Abstract
Approximately 12% of Americans do not consume the recommended level of zinc and could be at risk for marginal zinc deficiency. Zinc functions in antioxidant defense and DNA repair and could be important for prostate health. We hypothesized that marginal zinc deficiency sensitizes the prostate to oxidative stress and DNA damage. Rats were fed a zinc-adequate (ZA; 30 mg Zn/kg) or marginally zinc-deficient (MZD; 5–6 mg Zn/kg) diet for 6 weeks. MZD increased p53 and PARP expression but no change in 8-hydroxy-2′-deoxyguanosine levels was detected. To examine the susceptibility to exogenous oxidative stress, rats fed a ZA or MZD diet were assigned to exercising (EXE) or sedentary (SED) groups for 9 weeks. MZD or EXE alone did not affect oxidative DNA damage in the prostate; however, combined MZD + EXE increased DNA damage in the dorsolateral lobe. PARP and p53 expression was not further induced with MZD + EXE, suggesting that MZD interferes with DNA repair responses to stress. Finally, the addition of phytase to the MZD diet successfully restored zinc levels in the prostate and decreased DNA damage back to ZA levels. Overall, this study suggests that marginal zinc deficiency sensitizes the prostate to oxidative stress and demonstrates the importance of maintaining optimal zinc nutrition in physically active populations.
Keywords: Zinc deficiency, Prostate, DNA damage, 8-Hydroxy-2′-deoxyguanosine, Exercise, DNA repair, Free radicals
Approximately 12% of Americans do not consume the Estimated Average Requirement for zinc; thus a significant proportion of the U.S. population could be at risk for marginal zinc deficiency [1]. The prostate contains the highest concentration of zinc (Zn) of any soft tissue and secretes high amounts of zinc in prostatic fluid [2]. Zinc concentrations in malignant prostate tissues are about 10–25% of those found in healthy prostates [3], suggesting that high zinc concentrations may be essential for the maintenance of prostate health. However, the specific functions of zinc in the prostate and the mechanisms by which zinc maintains prostate health are still unclear.
Zinc is an important element in numerous transcription factors, antioxidant defense enzymes, and DNA repair proteins such as p53. In particular, zinc may function in cells to help maintain DNA integrity. Low cellular zinc increases oxidative stress and impairs DNA binding activity of p53 [4,5]. Thus, several different mechanisms may be involved in processes leading to impaired DNA integrity with zinc deficiency in vivo: (1) zinc deficiency may increase oxidative stress, which may directly cause DNA damage; (2) zinc deficiency may impair DNA damage repair responses [6].
One functional consequence of zinc deficiency is an increased susceptibility to exogenous oxidative stresses such as hyperoxia and endotoxin challenge [7,8]. We have previously shown that zinc deficiency increases oxidative stress and DNA damage in prostate epithelial cells in vitro [9]. We have also reported that severe zinc depletion (<1 ppm Zn in diet) in vivo increases oxidative stress and DNA damage [10]. Although marginal zinc deficiency is more physiologically relevant to human zinc deficiency than severe zinc deficiency [11], many of the studies have used only severe zinc depletion models. Very few in vivo studies have been done to examine the effects of marginal zinc deficiency on an animal's susceptibility to oxidative stress. The goal of this study was to examine the effects of marginal zinc depletion on DNA integrity in the prostate both alone and in the presence of chronic exercise as an exogenous oxidative stress. We hypothesized that physically active zinc-deficient animals may be more susceptible to oxidative stress and have impaired DNA repair, which would cause persistent DNA damage in the prostate.
In addition, because the prostate lobes differ markedly in zinc concentration, function, and embryological origin [1,12], we analyzed the dorsolateral and ventral lobes to examine lobe-specific effects. Finally, we added phytase to the marginally zinc-deficient diet to examine whether phytase supplementation of a low-zinc diet could reverse the deleterious effects of marginal zinc depletion on DNA integrity. Because the bioavailability of zinc in animal sources of foods is much higher than in plant sources of food, populations with negligible intake of animal proteins, such as vegetarians, may be at risk of marginal zinc deficiency. Phytate, which is present in high amounts in plant sources of food, is one of the major inhibitors of zinc absorption. Therefore, providing phytase supplementation to those populations susceptible to marginal zinc deficiency may increase zinc absorption and improve zinc status. This study is one of the first to examine the in vivo effects of dietary marginal zinc depletion and exercise on DNA integrity in the prostate and highlights the importance of maintaining adequate zinc status in physically active populations.
Material and methods
Animals
Marginal zinc depletion study
Male Sprague–Dawley rats (12/group, 5 weeks of age, ~110 g) from Charles River Laboratories (Wilmington, MA, USA) were acclimated for 1 week to the temperature- and humidity-controlled environment with a 12-h dark:light cycle. They were maintained in polycarbonate cages. Rats were randomly assigned to one of two dietary treatments: zinc-adequate diet (ZA; 30 mg Zn/kg) or marginally zinc-deficient diet (MZD; 5–6 mg Zn/kg).
Zinc and chronic exercise interaction study
Male Sprague–Dawley rats (12/group, 4 weeks of age, 125–150 g) from Charles River Laboratories were housed individually in polycarbonate cages and acclimated for 2 weeks to the temperature- and humidity-controlled environment with a 12-h dark:light cycle. The rats were then divided into voluntary wheel-running (EXE) or sedentary (SED) groups based on equal average body weight. Rats in the EXE group were assigned to cages equipped with 345-mm (diameter) running wheels (Mini Mitter Co., Bend, OR, USA) and were allowed unrestricted access to the running wheels. Each cage was fitted with a magnetic switch to allow for the counting of wheel revolutions using Vital View 3000 software (Mini Mitter). Animals were fed a ZA (30 mg Zn/kg) or MZD (5–6 mg Zn/kg) diet. To further examine the effects of phytase on zinc status and DNA integrity in the prostate, 1500 phytase units/kg diet (Natuphos, BASF Corp., Florham Park, NJ, USA) was added to the MZD diet and fed to the rats in both the EXE and the SED groups (MZD + P).
For the marginal zinc-depletion study, diets were based on modified AIN-93M diets formulated with egg white rather than casein (Research Diets, Inc., New Brunswick, NJ, USA) [10]. For the zinc and chronic exercise study, diets were prepared according to the LM-485 formulation provided by soy protein rather than casein (Research Diets) [13]. Zinc is provided as zinc carbonate in all diets. Deionized water was provided as drinking water. Rats were killed after anesthesia with isoflurane overdose (1–5%; Henry Schein, Melville, NY, USA). Diet intakes and body weights were measured twice per week. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the U.S. Army Research Institute of Environmental Medicine and Oregon State University, and the animals were maintained in accordance with the IACUC's guidelines for the care and use of laboratory animals.
Tissue and blood collection
Blood samples were collected by cardiac puncture into trace-element-free vials containing EDTA. Plasma was separated immediately and frozen at −80°C until analysis. Samples of prostate (whole prostate or microdissected dorsolateral and ventral prostate lobes) were dissected, and half was immediately snap frozen at −80°C and the other half was stored in RNAlater (Ambion, Austin, TX, USA) until analysis.
Zinc analysis
Plasma, ventral and dorsolateral lobe, and whole prostate (without microdissection) (n=12) zinc concentrations were determined by inductively coupled plasma–optical emission spectroscopy (ICP-OES; Teledyne Leeman Labs, Hudson, NH, USA) with a minor modification of a previously described method [14]. Plasma or prostate was digested in 1 ml 69–70% nitric acid overnight. After digestion, the samples were diluted 10 times with water treated with Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed by ICPOES against known standards [10].
DNA damage analysis
Comet assay in peripheral blood cells
Single-strand breaks in peripheral blood cells (n=10) were determined by alkali single-cell gel electrophoresis (comet assay) as described by Singh et al. [15]. Blood cell pellets isolated from fresh blood were mixed with low-melting-point agarose and applied onto microscope slides (Trevigen, Gaithersburg, MD, USA). DNA in lysed cells was allowed to unwind in alkali buffer (0.3 M NaOH, 1 mM EDTA) for 20 min. Samples then underwent electrophoresis for another 20 min at 300 mA, 25 V. Immediately before being scored, nuclear material was stained with 20 μl/circle ethidium bromide (20 mg/l). Comet measurements were made by image analysis using a Nikon E400 fluorescence microscope and Comet Assay III software (Perceptive Instruments, UK). Images of 100 randomly selected nuclei (50 nuclei from each of two replicate slides) were analyzed from each sample. The comet measurements of tail moment were recorded and used to indicate DNA damage in rat peripheral blood cells. One comet slide was made for each rat, and on each slide 50 comets were scored blindly for tail moments. Results are presented as fold of the tail moments compared to the zinc-adequate group.
ELISA of 8-hydroxy-2-deoxyguanosine in the prostate
DNA was isolated using a chaotropic sodium iodide method as described by Helbock et al. [16]. 8-OHdG levels (n=4) were measured in DNA extracted from rat prostate using an 8-OHdG ELISA kit (Japan Institute for the Control of Aging, Tokyo, Japan) following the manufacturer's instructions. Briefly, 50 μl of isolated DNA sample or standard was added to each well, and the plate was read at 450 nm using a Spectra Max plate reader (Molecular Device, Sunnyvale, CA, USA).
Quantitative real-time PCR analysis
The mRNA abundance of p53 and PARP in the prostate was measured by quantitative real-time PCR (qRT-PCR). Total RNA was extracted from prostate tissue using an RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA, USA). First-strand cDNA was reverse transcribed from the isolated RNA using the Superscript first-strand synthesis system (Invitrogen, Carlsbad, CA, USA). The primers for the measured transcripts were p53 forward, 5′-GCGTTGCTCTGATGGTGA-3′; p53 reverse, 5′-CAGCGTGATGATGGTAAGGA-3′; PARP forward, 5′-TGTGAACTCCTCTGCACCAG-3′; PARP reverse, 5′-AGCTGAGGC AGACACATCC-3′; 18S forward, 5′-GGACCAGAGCGAAAGCATTTGC-3′; 18S reverse, 5′-CGCCAGTCGGCATCGTTTATG-3′. The annealing temperature for all transcripts was 58°C, and the cycle number was 40. The PCRs were performed using the DyNAmo HS SYBR Green qPCR kit (New England BioLabs, Ipswich, MA, USA) as described by the manufacturer. A standard curve was generated from serial dilutions of purified plasmid DNA that encoded the gene of interest. Data represent averaged copy number normalized to the 18S housekeeping gene.
Western analysis of DNA repair proteins
Nuclear and cytosolic proteins were extracted from frozen prostate using the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA) as described by the manufacturer. Protein concentrations were determined using the DC Protein Assay (Bio-Rad Laboratories). Proteins (30 μg/lane) were separated by SDS–PAGE on a 4–12% Bis– Tris gel (Invitrogen) and transferred to nitrocellulose membrane (Bio-Rad Laboratories). Equal protein loading was confirmed with β-actin levels. After the membrane was blocked, the primary antibodies used for detection were mouse anti-p53 (Ab-1; Calbiochem, San Diego, CA, USA), mouse anti-poly(ADP ribose) polymerase (PARP; BD Pharmingen, San Jose, CA, USA), and mouse anti-β-actin (Sigma–Aldrich, St. Louis, MO, USA). Bound antibodies were detected using either goat anti-mouse IgG–HRP or goat anti-rabbit IgG–HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and developed with SuperSignal West Femto chemiluminescence substrate (Pierce, Rockford, IL, USA). Images were acquired on an Alpha Innotech photodocumentation system (Alpha Innotech, Hayward, CA, USA) and analyzed using Image J v1.37 software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed with the use of Prism (version 4.0; GraphPad Software, San Diego, CA, USA). Student's t test was used for two-group comparisons. Main effects of exercise, diet, and interaction factors were analyzed by two-way ANOVA. Pair-wise comparisons between groups with the same exercise level or the same diet were analyzed by Bonferroni's post hoc test. Equal variances among groups were tested by Bartlette's test, and logarithm data transformation was taken into account for unequal variances. Differences were considered statistically significant at P<0.05. Values are means±SEM (standard error of the mean) unless otherwise indicated.
Results
Body weights
Marginal zinc depletion did not change the food intake in any of the studies (data not shown). By the end of the study, average body weights in the EXE group (322.3±5.4 g) were significantly lower than in the SED group (360.7±5.2 g, P<0.05), and the body weights in the MZD group (312.8±5.3 g) were significantly lower than in the ZA group (368.7±6.8 g, P<0.001).
Effects of marginal zinc depletion on DNA damage and DNA repair
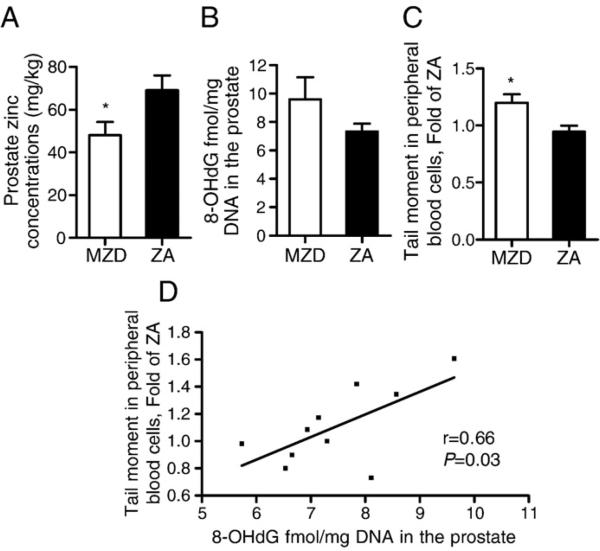
Marginal zinc depletion alone for 6 weeks significantly reduced plasma zinc concentrations by 22% (MZD vs ZA, 2.54±0.22 vs 3.19± 0.22 μg/ml; P<0.05) and prostate zinc concentrations by 30% compared to the ZA rats (Fig. 1A, P<0.05).
Fig. 1.
Effects of marginal zinc depletion on (A) zinc concentrations, (B) oxidative DNA damage in the whole prostate, and (C) DNA strand breaks in peripheral blood cells of rats. Rats were fed an MZD or ZA diet for 6 weeks. Oxidative DNA damage was measured by 8-OHdG ELISA and DNA strand breaks were measured by comet assay. *P<0.05, significantly different from the ZA group. (D) Prostate 8-OHdG concentrations were plotted as a function of tail moment of peripheral blood cells, with correlation r and P value. Values are means±SEM (n=10).
To examine the effects of marginal zinc depletion on DNA integrity, two types of DNA damage were measured: DNA strand-breaks in peripheral blood cells by comet assay and oxidative DNA damage in prostates by 8-OHdG ELISA. The tail moments of peripheral blood cells in the MZD group were 20% higher than in the ZA group (Fig. 1C, P<0.05), and the prostate 8-OHdG concentrations in the MZD group were 32% higher (NS) than in the ZA group (Fig. 1B, P=0.20). However, the 8-OHdG concentrations in the prostate were strongly and significantly correlated with the tail moments of peripheral blood cells (Fig. 1D, r=0.66, P=0.03).
Because DNA repair functions are crucial for maintaining DNA integrity, we examined the effects of marginal zinc depletion on the DNA repair proteins PARP and p53 in the prostate. PARP is a zinc-containing protein and plays an essential role in DNA repair [17]. The abundance of PARP and p53 mRNA in the prostates of the MZD rats was significantly higher than in the ZA rats (Figs. 2A and 2B, P<0.05). Consistent with the mRNA expression, both p53 and PARP protein levels in the prostates of the MZD rats were significantly higher than in the ZA rats (Figs. 2C and 2D, P<0.05). These data indicate that marginal zinc depletion activates DNA repair activities in the prostate and provide indirect confirmation of DNA damage stress in the prostate with marginal zinc deficiency. Activation of these repair systems might also partially account for why we did not observe significant increases in oxidative DNA damage in the prostates of the MZD rats.
Fig. 2.
Effects of marginal zinc depletion on (A, B) RNA transcript and (C, D) protein levels of the DNA repair proteins p53 and PARP in the whole prostate. Rats were fed an MZD or ZA diet for 6 weeks. (A, B) Results of qRT-PCR are presented as relative transcript copy number normalized to 18S transcripts, with fold of ZA. (C, D) Relative abundance of each protein was determined by densitometry analysis with normalization to β-actin. Representative Western blots are shown. Values are means±SEM (n=6). *P<0.05, significantly different from the ZA group.
Effects of marginal zinc depletion on chronically exercising rats
We performed a second marginal zinc-depletion study in voluntary wheel-running rats and examined the combined effects of marginal zinc depletion and chronic exercise on DNA integrity in the prostate. Zinc concentrations in plasma and prostate dorsolateral lobes of the MZD rats were significantly lower than in the ZA rats (Table 1, P<0.05). In contrast, zinc concentrations in prostate ventral lobes were not affected by marginal zinc depletion, indicating that prostate dorsolateral lobes, which retain higher zinc concentrations than the ventral lobes, were more susceptible to dietary marginal zinc depletion. Exercise alone had no effect on tissue zinc concentrations (Table 1, Exercise, P>0.05).
Table 1.
Zinc concentrations in plasma and prostate ventral and dorsolateral lobes of the EXE and SED rats fed the MZD or ZA dieta
| Exercise (EXE) |
Sedentary (SED) |
P value |
|||||
|---|---|---|---|---|---|---|---|
| MZD | ZA | MZD | ZA | Interaction | Exercise | Zinc | |
| Plasma (μg/ml) | 0.52 ± 0.06*** | 1.18 ±0.06 | 0.60 ± 0.04*** | 1.06 ±0.05 | 0.06 | 0.61 | <0.0001 |
| Prostate ventral lobe (mg/kg) | 12.9 ±1.2 | 12.9 ±0.4 | 10.9 ±0.9 | 12.0 ±1.5 | 0.62 | 0.19 | 0.62 |
| Prostate dorsolateral lobe (mg/kg)b | 58.3 ±1.2** | 237.1 ±1.1 | 71.7 ±1.2* | 177.4 ±1.1 | 0.22 | 0.83 | 0.0002 |
P<0.05
P< 0.01
P< 0.001, significantly different from the ZA rats within the same EXE or SED group.
Values are means ±SEM (n = 6). Main effects of exercise, zinc, and interaction were determined by two-way ANOVA with Bonferroni post hoc test.
Data were logarithm transformed to account for the unequal variance among groups before two-way ANOVA was performed.
We then evaluated oxidative DNA damage in the dorsolateral and ventral lobes and found that 8-OHdG concentrations in prostate dorsolateral lobes of the MZD rats were higher than in the ZA rats in the EXE group (206% of the ZA rats, Fig. 3A, P<0.05) but not in the SED group. Neither marginal zinc deficiency nor chronic exercise alone had any effect on prostate oxidative DNA damage (P>0.05), but a combination of both affected 8-OHdG concentrations in the dorsolateral lobes (interaction P=0.06). In addition, 8-OHdG concentrations in prostate ventral lobes were not affected by marginal zinc depletion or chronic exercise (Fig. 3B, P>0.05). Collectively, these results indicate that marginal zinc depletion increases rats' susceptibility to chronic exercise-induced oxidative stress and impairs DNA integrity specifically in prostate dorsolateral lobes. Because zinc concentrations and 8-OHdG concentrations were altered only in prostate dorsolateral lobes, we examined DNA repair proteins specifically in the dorsolateral lobes. Within the SED group, p53 and PARP mRNA abundance in the MZD rats was significantly higher than in the ZA rats (Figs. 4A and 4B, P<0.05), which is consistent with what we found in the whole prostates of the marginal zinc depletion study (Figs. 2A and 2B). Although chronic exercise alone did not significantly increase p53 expression (ZA of EXE vs ZA of SED, P=0.17), it did significantly increase PARP expression (P<0.05). Because the interaction factors of diet and exercise were not significant for either p53 or PARP expression (P>0.05, Figs. 4A and 4B) we combined the rats with the same diets. The main effects of diets were 1.65±0.10 (MZD) vs 1.13±0.10 (ZA) for p53 and 1.72±0.10 (MZD) vs 1.31± 0.14 (ZA) for PARP. Interestingly, despite increased DNA damage, marginal zinc depletion in the EXE group failed to further increase p53 and PARP gene expression (within the EXE group, MZD vs ZA, P>0.05). Thus, the ability of the prostate cells to respond to additional stressors and DNA damage may have been compromised. Collectively, these results suggest that marginal zinc depletion may compromise the protective responses of the prostate to chronic-exercise-induced oxidative stress and interfere with the activation of DNA repair protein expression.
Fig. 3.
Effects of marginal zinc depletion and chronic exercise on 8-OHdG levels in (A) dorsolateral and (B) ventral prostate lobes. Voluntary wheel-running or sedentary rats were fed an MZD or ZA diet for 9 weeks. Oxidative DNA damage was measured by 8-OHdG ELISA. Values are means±SEM (n=4). *P<0.05, significantly different between groups with the same exercise levels. #P<0.05, significantly different between groups with the same diet. P values for the main effects of exercise, zinc, and interaction are listed.
Fig. 4.
Effects of marginal zinc depletion and chronic exercise on the expression of the DNA repair genes (A) p53 and (B) PARP in the prostate dorsolateral lobes of rats. Voluntary wheel-running or sedentary rats were fed an MZD or ZA diet for 9 weeks. Values are means±SEM (n=6). *P<0.05, **P<0.01, significantly different between groups with the same exercise levels. #P<0.05, significantly different between groups with the same diet. P values for the main effects of exercise, zinc, and interaction are listed.
Effects of phytase supplementation on prostate DNA integrity
We found that the addition of phytase to the MZD diet increased zinc concentrations in the prostate dorsolateral lobes to the levels in the ZA rats regardless of group (EXE or SED, Fig. 5A, P<0.05). Combining the rats within the same diet, the main effects of diet on the zinc concentrations of prostate dorsolateral lobes were 57.5±1.2 (MZD) vs 151.4±1.2 (MZD + P) vs 199.5±1.12 mg (ZA) Zn/kg. However, phytase supplementation did not alter Fe or Cu concentrations in the plasma and prostate lobes (data not shown; diet factor, P>0.05). Along with elevated zinc concentrations in prostate dorso- lateral lobes, oxidative DNA damage was significantly reduced to the ZA levels in the MZD + P rats within the EXE group (Fig. 5B, P<0.05). These results indicate that phytase supplementation effectively reverses the deleterious effects of marginal zinc depletion on DNA integrity in the prostate dorsolateral lobes.
Fig. 5.
Effects of phytase supplementation on (A) zinc concentrations and (B) 8-OHdG levels in the prostate dorsolateral lobes. Voluntary wheel-running or sedentary rats were fed an MZD, MZD + P, or ZA diet for 9 weeks. Oxidative DNA damage was measured by 8-OHdG ELISA. Values are means±SEM (n=4). *P<0.05, **P<0.01, significantly different within the same exercise levels. #P<0.05, significantly different between groups with the same diet.P values for the main effects of exercise, diet, and interaction are listed.
Discussion
This study shows that neither marginal zinc deficiency nor chronic exercise alone caused oxidative damage in the prostate; however, the combined effects of low zinc with chronic exercise significantly increased oxidative DNA damage in the dorsolateral lobes of the prostate. Importantly, the dorsolateral lobe is the region that accumulates the highest levels of zinc in the prostate and is also the region most susceptible to prostate cancer [1,18]. The interactive effect of marginal zinc depletion and chronic exercise could be due to increases in oxidative stress in combination with the impairment of DNA damage responses through interference with the activation of p53 and PARP. In addition, we also show that phytase supplementation effectively maintained zinc levels in the prostate and prevented the impairment of DNA integrity in the prostate of rats fed marginal zinc-deficient diets. Overall, these results suggest that zinc deficiency may markedly affect one's response to exogenous oxidative stresses and DNA damaging agents and highlight the importance of maintaining optimal zinc nutrition in physically active populations to prevent oxidative DNA damage in the prostate.
Prostates contain the highest zinc concentration of any soft tissue [2]. It has been proposed that maintaining high zinc content is essential for prostate health, and losing the ability to accumulate high zinc levels may contribute to the prostate malignancy [19–23]. However, prostate lobes have substantially different zinc concentrations and also have different susceptibility to malignancy. In human prostates, the peripheral zone contains much higher zinc than the other zones, and 80% of prostate malignancies develop in this zone [18]. The dorsolateral lobe of rat prostate is embryologically homo-logous to the peripheral zone of the human prostate [1] and retains higher levels of zinc than the ventral lobe [12]. Therefore, dorsolateral and ventral lobes may have differential sensitivity to marginal zinc depletion. This study confirms that there is a differential response to dietary zinc depletion between the dorsolateral and the ventral lobes of the prostate. The ventral lobe is able to maintain zinc concentrations during marginal zinc depletion, whereas the dorsolateral lobe preserves only 30–40% of its normal zinc concentration. Moreover, marginal zinc depletion and chronic exercise together significantly increase oxidative DNA damage only in the dorsolateral lobe and not in the ventral lobe. Altogether, the prostate dorsolateral lobe, not ventral lobe, is susceptible to marginal zinc depletion, oxidative stress, and oxidative DNA damage. One factor that may determine susceptibility to zinc deficiency is the prostate's ability to maintain zinc homeostasis. It has been proposed that the dysregulation of zinc transporters in the prostate may impair zinc homeostasis and contribute to prostate malignancies [3,20,23]. Downregulation of zinc transporters, including ZnT1, ZnT3, Zip1, and Zip2, has been found to be associated with the low intracellular zinc content in human prostate cancer tissues or prostate epithelial cancer cell lines [19–21,23–26], and redistribution of ZIP3 was found in the tumorigenic human prostate epithelial cell line RWPE2 [20]. Examination of the mechanisms regulating zinc concentrations and compartmentalizing in the prostate during zinc deficiency and prostate cancer progression is an important area for future studies.
Previous studies done in our laboratory have shown that dietary zinc depletion increases DNA strand breaks in rat peripheral blood cells through increases in oxidative stress and interference of DNA repair proteins including p53 and PARP [10]. In vitro we have found that p53 binding activity is impaired in prostate epithelial cells with zinc depletion [9,27]. However, this study found that in the prostate, dietary marginal zinc depletion alone did not significantly increase oxidative DNA damage, despite a 30% reduction in prostate zinc concentrations. Similarly, chronic exercise alone did not increase oxidative DNA damage in the prostate. These results are not entirely surprising because of the possibly health-beneficial effects of chronic exercise. Indeed, vigorous exercise increases oxidative damage and the generation of ROS/RNS [28–31] by enhancing oxygen consumption and inducing inflammatory responses as a result of tissue injury [32]. However, it has been shown that long-term chronic exercise training may beneficially reduce mitochondrial hydrogen peroxide [33,34] in the muscle tissue. Therefore, chronic exercise alone may not impair DNA integrity. On the other hand, chronic moderate exercise may induce adaptation responses in the body by increasing the expression of antioxidant enzymes such as CuZnSOD [33,35,36]. It has been known that Zn is an essential component of CuZnSOD, and marginal zinc depletion might diminish the beneficial effects of exercise by compromising these adaptive responses. Closer examination of adaptive stress responses with zinc deficiency are an important area for future research.
Although neither marginal zinc deficiency nor chronic exercise alone increased oxidative DNA damage, a combination of both factors increased oxidative DNA damage in the prostate dorsolateral lobe. One possible mechanism is that the collective oxidative stress caused by both marginal zinc depletion and chronic exercise exceeds the capacity of antioxidant defense and DNA repair, resulting in oxidative DNA damage. It has been established that zinc has an antioxidant capacity and that zinc depletion in rats generates carbon-centered free radicals in lung microsomes [37] and oxidatively modified proteins and lipids in various tissues [38–41]. Studies in our lab have shown that both severe [10] and marginal zinc depletion [42] increases F2-isoprostanes in rat plasma. The mechanisms for the antioxidant functions of zinc include: (1) zinc protects sulfhydryl groups from oxidation, thereby maintaining normal function of proteins such as 5-aminolaevulinate dehydratase [43]; (2) zinc maintains the reductive intracellular environment through the modulation of thiol status [40,44]; (3) zinc antagonizes the activity of bivalent transition metals, including iron and copper, and prevents deleterious free radical reactions (e.g., Fenton reaction); (4) zinc regulates the expression of metallothionein [45,46] and CuZnSOD activity.
A second possible mechanism for the interactive effects of marginal zinc depletion and chronic exercise on DNA integrity could be related to DNA repair functions, which are insufficiently activated and unable to respond to DNA damage. This study specifically examined the DNA repair proteins p53 and PARP in the prostate. p53 is a zinc-containing protein and functions in DNA repair, cell proliferation, and cell death [47]. Zinc is located in the DNA binding domain of p53 and is essential for the DNA binding activity of p53. Although p53 expression is induced in zinc-depleted cells and animals [4,47], the DNA binding activity is impaired by zinc deficiency [9,27]. PARP is also a zinc-containing protein, and PARP-like zinc finger is essential for the recognition of DNA strand breaks and DNA binding of PARP [48]. PARP binds to DNA single-strand breaks, which are created during base excision repair (BER), through its zinc-finger motif and recruits other DNA repair factors (e.g., XRCC and DNA ligase) to the nick to finish BER [48,49]. One human study revealed a positive correlation between cellular poly(ADP-ribosyl)ation capacity and zinc status in PBMC, indicating that zinc could be required for PARP activity [50]. PARP is activated by the accumulation of DNA strand breaks and 8-OHdG in nuclear DNA and plays pivotal roles in DNA-repair and cell check-point pathways [17,51]. If cells possess normal DNA damage responses, increases in oxidative stress and DNA lesions will effectively activate p53 and PARP expression to repair or eliminate DNA damage. With marginal zinc depletion, we measured increased expression in p53 and PARP. However, we did not detect significant increases in p53 and PARP in the marginal zinc-depleted EXE rats in comparison with the zinc-adequate EXE rats (Figs. 4A and 4B), although oxidative DNA damage was substantially increased by marginal zinc depletion in the EXE rats. This inconsistent alteration between DNA lesion and DNA repair proteins suggests that marginal zinc depletion might inhibit the further synthesis of p53 and PARP during the DNA damage response, which could be due to the shortage of available zinc for incorporation into the p53 and PARP proteins. However, further mechanistic studies are required to test this hypothesis. Regardless, these data indicate that marginal zinc depletion may have impaired DNA damage responses in the prostates of chronically exercising rats and increased the susceptibility of the prostate to oxidative DNA damage.
It is important to note that marginal zinc depletion by itself may affect running performance. In this study, we did not observe any difference in daily running distance between the MZD and the MZD + P or ZA groups until the end of the study. By week 6, the MZD group had less daily running distance than the MZD + P or ZA groups, which were not different from each other [13]. However, we would expect that the decreased exercise level would more likely to decrease, not increase, DNA lesions, as found in this study. To address the question about whether marginal zinc depletion has different effects on DNA damage in combination with different levels of exercise, future studies using strictly controlled levels of exercise need to performed.
High phytate consumption markedly decreases the bioavailability of dietary zinc because phytate forms an insoluble complex with zinc that is difficult to absorb in the gastrointestinal tract. Phytate is a major factor that accounts for why vegetarians or people in developing countries are at high risk of zinc deficiency. It has been demonstrated that dietary microbial phytase supplementation in animals fed zinc-deficient diets improves zinc status [13,52–55]. This study further shows that phytase supplementation improved zinc status and reversed the effects of marginal zinc depletion on DNA integrity in the prostate and suggests that phytase supplementation might be an effective intervention strategy to prevent zinc-deficiency-induced DNA damage in populations habitually consuming high-phytate, low-zinc diets.
Overall, this study shows an important interactive effect of marginal zinc deficiency and chronic exercise on impairing DNA integrity, suggesting that marginal zinc depletion sensitizes the prostate to oxidative stress and increases oxidative DNA damage. This study also points out the importance of maintaining optimal zinc nutriture in physically active populations and highlights the importance of zinc in prostate health.
Acknowledgments
We gratefully acknowledge Dr. David Yu and the Cancer Chemoprotection Core at the Linus Pauling Institute and the W.M. Keck Collaboratory at Oregon State University for their assistance in conducting these studies. This study was funded by Oregon AES (OR00735) and the Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210) and by the U.S. Army MRMC. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense.
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- BER
base excision repair
- EXE
voluntary wheel running group
- ZA
zinc-adequate group
- MZD
marginally zinc-deficient group
- MZD + P
marginally zinc-deficient group with phytase supplementation
- PARP
poly(ADP ribose) polymerase
- SED
sedentary
References
- 1.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can. J. Med. Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol. Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oteiza PI, Clegg MS, Keen CL. Short-term zinc deficiency affects nuclear factor-kappaB nuclear binding activity in rat testes. J. Nutr. 2001;131:21–26. doi: 10.1093/jn/131.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Ho E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Iizuka Y, Furusawa S, Ishikawa M, Satoh S, Takayanagi M. Role of Zn2+ in oxidative stress caused by endotoxin challenge. Eur. J. Pharmacol. 2002;451:309–316. doi: 10.1016/s0014-2999(02)02223-9. [DOI] [PubMed] [Google Scholar]
- 8.Taylor CG, Bray TM. Effect of hyperoxia on oxygen free radical defense enzymes in the lung of zinc-deficient rats. J. Nutr. 1991;121:460–466. doi: 10.1093/jn/121.4.460. [DOI] [PubMed] [Google Scholar]
- 9.Yan M, Song Y, Wong CP, Hardin K, Ho E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J. Nutr. 2008;138:667–673. doi: 10.1093/jn/138.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno RS, Song Y, Leonard SW, Mustacich DJ, Taylor AW, Traber MG, Ho E. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J. Nutr. Biochem. 2007;18:509–518. doi: 10.1016/j.jnutbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Sandstead HH. Is zinc deficiency a public health problem? Nutrition. 1995;11:87–92. [PubMed] [Google Scholar]
- 12.Iguchi K, Usui S, Inoue T, Sugimura Y, Tatematsu M, Hirano K. High-level expression of zinc transporter-2 in the rat lateral and dorsal prostate. J. Androl. 2002;23:819–824. [PubMed] [Google Scholar]
- 13.Scrimgeour AG, Marchitelli LJ, Whicker JS, Song Y, Ho E, Young AJ. Phytase supplementation increases bone mineral density, lean body mass and voluntary physical activity in rats fed a low-zinc diet. J. Nutr. Biochem. 2009 Jul 1; doi: 10.1016/j.jnutbio.2009.03.015. [Electronic publication ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Verbanac D, Milin C, Domitrovic R, Giacometti J, Pantovic R, Ciganj Z. Determination of standard zinc values in the intact tissues of mice by ICP spectrometry. Biol. Trace Elem. Res. 1997;57:91–96. doi: 10.1007/BF02803873. [DOI] [PubMed] [Google Scholar]
- 15.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 16.Helbock HJ, Beckman KB, Ames BN. 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 17.Soldatenkov VA, Smulson M. Poly(ADP-ribose) polymerase in DNA damage-response pathway: implications for radiation oncology. Int. J. Cancer. 2000;90:59–67. doi: 10.1002/(sici)1097-0215(20000420)90:2<59::aid-ijc1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Gyorkey F, Min KW, Huff JA, Gyorkey P. Zinc and magnesium in human prostate gland: normal, hyperplastic, and neoplastic. Cancer Res. 1967;27:1348–1353. [PubMed] [Google Scholar]
- 19.Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, Skacel M, Tubbs R, Bagasra O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol. 2003;11:253–260. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;6:10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iguchi K. Zinc and metallothionein levels and expression of zinc transporters in androgen-independent subline of LNCaP cells. J. Androl. 2004;25:154–161. doi: 10.1002/j.1939-4640.2004.tb02771.x. [DOI] [PubMed] [Google Scholar]
- 22.Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 23.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200:187–195. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 25.Beck FW, Prasad AS, Butler CE, Sakr WA, Kucuk O, Sarkar FH. Differential expression of hZnT-4 in human prostate tissues. Prostate. 2004;58:374–381. doi: 10.1002/pros.10344. [DOI] [PubMed] [Google Scholar]
- 26.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 27.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J. Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 28.Mastaloudis A, Yu TW, O'Donnell RP, Frei B, Dashwood RH, Traber MG. Endurance exercise results in DNA damage as detected by the comet assay. Free Radic. Biol. Med. 2004;36:966–975. doi: 10.1016/j.freeradbiomed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Tanimura Y, Shimizu K, Tanabe K, Otsuki T, Yamauchi R, Matsubara Y, Iemitsu M, Maeda S, Ajisaka R. Exercise-induced oxidative DNA damage and lymphocytopenia in sedentary young males. Med. Sci. Sports Exerc. 2008;40:1455–1462. doi: 10.1249/MSS.0b013e31817242cf. [DOI] [PubMed] [Google Scholar]
- 30.Mastaloudis A, Morrow JD, Hopkins DW, Devaraj S, Traber MG. Anti-oxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic. Biol. Med. 2004;36:1329–1341. doi: 10.1016/j.freeradbiomed.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 31.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen CK, Packer L, Hñninen O. Handbook of Oxidants and Antioxidants in Exercise. Elsevier; Amsterdam: 2000. [Google Scholar]
- 33.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am. J. Physiol. 2005;289:R1564–1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 34.Venditti P, Masullo P, Di Meo S. Effect of training on H2O2 release by mitochondria from rat skeletal muscle. Arch. Biochem. Biophys. 1999;372:315–320. doi: 10.1006/abbi.1999.1494. [DOI] [PubMed] [Google Scholar]
- 35.Leeuwenburgh C, Hansen PA, Holloszy JO, Heinecke JW. Oxidized amino acids in the urine of aging rats: potential markers for assessing oxidative stress in vivo. Am. J. Physiol. 1999;276:R128–135. doi: 10.1152/ajpregu.1999.276.1.R128. [DOI] [PubMed] [Google Scholar]
- 36.Caillaud C, Py G, Eydoux N, Legros P, Prefaut C, Mercier J. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic. Biol. Med. 1999;26:1292–1299. doi: 10.1016/s0891-5849(98)00342-6. [DOI] [PubMed] [Google Scholar]
- 37.Bray TM, Kubow S, Bettger WJ. Effect of dietary zinc on endogenous free radical production in rat lung microsomes. J. Nutr. 1986;116:1054–1060. doi: 10.1093/jn/116.6.1054. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan JF, Jetton MM, Hahn HK, Burch RE. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am. J. Clin. Nutr. 1980;33:51–56. doi: 10.1093/ajcn/33.1.51. [DOI] [PubMed] [Google Scholar]
- 39.Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc deficiency-induced changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology. 2002;175:223–234. doi: 10.1016/s0300-483x(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 40.Shaheen AA, el-Fattah AA. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int. J. Biochem. Cell Biol. 1995;27:89–95. doi: 10.1016/1357-2725(94)00053-0. [DOI] [PubMed] [Google Scholar]
- 41.Canali R, Vignolini F, Nobili F, Mengheri E. Reduction of oxidative stress and cytokine-induced neutrophil chemoattractant (CINC) expression by red wine polyphenols in zinc deficiency induced intestinal damage of rat. Free Radic. Biol. Med. 2000;28:1661–1670. doi: 10.1016/s0891-5849(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress and antioxidant defenses in peripheral blood of rats. J. Nutr. 2009;139:1626–31. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs PN, Gore MG, Jordan PM. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem. J. 1985;225:573–580. doi: 10.1042/bj2250573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills BJ, Lindeman RD, Lang CA. Differences in blood glutathione levels of tumor-implanted or zinc-deficient rats. J. Nutr. 1981;111:1586–1592. doi: 10.1093/jn/111.9.1586. [DOI] [PubMed] [Google Scholar]
- 45.Liu CG, Zhang L, Jiang Y, Chatterjee D, Croce CM, Huebner K, Fong LY. Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res. 2005;65:7790–7799. doi: 10.1158/0008-5472.CAN-05-1345. [DOI] [PubMed] [Google Scholar]
- 46.Sun JY, Jing MY, Wang JF, Zi NT, Fu LJ, Lu MQ, Pan L. Effect of zinc on biochemical parameters and changes in related gene expression assessed by cDNA microarrays in pituitary of growing rats. Nutrition. 2006;22:187–196. doi: 10.1016/j.nut.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Fanzo JC, Reaves SK, Cui L, Zhu L, Wu JY, Wang YR, Lei KY. Zinc status affects p53, gadd45, and c-fos expression and caspase-3 activity in human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2001;281:C751–757. doi: 10.1152/ajpcell.2001.281.3.C751. [DOI] [PubMed] [Google Scholar]
- 48.Petrucco S, Percudani R. Structural recognition of DNA by poly(ADP-ribose) polymerase-like zinc finger families. FEBS J. 2008;275:883–893. doi: 10.1111/j.1742-4658.2008.06259.x. [DOI] [PubMed] [Google Scholar]
- 49.Flohr C, Burkle A, Radicella JP, Epe B. Poly(ADP-ribosyl)ation accelerates DNA repair in a pathway dependent on Cockayne syndrome B protein. Nucleic Acids Res. 2003;31:5332–5337. doi: 10.1093/nar/gkg715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunzmann A, Dedoussis G, Jajte J, Malavolta M, Mocchegiani E, Burkle A. Effect of zinc on cellular poly(ADP-ribosyl)ation capacity. Exp. Gerontol. 2008;43:409–414. doi: 10.1016/j.exger.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Oka S, Ohno M, Tsuchimoto D, Sakumi K, Furuichi M, Nakabeppu Y. Two distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAs. EMBO J. 2008;27:421–432. doi: 10.1038/sj.emboj.7601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rimbach G, Pallauf J. Enhancement of zinc utilization from phytate-rich soy protein isolate by microbial phytase. Z. Ernahrungswiss. 1993;32:308–315. doi: 10.1007/BF01611169. [DOI] [PubMed] [Google Scholar]
- 53.Yonekura L, Suzuki H. Effects of dietary zinc levels, phytic acid and resistant starch on zinc bioavailability in rats. Eur. J. Nutr. 2005;44:384–391. doi: 10.1007/s00394-004-0540-9. [DOI] [PubMed] [Google Scholar]
- 54.Pagano AR, Yasuda K, Roneker KR, Crenshaw TD, Lei XG. Supplemental Escherichia coli phytase and strontium enhance bone strength of young pigs fed a phosphorus-adequate diet. J. Nutr. 2007;137:1795–1801. doi: 10.1093/jn/137.7.1795. [DOI] [PubMed] [Google Scholar]
- 55.McClung JP, Stahl CH, Marchitelli LJ, Morales-Martinez N, Mackin KM, Young AJ, Scrimgeour AG. Effects of dietary phytase on body weight gain, body composition and bone strength in growing rats fed a low-zinc diet. J. Nutr. Biochem. 2006;17:190–196. doi: 10.1016/j.jnutbio.2005.07.003. [DOI] [PubMed] [Google Scholar]