Abstract
Male bonnet monkeys (Macaca radiata) were subjected to the Variable Foraging Demand (VFD) early stress paradigm as infants, MRI scans were completed an average of four years later, and behavioral assessments of anxiety and ex-vivo corpus callosum (CC) measurements were made when animals were fully matured. VFD rearing was associated with smaller CC size, CC measurements were found to correlate with fearful behavior in adulthood, and ex-vivo CC assessments showed high consistency with earlier MRI measures. Region of Interest (ROI) hippocampus and whole brain voxel- based morphometry assessments were also completed and VFD rearing was associated with reduced hippocampus and inferior and middle temporal gyri volumes. Animals were also characterized according to serotonin transporter genotype (5-HTTLPR), and the effect of genotype on imaging parameters was explored. The current findings highlight the importance of future research to better understand the effects of stress on brain development in multiple regions, including the corpus callosum, hippocampus, and other regions involved in emotion processing. Nonhuman primates provide a powerful model to unravel the mechanisms by which early stress and genetic makeup interact to produce long-term changes in brain development, stress reactivity, and risk for psychiatric disorders.
Keywords: stress, monkeys, corpus callosum, hippocampus, brain abnormalities, 5-HTTLPR
1. Introduction
Childhood stress has been associated with increased risk of developing a variety of stress-related mood and anxiety disorders (Heim et al., 2000). Moreover, numerous studies have shown that early-life stress produces long-term changes in brain structure and function. The effect of stress on hippocampus has been the focus of a number of structural neuroimaging studies. The hippocampus is involved in declarative memory and spatial/contextual learning, with afferent and efferent connections to limbic structures that regulate mood and cognition (Schmidt and Duman, 2007). Hippocampal pyramidal cells are rich in glucocorticoid receptors, which are thought to mediate their vulnerability to stress (McEwen and Magarinos, 2001). Childhood adverse events have been associated with small hippocampal size in adults (Bremner et al., 1997; Stein et al., 1997; Driessen et al., 2000; Vythilingam et al., 2002) but not in children (De Bellis et al., 1999; Carrion et al., 2001; De Bellis et al., 2001; De Bellis, 2002; Tupler and De Bellis, 2006; Woon and Hedges, 2008), suggesting that hippocampal volumetric differences may not be apparent until adulthood (Karl et al., 2006). In contrast, clinical studies have consistently reported smaller corpus callosum (CC) area in children exposed to early-life stress (De Bellis et al., 1999; De Bellis et al., 2001; De Bellis, 2002; Teicher et al., 2004), with conflicting findings in adults (Villarreal et al., 2004; Kitayama et al., 2007). These data suggest that in contrast to structural changes in the hippocampus that may only become evident many years after exposure to early-life stress, differences in CC size are present early and possibly persist throughout life. Reductions in CC size have also been reported in nonhuman primates exposed to early-life stress (Sanchez et al., 1998; Coe et al., 2002), with changes in CC size correlated with cognitive deficits in nonhuman primates (Sanchez et al., 1998). The association between CC size and behavioral phenotypes, however, has not yet been characterized in nonhuman primates.
In light of the findings of reduced CC area in patients with childhood maltreatment and the association of early-life stress with lower CC size in nonhuman primates, we examined the behavioral effects of CC structure in male bonnet macaques subject to variable foraging demand (VFD) rearing. VFD is an early-life stress paradigm in which infant bonnet macaques are reared by mothers undergoing an experimentally-induced “perception” of food uncertainty (Gorman et al., 2002). VFD and non-VFD mothers and infants differ on a number of behavioral and biological indices that persist throughout development, including disrupted measures of maternal-infant attachment (Andrews and Rosenblum, 1991), increased stress reactivity (Rosenblum et al., 2001), synchronized maternal-infant elevation of cerebrospinal fluid (CSF) concentrations of corticotropin releasing factor (CRF; Coplan et al., 2005), and reduced neuronal integrity (Mathew et al., 2003; Coplan et al., 2010b).
In this study, in addition to MRI measurements of the CC, hippocampus was measured by both voxel-based morphometry (VBM) and ROI-approaches. VBM is an automated method allowing identification of anatomical differences in the whole brain without the pre-specification of regions of interest (Karl et al., 2006). Preliminary application of VBM methods to adults with PTSD have confirmed prior ROI analyses and identified additional novel areas that appear to be altered in patients with PTSD (Chen et al., 2006; Emdad et al., 2006; Jatzko et al., 2006; Li et al., 2006). Therefore, in addition to the a priori planned ROI analyses, an exploratory whole-brain VBM analysis was performed to screen for additional regions affected by early-life stress.
Over the last few years there has been a burgeoning of research demonstrating that long-term changes associated with early stress can be modified by polymorphic variation in the serotonin transporter gene (SLC6A4). In humans and nonhuman primates, there are two common functional alleles of 5-HTTLPR, the short (“s”) allele and long (“l”) allele (Dannlowski et al., 2007). Although the exact mechanism by which 5-HTTLPR modifies stress reactivity is unknown, the association of the “s” allele with vulnerability to stress is robust. In nonhuman primates, 5-HTTLPR genotype does not predict the phenotype of infants reared under optimal conditions (e.g. parent-reared). However, for nonhuman primate infants reared under more stressful conditions (e.g. peer-reared), those with an “s” allele show greater emotional distress, elevated hypothalamic pituitary adrenal (HPA) axis response to stress, lower basal serotonergic function, and elevated CSF CRF (Bennett et al., 1998; Champoux et al., 2002; Barr et al., 2003; Coplan et al., 2010a).
In the current investigation it was hypothesized that: 1) VFD rearing would be associated with smaller CC and hippocampus, and 2) these brain changes would be related to individual differences in behavioral measures of emotional reactivity. Exploratory analyses were also conducted to determine the effects of 5-HTTLPR genotype on hippocampus volume and corpus callosum area.
2. Materials and Methods
2.1 Subjects
Imaging and genetics data were available for 23 adult male Bonnet Macaques (Macaca radiata): 14 VFD-reared and 9 normal reared. At time of MRI scanning, there were no statistical differences between the groups in age (VFD: 59.1 ± 31.1 months; non-VFD: 66.3 ± 31.7; t = 0.53, ns) or weight (VFD: 4.85 ± 1.3 kg; non-VFD: 5.2 ± 0.6, t=0.46, ns).
2.2 Procedures
Primates were socially-housed in the SUNY-Downstate Nonhuman Primate Facility. The study was approved by the Institutional Animal Care and Use Committees of SUNY-Downstate, Mount Sinai Medical School, and Veterans Administration West Haven Connecticut campus.
2.2.1 Rearing Procedures
Mother-infant dyads were group-housed in pens of 5-7 dyads each, and stabilized for at least four weeks prior to VFD onset. After infants reached at least 2-months of age, dyads were subjected to a standard VFD procedure that involved 8 alternating 2-week blocks in which maternal food was readily obtained (low foraging demand; LFD) or more difficult to access (high foraging demand; HFD). During the HFD conditions, the mothers had to find the food by digging through clean wood-chips. Adequate food was always available under both conditions, and there were no differences in weight between VFD and non-VFD mothers or infants. However, the unpredictability of foraging conditions prevented VFD mothers from attending to the needs of their infants, thereby achieving the early-life stress paradigm through the disruption of normative patterns of maternal rearing and infant attachment (Coplan et al., 2005). After infancy, there were no experimental manipulations to confound the VFD-rearing effects.
2.2.2 Scanning Procedure
On the day of the brain scan, study subjects were ushered into familiar carrying cages and transported to Mount Sinai Medical Center in a dedicated animal transport van with air-conditioning. The monkeys’ heads were positioned in a Styrofoam headrest inside a human knee coil and taped snugly over the forehead to minimize movement. Subjects remained anesthetized throughout scanning and were continuously monitored by pulse oximeter.
MRI data were acquired in a 3-T Siemens Allegra scanner. The protocol for the structural scans consisted of a three-plane sagittal localizer from which all other structural scans were prescribed. The following structural scans were acquired: Axial 3D-MPRAGE (TR=2500ms, TE=4.4ms, FOV=21cm, matrix size= 256×256, 208 slices with thickness= 0.82mm); Turbo spin echo T2-weighted Axial (TR=5380ms, TE=99ms, FOV=18.3cm × 21cm, matrix=512×448, Turbo factor=11, 28 slices, thickness=3mm, skip 1mm).
2.2.3 MRI Data Pre-processing and Analysis
All MRI ROI and ex-vivo data analyses were completed by raters blind to subjects’ rearing and genotype. The axial MPRAGE series were imported into ANALYZE AVW 7.0 software platform. In order to isolate whole brain from its surroundings, skull, surface CSF, and meninges were stripped using a combination of tools including image thresholding, region growing, and manual tracing.
To isolate cortical grey matter, a level set grey-white segmentation algorithm was applied (Zeng et al., 1999). This method takes advantage of the nearly constant thickness of cortical mantle and of unvarying surface boundaries of cortex: the outer surface given by CSF/grey matter boundary and the inner one given by white/grey matter boundary. The two surfaces were evolved simultaneously, each using its own image derived information while maintaining coupling between the inner and outer surfaces at all times. A final representation of the cortical surface and an automated segmentation of cortex were output in ANALYZE format.
CC area measurements were obtained at the midsagittal slice as determined by the presence of the septum pellucidum. The CC was outlined and segmented into seven sections according to Witelson (1989) using a semiautomated program. Intra-rater reliability performed on all subregions of the CC for 10 ratings was 0.90 (P < 0.0001) for subregion 1 area, 0.94 (P < 0.0001) for subregion 2 area, 0.91 (P < 0.001) for subregion 3 area, 0.95 (P < 0.0001) for subregion 4 area, 0.97 (P < 0.0001) for subregion 5 area, 0.98 (P < 0.0001) for subregions 6 and 7 area. The hippocampi were manually traced using a detailed set of guidelines developed by Schumann et al. (2004) and adjusted, when necessary, to the bonnet macaque brain morphology using a primate brain atlas (Witelson, 1989). The MPRAGEs were reoriented to the hippocampal axis, i.e., horizontal axis was parallel to a line from rostral to caudal pole of the hippocampus. The tracings were performed in oblique coronal slices, but were also checked in sagittal and axial views. Repeated measurements were performed in a random order on 5 subjects, and both intra-rater and inter-rater reliability gave an ICC > 0.93 for right/left hippocampus.
An additional structural MRI data analysis was performed using VBM SPM5 (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm). Briefly, a single subject raised under normative conditions was chosen as the initial template. All images were registered through linear (zooms, rotations, translations and shears) deformations to the single subject template. An average image, called template henceforth, was created with the obtained deformed images. Afterwards, the same original images were linearly deformed to the created template and this step was iterated 20 times to minimize the bias caused by utilization of a single subject as the initial template. On the 22nd step, original images were linearly and also non-linearly registered to the final template. A brain mask containing gray matter, white matter and CSF, was manually delineated for the template and used to eliminate skull and meninges from the final registered images. In order to preserve brain volume, images were scaled using the Jacobian matrix, so that the total amount of grey matter in the resulting images remains the same as it would be in the original images. The obtained images were finally smoothed with a Gaussian filter at Full Width at Height Maximum equal to 4 mm.
2.2.4 Behavioral Measures of Emotional Reactivity
Twelve animals that participated in this MRI study were subject to behavioral testing approximately two years after neuroimaging as part of an interlocking research protocol. The animals were exposed briefly to an intruder, a fear-stimulus which is a variation of a previously detailed masked intruder test (Rosenblum et al., 2001). Emotional Responsivity was rated blind to rearing and genotype status using a 3-point scoring scale. To receive a score of three for intruder distress, subjects exhibited defensive behaviors including; fang-baring, growling, direct eye-contact, pilo-erection, ear flexing, cage shaking, mouth gaping and lip smacking. The defensive response represents an interpretation of the intruder threat as proximal. The least distressed response received a score of “one” which was characterized by an animal that was minimally defensive, relaxed and timid in response to the intruder, averting eye contact and receding to the back of the cage, suggesting a perception of distal threat. A score of two describes a subject with intermediate levels of behavior, alternating between defensive and relaxed behaviors. Each subject was observed by two observers blind to rearing condition and genotype. 100% between-observer concordance was observed for the behavioral scoring whose duration was 60 seconds.
2.2.5 Ex-vivo measurements of corpus callosum
Ex-vivo cross-sectional CC area measurements were obtained from photographs of the fixed tissue in the mid-sagittal plane, using the same measurement procedures as utilized for the MR images. The images were converted to ANALYZE format and the CC was outlined and segmented into seven sections according to the procedures developed by Witelson (1989) using a semiautomated program.
2.2.6 5-HTTLPR Genotyping
Genotypes were determined by PCR amplification followed by size fractionation on a 2% agarose gel. Primers used were CAG CAC CTA ACC CCC TAA TGT CCC TG and GAT TCT GGT GCC ACC TAG ACG CCA G. Each 10 μl reaction contained 20ng DNA, 1uM of each primer, 1 M betaine, 10 uM dNTPs, and 0.1unit KlenTaq polymerase, in manufacturer's PC2 buffer. Cycling parameters were 95°C for 5 minutes followed by 30 cycles at 95°/72o for 30 and 60 seconds respectively, using an MJR thermal cycler.
2.2.7 Statistical Analyses
Prior to conducting the ROI and analyses, the distribution of the outcome measures was examined using Shapiro Wilk's test of normality. Skewed distributions were corrected using standardized transformations. A 2 (rearing: VFD vs. non-VFD) × 2 (genotype: l/l vs. s/l or s/s) factorial analysis of variance (ANOVA) was conducted to examine group differences on total CC area and hippocampus volume, and exploratory analyses were conducted to examine group differences within subregions of the CC. Given the previous report of an effect of heritability on the hippocampal volume in nonhuman primates (Lyons et al., 2001), we performed a one-way ANOVA for paternity effects on half-siblings within each of the rearing groups. The one-way ANOVA was performed within groups as there was a confound of rearing status and paternity. There were no significant paternity effects on the CC area or the hippocampal volume. Age, weight, total white matter and brain volume were examined as covariates, but none of these variables were significant, except total brain volume. As a consequence, brain volume was retained in the hippocampus and callosal ROI analysis. Significance was set at p ≤ 0.05, two tailed, for all ROI analyses, and means and standard deviations are reported in the text and tables. Given the small sample size, only descriptive summary statistics are provided to examine interactions between genotype and rearing condition.
For the VBM analyses, statistical maps were created through a 2 (rearing: VFD vs. non-VFD) X 2 (genotype: l/l vs. s/l or s/s) ANOVA design covarying for total intracranial volume. As we had an a priori-expectation of finding reduced hippocampal and CC volume in the VFD group in the VBM analyses, a lower threshold of p< 0.005 uncorrected was used to display the maps. The ensuing detected volumetric differences were further assessed with small volume correction (SVC) using a sphere radius of 2 mm, corresponding to a volume of 33.51 mm3 ((4/3)*3.14*(2 mm)). A voxel-level FDR-corrected p< 0.05 was used as a criterion for significance. Given the small number of subjects, the statistical threshold was set at a relatively liberal level for the exploratory VBM analysis (p< 0.005, uncorrected for multiple comparisons).
3. Results
3.1 ROI Results
3.1.1 Corpus Callosum Cross-Sectional Area
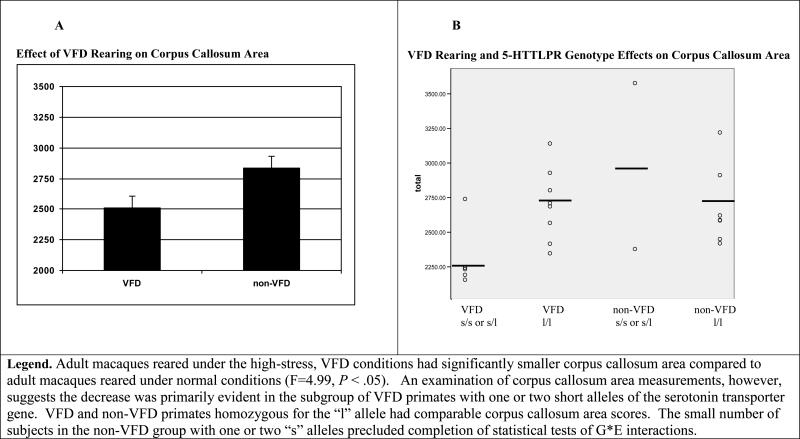
A significant main effect for rearing condition was found, with smaller mid-sagittal cross-sectional area observed in VFD-reared animals (F1,17=5.43, P <0.032; VFD: 90.0 mm2 ± 11.9; non-VFD: 98.4 mm2 ± 14.6) (see Fig.1).
Figure 1. Corpus Callosum Mid-Sagittal Cross Sectional Area in VFD-Reared and non-VFD Reared Non-Human Primates.
Adult macaques reared under the high-stress, VFD conditions had significantly smaller corpus callosum area compared to adult macaques reared under normal conditions (F=4.99, P < .05). An examination of corpus callosum area measu rements, however, suggests the decrease was primarily evident in the subgroup of VFD primates with one or two short alleles of the serotonin transporter gene. VFD and non-VFD primates homozygous for the “l” allele had comparable corpus callosum area scores. The small number of subjects in the non-VFD group with one or two “s” alleles precluded completion of statistical tests of G*E interactions.
3.1.2 Hippocampal Volume
After controlling for total brain volume, a significant main effect of rearing was found on the left side, with smaller hippocampi in VFD-reared animals (F1,17=8.21, P < 0.01; VFD: 0.43 cm3 ± 0.04; non-VFD: 0.46 cm3 ± 0.02). There was no effect of rearing on right hippocampal volume (F1,17=0.36, ns; VFD: 0.49 cm3 ± 0.05; non-VFD: 0.47 cm3 ± 0.05).
3.2 VBM Results
3.2.1 Corpus Callosum
The VBM analysis provides an assessment of total CC volume, not just mid-sagittal cross-sectional area. No significant main effect for rearing condition was observed for CC volume.
3.2.2 Hippocampal Volume
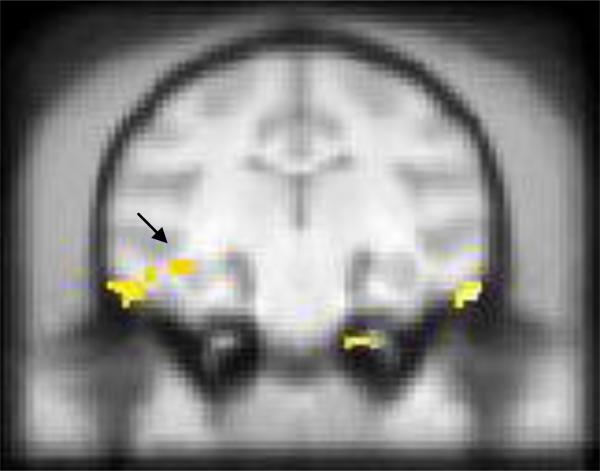
Consistent with the ROI analysis results, there was a significant rearing effect on left (pFDR_corrected: 0.010, Z-score: 2.67), but not right hippocampal volume, with smaller left hippocampal volume in VFD-reared animals.
3.3 Brain-Behavior Analyses
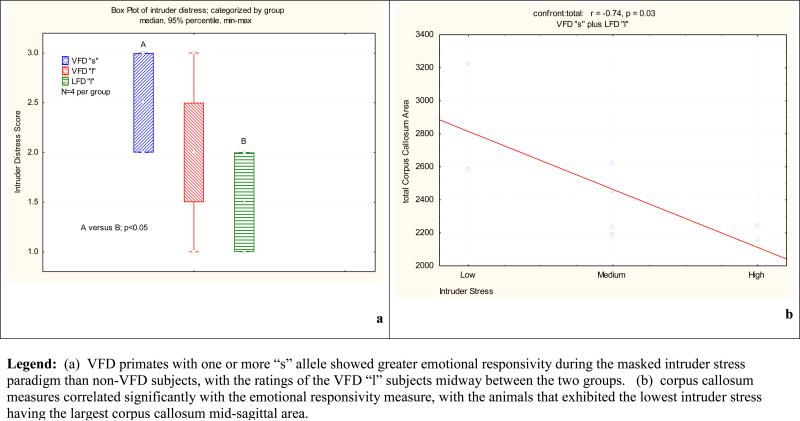
To assess whether changes in CC size correlated with fearful behavior in adulthood, responses to the Masked Intruder Stress Paradigm were assessed in 12 animals used in the initial MRI study. The animals were exposed briefly to a masked intruder and fearful responses were scored by raters who were blind to the rearing and genotype of the animals (Rosenblum et al., 2001). The emotional responsivity measure correlated significantly with the ROI CC assessments (R=-0.74, P < 0.01), such that the animals who exhibited the lowest intruder stress had the largest CC mid-sagittal area.
3.4 Ex-vivo Corpus Callosal Measurements
Upon completion of the behavioral work, animals were sacrificed and tissue was collected for further histological and biochemical characterization of structural changes noted in the imaging studies. In addition, ex-vivo cross-sectional CC area measurements were obtained from photographs of the fixed tissue in the mid-sagittal plane, using the same measurement procedures as utilized for the MR images The MRI in-vivo and postmortem ex-vivo total CC area measurements were highly correlated (r=0.82, P < 0.001), suggesting that differences in CC size are stable across adulthood. A significant main effect for rearing condition was also found with the ex-vivo measurements with significantly smaller mid-sagittal cross-sectional CC area in regions 3 (F=4.53, P < 0.05), 4 (F=4.55, P < 0.05), and 7 (F=5.70, P <0.05), and trends toward reduced CC area in regions 2 (F=3.68, P < 0.10) and 6 (F=3.19, P < 0.10).
3.5 Genotype Exploratory Results
3.5.1 Allele Frequencies
Six (43%) of the fourteen VFD-reared subjects carried the “s” allele, either as “sl” or in one case, as an “ss” genotype. Two of the nine non-VFD subjects (22%) were “sl” genotype. All remaining subjects were homozygous for the “l” allele. The proportion of subjects with the “s” allele did not vary by rearing group (Fisher's Exact Test, P >0.4).
3.5.2 ROI Results
The means, standard deviations, and F-values for the CC area analyses are included in Table 2. VFD-reared animals with one or two “s” alleles had the smallest cross-sectional CC area (see Fig. 1). Over 80% (5/6) of the VFD subjects with one or two “s” alleles had smaller CC cross-sections than did any of the animals in the other groups. Alternatively, no genotype effect was observed for left or right hippocampal volume.
Table 2.
Corpus Callosum Area: Effects of Genotype and Early Rearing Conditions
| VFD Rearing | Non-VFD Rearing | F-Value* Genotype | F-Value* Early Rearing | |||
|---|---|---|---|---|---|---|
| l/l | s/l or s/s | l/l | s/l or s/s | |||
| N | 8 | 6 | 7 | 2 | ||
| Total Corpus Callosum Area | 96.6 ± 9.4 | 81.1 ± 8.8 | 96.1 ± 10.2 | 106.6 ± 30.3 | .011, ns | 5.43, P < .032 |
| Region 1 - Rostrum 1 | 0.9 ± 0.5 | 0.6 ± 0.2 | 1.1 ± 0.9 | 0.7 ± 0.2 | 0.24, ns | 0.31, ns |
| Region 2 - Genu 2 | 23.4 ± 1.6 | 19.9 ± 1.8 | 24.0 ± 3.7 | 22.7 ± 3.0 | 2.71, ns | 3.00 |
| Region 3 - Rostral Body | 14.9 ± 2.3 | 12.8 ± 2.4 | 15.0 ± 1.3 | 17.1 ± 4.9 | 0.0, ns | 3.56, P < .07 |
| Region 4 - Ant. Midbody | 12.9 ± 1.7 | 10.8 ± 1.7 | 12.5 ± 2.5 | 14.8 ± 5.5 | 0.06, ns | 3.33, P<0.09 |
| Region 5 - Post. Midbody 3 | 12.8 ± 1.8 | 11.3 ± 1.3 | 12.8 ± 2.1 | 15.4 ± 6.1 | 0.12, ns | 2.40, ns |
| Region 6 - Isthmus 2 | 10.5 ± 1.8 | 8.3 ± 1.1 | 10.0 ± 1.3 | 12.9 ± 4.8 | 2.71, ns | 4.70, P < .05 |
| Region 7 - Splenium | 21.2 ± 3.3 | 17.5± 3.2 | 20.8 ± 2.1 | 22.9 ± 6.3 | 0.0, ns | 2.28,ns |
Notes: Callosal measurements means and standard deviations are reported in mm .
= square root transformed values used for analyses, raw scores presented in Table.
= log transformed values used for analyses, raw scores presented in Table.
= rank transformed values used for analyses, raw scores presented in Table.
df=1,17
3.5.3 VBM Results
A significant main effect for genotype (pFDR_corrected: 0.002, Z-score: 3.43) was found, and VFD-reared primates with one or two “s” alleles had reduced posterior CC volume when compared to the other animals. However, no genotype effect was observed for left or right hippocampal volume.
3.5.4 Whole-Brain Exploratory Analysis
There were significant genotype effects on multiple subcortical and cortical brain regions (see Table 1). Primates who carried at least one “s” allele had smaller volumes of cuneus bilaterally, left fusiform gyrus, and right intraparietal sulcus. Moreover, greater volumes of right amygdala, right striatum, bilateral middle temporal gyrus, right superior temporal gyrus, and right inferior frontal gyrus volumes were observed in the primates with one or two “s” alleles. As these analyses were not adjusted for multiple comparisons due to the small sample, these results should be considered preliminary.
Table 1.
Brain regions, P and Z scores corresponding to significant effects for rearing conditions, genotype, and genotype by early condition interaction.
| Brain Area | SPM Analysis | |
|---|---|---|
| P value (uncorrected) | Z-score | |
| Main effect for rearing condition | ||
| Non-VFD > VFD | ||
| Hippocampus (left) | 0.01 | 2.67 |
| Right inferior temporal gyrus | 0.001 | 4.60 |
| Left middle temporal gyrus | 0.005 | 2.61 |
| VFD > non-VFD | ||
| No differences | --------- | --------- |
| Main effect for genotype | ||
| Genotype “ll > “ss/sl” | ||
| Corpus Callosum | 0.002 | 3.43 |
| Left cuneus | 0.001 | 3.04 |
| Right cuneus | 0.002 | 2.96 |
| Left fusiform gyrus | 0.003 | 2.76 |
| Right intraparietal sulcus | 0.003 | 2.19 |
| Genotype “ss/sl > “ll” | ||
| Right superior temporal gyrus | 0.001 | 3.90 |
| Right inferior frontal gyrus/orbitofrontal gyrus | 0.001 | 3.21 |
| Left middle temporal gyrus | 0.004 | 2.95 |
| Right middle temporal gyrus | 0.002 | 2.83 |
| Right striatum | 0.002 | 2.81 |
| Right amygdala | 0.003 | 2.24 |
3.5.5 Brain-Behavior Analyses
: The 12 monkeys, assessed using the intruder stress test, were: 4 VFD animals with one or more “s” alleles, 4 VFD animals who were homozygous for the “l” allele, and 4 non-VFD animals who were also homozygous for the “l” allele. As shown in Figure 3a, VFD-reared primates with one or two “s” alleles showed significantly greater emotional responsivity than non-VFD animals, with ratings of the VFD “l” subjects midway between the two groups.
Figure 3. Behavioral Ratings of Emotional Responsivity.
(a) VFD primates with one or more “s” allele showed greater emotional responsivity during the masked intruder stress paradigm than non-VFD subjects, with the ratings of the VFD “l” subjects midway between the two groups. (b) corpus callosum measures correlated significantly with the emotional responsivity measure, with the animals that exhibited the lowest intruder stress having the largest corpus callosum mid-sagittal area.
4. Discussion
Few nonhuman primate studies have examined the effect of early-life stress on the corpus callosum (CC) and the hippocampus (Table 3). In the current study, bonnet macaques subjected to early-life stress were found to have a smaller mid-sagittal CC cross sectional area than monkeys reared under normal conditions. This is consistent with earlier studies in prepubescent nonhuman primates (Sanchez et al., 1998; Coe et al., 2002), but not with a recently published report that found no differences in CC (Spinelli et al., 2009). Given the gender differences in the CC (Bachmann et al., 2003; Suganthy et al., 2003) and the gender-by-early stress effect on the CC (Coe et al., 2002; see Table 3), it is possible that Spinelli and collaborators (2009) were not able to detect a rearing effect on the CC as a result of using a mixed-gender cohort. In addition, they were not able to investigate the sex-by-rearing interaction due to a limited number of subjects.
Table 3.
Summary of nonhuman primate structural imaging studies that examined early-life stress effect on the corpus callosum cross-sectional area and the hippocampus volume.
| Study | Monkey species | Approximate age of MRI acquisition | Approximate ELS timing | No. of subjects in the ELS group | No. of subjects in the control group | Type of ELS | Findings |
|---|---|---|---|---|---|---|---|
| Sanchez et al., 1998 | Rhesus | 18 months | Age 2-12 months | 9 males | 11 males | Nursery rearing | Reduced CC area in ELS group No HC differences |
| Coe et al., 2001 | Rhesus | 7-11 months | Day 90-140 of post-conception (30% of pregnancy) | 6 males 5 females | 3 males 2 females | Acoustical startle | Reduced CC area in the males of ELS group Enlarged CC area in the females of ELS group |
| Spinelli et al., 2009 | Rhesus | 24-32 months | Age 0-6 months | 6 males 7 females | 7 males 8 females | Peer rearing | No CC differences Significant main effect of sex on CC (female > male) No HC differences |
| Current study | Bonnet | 59-66 months | Age 2-6 months | 14 males | 9 males | Variable foraging demand | Reduced CC area in ELS group Reduced left but not right HC volume in ELS group |
| Lyons et al., 2001 | Squirrel | 43-71 years | Age 3-6 months | 6 males 7 females | LFD: 7 males 6 females HFD: 7 males 6 females |
Intermittent social separation | No HC differences Heritability effect on HC |
Abbreviations: Early-life stress (ELS); Corpus callosum (CC); Hippocampus (HC); High foraging demand (HFD); Low foraging demand (LFD);
Corpus callosum measurements in the current study were stable throughout life and correlated with behavioral indices of fearfulness as assessed using the intruder stress paradigm. This finding is consistent with human studies which have reported smaller CC area (De Bellis et al., 1999) and reduced fractional anisotropy, a measure of white matter integrity (Jackowski et al., 2008), to be associated with greater PTSD and anxiety symptoms in children. It is currently unclear, however, whether changes in CC are directly involved in regulating fearful behavior, or simply correlate with it.
As has been discussed elsewhere (Jackowski et al., 2008), the medial and posterior portions of the CC contain interhemispheric projections from the auditory cortices, posterior cingulate, insula, and somatosensory and visual cortices to a lesser extent. It also includes connections from the inferior parietal lobe to the contralateral inferior parietal lobe, superior temporal sulcus, cingulate, retrosplenial cortex, and parahippocampal gyrus (Pandya and Seltzer, 1986). Several of the regions with interhemispheric projections through the medial and posterior CC have connections with prefrontal cortical areas, and are involved in circuits that mediate the processing of emotional stimuli and various memory functions -- core disturbances associated with a history of early trauma.
This is the first imaging study to show structural hippocampal deficits in nonhuman primates subject to early-life stress. These results are in contrast with previously reported two prepubescent rhesus monkey and one early-adult squirrel monkey studies which showed no differences in the hippocampal volume (Sanchez et al., 1998; Lyons et al., 2001; Spinelli et al., 2009; see Table 3). Stress is known to affect the trajectory of brain development. In rodents, early-life stress seems to affect the hippocampus though the effects are seen only in adulthood and not during development (Andersen and Teicher, 2004). These findings parallel human studies which showed an association between childhood adverse events and small hippocampal size in adults (Bremner et al., 1997; Stein et al., 1997; Driessen et al., 2000; Vythilingam et al., 2002) but not in children (De Bellis et al., 1999; Carrion et al., 2001; De Bellis et al., 2001; De Bellis, 2002; Tupler and De Bellis, 2006; Woon and Hedges, 2008). In the current study, bonnet macaques were scanned after puberty at about four years of age. Thus, the difference in age can potentially account for the conflicting results between our study and the previous rhesus monkey studies that examined animals of less than 3 years of age (Sanchez et al., 1998; Spinelli et al., 2009). Lastly, the use of different form of stress (i.e. intermittent social separation vs. VFD) and different type of monkeys (i.e. squirrel vs. bonnet macaques) may explain the lack of rearing effect on the hippocampus in the adult squirrel monkey study (Lyons et al., 2001).
Consistent with previous studies in adults with PTSD (Kitayama et al., 2005; Smith, 2005; Karl et al., 2006), the nonhuman primates subjected to early stress had smaller left hippocampal volume than the primates reared under normal conditions. The mechanisms responsible for the lateralization of hippocampal findings in humans and nonhuman primates, however, are not currently understood. Preclinical studies of the effects of stress suggest a minimum of four mechanisms by which hippocampal atrophy may result from stress: dendritic atrophy, synaptic remodeling (synaptic overproduction and pruning), increased neuronal death, and decreased rates of neurogenesis (Sapolsky et al., 1985; Watanabe et al., 1992; Gould and Cameron, 1996; Sapolsky, 1996; Andersen and Teicher, 2004).
Reductions in middle and inferior temporal gyri were also noted in association with VFD rearing. The middle temporal gyrus and inferior temporal gyrus subserve language and semantic memory processing, visual perception, and multimodal sensory integration (Onitsuka et al., 2004). Structural and functional changes in these regions have not been systematically examined or reported (Karl et al., 2006; Etkin and Wager, 2007), and further examination of these regions is required to verify the significance of these findings.
Given the limited number of subjects, the genetic findings should be considered preliminary. In the current study, the exploratory examination of the genetic and rearing effects suggest that CC reduction was most marked in VFD primates with one or two “s” alleles of the serotonin transporter gene (5-HTTLPR). This pattern of findings was observed in the ROI analysis of mid-sagittal cross sectional CC area, and by the VBM analysis examining CC volume. Replication of these findings in larger samples that will allow for statistical testing of these associations is needed. Additional work is also needed to characterize the histological and biochemical processes that may be responsible for these effects on CC development. Alternatively, no genotype or gene-by-rearing effect was evident in our hippocampal measures. Future work is needed to assess whether gene-by-rearing interaction affects more subtle histological or biochemical processes in the hippocampus.
Several limitations of the present study warrant mentioning. The MRI scans were performed only in male subjects, so we do not know whether females are similarly affected. Additionally the small number of “s” allele subjects in the non-VFD group is another limitation of the study and consequently, a rearing condition by-genotype analysis could not be performed. The small sample size and subsequent inability to control for multiple comparisons in the exploratory VBM analyses is another weakness of the current investigation. Use of more sophisticated VBM analyses to examine gray and white matter separately is warranted in the future, together with postmortem studies to determine if the CC findings are associated with changes in axon number, size, myelination, or both. Additional histological and biochemical characterizations are needed to identify molecular mechanisms responsible for gross morphological changes noted here and to help understand how 5-HTTLPR and early-life stress modify both structure and function of circuits that control stress reactivity.
This report describes the first longitudinal study to demonstrate a stable structural brain change in white matter that correlate with fearfulness. Nonhuman primates provide a powerful model in which to combine neuroimaging, behavioral, and postmortem analyses to delineate the mechanisms by which early stress and genetic makeup interact to produce long-term changes in brain development, stress reactivity, and risk for psychiatric disorders. Improved understanding of these processes will likely lead to the development of novel strategies for the prevention and treatment of neuropsychiatric disorders.
Figure 2. Voxel Based Morphometry Analyses.
VFD rearing was also associated with reduced left hippocampal volume (arrow).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andrews MW, Rosenblum LA. Attachment in monkey infants raised in variable- and low-demand environments. Child Development. 1991;62:686–93. [PubMed] [Google Scholar]
- Bachmann S, Pantel J, Flender A, Bottmer C, Essig M, Schroder J. Corpus callosum in first-episode patients with schizophrenia--a magnetic resonance imaging study. Psychological Medicine. 2003;33:1019–27. doi: 10.1017/s0033291703008043. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD, Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL, Bennett AJ, Heils A, Long JC, Lorenz JG, Shoaf SE, Linnoila MV, Heinz A, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Weinberger DR, Linnoila M. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcoholism: Clinical and Experimental Research. 2003;27:812–7. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD, Heinz A, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Weinberger DR, Linnoila M. Early experience and serotonin transporter gene variation interact to influence primate CNS function In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. American Journal of Psychiatry. 1998;155:118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–51. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–63. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, Yan L, Zhang J, Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Research. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. Epub 2005 Dec 20. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Developmental Psychobiology. 2002;41:178–85. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Altemus M, Mathew SJ, Smith EL, Sharf B, Coplan PM, Kral JG, Gorman JM, Owen MJ, Nemeroff CB, Rosenblum LA, Trost RC, Scharf BA, Martinez J, Monn JA, Schoepp DD. Synchronized maternal-infant elevations of primate CSF CRF concentrations in response to variable foraging demand. CNS Spectrum. 2005;10:530–6. doi: 10.1017/s109285290001018x. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Kaufman J, Gelernter J, Smith EL, Perera TD, Dwork AJ, Kaffman A, Gorman JM, Rosenblum LA, Owens MJ, Nemeroff CB. Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: A pilot study. Psychoneuroendocrinology. 2010a doi: 10.1016/j.psyneuen.2010.07.011. doi:10.1016/j.psyneuen.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR, Smith EL, Dwork AJ, Perera TD, Pantol G, Carpenter D, Rosenblum LA, Shungu DC, Gelernter J, Kaffman A, Jackowski A, Kaufman J, Gorman JM. The role of early life stress in development of the anterior limb of the internal capsule in nonhuman primates. Neuroscience Letters. 2010b;480:93–6. doi: 10.1016/j.neulet.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T. Serotonergic genes modulate amygdala activity in major depression. Genes, Brain and Behavior. 2007 doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology. Part II: Brain development Biological Psychiatry. 1999;45:1271–84. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Harenski KA. Anterior cingulate N-acetylaspartate/creatine ratios during clonidine treatment in a maltreated child with posttraumatic stress disorder. Journal of Child and Adolescent Psychopharmacology. 2001;11:311–6. doi: 10.1089/10445460152595649. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–70. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–22. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Emdad R, Bonekamp D, Sondergaard HP, Bjorklund T, Agartz I, Ingvar M, Theorell T. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychotherapy and Psychosomatics. 2006;75:122–32. doi: 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Mathew S, Coplan J. Neurobiology of early life stress: nonhuman primate models. Seminars of Clinical Neuropsychiatry. 2002;7:96–103. doi: 10.1053/scnp.2002.31784. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Developmental Neuroscience. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LH, Krystal JH, Kaufman J. Corpus Callosum in Maltreated Children with PTSD: A Diffusion Tensor Imaging Study. Psychiatry Research Neuroimaging. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzko A, Rothenhofer S, Schmitt A, Gaser C, Demirakca T, Weber-Fahr W, Wessa M, Magnotta V, Braus DF. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. Journal of Affective Disorders. 2006;94:121–6. doi: 10.1016/j.jad.2006.03.010. Epub 2006 May 15. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience Biobehavioral Reviews. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. Epub 2006 May 26. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of Affective Disorders. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Brummer M, Hertz L, Quinn S, Kim Y, Bremner JD. Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: a preliminary study. Journal of Nervous and Mental Disorders. 2007;195:1027–9. doi: 10.1097/NMD.0b013e31815c044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen S, Liu J, Zhang J, He Z, Lin X. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Canadian Journal of Psychiatry. 2006;51:431–7. doi: 10.1177/070674370605100704. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Archives of General Psychiatry. 2001;58:1145–51. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Perera T, Lisanby SH, Rosenblum LA, Gorman JM, Coplan JD. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biological Psychiatry. 2003;54:727–35. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Human Psychopharmacology. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. American Journal of Psychiatry. 2004;161:1603–11. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commisural fibers. In Two Hemispheres - One Brain: Functions of the Corpus Callosum. In: Lepore F, Ptito M, Jasper HH, editors. Neurology and Neurobiology. New York. Vol. 17. Alan R. Liss, Inc.; 1986. pp. 47–74. [Google Scholar]
- Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Developmental Psychobioly. 2001;39:40–5. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. Journal of Neuroscience. 1985;5:1222–7. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural Pharmacology. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66:658–65. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Suganthy J, Raghuram L, Antonisamy B, Vettivel S, Madhavi C, Koshi R. Gender and age-related differences in the morphology of the corpus callosum. Clinical Anatomy. 2003;16:396–403. doi: 10.1002/ca.10161. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56:80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented Hippocampal Volume in Children and Adolescents with Posttraumatic Stress Disorder. Biological Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, Brooks WM. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Research. 2004;131:227–35. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588:341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–36. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Zeng X, Staib LH, Schultz RT, Duncan JS. Segmentation and measurement of the cortex from 3-D MR images using coupled-surfaces propagation. IEEE Transactions on Medical Imaging. 1999;18:927–37. doi: 10.1109/42.811276. [DOI] [PubMed] [Google Scholar]