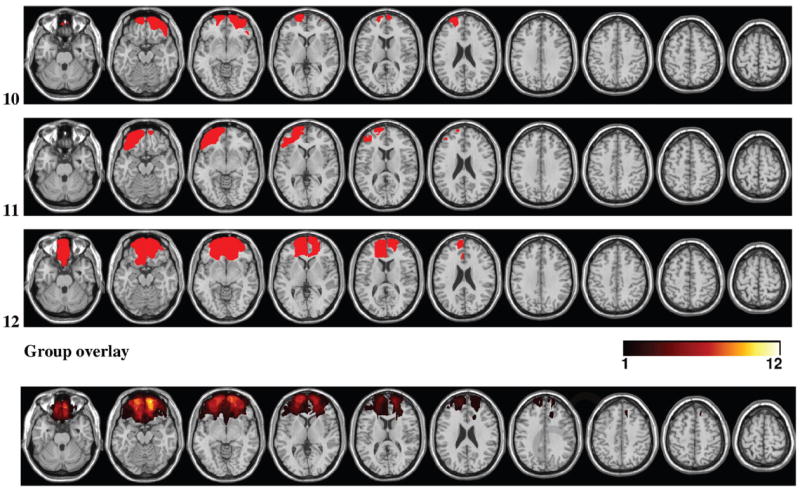
Lesion reconstructions for the OFC group. Individual patients (1–12) and group overlay (bottom row). The color code for the group overlay indicates the number of patients with damaged tissue in that area.
Task and Procedure
The SST consisted of blocks of 70 lateralized presented go stimuli where arrows pointing to the right required a right button press (with the right hand index finger), and left-pointing arrows required a left button press (with the left hand index finger). The primary go task thereby corresponds to a Simon task (Simon & Rudell, 1967) with congruent and incongruent (50% of go trials each) correspondence of stimulus location and response side (Figure 2). Each block contained 30 stop trials (30% of all trials), in which an upward-pointing arrow followed the go stimulus indicating to withhold the button press (Figure 2). Stimulus duration was 150 msec and intertrial intervals ranged from 1600 to 2000 msec. To set the timing of the stop signal delay (SSD), we followed a procedure suggested by Pliszka, Liotti, and Woldorff (2000). The procedure starts with a fixed range of possible SSDs, which is then adapted after each block to yield an approximate inhibition rate of 50% for all participants. The SSD was initially set to vary randomly between 50 and 550 msec with the restriction that the SSD would equally likely be within each of the five 100-msec time bins in this range (i.e., 50–150, 150–250, 250–350, 350–450, and 450–550 msec). Whereas the inhibition probability for SSDs within the 50–150 msec bin will be very high, the inhibition probability for SSDs within the 450–550 msec bin will be very low. Approximately equal distributions for successful and failed inhibitions were ensured through individually tailoring inhibitory difficulty by correcting the SSD according to RTs in correct go trials in the preceding block. Specifically, after each block, the difference between mean go RT of the previous block and maximum SSD (550 msec) was added to the SSD range for the following block. For instance, with a given mean RT of 640 msec, this would increase the SSD range by 640 – 550 = 90 msec and result in a new SSD range of 140–640 msec. Additional blocks were run until participants had at least 30 successful and 30 failed inhibitions. All stimulus presentations and response recordings were controlled using E-prime software, version 2.0 (Psychology Software Tools, Pittsburgh, PA). Participants were seated 1 m from a 24-in. computer screen.
Figure 2.
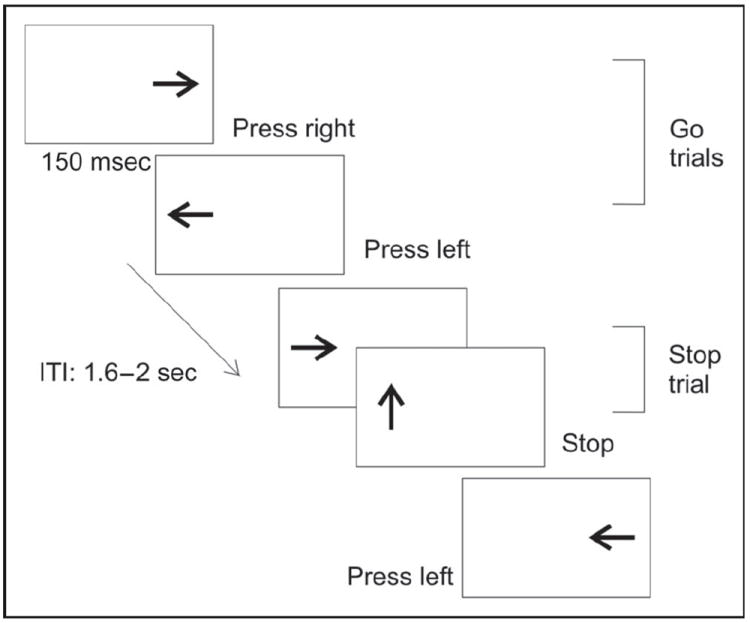
Visual SST. In go trials, participants were asked to respond to right- or left-pointing arrows with a right- or left-hand response, respectively. Stimuli were presented laterally, resulting in a Simon task with congruent go trials (first two examples) and incongruent go trials (last example). In stop trials, participants had to cancel the response when an upright arrow followed the right or left pointing arrows. The time interval between offset of the go signal and onset of the stop signal varied randomly within five SSD intervals that were initially 50–150 msec, 150–250 msec, 250–350 msec, 350–450 msec, and 450–550 msec. In each new block, mean go RT of the previous block subtracted by 550 msec was added to the five SSD intervals of the following trial.
EEG Recordings and Preprocessing
EEG data were acquired using a 128-channel HydroCel Geodesic Sensor Net and Net Amps 300 amplifier (Electrical Geodesics, Eugene, OR). Impedance was maintained below 50 kΩ, with 100 kΩ as an upper limit (Ferree, Luu, Russell, & Tucker, 2001). Recordings were initially referenced to Cz and off-line re-referenced to an average reference. EEG signals were sampled at 250 Hz with a 24-bit analog-to-digital converter and a DC to 125 Hz bandpass. Off-line, the EEG data were filtered with a high-pass filter of 0.5 Hz and a low-pass filter of 30 Hz. Analyses were performed with custom-written scripts in MATLAB (Natick, MA) and the EEGLAB toolbox (Delorme & Makeig, 2004). Vertical and horizontal eye movements were corrected based on an independent component analysis as implemented in EEGLAB, and bad channels identified by abnormally distributed data (kurtosis more than 3 SD away from mean) were removed and replaced with interpolated data (spherical spline interpolation). To improve signal to noise (see below) analysis of the ERPs focused on seven clusters of four electrodes each. Trials with remaining artifacts at those channels were rejected from further analysis if the amplitude exceeded 60 μV within 1 sec before and after stimulus onset relative to baseline.
Data Analyses
Behavioral Analyses
RTs and percentage of errors were computed separately for congruent and incongruent stimuli and submitted to a repeated-measures ANOVA with the within-subject factor Congruency and the between-subject factor Group (OFC lesion patients, OFC, vs. controls, CTR). The SSRT and average SSD were also assessed separately for congruent and incongruent stimuli. An estimation of the SSRT in successful inhibitions was calculated by subtracting the participant’s average SSD from the nth percentile of the RT distribution of correct go responses with n being the probability of failed inhibitions (Band et al., 2003). We additionally computed the percentage of inhibitions for three stop signal bins separately and calculated the slope of the resulting function. As the SSDs were different between participants, we divided the stop trials in three bins (SSD < 350 msec, 350–550 msec, and SSD > 550 msec), which yielded roughly equal number of trials in each bin. We computed the percentage of inhibited trials for each bin and calculated the slope of the change from Bins 1 to 2 and Bins 2 to 3 by relating the difference in inhibition rate to difference in the individual’s average SSDs for the respective bins. We computed post-stop error slowing as the RT difference between correct go trials after stop errors and those after correct go trials.
ERP Analyses
Data of go and stop trials were epoched time-locked to stimulus onset in segments from −300 to 1000 msec with a baseline from −100 to 0 msec. Additionally, response-locked ERPs from correct go trials and incorrect stop trials were epoched in segments from −300 to 1000 msec with a baseline from −300 to 0 msec. To increase signal-to-noise ratio, stop trials after congruent and incongruent go stimuli were collapsed. To assess statistical differences between the conditions, average amplitudes in the time windows of interest were subjected to repeated-measures ANOVAs. The time windows of interest were based on previous studies (Ramautar et al., 2006; Schmajuk et al., 2006) and visual inspection of the peak latency of the components of interest. Analyzed time windows for the different ERP components were as follows: go P3: 350–450 msec, stop N2: 200–250 msec, stop P3: 300–400 msec, ERN: 60–140 msec, Pe: 200–400 msec. To reduce the number of statistical comparisons and to improve the signal-to-noise ratio, we clustered electrodes over ROIs covering frontal to parietal areas (Figure 3). For visualization only, the data were low-pass filtered with 15 Hz.
Figure 3.
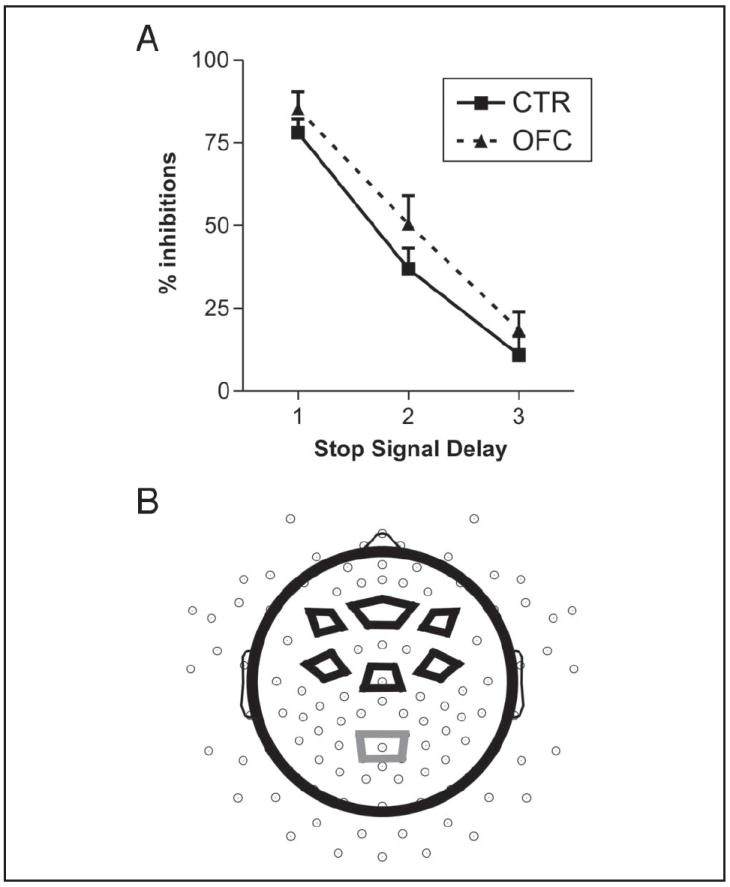
Behavioral results and ROI electrode clusters. (A) Inhibition rate for OFC patients and controls (CTR), separately for three SSD bins (1 = <350 msec, 2 = 350–550 msec, and 3 = >550 msec). Patients and controls did not differ in the slope between Bins 1 and 2 or Bins 2 and 3. (B) Visualization of the electrode clusters used for statistical analyses. Three electrode clusters were established along the anterior–posterior axis (frontal, central, and parietal), and three clusters along the right–left axis (right, midline, and left), except for only a midline parietal cluster. Go P3 was analyzed at the centroparietal cluster (gray lines), and stop N2 and P3 were analyzed at the six frontal and central clusters (black lines).
Time–Frequency Analyses
To study group differences in the theta and beta frequency bands, single trial data were convolved with a complex Morlet wavelet:
with the relation f0/σf (where σf = 1/(2πσt)) set to 6.7 (Tallon-Baudry, Bertrand, Delpuech, & Permier, 1997). We computed and averaged changes in time-varying energy (square of the convolution between wavelet and signal) in the studied frequencies (from 1 to 25 Hz; linear increase) with respect to baseline, separately for each participant. Selection of the analyzed theta (4–8 Hz) and beta (13–25 Hz) frequency band was based on literature (Marco-Pallares et al., 2008).
To analyze power changes in stop trials, we computed amplitude changes relative to the prestimulus baseline (−150 to 50 msec) between stop stimulus onset until 1800 msec in consecutive time windows for the frequency bands of interest. For theta, we analyzed 9 time windows of 200 msec, and for beta, we analyzed 37 time windows of 48 msec. The lengths of the chosen time periods were different to account for different cycle length of theta and beta oscillations. We focused on power changes over central midline electrodes, based on previous literature (Cavanagh, Cohen, & Allen, 2009; Marco-Pallares et al., 2008; Yeung, Bogacz, Holroyd, Nieuwenhuis, & Cohen, 2007). For each time window, we performed a t test comparing OFC patients and controls, separately for failed inhibitions (FI), successful inhibitions (SI), and go trials (GO). To account for multiple comparisons, we considered significant only those effects that remained at p < .05 for at least three consecutive time windows (i.e., 600 msec for theta effects and 144 msec for beta effects). Time windows of significant effects were further analyzed for Condition × Group interactions by means of repeated-measures ANOVA (see Cohen, van Gaal, Ridderinkhof, & Lamme, 2009; Marco-Pallares et al., 2008, for a similar approach).
For all statistical effects involving more than one degree of freedom in the numerator, the Huynh–Feldt correction was applied to correct for possible violations of the sphericity assumption (Huynh & Feldt, 1976). We report the uncorrected degrees of freedom and the corrected probabilities.
RESULTS
Behavioral Data
Behavioral results are summarized in Table 3. In total, 76–140 correct congruent trials and 68–140 correct incongruent trials were included in the analyses. Both patients and controls were slower and made more errors in incongruent compared with congruent go trials (main effect Congruency for RTs: F(1, 24) = 17.7, p < .01, and for error rate: F(1, 24) = 20.9, p < .01). Patients and controls did not differ in their performance in the go trials (main effects and interactions: p > .2).
Table 3.
Behavioral Results in the SST for Patients and Controls
| CTR
|
OFC
|
|||
|---|---|---|---|---|
| CON | INC | CON | INC | |
| RT go signals (msec) | 599 (131) | 634 (152) | 658 (139) | 699 (131) |
| Errors (%) | 0.63 (1.0) | 3.76 (3.88) | 0.63 (1.26) | 3.16 (3.65) |
| SSD (SI) | 336 (109) | 333 (103) | 376 (99) | 383 (106) |
| SSD (FI) | 503 (108) | 526 (105) | 563 (124) | 560 (114) |
| RT stop errors | 522 (75) | 579 (116) | 568 (114) | 606 (100) |
| Inhibitions (%) | 45 (9.8) | 50 (14.1) | 49 (11.7) | 55 (13.3) |
| SSRT | 179 (30) | 179 (25) | 171 (13) | 176 (41) |
| Post-stop error slowing | 39 (58) | 12 (80) | ||
| Post-stop inhibition slowing | 28 (52) | 14 (34) | ||
Behavioral results for controls (CTR) and patients with OFC lesions (OFC), separately for congruent (CON) and incongruent (INC) trials. SSD = stop signal delay (msec) for successful (SI) and failed inhibitions (FI); SSRT = stop signal RT (msec); Errors = response hand errors. Standard deviance is given in brackets.
The tailoring of SSDs was successful in that participants inhibited on average about 50% of the stop trials with no differences between congruent and incongruent trials (F < 1) or between groups (F < 1). In accordance with the horse race model, RTs of stop errors were shorter than RTs of correct go trials as can be assessed from Table 3. This held true for both congruent (p < .001) and incongruent trials (p < .001) and did not differ between groups (p > .2). The difference in RTs was found in every single participant (except one control who was similarly fast in stop error and correct go trials, but only in congruent trials). The groups did not differ in their SSRT (F < 1), which was estimated to be about 179 msec in controls and 175 msec in OFC patients with no difference between congruency conditions (F < 1). Patients and controls did not differ in their average SSD in inhibited or noninhibited stop trials (both main effect Group and interaction Group × Inhibition: p > .1), but for both groups the delay in successful inhibitions was about 200 msec faster, F(1, 24) = 660.3, p < .001. A significant threefold interaction of Group × Inhibition × Congruency, F(1, 24) = 4.8, p = .038, reflected an enhanced SSD in incongruent compared with congruent failed inhibitions in the control group, whereas no such congruency effect was observed in the patients. As can be observed in Figure 3A, the two groups did not differ in the slope of their inhibition functions (Slope Bins 1–2: p > .4, Bins 2–3: p > .7). Although the difference between RTs to the subsequent go signal after stop errors and after correct go trials was significant for controls (39 msec; p = .03), but not for OFC patients (12 msec; p = .6), and patients thus showed nominally reduced post-stop error slowing compared with controls, the interaction of Group and Sequence (post-error vs. post-correct) did not reach significance (p > .3). Both groups also slowed down after inhibited stop trials compared with go trials, F(1, 24) = 6.0, p = .022 (see Table 3), but did not differ significantly in the amount of post-stop inhibition slowing (F < 1). Moreover, RTs after inhibited and failed stop trials did not differ significantly in either group (ps > .1).
ERP Results in Go Trials
We focused on the P3 and restricted the statistical analysis to the electrode cluster where it is best observed, that is, on a parietal cluster centered around Pz (see Figure 3B). The statistical analysis of the P3 (mean amplitude = 350–450 msec) included the within-subject factor Congruency and the between-subject factor Group. The OFC group had an overall trend level increase in P3 amplitude compared with controls, F(1, 24) = 3.4, p = .08 (Figure 4), and a significant enhancement in congruent trials, t(24) = −2.3, p = .03 (interaction Congruency × Group: F(1, 24) = 3.3, p = .08). No main effect of Congruency was observed (F < 1).
Figure 4.
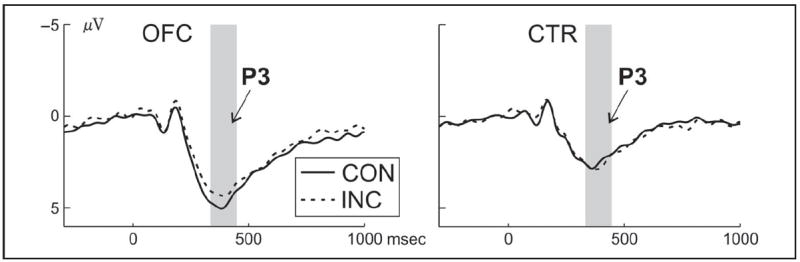
ERP results for go trials. ERPs for congruent (CON, solid lines) and incongruent (INC, dashed lines) go trials at the centroparietal cluster, separately for OFC patients (left column) and controls (right column). The time window of interest for the P3 is highlighted in gray.
ERP Results in Stop trials
For stop ERPs, we analyzed the frontocentral stop N2 and P3. Again, statistical analyses were restricted to the electrode clusters at which the components were maximal. For both the stop N2 (200–250 msec) and P3 (300–400 msec), we subjected the mean amplitude at frontal and central electrode clusters to a repeated-measures ANOVA with the within-subject factors Inhibition, Anterior (frontal vs. central), and Laterality (left, midline, right) and the between-subject factor Group. Interactions between Group and Inhibition, Laterality, or Anterior were explored by performing follow-up analyses on separate levels of the relevant factor.
The stop N2 was larger in failed compared with successful inhibitions, F(1, 24) = 4.3, p = .049 (Inhibition × Anterior and Inhibition × Laterality: p < .05), particularly at the central midline clusters (main effect Inhibition: F(1, 24) = 5.8, p = .02). OFC patients showed a trend toward a reduced N2 compared with controls, F(1, 24) = 3.6, p = .07 (Anterior × Group: p = .06), which yielded significance at the central Anterior location, F(1, 24) = 5.1, p = .03 (Figure 5A). The stop P3 (Figure 5A and B) did not differ between failed and successful inhibitions (main effect Inhibition: F < 1), but OFC patients showed by trend an enhanced P3 compared with controls, F(1, 24) = 3.8, p = .06 (Anterior × Group: p = .05), which was significant at the central Anterior level, F(1, 24) = 4.7, p = .04).
Figure 5.
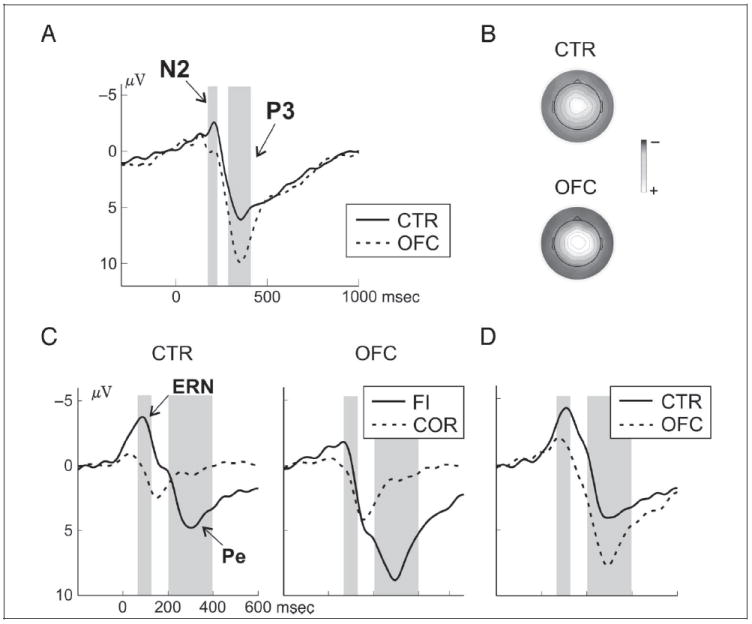
ERP results for stop trials. (A) ERPs at the central midline cluster for patients and controls in successful (SI) inhibitions. The N2, larger in controls than patients, and the P3, larger in patients than controls, are highlighted with gray boxes. (B) The P3 topography (300–400 msec) for the two groups is shown with a color scale from 5 to −10 μV. (C) Response-locked ERPs at the central midline cluster in controls (left column) and patients (right column), separately for correct go trials (dashed lines) and failed inhibition trials (solid lines). The analyzed time windows for the ERN and the Pe are marked with gray boxes. (D) Difference waves (failed inhibition trials − correct go trials) highlighting the ERN and the Pe in the two groups.
Response-locked ERPs
In both groups, we observed an ERN and a Pe after failed inhibitions, but OFC patients showed a diminished ERN and an increased Pe (Figure 5C and D). For both components, mean amplitude values from a central midline electrode cluster were subjected to repeated-measures ANOVA with the factors Accuracy (stop trial failed inhibition vs. correct go trial) as within-subject factor and Group as between-subject factor. The ERN-analysis (60–140 msec) showed an interaction of Accuracy × Group, F(1, 24) = 6.98, p = .01, because of a smaller amplitude difference between correct go trials and failed inhibitions in OFC patients (p = .048) than in controls (p < .001). Regarding the Pe (200–400 msec), an interaction of Accuracy × Group, F(1, 24) = 4.44, p = .046, reflected an enhanced amplitude difference between failed stop trials and correct go trials in the OFC group (p < .001) compared with controls (p = .008).
As outlined in the Introduction, previous inconsistent results of an altered ERN in OFC patients have been explained with differences in the lesion location, specifically whether the lesions affected subgenual cingulate cortex or not. The present OFC sample included patients with BA 25/32 damage. To examine whether the ERN reduction can be attributed to BA 25/32 damage, we first determined lesion overlap with Brodmann’s areas based on the atlas implemented in MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron/). A Brodmann’s area was considered damaged only if the overlap exceeded 0.2 cm3. The difference in ERN amplitude between single patients and the mean of the control group were then computed using the Crawford and Howell (1998) method as recommended by Crawford and Garthwaite (2012). Three patients had an ERN that was statistically different from controls, and all had BA 25/32 damage; three of the remaining nine patients with a normal ERN also had BA 25/32 damage.
Time–Frequency Results in Go and Stop Trials
We did not find any significant group differences in the theta band, neither for stop- nor go trials. The lack of theta effects did not depend on the threshold of three significant time windows as there were neither any group differences without this threshold. In the beta band, we observed specific group differences after stop trials. By directly comparing the two groups (see Methods section), OFC patients showed an increased beta response after error trials between 1250 and 1630 msec (Figure 6A and B) and after successful inhibitions between 1440-1680 msec. OFC patients showed a stronger beta decrease in go trials around the response, that is, between 580 and 830 msec. To test for interactions, we subjected the mean power change between 1250 and 1600 msec to a repeated-measures ANOVA with the factors Group and Condition (FI, SI, and GO), which confirmed an interaction of Condition and Group, F(2, 48) = 6.04, p < .01 (Figure 6C). As can be observed, both groups showed a beta increase after stop trials, particularly after failed inhibitions (Figure 6C), but this beta increase was more pronounced and persisted longer in the OFC patients compared with the controls (Figure 6A). To ensure that the results are not caused by the selected time windows, we also assessed group differences in the post-error beta increase for every sample, which yielded very similar results (significant group differences between 1420 and 1580 msec). It should be noted that group differences in the beta effect did not survive Bonferroni or false discovery rate correction for multiple comparisons.
Figure 6.
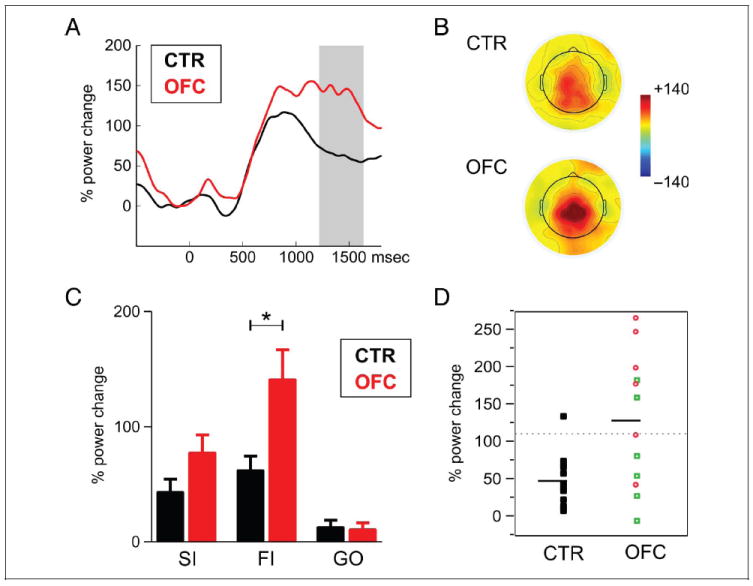
Time–frequency results for stop trials. (A) Beta power (13–25 Hz) changes in failed inhibitions (FI), separately for controls (black) and patients (red). Both groups showed increased beta after errors, which was more pronounced and persistent in patients (time window of significant differences is indicated with gray box). (B) Shown is the topography of the beta increase (1250–1600 msec) for the two groups in FI trials. (C) Beta power change between 1250 and 1600 msec after the stop or go signal, separately for successful inhibition (SI), failed inhibition (FI), and go trial (GO) in controls (black) and patients (red). (D) Beta power change in FI for individual participants in the two groups. The dotted line indicates the point of significantly increased (p < .05) beta values compared with controls. Red circles denote patients whose lesion extended to BA 25/32, whereas green squares indicate patients whose lesion spared BA 25/32. The black horizontal line indicates the mean of the OFC patient and control groups.
To further investigate the beta findings, we examined the lesion specificity in the patients who showed this prolonged beta response. In Figure 6D, the beta response after stop errors is shown for each individual participant with color coding of patients whose lesion included BA 25 or BA 32 (shown in red circles) and those that did not (green squares). As can be observed, five of six patients with BA 25/32 lesion had a significantly increased beta response, whereas only two of six without BA 25/32 lesion showed increased beta power. This can also be observed from the results of a lesion subtraction analysis that we performed (Karnath, Fruhmann Berger, Kuker, & Rorden, 2004), depicted in Figure 7. For this analysis, a lesion overlap image was created of the group of participants who showed a deficit (here: an increased beta response) and an image of those that did not. In the second step, one image was subtracted from the other, and the resulting image provided the relative lesion probability in the “impaired” subgroup relative to the other. It should be stressed that the results have to be interpreted very cautiously and can only provide a hint for future studies as the number of patients is quite small. This approach is not a voxel-wise statistical analysis (Karnath et al., 2004) either as such analysis would not be warranted by this small sample. This said, one can notice that the subgenual cingulate gyrus and medial OFC were lesioned more often in those who showed an increased beta response compared with those who did not.
Figure 7.
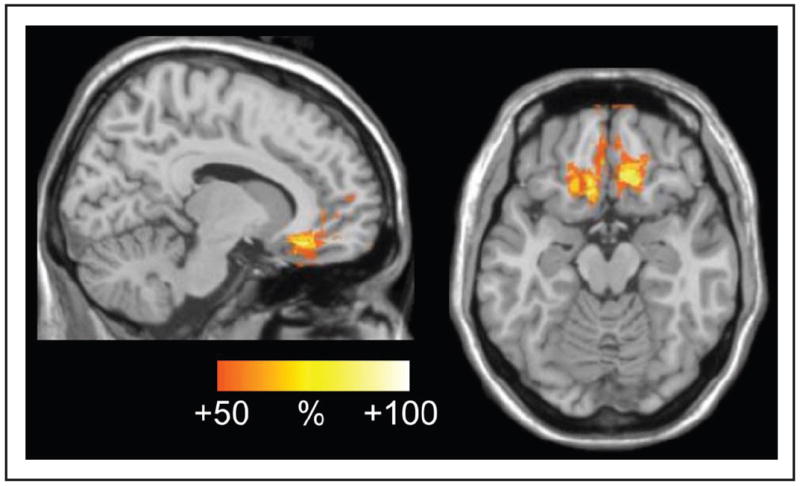
Lesion subtraction analysis. Plot of the subtracted superimposed lesions of the patients with increased beta response minus group of patients showing no change in beta power compared with healthy controls. The overlap represents regions that were damaged at least 50% more frequently in patients with increased beta response than in the others.
DISCUSSION
We investigated the behavioral and electrophysiological correlates of response inhibition and performance monitoring in a challenging SST in patients with focal OFC lesions and in healthy controls. OFC lesion patients did not show any behavioral impairment, but several altered neurophysiological responses. Specifically, the stop N2 and ERN were found to be diminished, whereas the stop P3, the Pe, and the post-stop error beta increase were augmented in OFC patients. The results demonstrate that the OFC is not critically involved in response inhibition per se, but that it modulates measures of action monitoring and action outcome evaluation. The results are discussed with respect to current models of inhibitory control and action monitoring.
Behavioral Results
There were no significant behavioral differences in the SST between the OFC-lesioned patients and the healthy control participants. The experimental manipulation of stimulus congruency (i.e., location of go signal in relation to required response hand) had similar effects on the various performance measures in both groups. As expected, the incongruent condition resulted in augmented go signal error rates and RTs. OFC patients and controls responded to go signals with similar speed and high accuracy. The on-line response-tracking algorithm employed to individually adjust stop signal intervals was effective in obtaining approximately 50% successful inhibitions in both groups. The similarity across groups in both SSRT and the slope of the inhibitory function over SSDs indicates that circumscribed OFC lesions do not impair response suppression in the SST. Null findings are generally difficult to interpret though, and one might argue that we were lacking power to detect any behavioral group differences with our small sample size. However, the SSRTs of the two groups were virtually identical (Table 3) and not comparable to group differences found with, for example, ADHD children (Schachar et al., 2007; Pliszka et al., 2000). Moreover, previous studies on patients with focal frontal lesions were able to detect behavioral differences despite having similar or even smaller sample sizes (Krämer et al., 2013; Voytek et al., 2010). The result fits with current models of motor response inhibition, which do not ascribe a critical role to the OFC (Rudebeck et al., 2013; Aron, 2011; Munakata et al., 2011) and in accordance with prior reports of normal performance in OFC-lesioned patients in the SST (Aron et al., 2003) and the go/no-go task (Swick, Ashley, & Turken, 2008).
Although the patient group nominally showed less post-stop error slowing compared with the control group, the difference did not reach significance. The finding suggests that the OFC-lesioned patients were able to flexibly adjust behavior in response to stop errors. It should be mentioned though that it has been debated whether post-error slowing in general, and specifically in the SST, reflects cognitive control and behavioral adjustments or simply priming (Beyer et al., 2012; Verbruggen et al., 2008).
Despite similar task performance, there were differences between OFC patients and controls in several concomitantly recorded electrophysiological parameters. The results showed systematic group differences in the magnitude of ERP components related to action monitoring and action outcome evaluation.
OFC Lesions Reduce Stop N2 and ERN
Stop trials elicited a frontally distributed negative polarity potential, previously denoted the stop N2. OFC lesion patients showed by trend a generally decreased stop N2, but the stop N2 amplitude in both groups was larger in unsuccessful compared with successful inhibition trials. The latter finding is difficult to reconcile with a pure inhibitory function of the N2. The stop N2 has also been linked with conflict or action monitoring rather than response inhibition (Huster et al., 2013), although much of the support for this interpretation comes from go/no-go studies and not SSTs. According to this interpretation, the enhanced stop N2 to failed response suppressions would indicate greater response conflict compared with successful inhibitions. An enhanced stop N2 in failed relative to successful stop trials has been suggested to reflect a greater significance of stop signals in trials in which participants fail to cancel the imminent response to the go signal (Ramautar et al., 2006). In this study, the results may indicate that both study groups perceived greater response conflict and/or assigned a greater signal value or meaningfulness to the failed compared with the successful inhibition trials, but this effect was generally dampened in OFC patients. It should be noted that this effect yielded significance only on a trend level. Future studies are therefore needed to replicate this finding. Alternatively, the stop N2 in error trials might essentially reflect the ERN or at least overlap with the ERN, which would explain the increased N2 in failed compared with successful inhibitions. Future studies should examine more systematically to what extent the stop N2 and ERN reflect distinct processes (which are possibly overlapping in stop error trials) or rather a more general action monitoring system.
Response-locked analyses suggested diminished error monitoring in the patients manifesting in decreased ERN following failed inhibitions compared with the controls. In contrast, OFC patients had increased amplitude of the subsequent Pe component (discussed below). Both the ERN and the Pe are considered neurophysiological markers for error monitoring processes, but are thought to reflect dissociable aspects of monitoring (Burle, Roger, Allain, Vidal, & Hasbroucq, 2008; Overbeek et al., 2005; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, et al., 2001). The ERN has been thought to index early automatic error detection resulting from a comparison process discerning a mismatch between the actual and the intended movement or activity in a conflict monitoring system that is not specific to errors (Botvinick, Cohen, & Carter, 2004; Yeung, Botvinick, & Cohen, 2004). The latter account would be in agreement with the functional role of the dorsal ACC, which has been identified as the primary neural generator for the ERN (Stemmer, Segalowitz, Witzke, & Schonle, 2004; Ito, Stuphorn, Brown, & Schall, 2003). ACC has been found to play a critical role in conflict monitoring and error detection (Rushworth, Kennerley, & Walton, 2005; Gehring & Fencsik, 2001), but findings of ACC activation also in the absence of overt error indicate that it contributes to prevention of errors through continuous action monitoring (Huettel & McCarthy, 2004; Ullsperger & von Cramon, 2001).
Ventromedial sectors of the OFC are connected with the dorsal ACC via the cingulum bundle (Schmahmann et al., 2007). Moreover, the ventromedial pFC encompasses the rostral ACC, which is involved in affective processes (Bush et al., 2000). Notably, some studies suggest that the ERN may be at least partly distinguishable from purely cognitive factors and may rather reflect the emotional reaction to committing an error (Hajcak & Foti, 2008; Vocat, Pourtois, & Vuilleumier, 2008; Gehring & Willoughby, 2002). In this study, the altered ERN may reflect a direct disruption of the OFC/rostral-ventral “affective” subdivision of ACC and/or a distant modulatory influence of the OFC over the rostral-dorsal ACC. In support of the latter, error-related intracranial potentials have been recorded at shorter latencies in the OFC compared with the rostral ACC (and dorsolateral pFC; Brazdil et al., 2002). In support of the reduced ERN reflecting a direct disruption of ACC, we found that all patients with an ERN that differed from controls had a lesion that encompassed the BA 25/32 (subgenual cingulate gyrus), whereas only a third of the patients with a normal ERN had damage in this area. It should be mentioned as a cautionary note that any claim about Brodmann’s areas affected by lesions should be interpreted carefully as it is not possible to directly infer upon cytoarchitectonic structure based on the MRI data.
Our results are concordant with Turken and Swick, who reported an attenuated ERN but preserved Pe in four patients with extensive OFC lesions performing a variant of the Stroop paradigm (Turken & Swick, 2008). As found in our OFC sample, the patients had normal post-error slowing, but three had lower than normal error correction rates. All patients had lesions that included perigenual ACC, but that largely spared the dorsal “cognitive” sector of ACC. Ullsperger et al. did not observe changes in the ERN or Pe in patients with frontopolar damage encompassing the anterior part of the OFC, but sparing the perigenual ACC (Ullsperger et al., 2002). Altogether, the studies suggest that OFC damage extending to peri- or subgenual ACC diminishes the ERN, possibly via changes in modulatory effects on the performance monitoring system subserved by dorsal ACC.
OFC Lesions Augment Stop P3, Pe, and Post-stop Error Beta Response
Evidence suggests that the Pe can be distinguished from the ERN both in functional and neuroanatomical terms. Although both the ERN and the Pe are modulated by error awareness, the Pe awareness effect has been suggested to reflect a more binary decision (i.e., error or correct) regarding the error whereas the magnitude of the ERN is more sensitive to the subjective level of confidence that an error was committed (Navarro-Cebrian, Knight, & Kayser, 2013; Shalgi & Deouell, 2012). Dipole source localization indicated that the Pe had generators in the posterior cingulate cortex (PCC; see also O’Connell et al., 2007). The results from this study support the notion that the ERN and Pe are dissociated as OFC-lesioned patients had attenuated ERN whereas the ensuing Pe was augmented compared with healthy controls. In line with the interpretation offered by Turken and Swick for their finding of dissociable ERN and Pe effects in OFC patients (Turken & Swick, 2008), the increased Pe despite an attenuated preceding ERN suggests that error signals were indeed processed by the action monitoring system. A likely participating node in this network would be the PCC (Vocat et al., 2008), which was unaffected in our OFC-lesioned group. Recent studies suggest that PCC neurons encode environmental outcomes and have a role in the dynamic allocation of neural resources to motivationally significant information (Pearson & Platt, 2013; Hayden, Nair, McCoy, & Platt, 2008). We suggest that the enhanced Pe may be because of altered OFC–PCC functional connectivity.
Comparison of experimental antecedents, morphology, and topographical distribution of the Pe and the P3, the latter being a slow positive deflection typically evoked by any motivationally significant event, suggests that both components share several characteristics. In their review of the functional meaning of the Pe, Overbeek and co-workers claimed that it may constitute a P3 associated with the motivational significance of the error (Overbeek et al., 2005). Following this interpretation, the increased Pe in our OFC-lesioned patients implies that they perceived failures as more motivationally important.
In go as well as stop trials, the N2 was succeeded by a prominent P3 component. OFC patients tended to have an enhanced frontocentral P3 to go trials compared with controls, which was significant for congruent trials. In contrast to the stop N2, the stop P3 was not modulated by the result of the stop process, as it did not significantly differ between failed and successful response cancellations in either group. Independent of inhibition outcome, OFC patients displayed significantly augmented P3 amplitudes at central electrode clusters compared with controls. It has been proposed that the anterior midline stop P3 occurs too late to reflect response inhibition itself, although inferring the exact timing of an underlying process from the component’s peak latency, for instance, is problematic (Luck, 2005). In this study, the positive deflection only started around 300 msec, which is well after the SSRT. The stop P3 might rather correspond to an aftereffect of inhibition such as the evaluation of the inhibitory process or its outcome (Huster et al., 2013; Bruin, Wijers, & van Staveren, 2001). The present finding of a slightly enhanced stop P3 regardless of inhibition outcome suggests that the OFC is involved in evaluative and updating processes associated with the result of the stop process, although this effect reached significance only for the central electrode cluster. Our ERP findings are in agreement with the view that the orbitofrontal and cingulate cortex, both of which have strong reciprocal connections with the BG (Huster et al., 2013; Rosenbloom, Schmahmann, & Price, 2012; Tekin & Cummings, 2002), play key roles in representing links between actions and outcomes (Hayden & Platt, 2010; Schoenbaum, Roesch, Stalnaker, & Takahashi, 2009), as well as in implementing behavioral change following unfavorable outcomes (Pearson & Platt, 2013). Given the OFC’s role in motivational processing, one might have also expected a reduced instead of an enhanced Pe and P3. However, the SST is challenging as two contradictory subtasks (the primary go task and the stop task on a subset of trials) have to be performed. Any evaluation of the motivational significance thus has to take the context into account to balance performance in the go task and the stop task. It might be the evaluation of performance with respect to the current motivational state and context that the OFC is needed for (Rudebeck et al., 2013), resulting in an overall increased P3 and Pe in pFC lesion patients. This is, however, clearly speculative and further studies are needed that directly manipulate motivational significance to clarify the functional changes resulting from OFC damage.
It has recently been suggested that oscillatory activity in the beta frequency range is important for long-range communication within an inhibition network that includes pFC and BG nodes (Swann et al., 2012; Aron, 2011). In this study, both patients with OFC damage and controls showed increased central beta power in stop versus go trials and further increased beta after failed compared with successful inhibitions about 1200–1600 msec post-stop signal onset. Beta increase after movement termination is a well-established phenomenon that has been associated with deactivation or inhibition in the motor cortex (Neuper & Pfurtscheller, 2001). Recently, studies have reported increased beta power after action errors. Marco-Pallares and colleagues reported a late beta increase with a frontocentral maximum following choice errors in an Eriksen flanker task (Marco-Pallares et al., 2008). Beyer et al. observed enhanced frontal beta power after failed inhibitions in an SST (Beyer et al., 2012). The beta increase in these studies had a different topography than the typical motor event-related synchronization, suggestive of prefrontal midline sources such as pre-SMA or ACC. The effects were interpreted as reflecting an inhibitory mechanism underlying adaptive control after action errors or as indexing an immediate reaction to the error. In this study, we replicated the increased beta power after stop errors. As in the study by Beyer et al. (2012), the beta increase was transient in the controls and returned toward baseline around the onset of the next trial (which was on average around 1300 msec after stop signal onset). Interestingly, the beta increase after stop errors in this study was stronger and persisted longer in the OFC compared with the control group. As can be assessed from Figure 6D, 7 of 12 patients showed an increased beta response compared with controls, which resulted in a group difference that was overall not very strong and did not survive false discovery rate or Bonferroni correction for multiple comparisons. Clearly, future studies are needed to replicate this finding. However, single-subject and lesion subtraction analyses suggested systematic anatomical differences possibly accounting for group heterogeneity. Specifically, patients with a significantly enhanced beta response had more often lesions affecting BA 25/32 than those who had an average beta response. Again, given the small sample size, caution is warranted in interpreting this finding, which might rather serve to generate hypotheses for future studies. Regardless of the precise functional significance of the post-error beta increase, the augmented beta of the OFC patients might reflect altered modulation of activity in a frontal-BG network, possibly related to action outcome evaluation or adaptive control after errors.
Limitations and Conclusions
This study cannot resolve whether increased stop error beta and Pe as well as stop P3 in the OFC patients reflect late cognitive mechanisms compensating for disrupted early conflict or error processing or, alternatively, disinhibition of neural activity because of deficient modulation by the OFC. It should be noted that enhanced magnitudes of both early sensory and later cognitive ERPs have been reported in previous studies of patients with OFC damage (Solbakk, Reinvang, Svebak, Nielsen, & Sundet, 2005; Rule, Shimamura, & Knight, 2002; Hartikainen, Ogawa, Soltani, & Knight, 2001) and were suggested to reflect disinhibition of neural processes resulting from impaired OFC-mediated top–down control. Although different interpretations for altered electrophysiological responses may not be incompatible, it is of interest that OFC damage had differential effects on EEG indices of cognitive processes elicited by distinct task parameters in this study.
In summary, patients with chronic focal OFC damage and healthy controls exhibited similarities in several performance measures as well as in qualitative characteristics of electrophysiological responses to the SST. Although response inhibition itself was not impaired, as indicated by the normal SSRT, findings of diminished stop N2 and ERN suggest that OFC injury impacted action monitoring. Moreover, the increased stop P3, Pe, and post-stop error beta response suggest that OFC injury affected action outcome evaluation, possibly through interactions with the cingulate cortex. The findings of this study support the notion that the OFC is not only recruited in processing of tangible behavioral reinforcers such as food and money, but also in processing of more abstract reinforcers such as performing correctly (Elliott & Deakin, 2005). Importantly, the findings add human lesion, behavioral, and electrophysiological support to the gradually accruing contention (e.g., Rudebeck et al., 2013; Schoenbaum et al., 2009) that the OFC serves a critical role in outcome expectation and evaluation of its motivational value rather than in behavioral inhibition.
Acknowledgments
The study was supported by the Research Council of Norway (186504/V50 to A. K. S.), the South-Eastern Norway Regional Health Authority (2008047 to A. K. S.), and the German Research Foundation (KR 3691/1-1 to U. M. K.). We would like to thank Clay Campbell Clayworth and Håkon Engen for support in establishing routines for lesion reconstructions and Dr. Paulina Due-Tønnessen for evaluating the structural MRI scans.
References
- Aron AR. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;27:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Beyer F, Munte TF, Fischer J, Krämer UM. Neural aftereffects of errors in a stop-signal task. Neuropsychologia. 2012;50:3304–3312. doi: 10.1016/j.neuropsychologia.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Roman R, Falkenstein M, Daniel P, Jurak P, Rektor I. Error processing—Evidence from intracerebral ERP recordings. Experimental Brain Research. 2002;146:460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA, van Staveren AS. Response priming in a go/nogo task: Do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clinical Neurophysiology. 2001;112:1660–1671. doi: 10.1016/s1388-2457(01)00601-0. [DOI] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T. Error negativity does not reflect conflict: A reappraisal of conflict monitoring and anterior cingulate cortex activity. Journal of Cognitive Neuroscience. 2008;20:1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. Journal of Neuroscience. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Current Opinion in Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Cohen MX, van Gaal S, Ridderinkhof KR, Lamme VA. Unconscious errors enhance prefrontal-occipital oscillatory synchrony. Frontiers in Human Neuroscience. 2009;3:54. doi: 10.3389/neuro.09.054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Single-case research in neuropsychology: A comparison of five forms of t test for comparing a case to controls. Cortex. 2012;48:1009–1016. doi: 10.1016/j.cortex.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. Clinical Neuropsychology. 1998;12:482–486. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone S, Barry R. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain and Cognition. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological brain research. Tilburg: Tilburg University Press; 1990. pp. 192–195. [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. Journal of Neuroscience. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Ogawa KH, Soltani M, Knight RT. Effects of emotional stimuli on event-related potentials and reaction times in orbitofrontal patients. Brain and Cognition. 2001;47:339–341. [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60:19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. Journal of Neuroscience. 2010;30:3339–3346. doi: 10.1523/JNEUROSCI.4874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International Journal of Psychophysiology. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in randomized block and splitsplot designs. Journal of Statistics Education. 1976;1:69–82. [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jung J, Bayle D, Jerbi K, Vidal JR, Henaff MA, Ossandon T, et al. Intracerebral gamma modulations reveal interaction between emotional processing and action outcome evaluation in the human orbitofrontal cortex. International Journal of Psychophysiology. 2011;79:64–72. doi: 10.1016/j.ijpsycho.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cerebral Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biological Psychology. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Cunillera T, Camara E, Marco-Pallares J, Cucurell D, Nager W, et al. The impact of COMT and DRD4 genotypes on neurophysiological correlates of performance monitoring. Journal of Neuroscience. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer UM, Knight RT, Münte TF. Electrophysiological evidence for different inhibitory mechanisms when stopping or changing a planned response. Journal of Cognitive Neuroscience. 2011;23:2481–2493. doi: 10.1162/jocn.2010.21573. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Solbakk AK, Funderud I, Løvstad M, Endestad T, Knight RT. The role of the lateral prefrontal cortex in inhibitory motor control. Cortex. 2013;49:837–849. doi: 10.1016/j.cortex.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in obsessive-compulsive disorder. Brain and Cognition. 2004;54:257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luu P, Tucker DM. Regulating action: Alternating activation of midline frontal and motor cortical networks. Clinical Neurophysiology. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Camara E, Münte TF, Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. Journal of Cognitive Neuroscience. 2008;20:1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Cebrian A, Knight RT, Kayser AS. Error-monitoring and post-error compensations: Dissociation between perceptual failures and motor errors with and without awareness. Journal of Neuroscience. 2013;33:12375–12383. doi: 10.1523/JNEUROSCI.0447-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clinical Neurophysiology. 2001;112:2084–2097. doi: 10.1016/s1388-2457(01)00661-7. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MF. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. European Journal of Neuroscience. 2012;35:997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, et al. The role of cingulate cortex in the detection of errors with and without awareness: A high-density electrical mapping study. European Journal of Neuroscience. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing—On the functional significance of the Pe vis-a-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pearson JM, Platt ML. Change detection, multiple controllers, and dynamic environments: Insights from the brain. Journal of the Experimental Analysis of Behavior. 2013;99:74–84. doi: 10.1002/jeab.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biological Psychology. 2006;72:96–109. doi: 10.1016/j.biopsycho.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Schmahmann JD, Price BH. The functional neuroanatomy of decision-making. Journal of Neuropsychiatry & Clinical Neurosciences. 2012;24:266–277. doi: 10.1176/appi.neuropsych.11060139. [DOI] [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, v Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behavioural Brain Research. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: Conjunctive brain activations across different versions of Go/NoGo and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience. 2013;16:1140–1147. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Kennerley SW, Walton ME. Cognitive neuroscience: Resolving conflict in and over the medial frontal cortex. Current Biology. 2005;15:R54–R56. doi: 10.1016/j.cub.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderecka M, Grabowska A, Szewczyk J, Gerc K, Chmylak R. Response inhibition of children with ADHD in the stop-signal task: An event-related potential study. International Journal of Psychophysiology. 2012;85:93–105. doi: 10.1016/j.ijpsycho.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Deouell LY. Is any awareness necessary for an Ne? Frontiers in Human Neuroscience. 2012;6:124. doi: 10.3389/fnhum.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Rudell AP. Auditory S-R compatibility: The effect of an irrelevant cue on information processing. Journal of Applied Psychology. 1967;51:300–304. doi: 10.1037/h0020586. [DOI] [PubMed] [Google Scholar]
- Solbakk AK, Reinvang I, Svebak S, Nielsen CS, Sundet K. Attention to affective pictures in closed head injury: Event-related brain potentials and cardiac responses. Journal of Clinical and Experimental Neuropsychology. 2005;27:205–223. doi: 10.1080/13803390490515739. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Witzke W, Schonle PW. Error detection in patients with lesions to the medial prefrontal cortex: An ERP study. Neuropsychologia. 2004;42:118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59:2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30-70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: A review and update on advancing the use of the Glasgow Outcome Scale. Journal of Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D. The effect of orbitofrontal lesions on the error-related negativity. Neuroscience Letters. 2008;441:7–10. doi: 10.1016/j.neulet.2008.05.115. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY, Muller NG. Interactions of focal cortical lesions with error processing: Evidence from event-related brain potentials. Neuropsychology. 2002;16:548–561. doi: 10.1037//0894-4105.16.4.548. [DOI] [PubMed] [Google Scholar]
- Ursu S, Carter CS. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: Implications for neuroimaging studies of decision-making. Brain Research, Cognitive Brain Research. 2005;23:51–60. doi: 10.1016/j.cogbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience and Biobehavioral Reviews. 2009a;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance. 2009b;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, Liefooghe B, Vandierendonck A. Short-term aftereffects of response inhibition: Repetition priming or between-trial control adjustments? Journal of Experimental Psychology: Human Perception and Performance. 2008;34:413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: A spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46:2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barceló F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: Guidelines for their use. Journal of Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Nieuwenhuis S, Cohen JD. Theta phase resetting and the error-related negativity. Psychophysiology. 2007;44:39–49. doi: 10.1111/j.1469-8986.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]


