Abstract
Many microbial pathogens use specialized secretion systems to inject proteins referred to as effectors directly into eukaryotic host cells. These effectors directly target various eukaryotic signaling pathways and cellular processes, often by mimicking the activity of host cell proteins. Effectors of pathogenic Escherichia coli and Salmonella typhimurium can also act as molecular scaffolds that not only recruit but also directly regulate the activity and localization of multiple eukaryotic proteins. By assembling and localizing disparate signaling pathways, the bacteria can reengineer host cell processes to generate novel processes not previously observed in eukaryotic cells.
Numerous Gram-negative bacteria, including pathogens and endosymbionts, use complex secretion systems to deliver proteins directly into the host cell cytoplasm. These machines serve as channels to deliver tens of proteins across multiple lipid membranes, including the bacterial inner and outer membranes and the host cell plasma membrane. Once injected into target cells, the effectors target various host cell processes to promote bacterial spread and survival.
The majority of effectors studied to date appear to encode a single function, often resulting in the activation or inhibition of eukaryotic signaling pathways. Effectors may exhibit activities associated with mammalian regulators like Rho GAPs (GTP activating proteins) (1), Rho GEFs (GTP exchange factors) (2), and E3 ubiquitin ligases (3), or novel catalytic functions such as phosphothreonine lyase (4) or serine/threonine acetylation activity (5). Notably, even when effectors exhibit previously characterized eukaryotic functions, they often do so by novel means, a result of convergent evolution.
Type III secreted effectors can also manipulate host cell processes by acting as molecular scaffolds that link and modulate the activity of eukaryotic host cell proteins involved in unrelated cellular processes (6–8). In this way, the bacteria can reengineer the basic wiring of eukaryotic cells to confer novel phenotypes. Selyunin and colleagues revealed an example of this phenomenon through studies with EspG, a type III secreted effector common to both enteropathogenic and enterohemorrhagic E. coli (8).
Enteropathogenic and enterohemorrhagic E. coli are important sources of worldwide diarrheal illness. These extracellular pathogens are noted for their ability to disrupt the intestinal epithelial barrier function and trigger the formation of filamentous actin “pedestals” beneath bound bacteria. While attached to the intestinal epithelium, the bacteria use a type III secretion system to deliver on the order of 50 to 60 proteins, including EspG (9), directly into host cells (10). Selyunin and colleagues observed that the heterologous expression of EspG in mammalian cells resulted in the disruption of vesicular trafficking and fragmentation of the Golgi apparatus, the site at which this effector primarily localizes under these conditions. These phenotypes may account for the EspG-dependent mislocalization of plasma membrane anion (11) and aquaporin (12) channel proteins observed during the course of an infection with enteropathogenic E. coli and its close relative, Citrobacter rodentium, respectively.
To gain insights into the molecular mechanism of action of EspG, Selyunin and colleagues conducted a yeast two-hybrid screen, which demonstrated that EspG can interact with members of two distinct protein families, p21-activated kinases (PAKs) and ADP-ribosylating GTPases (ARFs). Interactions with members of each family were confirmed through coimmunoprecipitation assays. PAKs are serine/threonine kinases involved in regulating many cellular processes, including cytoskeletal dynamics, such as cell motility, as well as apoptosis, and cell cycle progression (13). ARFs mediate membrane trafficking events between different cellular components including the Golgi apparatus and plasma membrane (14). These two proteins normally do not interact.
To investigate the consequence of the interactions of EspG with ARF and PAK, the investigators solved the crystal structure of EspG in complex with regions of each of the two proteins. EspG recognizes the Iα3 helix in the PAK N-terminal auto-inhibitory domain, which normally binds to the C-terminal kinase domain to maintain it in an inactive configuration. This inhibition is relieved by conformational changes triggered upon binding of the N-terminal domain by activated Cdc42 or Rac1 GTPase (15). Through a series of elegant biochemical and structural studies, Selyunin and colleagues established that binding of the Iα3 helix by EspG triggers a novel allosteric activation of the downstream kinase domain, resulting in activation of PAK. Concurrent structural studies conducted by Germane and Spiller also revealed that EspG binds to the autoinhibitory domain of PAK (16).
The crystal structure of EspG in complex with ARF6 also revealed insights into the molecular action of EspG. EspG binds GTP, but not GDP-bound ARF. This binding blocks the recruitment of GTPase activating proteins (GAPs) to ARFs, inhibiting the conversion of GTP to GDP that is presumably required for ARF activity. Selyunin et al. hypothesize that this disruption of the ARF GDP/GTP cycle results in the observed EspG-mediated inhibition of Golgi trafficking, a phenotype previously reported with brefeldin A, a fungal toxin that also inhibits the GTPase activity of ARF1 (17).
ARF6 and PAK2 interacted with distinct and nonoverlapping regions of EspG, suggesting that the two proteins could bind simultaneously. Indeed, all three proteins could form a tripartite complex in solution. Furthermore, consistent with the localization of EspG to the Golgi apparatus, the EspG-PAK-ARF complex could also form on Golgi mimetic liposomes (Fig. 1). The assembly of this complex was dependent on ARF, a protein anchored to the Golgi membrane through its N-terminal myristoylation modification. ARF recruited EspG, which in turn recruited PAKs. Thus, the specific positioning of the EspG complex on the Golgi membrane is a function of binding to one of the host cell proteins targeted by this bacterial effector. The localization of ARF and EspG to the Golgi apparatus was sufficient to mediate the observed Golgi fragmentation phenotype, because it did not require PAK localization. The functional relevance of the recruitment of activated PAK to these complexes remains to be determined, but based on the phenotypes associated with activities of other catalytic bacterial scaffolds described below, it is possible that the coordinated regulation of PAK and ARF by EspG at the Golgi may generate a novel cellular process.
Fig. 1.
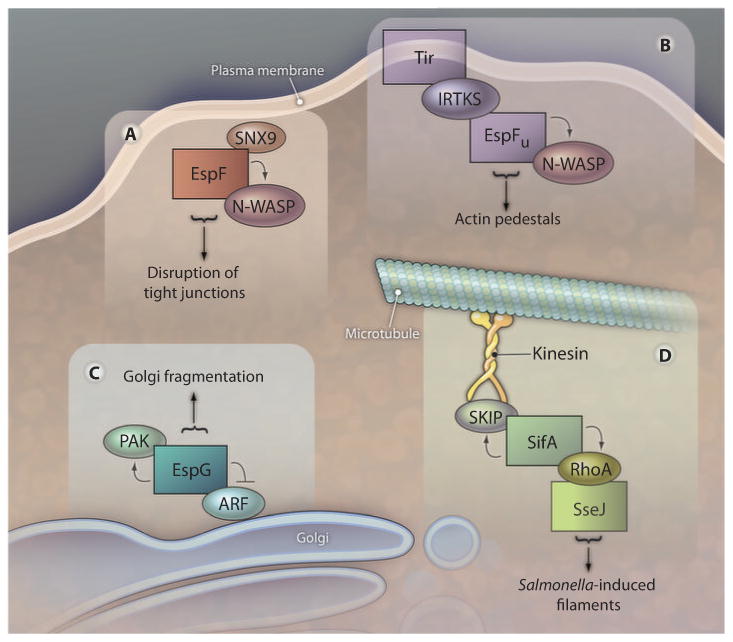
Type III secreted scaffolds nucleate various higher-order complexes that result in novel eukaryotic host cell responses. Interactions with some effectors (represented as boxes) result in activation of host cell proteins (such as PAK, N-WASP, and RhoA), whereas others, such as ARF, are inactivated. The assembled complexes have been implicated in the processes designated below the arrows.
Two other E. coli type III effectors, EspF (6) and EspFU (18, 19), can also serve as molecular scaffolds. These homologous effectors are implicated in the disruption of epithelial tight junctions (20) and the formation of filamentous actin pedestals (21, 22), respectively. Both proteins contain multiple 47-residue repeats that bind and activate N-WASP, a host protein that controls actin assembly (23). Like EspG, each repeat also binds a second host protein: The EspF repeat binds sorting nexin 9 (SNX9), a protein implicated in late-stage vesicle formation (24), whereas the EspFU repeat binds to IRTKS and/or IRSp53, proteins that appear to link membrane deformation to actin remodeling (25) (Fig. 1). Reflecting an additional layer of complexity, IRTKS and IRSp53 recognize a second E. coli type III secreted effector, Tir (26). Given that Tir is a transmembrane effector that is concentrated at sites of bacterial attachment, IRTKS or IRSp53 (or both) thus mediate the recruitment of the ternary complex to the precise location where actin polymerization is to be triggered. Thus, bacterial scaffolds have the capacity to mediate the formation and specific localization of complexes with novel function.
Studies with Salmonella effectors provide a more developed understanding of how a bacterial catalytic scaffold can mediate the formation of higher-order complexes to reengineer the basic wiring of eukaryotic host cells. Salmonella, in contrast to enteropathogenic and enterohemorrhagic E. coli, are facultative intracellular pathogens. Salmonella uses two type III secretion systems during the course of an infection. The first type III secretion system (SPI1) mediates the uptake of these bacteria into normally nonphagocytic epithelial cells, where they reside in phagososomes. The second type III secretion system (SPI2) acts to remodel and maintain the integrity of these Salmonella-containing vacuoles (SCVs). During the course of the infection, the SCVs fuse with endosomal compartments and form Salmonella-induced filaments. Heterologous expression of two SPI2 effectors, SifA and SseJ, is sufficient to mediate the formation of Salmonella-induced filaments, even in the absence of infection (7).
SifA, a member of a growing family of bacterial RhoGEFs (27), is a catalytic scaffold (7). The SifA C terminus, which encodes the RhoGEF, binds and activates RhoA. In turn, RhoA presumably recruits SseJ, a glycerophospholipid acetyltransferase (28) that is activated specifically when bound to RhoA-GTP (29). SseJ modifies the cholesterol content of the SCV, presumably to promote the formation of Salmonella-induced filaments (30). Meanwhile, the N-terminal domain of SifA binds SKIP (SifA and kinesin interacting protein), a protein that simultaneously binds kinesin, a microtubule motor protein (Fig. 1). By bridging interactions between SifA and kinesin, SKIP facilitates the formation and elongation of Salmonella-induced filaments along microtubules (31), demonstrating another way by which bacteria target the establishment of novel higher-order complexes to distinct host subcellular locations.
In summary, a new paradigm for understanding the clever means by which bacteria manipulate eukaryotic host cells is emerging. EspG is the most recent example of bacterial effectors that can act as a scaffold to manipulate eukaryotic host cell proteins. The ability of a single effector, EspG, to simultaneously activate and inhibit the activity of two unrelated eukaryotic proteins demonstrates another ingenious way that bacteria have evolved to usurp eukaryotic host cell processes to their own advantage. Many questions remain regarding the role of EspG in the context of an E. coli infection when it is delivered into host cells along with presumably tens of additional E. coli type III secreted effectors. Nevertheless, the observation that interactions between EspG and PAK result in activation and relocalization of the kinase suggests that the corecruitment and regulation of PAK and ARF to the Golgi apparatus by EspG probably results in a novel activity, which future studies will hopefully reveal.
Acknowledgments
Funding: This work was supported by NIH R01 64285 to C.F.L. and NIH R01 AI46454 to J.M.L.
References and Notes
- 1.Fu Y, Galán JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 2.Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galán JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 3.Rohde JR, Breitkreutz A, Chenal A, Sansonetti PJ, Parsot C. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe. 2007;1:77–83. doi: 10.1016/j.chom.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 6.Alto NM, Weflen AW, Rardin MJ, Yarar D, Lazar CS, Tonikian R, Koller A, Taylor SS, Boone C, Sidhu SS, Schmid SL, Hecht GA, Dixon JE. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–1278. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohlson MB, Huang Z, Alto NM, Blanc MP, Dixon JE, Chai J, Miller SI. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe. 2008;4:434–446. doi: 10.1016/j.chom.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selyunin AS, Sutton SE, Weigele BA, Reddick LE, Orchard RC, Bresson SM, Tomchick DR, Alto NM. The assembly of a GTPasekinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–111. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott SJ, Krejany EO, Mellies JL, Robins-Browne RM, Sasakawa C, Kaper JB. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect Immun. 2001;69:4027–4033. doi: 10.1128/IAI.69.6.4027-4033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobe T, Beatson SA, Taniguchi H, Abe H, Bailey CM, Fivian A, Younis R, Matthews S, Marches O, Frankel G, Hayashi T, Pallen MJ. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A. 2006;103:14941–14946. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttman JA, Samji FN, Li Y, Deng W, Lin A, Finlay BB. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol. 2007;9:131–141. doi: 10.1111/j.1462-5822.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 13.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 15.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 16.Germane KL, Spiller BW. Structural and functional studies indicate that the EPEC effector, EspG, directly binds p21-activated kinase. Biochemistry. 2011;50:917–919. doi: 10.1021/bi1020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chardin P, McCormick F. Brefeldin A: The advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 18.Vingadassalom D, Kazlauskas A, Skehan B, Cheng HC, Magoun L, Robbins D, Rosen MK, Saksela K, Leong JM. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc Natl Acad Sci U S A. 2009;106:6754–6759. doi: 10.1073/pnas.0809131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss SM, Ladwein M, Schmidt D, Ehinger J, Lommel S, Städing K, Beutling U, Disanza A, Frank R, Jänsch L, Scita G, Gunzer F, Rottner K, Stradal TE. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe. 2009;5:244–258. doi: 10.1016/j.chom.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Elliott SJ, O'Connell CB, Koutsouris A, Brinkley C, Donnenberg MS, Hecht G, Kaper JB. A gene from the locus of enterocyte effacement that is required for enteropathogenic Escherichia coli to increase tight-junction permeability encodes a chaperone for EspF. Infect Immun. 2002;70:2271–2277. doi: 10.1128/IAI.70.5.2271-2277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garmendia J, Carlier MF, Egile C, Didry D, Frankel G. Characterization of TccP-mediated N-WASP activation during enterohaemorrhagic Escherichia coli infection. Cell Microbiol. 2006;8:1444–1455. doi: 10.1111/j.1462-5822.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 22.Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 24.Lundmark R, Carlsson SR. Sorting nexin 9 participates in clathrin-mediated endocytosis through interactions with the core components. J Biol Chem. 2003;278:46772–46781. doi: 10.1074/jbc.M307334200. [DOI] [PubMed] [Google Scholar]
- 25.Millard TH, Dawson J, Machesky LM. Characterisation of IRTKS, a novel IRSp53/MIM family actin regulator with distinct filament bundling properties. J Cell Sci. 2007;120:1663–1672. doi: 10.1242/jcs.001776. [DOI] [PubMed] [Google Scholar]
- 26.de Groot JC, Schluter K, Carius Y, Quedenau C, Vingadassalom D, Faix J, Weiss SM, Reichelt J, Standfus-Gabisch C, Lesser CF, Leong JM, Heinz DW, Bussow K, Stradal TE. Structural basis for complex formation between human IRSp53 and the translocated intimin receptor Tir of enterohemorrhagic E. coli. Structure. doi: 10.1016/j.str.2011.06.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Sutton SE, Wallenfang AJ, Orchard RC, Wu X, Feng Y, Chai J, Alto NM. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat Struct Mol Biol. 2009;16:853–860. doi: 10.1038/nsmb.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlson MB, Fluhr K, Birmingham CL, Brumell JH, Miller SI. SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice. Infect Immun. 2005;73:6249–6259. doi: 10.1128/IAI.73.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christen M, Coye LH, Hontz JS, LaRock DL, Pfuetzner RA, Megha SI, Miller SI. Activation of a bacterial virulence protein by the GTPase RhoA. Sci Signal. 2009;2:ra71. doi: 10.1126/scisignal.2000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder N, Mota LJ, Méresse S. Salmonella-in.duced tubular networks. Trends Microbiol. 2011;19:268–277. doi: 10.1016/j.tim.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Boucrot E, Henry T, Borg JP, Gorvel JP, Méresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–1178. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]


