Abstract
TLR5-deficient mice have been reported to develop spontaneous intestinal inflammation and metabolic abnormalities. However, we report that TLR5-deficient mice from two different animal colonies display no evidence of basal inflammatory disease, metabolic abnormalities, or enhanced resistance to Salmonella infection. In contrast, the absence of TLR5 hindered the initial activation and clonal expansion of intestinal flagellin-specific CD4 T cells following oral Salmonella infection. Together, these data demonstrate that a basal inflammatory phenotype is not a consistent feature of TLR5-deficient mice, and document a novel role for TLR5 in the rapid targeting of flagellin by intestinal pathogen-specific CD4 T cells.
Introduction
Toll-like Receptors (TLRs) allow host recognition of microbe-associated molecular patterns (MAMPS) and rapid initiation of an inflammatory response to invading pathogens (1, 2). Recent data also indicate that these innate immune receptors detect MAMPs expressed by commensal organisms and this recognition can be vitally important for maintaining immune homeostasis, particularly in the intestine (3). Lack of an individual TLR or adaptor molecules required for TLR signaling can therefore disrupt immune homeostasis and increase susceptibility to inflammatory diseases (4, 5).
TLR5 specifically recognizes flagellin, the major protein constituent of bacterial flagella, a conserved microbial structure known to be required for bacterial directed motility (6–10). Ligation of TLR5 initiates an innate immune response that is characterized by host cell production of inflammatory chemokines and cytokines, requiring Myd88, MAP kinase and NF-kB activation (9, 11, 12). Bacterial flagellins can also be recognized by non-TLR host cytosolic receptors, leading to Caspase-1 activation and IL-1β secretion (13), highlighting the importance of flagellin recognition for anti-bacterial immunity. The initial reports describing TLR5-deficient mice confirmed that TLR5 is required for the rapid inflammatory response induced after injection of soluble flagellin (14, 15), and also suggested that TLR5-deficient mice are more resistant to some flagellated pathogens (14), but also more susceptible to others (16).
In addition to this well-studied interaction of flagellin with host innate immune responses, flagellin also happens to be a protein antigen that is specifically targeted by the adaptive immune system during bacterial infection and inflammatory disease (17–19). Thus, the potential exists that host expression of a flagellin receptor could serve to modulate antigen-specific CD4 T cell responses to flagellin peptides (7). However, one recent study has argued against this possibility since flagellin-specific IgG responses were found to develop normally in the absence of TLR5 (20).
Other reports have suggested that TLR5 might play an important role in immune homeostasis, particularly in regulating host responses to intestinal microbial flora (9, 21). For example, TLR5-deficient mice were reported to develop spontaneous colitis due to an inflammatory response that was initiated by enteric flora and required TLR4 expression (22). Furthermore, TLR5-deficient mice were also reported to be innately resistant to infection with Salmonella typhimurium (23). Indeed, this resistance was most likely due to the basal intestinal inflammatory response described in TLR5-deficient mice, since it did not require host recognition of Salmonella flagellin (23). More recently, a re-derived line TLR5-deficient mice were reported to suffer from a profound metabolic syndrome, including hyperphagia, hypertension, resistance to insulin, and increased fat-pad mass (24). Together, these various reports strongly suggest that TLR5-mediated homeostatic responses to natural enteric flora are vitally important for maintaining overall host metabolism and also avoiding the initiation of harmful intestinal inflammation.
In this study, we have used a range of assays to examine several important metabolic and intestinal inflammatory variables and report the absence of basal inflammatory or metabolic defects in TLR5-deficient mice from two independent animal facilities. In marked contrast, TLR5-deficient mice displayed a prominent defect in the activation of flagellin-specific mucosal CD4 T cell responses following oral infection with flagellated Salmonella. Our data therefore demonstrate that TLR5 plays a crucial role in shaping the adaptive immune response to a flagellated pathogen, but suggest that any role for TLR5 in initiation of basal inflammatory or metabolic defects is likely to require unique floral components or other additional factors.
Materials and Methods
Mouse and bacterial strains
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and the Jackson Laboratory (Bar Harbor, ME) and used at 6–20 weeks of age. TLR5-deficient mice were bred at the University of Minnesota and the University of Birmingham, UK, from a line originally developed in the Akira laboratory (14). RAG-deficient SM1 TCR transgenic mice expressing CD90.1 or CD45.1 alleles have previously been described (25–28). We have previously shown that all peripheral T cells in SM1 mice have a naïve phenotype (28) and recognize a peptide close to the carboxy-terminus of Salmonella flagellin (427–441) in the context of I-Ab (18, 26). Myd88-deficient mice were provided by Dr. Way, University of Minnesota, and were bred at the University of Minnesota. All mice were cared for in accordance with University of Minnesota Research Animal Resource guidelines and Uk Home Office approval. Salmonella strain SL1344 was kindly provided by Dr. D. Xu, University of Glasgow, U.K. LPS-deficient S. typhimurium X4700 was generously provided by Dr. R. Curtiss, Arizona State University, AZ.
Examination of basal inflammatory and metabolic defects
Cohorts of wild-type and TLR5-deficient mice were weighed weekly to examine overall body mass. At approximately 10 or 20 weeks of age, blood was collected from groups of sex-matched wild-type and TLR5-deficient mice before cohorts from each group were sacrificed and organs harvested. Cecum and colon were observed closely for intestinal bleeding and overt signs of shrinkage or swelling before being weighed along with harvested spleens, livers, and fat pads. After weighing, spleens and livers were homogenized and whole organs plated onto LB agar to detect bacterial growth. SAA levels were examined in the sera using an ELISA kit according to manufacturers instructions (Biosource). Insulin and glucose levels were measured using commercially available assays and followed manufacturers instructions.
Purification and injection of flagellin
Flagellin was purified from S. typhimurium (X4700) using a modified acid-shock protocol (29, 30). Bacteria were grown at 37°C without shaking, before being washed and re-suspended in PBS/HCl (pH 2) for 30 minutes at room temperature. Bacterial supernatants were collected and ultracentrifugation and ammonium sulphate precipitation was used to purify flagellin. Residual LPS was removed by serial passage through multiple detoxigel columns (Pierce Biotechnology). Silver-stained SDS-PAGE gels were used to confirm purity of flagellin preparations and each batch of flagellin was found to be LPS-free using the Limulus assay. Groups of mice were injected intravenously with 10 μg Salmonella flagellin and serum collected to examine inflammatory cytokine production.
Salmonella infection and bacterial counts
S. typhimurium SL1344 or BRD509 (AroA−D−) were grown overnight in LB broth without shaking and diluted in PBS after estimation of bacterial concentration using a spectrophotometer. Mice were infected orally with 5x109 SL1344 after administration of 0.1ml 5% sodium bicarbonate. In all experiments the actual bacterial dose administered was confirmed by plating serial dilutions of the original culture onto MacConkey agar plates. Mice were monitored daily for signs of infection and were determined to be moribund if unresponsive to gentle prodding at which point they were euthanized as stipulated by our animal care protocol. To determine bacterial colonization in vivo, Peyer’s patches, mesenteric lymph nodes (MLN) and spleens from infected mice were homogenized in PBS and serial dilutions were plated onto MacConkey agar plates. After overnight incubation at 37°C, bacterial plates were counted and bacterial burdens calculated for each individual organ.
TCR transgenic adoptive transfers and analysis
Spleen and lymph node cells (inguinal, axillary, brachial, cervical, mesenteric, and peri-aortic) were harvested from SM1 mice and a single cell suspension was generated. An aliquot of this sample was stained using antibodies to CD4 and TCR Vβ2 in order to determine the percentage of TCR transgenic cells. Volumes were adjusted accordingly and 1x105–1x106 SM1 were injected intravenously into recipient C57BL/6 mice. At various time points after infection, Peyer’s patches, MLN, and spleens were harvested and a single cell suspension generated in complete EHAA medium. Samples were incubated on ice for 30 minutes in Fc block containing FITC-, PE-, PE-Cy5-, or APC-conjugated antibodies specific for CD4, CD11a, CD25, CD69, CD45.1, or CD90.1 (eBioscience and BD Bioscience). After staining, cells were fixed using paraformaldehyde and examined by flow cytometry using a FACS Canto. All flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Purification of dendritic cells and in vitro stimulation of T cells
Spleens were harvested from mice and incubated with collagenase D (37°C for 20 minutes) and EDTA to liberate dendritic cells. Magnetic anti-CD11c microbeads and multiple passes through selection columns (Miltenyi Biotech, Auburn, CA) were used to isolate CD11c+ spleen dendritic cells to 85–95% purity. Purified dendritic cells (1x105/well) were washed and placed in culture with SM1 T cells (1x105/well) plus titrated numbers of attenuated bacteria (BRD509) for 30 minutes. Cells were the harvested and washed before being cultured for 6 hours in medium containing antibiotics. Individual wells were harvested and stained for antibodies specific to CD4 and CD90.1 (to detect SM1 T cells) and surface activation molecules CD25 and CD69. Samples were acquired using a FACS Canto flow cytometer and data analyzed using FlowJo.
Statistical analysis
Data were first determined to be normally distributed and differences between groups examined using InStat (GraphPad Software, La Jolla, CA). Data in each group were compared using an unpaired t test and were considered significantly different with a p value of <0.05.
Results
TLR5-deficient mice do not develop basal inflammatory or metabolic defects
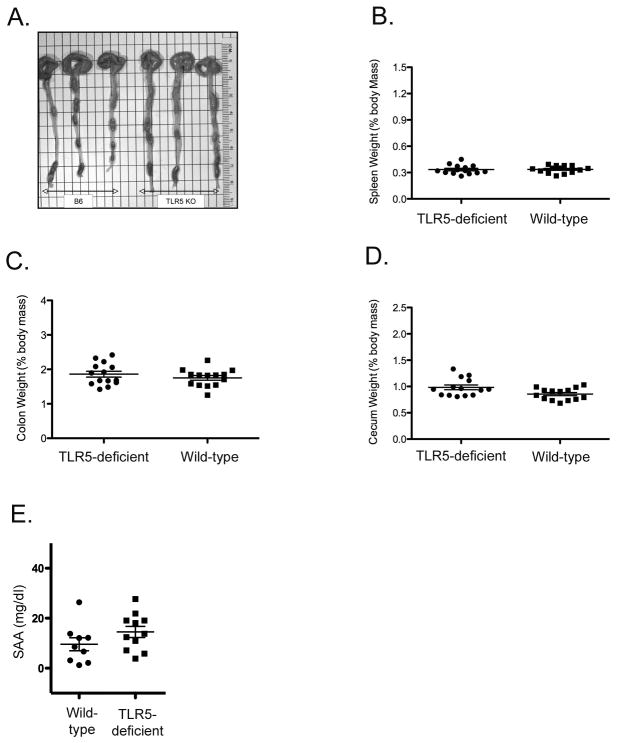
A recent study reported that 10–12% of TLR5-deficient mice developed spontaneous rectal prolapse and also had lower body weight, indicating the development of colitis (22). In the same study, analysis of the TLR5-deficient mice lacking prolapse found that 20% of these mice developed intestinal bleeding, around 30% had increased colon and spleen weight, and 40% had high levels of serum amyloid A (SAA) (22). In an effort to determine whether these findings are consistent among TLR5-deficient mouse colonies, we examined these variables in a TLR5-deficient colony at the University of Minnesota. Surprisingly, our analysis demonstrated that TLR5-deficient mice displayed no evidence of rectal prolapse, intestinal bleeding, contracted ceca, increased colon or spleen weight, or reduced cecum weight when compared to wild-type mice (Fig. 1, and data not shown). In addition, we found no evidence of rectal prolapse or increased spleen weight in an independent TLR5-deficient colony housed at the University of Birmingham, UK (data not shown). SAA levels were also found to be within a similar range for TLR5-deficient and wild-type mice at 10 weeks and 20 weeks of age (Fig. 1E, and data not shown). Furthermore, bacteria were absent from the spleen and liver of TLR5-deficient mice at Minnesota and Birmingham (data not shown), despite the fact that low level bacterial colonization in these organs was previously reported (22).
Figure 1. Lack of basal inflammatory phenotype in TLR5 Deficient mice.
Age-matched wild type and TLR5 deficient mice were euthanized at 10–12 weeks and examined for evidence of basal inflammation. (A) Photograph showing intestinal length from wild-type and TLR5-deficient mice. (B, C, D) Spleen, colon and cecum weight of wild-type and TLR5-deficient mice. Graphs show mean organ weight +/− SEM. (E) Serum Amlyoid A (SAA) levels for male wild-type and TLR5-deficient mice were determined by ELISA. Graph shows mean SAA levels +/− SEM for 9–11 mice per group. No significant difference in SAA levels was found when comparing wild-type and TLR5-deficient mice by unpaired t-test (p=0.1627).
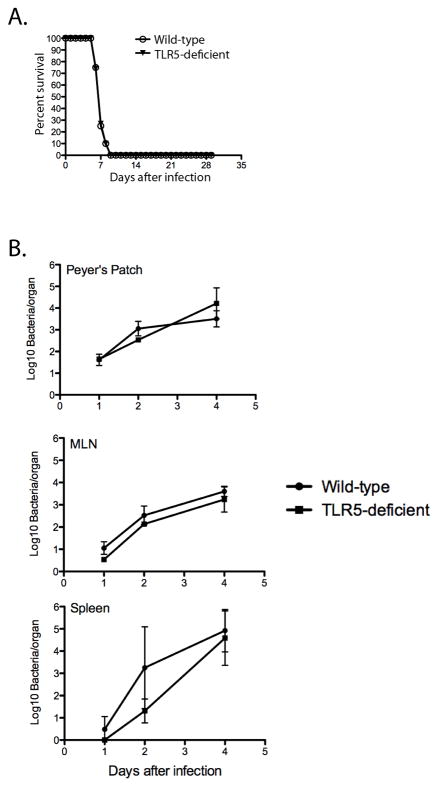
Given the previous correlation of basal intestinal inflammation and non-specific resistance to Salmonella (22, 23), we also examined the susceptibility of these mice to Salmonella infection and also monitored bacterial growth in the spleen. Consistent with our inability to detect a basal inflammatory state in TLR5-deficient mice, enhanced resistance to primary Salmonella infection was not observed, as measured by prolonged survival or reduced colonization after infection (Figure 2). Together these data demonstrate that a predisposition to spontaneous colitis, and non-specific resistance to Salmonella infection, is not a constant feature of TLR5-deficient mouse colonies.
Figure 2. TLR5-deficient mice and wild-type mice display equivalent susceptibility to oral infection with Salmonella.
Wild-type and TLR5-deficient mice were infected orally with 5x109 virulent Salmonella (SL1344) and (A) monitored daily and sacrificed when moribund or (B) bacterial burdens were examined on days 1, 2 and 4. Peyer’s patches, mesenteric lymph nodes and spleens were harvested and dilutions plated onto MacConkey agar plates to determine bacterial numbers at each time point. Survival graphs show the percentage of surviving mice in each group and represent 8–12 mice per group. Groups were found not to be statistically significant (p>0.05) using a Log-rank (Mantel-Cox) test. Lower plots show mean bacterial numbers +/− SD for 5 mice per group and are representative of 3 individual experiments. Groups were not statistically significant at any time point or in any organ by unpaired t-test (p>0.05).
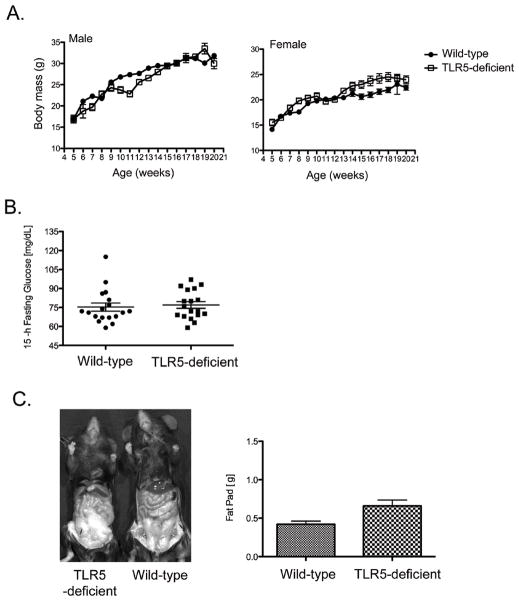
A more recent study reported that mice lacking TLR5 expression display a wide range of clinical and metabolic abnormalities, that bear some similarity to human metabolic syndrome (24). In this particular study, TLR5-deficient mice were found to have a 20% increase in body mass, significantly larger fat pads, elevation of blood glucose levels, and a substantial increase in basal insulin levels (24). Again, we examined many of these features in our two different TLR5-deficient colonies but were unable to detect significant differences in body mass, blood glucose levels, or basal insulin levels compared with wild type mice (Fig. 3, and data not shown). At 20 weeks of age, a small increase in fat pad size was observed in male TLR5-deficient mice (Fig. 3D). However, this was not detected in female mice, and did not appear to correlate with changes in any other measured parameter. Therefore, the development of basal inflammatory or metabolic disregulation is not a consistent feature of TLR5-deficient mouse colonies.
Figure 3. Absence of metabolic syndrome in TLR5-deficient mice.
(A) Groups of wild-type and TLR5-deficient mice were weighed at weekly intervals until 20 weeks of age. Data show mean body mass +/− SEM for male and female mice separately. Sex-matched groups were not significantly different at any time point by unpaired t-test (p>0.05). (B) Wild type and TLR5-deficient mice (20 weeks old) were fasted for 15 hours and blood glucose levels measured. (C) Abdominal adipose fat pads from 20 week old wild-type and TLR5-deficient mice were removed and weighed. A representative image (left) compares the abdominal region/adipose fat of a TLR5-deficient and wild-type male mouse, while the graph (right) shows mean fat pad weights +/− SEM. Fat pads were found to be significantly larger in 20 week old male TLR5-deficient mice compared with wild-type mice (p<0.05).
TLR5 is necessary for early targeting of intestinal CD4 T cell responses to a flagellated pathogen
Bacterial flagellin is a major antigenic target during infection and inflammatory disease (18, 19, 31–33), and it was therefore of interest to examine whether TLR5 deficiency had any effect on flagellin-specific T cell activation after infection with a flagellated pathogen. We previously generated a TCR transgenic mouse line using the TCR α and β chains of a T cell clone specific for Salmonella flagellin 427–441 in the context of I-Ab (18, 25). Naïve peripheral SM1 CD4 T cells can be used to monitor host T cell responses to bacterial flagellins in vitro and in vivo (7, 34, 35).
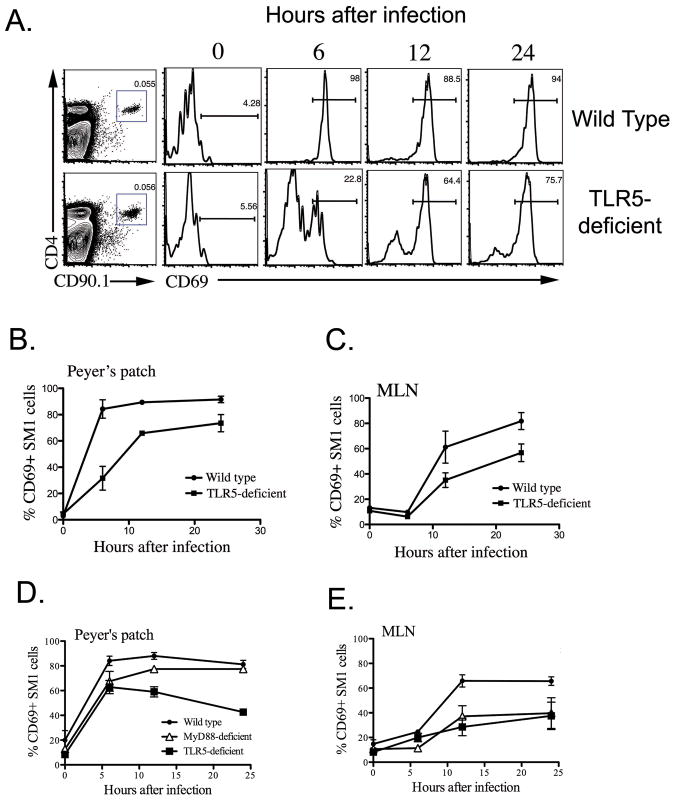
Wild-type and TLR5-deficient mice were adoptively transferred with flagellin-specific SM1 T cells, infected orally with Salmonella, and the activation and the expansion of SM1 T cells was directly examined in mucosal lymphoid tissues. As reported previously (36), almost all SM1 T cells in the Peyer’s patch of Wild-type mice were activated to express increased levels of CD69 within 6 hours of oral infection, and similar activation was detected a few hours later in the mesenteric lymph nodes (MLN) (Fig. 4). In marked contrast, only 25–30% of SM1 T cells were activated in the Peyer’s patch of TLR5-deficient mice, 6 hours after infection (Fig. 4). Furthermore, lower peak activation was detected at all time points examined in the Peyer’s patches and MLN of infected TLR5-deficient mice (Fig. 4). Similar deficient responses were also noted when CD25 was used as a marker of T cell activation (data not shown). Together these data demonstrate that TLR5 plays an obligatory role in the targeting of flagellin by intestinal Salmonella-specific CD4 T cells during oral infection. As SM1 T cells were ultimately activated in the Peyer’s patch and MLN of infected TLR5-deficient mice, we also examined whether delayed early activation affected clonal expansion of these cells at later time points. Indeed, clonal expansion of SM1 T cells was significantly lower in the Peyer’s patch and MLN of TLR5-deficient mice compared with wild-type mice, three days after oral infection (Fig. 5). Thus, TLR5 expression is required for the development of robust intestinal CD4 T cell responses to flagellin.
Figure 4. Deficiency in early flagellin-specific CD4 T cell activation after oral infection of TLR5-deficient mice with a flagellated pathogen.
Wild-type and TLR5-deficient mice were adoptively transferred with 800,000 SM1 T cells and infected orally with 5x109 virulent Salmonella (SL1344) the following day. At various time points after infection, Peyer’s patches and mesenteric lymph nodes were harvested from mice and SM1 T cells identified using antibodies specific for CD4 and CD90.1. The early activation of SM1 T cells was examined by determining the percentage of cells expressing CD69 following infection. (A) Representative FACS plots showing the detection of SM1 T cells in Peyer’s patches (Left plot) and subsequent activation of SM1 T cells by monitoring CD69 expression after gating on SM1 cells. (B–D) Graphs show mean percentage +/− SD of CD69+ SM1 T cells in Peyer’s patch and Mesenteric lymph node after infection.
Figure 5. TLR5 is required for optimal expansion of Salmonella flagellin-specific CD4 T cells.
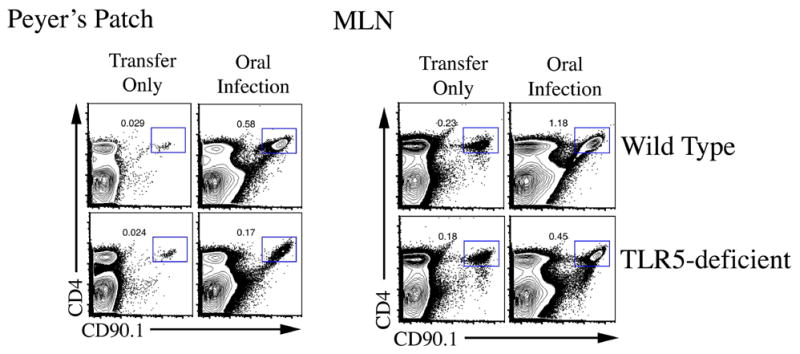
Wild-type and TLR5-deficient mice were adoptively transferred and infected as described in Figure 4. Three days after infection, Peyer’s patches and mesenteric lymph nodes were harvested and SM1 T cells identified using antibodies specific for CD4 and CD90.1. Plots show representative FACS plots for 3 mice per group.
We recently reported that TLR5 is able to function as an endocytic receptor enhancing antigen presentation of flagellin epitopes in an Myd88-independent manner after flagellin immunization (34). In order to determine if Myd88 was required for early SM1 T cell activation during Salmonella infection, we compared SM1 T cell activation in infected wild-type, Myd88-, and TLR5-deficient mice. Reduced SM1 T cell CD69 activation was again observed at all time points in the Peyer’s patch of TLR5-deficient mice (Fig. 4D). In contrast, similar activation was detected in wild-type and Myd88-deficient mice, although CD69 activation was lower in Myd88-deficient mice at the 6-hour time point (Fig. 4D). In marked contrast, SM1 CD69 expression was reduced in the MLN of both infected TLR5-, and Myd88-deficient mice (Fig. 4E). These data suggest that TLR5 can enhance Salmonella flagellin-specific T cell responses in the Peyer’s patch but may do so in an Myd88-independent manner while Myd88 signaling is likely to be important in the MLN.
TLR5-deficient dendritic cells do not activate flagellin-specific CD4 T cells after infection
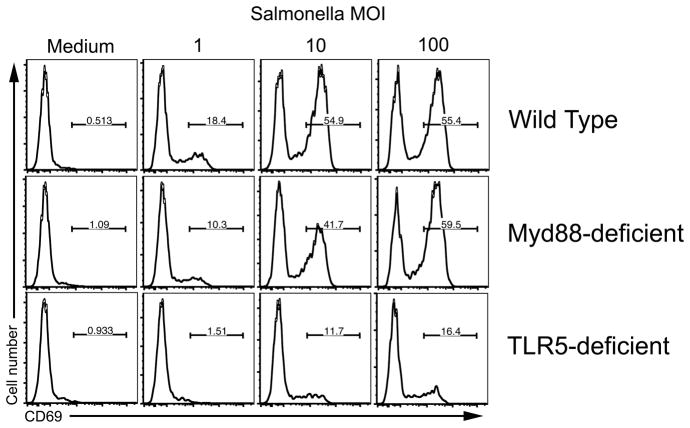
Given the differing results in the Peyer’s patch and MLN after infection, we decided to directly examine whether purified TLR5- and Myd88-deficient dendritic cells were capable of activating SM1 T cells in vitro in response to live Salmonella. As expected, dendritic cells from wild-type mice were able to activate SM1 T cells in vitro to increase expression of CD69 and CD25 (Fig. 6 and data not shown). However, TLR5-deficient dendritic cells poorly activated SM1 T cells in response to Salmonella infection and only at high MOIs (Fig. 6A). In contrast, dendritic cells from Myd88-deficient mice activated SM1 T cells to a similar degree and at similar MOIs as with wild-type dendritic cells (Fig. 6C). Thus, TLR5 expression is required in order to fully activate SM1 T cells in response to live bacteria in vitro while Myd88 expression is dispensable.
Figure 6. Dendritic cells require TLR5, but not Myd88, to activate flagellin specific T cells in response to Salmonella infection.
CD11c+ dendritic cells were purified from wild-type, TLR5-deficient, and Myd88-deficient mice and incubated with SM1 T cells in the presence of Salmonella (BRD509) at differing multiplicities of infection (MOI). Six hours after stimulation, cells were harvested and stained with antibodies specific for CD4, CD90.1 (to detect SM1 T cells), and CD69, before data was acquired using a flow cytometer. Representative CD69 histograms are shown for gated SM1 T cells from cultures with wild type, TLR5-deficient, or Myd88-deficient dendritic cells and are representative of three wells per group and two independent experiments.
Discussion
In contrast to previous observations (22–24), we were unable to detect any evidence of basal intestinal inflammation or metabolic irregularities in TLR5-deficient mice in two different animal facilities. The TLR5-deficient colony in Minnesota has been housed for over two years and never displayed any evidence of rectal prolapse or increased weight gain. Interestingly, both the TLR5-deficient colonies in Minnesota and Birmingham were derived from the same TLR5-deficient mouse line that was used in previous reports describing basal inflammation, and the genetic disruption is therefore identical. The most likely explanation for the discrepancy in TLR5-deficient basal disease is a difference in the enteric flora between institutional animal facilities or differences acquired during rederivation of these animals. Indeed, the basal metabolic deficits reported for TLR5-deficient mice were found to be alleviated by antibiotic treatment (24), indicating that bacterial flora is likely to drive this particular pathology. Thus, our data are still consistent with the possibility that TLR5-deficient mice have increased susceptibility to developing basal intestinal inflammatory defects, depending on the composition of the local enteric flora. Most importantly, however, our data demonstrate that these very obvious and severe inflammatory and metabolic deficiencies are not an obligatory phenotype of this particular mouse strain and require factors that are not present in animal facilities in Minnesota or Birmingham. Additional research will be required to identify the causative agent of this interesting and important process and determine the actual penetrance of a basal inflammatory phenotype in TLR5-deficient mice housed at other institutions.
Our primary infection experiments using TLR5-deficient mice also failed to demonstrate any substantial difference in the resistance of these mice to Salmonella. This finding is in broad agreement with previous studies where no difference in susceptibility to Salmonella infection was observed following i.p infection (14, 15), but differs markedly from studies using the oral infection route (23). However, it is very likely that the absence of a basal inflammatory defect in the intestine of our mouse colony allows a more accurate assessment of the role of TLR5 in resistance to Salmonella infection and we conclude therefore that TLR5 does not play a major role in initial innate immune defense against this particular pathogen. The presence of a basal inflammatory response might also explain the discrepancy between our data and a recent report examining flagellin-specific adaptive immune responses in TLR5-deficient mice (20). This study concluded that TLR5-deficient mice display no significant deficiency in the induction of flagellin-specific adaptive responses while our data suggest a significant deficiency exists after oral infection with a flagellated pathogen.
Our examination of adaptive immune responses in Salmonella-infected mice demonstrate that initial activation of flagellin-specific CD4 T cell responses is significantly delayed in the intestine of TLR5-deficient mice. We previously reported that very early activation of Peyer’s patch CD4 T cells requires the mobilization of CCR6+ dendritic cells to the epithelial layer, presumably to capture invading bacteria and process antigen (36). Given our current data, this early mobilization of antigen presenting cells may actually be driven by inflammatory signals that require TLR5 ligation at the epithelial surface. Indeed, flagellin stimulation is known to cause the production of CCL20 (37), the chemokine ligand for CCR6 (38). However, given the fact that T cell activation was lower in the Peyer’s patch of TLR5-deficient versus Myd88-deficient mice and during in vitro stimulation assays, we prefer an alternative explanation, namely that TLR5 simply functions as an endocytic receptor to enhance flagellin processing by mucosal antigen presenting cells in vivo. Indeed, we recently reported that TLR5 can function as an endocytic receptor after immunization with purified bacterial flagellin. If this pathway is also important in driving flagellin-specific T cell responses to oral infection, then TLR5 expression by intestinal antigen presenting cells should be required for directing the early adaptive response to flagellin. However, it is currently unclear whether Peyer’s patch or MLN dendritic cell subsets actually express TLR5. A recent report demonstrated that TLR5 expression by antigen presenting cells is required for the adjuvant effect of flagellin after immunization (39), thus supporting some aspects of our proposed model. However, irrespective of whether this model of TLR5 scavenging by antigen presenting cells during infection is correct, our data conclusively demonstrate that TLR5 expression is required for robust flagellin-specific CD4 T cell activation in the gut mucosa during early oral infection.
In conclusion, we report that TLR5-deficient mice do not develop basal inflammatory or metabolic defects or enhanced resistance to Salmonella infection. In contrast, TLR5 plays an obligatory role in directing CD4 T cell targeting to bacterial flagellins during oral infection with a flagellated pathogen.
Acknowledgments
The authors would like to acknowledge helpful discussions with the laboratory of Dr. S. Way in completion of these experiments.
This work was supported by grants from the National Institutes of Health, AI055743 and AI076278.
References
- 1.West AP, Koblansky AA, Ghosh S. Recognition and Signaling by Toll-Like Receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 2.Mackey D, McFall AJ. MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Mol Microbiol. 2006;61:1365–1371. doi: 10.1111/j.1365-2958.2006.05311.x. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Medzhitov R. Innate immune recognition of the indigenous microbial flora. Mucosal Immunol. 2008;1(Suppl 1):S10–14. doi: 10.1038/mi.2008.49. [DOI] [PubMed] [Google Scholar]
- 4.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Honko AN, Mizel SB. Effects of flagellin on innate and adaptive immunity. Immunol Res. 2005;33:83–101. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 7.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101:117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 9.Vijay-Kumar M, Gewirtz AT. Flagellin: key target of mucosal innate immunity. Mucosal Immunol. 2009;2:197–205. doi: 10.1038/mi.2009.9. [DOI] [PubMed] [Google Scholar]
- 10.Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. J Gastroenterol. 2009;44:803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 11.Ciacci-Woolwine F, I, Blomfield C, Richardson SH, Mizel SB. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 13.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 15.Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 17.Cookson BT, Bevan MJ. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognised by T cells from orally immunised mice. J Immunol. 1997;158:4310–4319. [PubMed] [Google Scholar]
- 18.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 19.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Akira S, Nunez G, Gewirtz AT. Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol. 2009;39:359–371. doi: 10.1002/eji.200838804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandoval DA, Seeley RJ. Medicine. The microbes made me eat it. Science. 328:179–180. doi: 10.1126/science.1188876. [DOI] [PubMed] [Google Scholar]
- 22.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijay-Kumar M, Aitken JD, Kumar A, Neish AS, Uematsu S, Akira S, Gewirtz AT. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSorley SJ, Asch S, Costalonga M, Rieinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173:4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 27.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan A, McSorley SJ. Pivotal advance: exposure to LPS suppresses CD4+ T cell cytokine production in Salmonella-infected mice and exacerbates murine typhoid. J Leukoc Biol. 2007;81:403–411. doi: 10.1189/jlb.0306194. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim GF, Fleet GH, Lyons MJ, Walker RA. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, Vella AT, McSorley SJ. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007;179:6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 31.Cookson BT, Cummings LA, Rassoulian Barrett SL. Bacterial antigens elicit T cell responses via adaptive and transitional immune recognition. Curr Opin Microbiol. 2001;4:267–273. doi: 10.1016/s1369-5274(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 32.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, Wrobel I, Quiros A, Vasiliauskas EA, Grill B, Israel D, Bahar R, Christie D, Wahbeh G, Silber G, Dallazadeh S, Shah P, Thomas D, Kelts D, Hershberg RM, Elson CO, Targan SR, Taylor KD, Rotter JI, Yang H. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41:29–38. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin AJ, McSorley SJ. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011 doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-Mediated Dendritic Cell Activation of Pathogen-Specific T Cells in Peyer’s Patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 39.Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009;182:7539–7547. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]